Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers
Abstract
1. Introduction
2. Materials and Methods
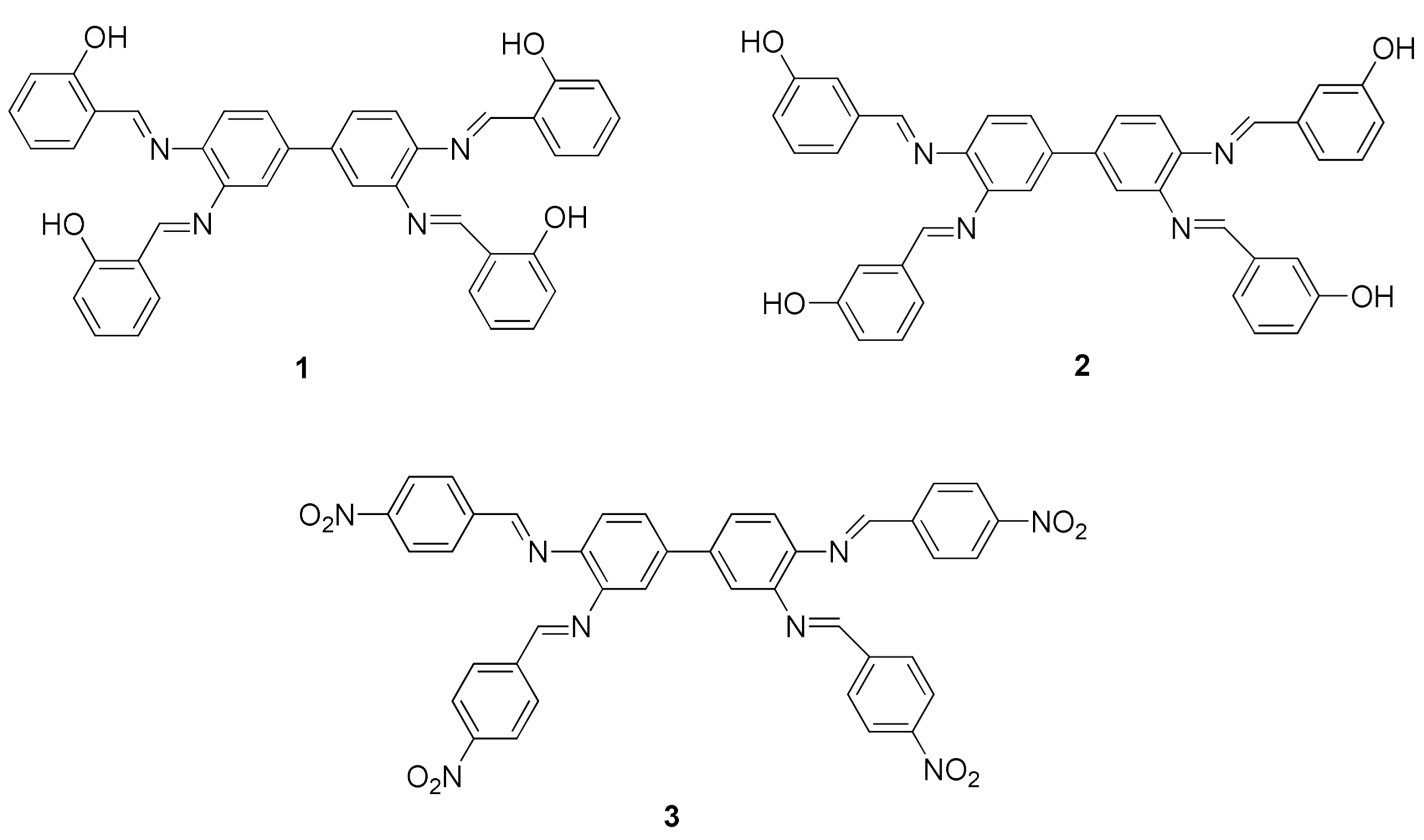
2.1. Synthesis of Tetra Schiff Bases 1–3
2.2. Preparation of PVC Films
2.3. Thermal Stability Test of PVC Films
2.3.1. Thermal Aging Test
2.3.2. Congo Red Test
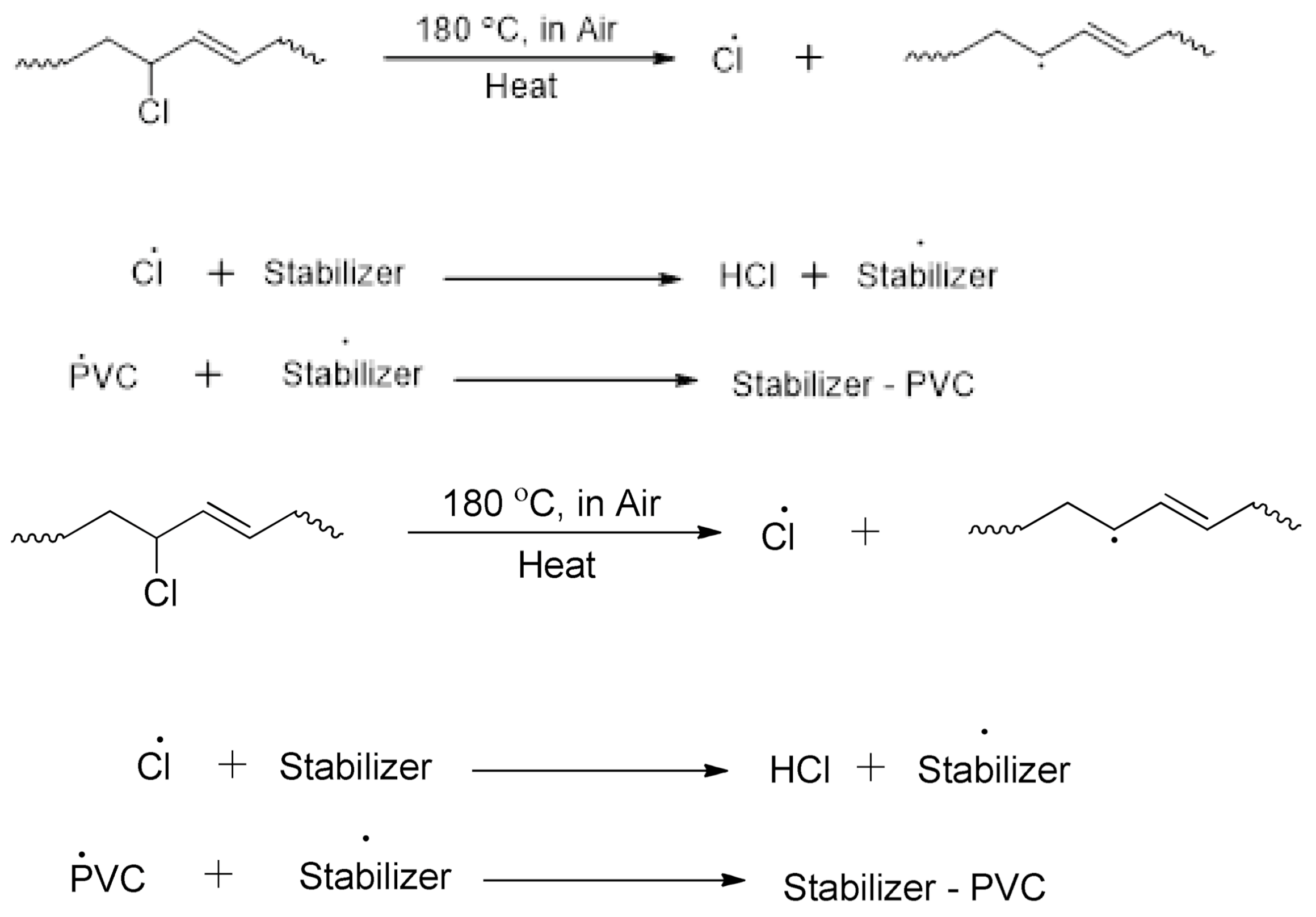
3. Results and Discussion
3.1. Synthesis of the Tetra Schiff Bases
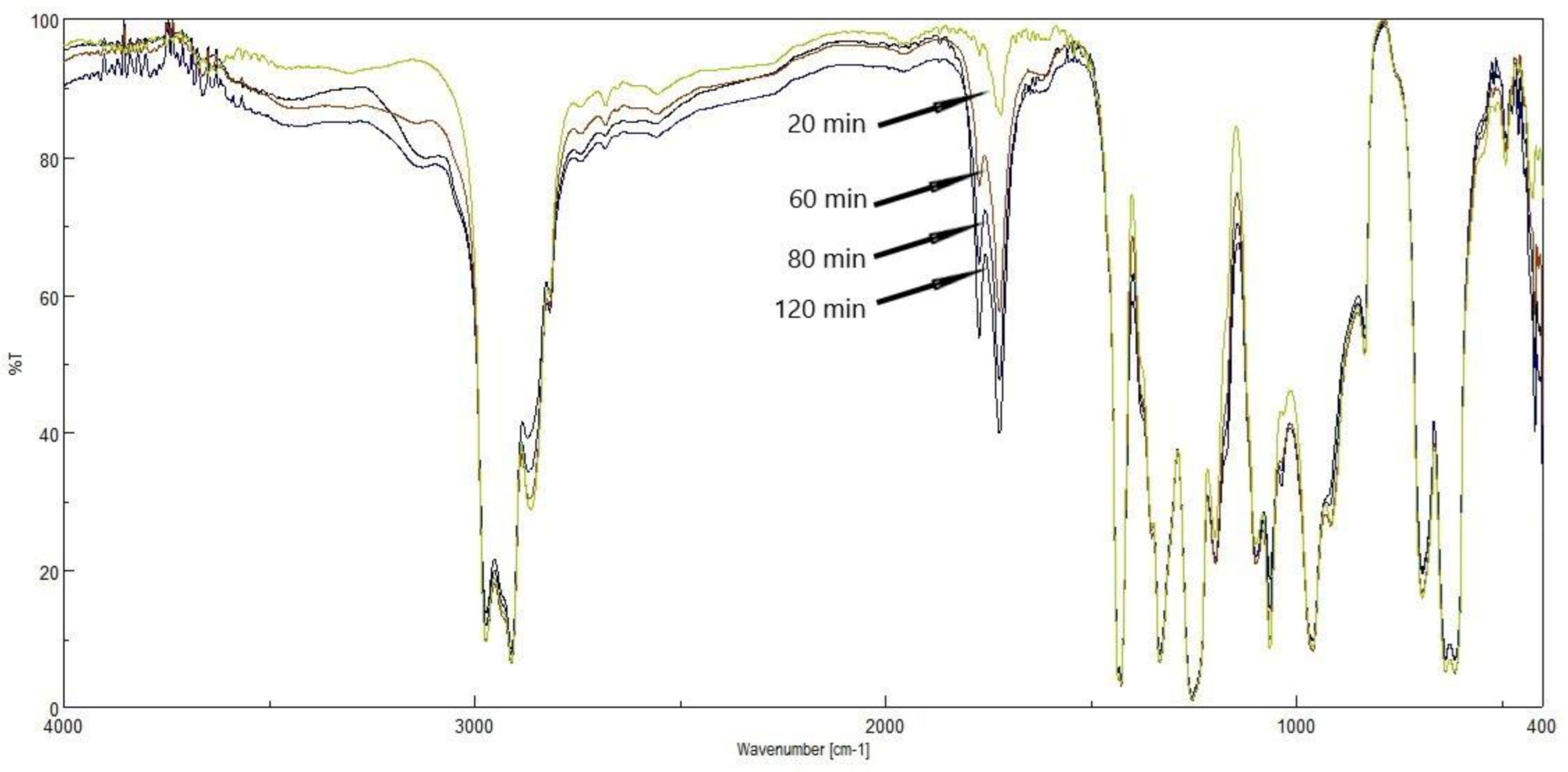
3.2. The FTIR Results of PVC Films
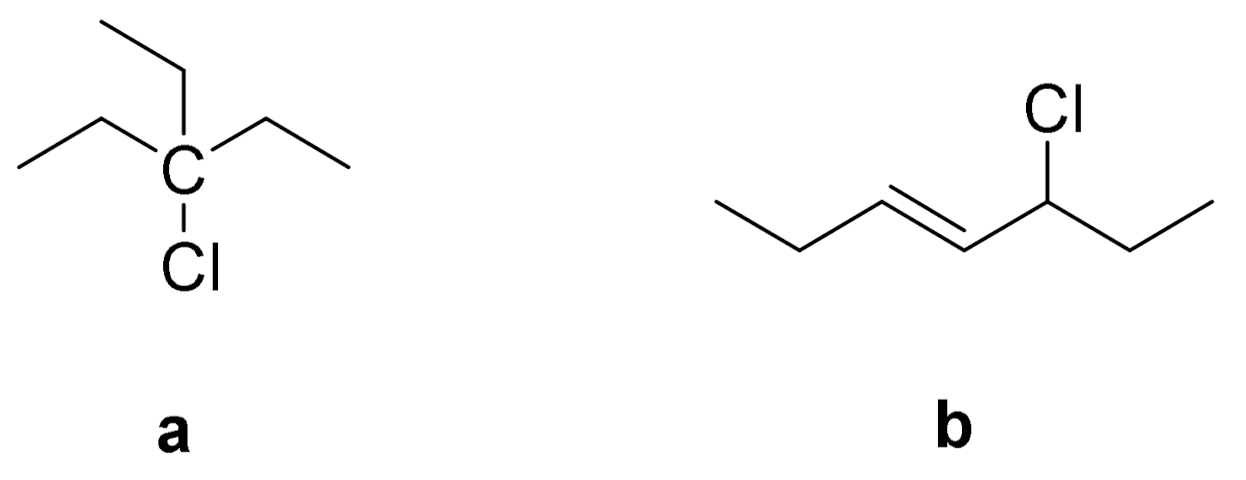
3.3. Mass Loss
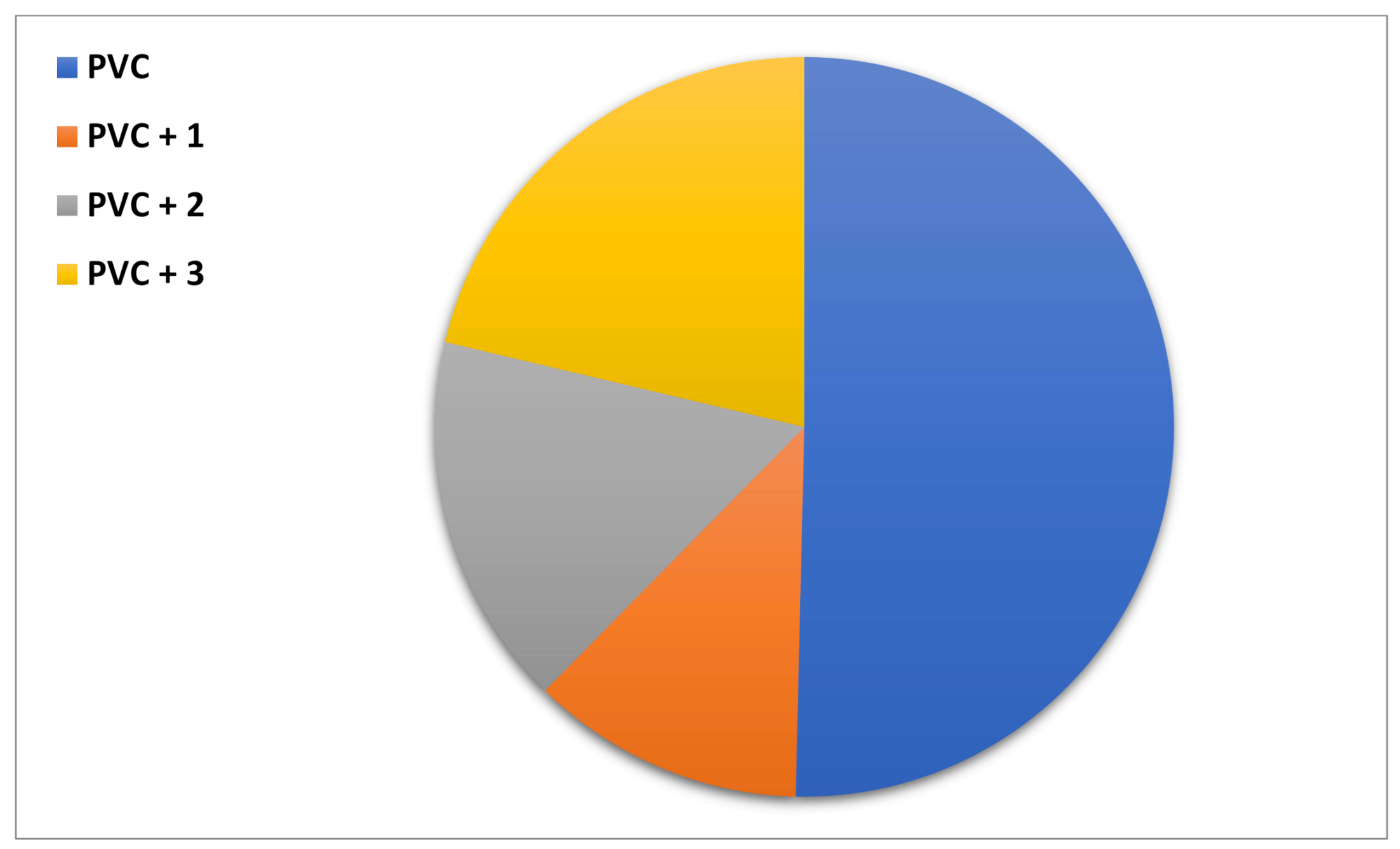
3.4. Congo Red Test
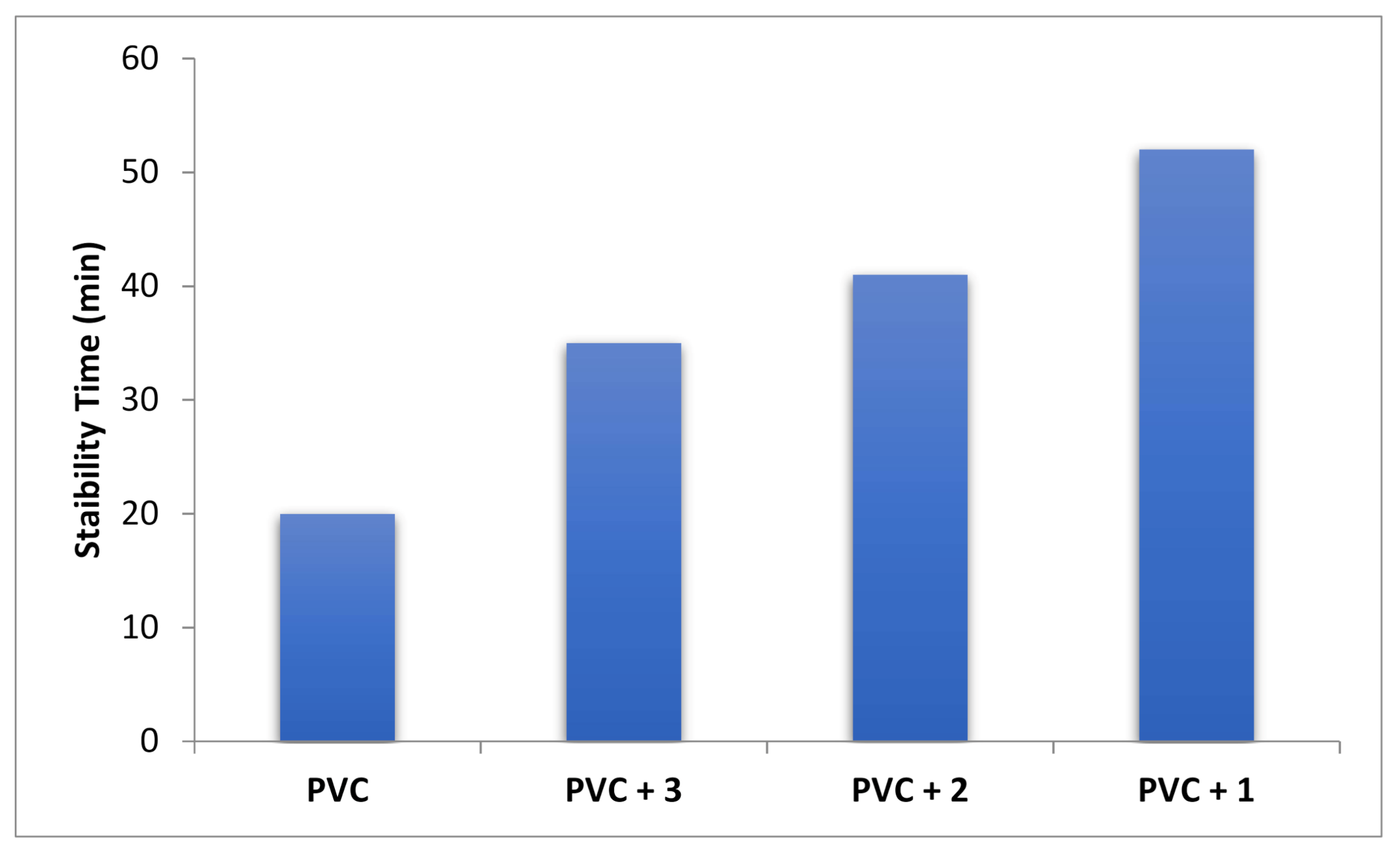
3.5. Oven-Aging Test
3.6. Mechanism of Improving PVC’s Thermal Stability by Additives (Scale of 100 µm)
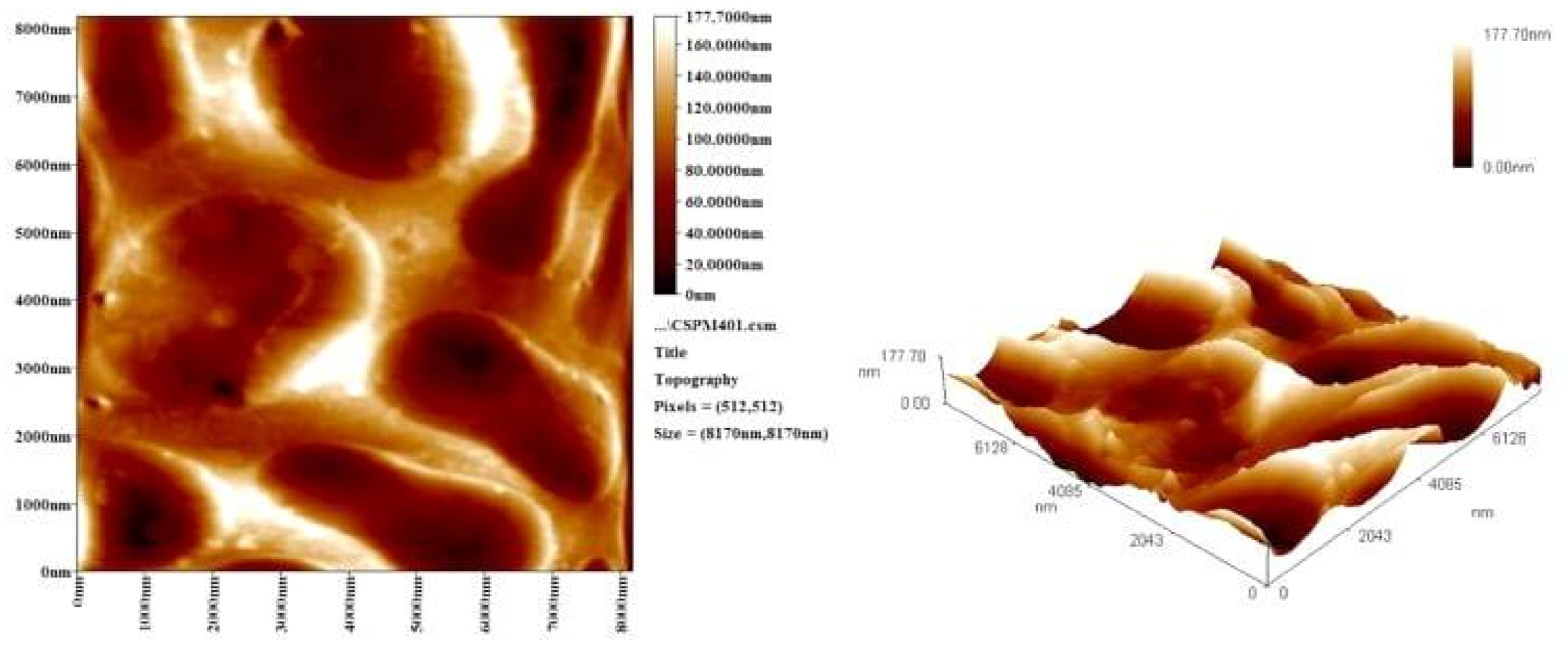
3.7. Atomic Force Microscope (AFM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Correa, C.A.; de Santi, C.R.; Leclerc, A. Green-PVC with full recycled industrial waste and renewably sourced content. J. Clean. Prod. 2019, 229, 1397–1411. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural Defects in Poly(vinyl chloride). J. Polym. Sci. Pol. Chem. 2005, 43, 2451–2467. [Google Scholar] [CrossRef]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Arkıs, E.; Balköse, D. Thermal stabilization of poly(vinyl chloride) by organotin compounds. Polym. Degrad. Stab. 2005, 88, 46–51. [Google Scholar] [CrossRef]
- Kalouskova, R.; Novotna, M.; Vymazal, Z. Investigation of thermal stabilization of poly(vinyl chloride) by lead stearate and its combination with synthetic hy- drotalcite. Polym. Degrad. Stab. 2004, 85, 903–909. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Wu, H.; Guo, S. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 2101–2109. [Google Scholar] [CrossRef]
- Wang, M.; Song, X.; Jiang, J.; Xia, J.; Li, S.; Li, M. Excellent hydroxyl and nitro- gen rich groups-containing tung-oil-based Ca/Zn and polyol stabilizers for enhanced thermal stability of PVC. Thermochim. Acta 2017, 658, 84–92. [Google Scholar] [CrossRef]
- Dong, T.; Li, D.; Li, Y.; Han, W.; Zhang, L.; Xie, G.; Sunarso, J.; Liu, S. Design and synthesis of polyol ester-based zinc metal alkoxides as a bi-functional thermal stabilizer for poly(vinyl chloride). Polym. Degrad. Stab. 2019, 159, 125–132. [Google Scholar] [CrossRef]
- Han, W.; Zhang, M.; Li, D.; Dong, T.; Ai, B.; Dou, J.; Sun, H. Design and Synthesis of a New Mannitol Stearate Ester-Based Aluminum Alkoxide as a Novel Tri–Functional Additive for Poly(Vinyl Chloride) and Its Synergistic Effect with Zinc Stearate. Polymers 2019, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New Tetra-Schiff Bases as Efficient Photostabilizers for Poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Huang, K.; Li, S.; Jianga, J.; Xia, J. Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: Preparation and thermal stabilization for PVC. RSC Adv. 2014, 4, 63576–63585. [Google Scholar] [CrossRef]
- Coltro, L.; Pitta, J.B.; Madaleno, E. Performance evaluation of new plasticizers for stretch PVC films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Satar, H.A.; Ahmed, A.A. Influence of Polyphosphates on the Physicochemical Properties of Poly (Vinyl Chloride) after Irradiation with Ultraviolet Light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Sabaa, M.W.; Oraby, E.H.; Naby, A.S.A.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-Term Effect of Ultraviolet Irradiation on Poly(vinyl chloride) Films Containing Naproxen Diorganotin(IV) Complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [PubMed]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of polyvinyl chloride containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [PubMed]


| Film | Degradation Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | 100 | 120 | |
| PVC |  |  | |||||
| PVC + 3 |  |  |  | ||||
| PVC + 2 |  |  |  | 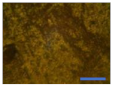 | |||
| PVC + 1 |  | 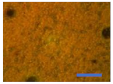 |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, D.S.; Kadhom, M.; Hadi, A.G.; Bufaroosha, M.; Salih, N.; Al-Dahhan, W.H.; Yousif, E. Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers. Chemistry 2021, 3, 288-295. https://doi.org/10.3390/chemistry3010021
Ahmed DS, Kadhom M, Hadi AG, Bufaroosha M, Salih N, Al-Dahhan WH, Yousif E. Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers. Chemistry. 2021; 3(1):288-295. https://doi.org/10.3390/chemistry3010021
Chicago/Turabian StyleAhmed, Dina S., Mohammed Kadhom, Angham G. Hadi, Muna Bufaroosha, Nadia Salih, Wedad H. Al-Dahhan, and Emad Yousif. 2021. "Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers" Chemistry 3, no. 1: 288-295. https://doi.org/10.3390/chemistry3010021
APA StyleAhmed, D. S., Kadhom, M., Hadi, A. G., Bufaroosha, M., Salih, N., Al-Dahhan, W. H., & Yousif, E. (2021). Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers. Chemistry, 3(1), 288-295. https://doi.org/10.3390/chemistry3010021








