Implantable Devices for the Treatment of Breast Cancer
Abstract
:1. Introduction
2. Breast Cancer and Typical Treatments
3. Implantable Device for Breast Cancer Treatment
3.1. Biopolymer-Based Implantable Devices
3.1.1. Biodegradable Implantable Devices
3.1.2. Nonbiodegradable Implantable Devices
3.2. Nanocomposite-Based Implantable Device
4. Challenges of Clinical Translation of Implantable Devices
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.J.; Yazan, L.S.; Abdullah, C.A.C. A Review on Current Nanomaterials and Their Drug Conjugate for Targeted Breast Cancer Treatment. Int. J. Nanomed. 2017, 12, 2373–2384. [Google Scholar] [CrossRef] [Green Version]
- Ghahremani, F.; Shahbazi-Gahrouei, D.; Kefayat, A.; Motaghi, H.; Mehrgardi, M.A.; Javanmard, S.H. AS1411 Aptamer Conjugated Gold Nanoclusters as a Targeted Radiosensitizer for Megavoltage Radiation Therapy of 4T1 Breast Cancer Cells. RSC Adv. 2018, 8, 4249–4258. [Google Scholar] [CrossRef] [Green Version]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-Mediated Bone Loss in Breast Cancer Bone Metastases Is Driven by an Interplay between Oxidative Stress and Induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Guan, X. Cancer Metastases: Challenges and Opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahverdiyev, A.M.; Parlar, E.; Dinparvar, S.; Bagirova, M.; Abamor, E.Ş. Current Aspects in Treatment of Breast Cancer Based of Nanodrug Delivery Systems and Future Prospects. Artif. Cells Nanomed. Biotechnol. 2018, 46, S755–S762. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- The American Association for Cancer Research. Breast Cancer Overview I. Available online: https://www.aacr.org/patients-caregivers/cancer/breast-cancer/ (accessed on 29 January 2022).
- Chew, S.A.; Danti, S. Biomaterial-Based Implantable Devices for Cancer Therapy. Adv. Healthc. Mater. 2017, 6, 1600766. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [Green Version]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum Dots as a Platform for Nanoparticle Drug Delivery Vehicle Design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef] [Green Version]
- Bikiaris, D.; Papageorgiou, G.Z.; Stergiou, A.; Pavlidou, E.; Karavas, E.; Kanaze, F.; Georgarakis, M. Physicochemical Studies on Solid Dispersions of Poorly Water-Soluble Drugs: Evaluation of Capabilities and Limitations of Thermal Analysis Techniques. Thermochim. Acta 2005, 439, 58–67. [Google Scholar] [CrossRef]
- Moorthi, C.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to Overcome Conventional Cancer Chemotherapy Limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar]
- Ranganathan, R.; Madanmohan, S.; Kesavan, A.; Baskar, G.; Krishnamoorthy, Y.R.; Santosham, R.; Ponraju, D.; Rayala, S.K.; Venkatraman, G. Nanomedicine: Towards Development of Patient-Friendly Drug-Delivery Systems for Oncological Applications. Int. J. Nanomed. 2012, 7, 1043–1060. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.M.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-Based Approaches in Anticancer Research. Int. J. Nanomed. 2012, 7, 4391–4408. [Google Scholar]
- Hu, C.M.J.; Zhang, L. Nanoparticle-Based Combination Therapy toward Overcoming Drug Resistance in Cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17. [Google Scholar] [CrossRef] [Green Version]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in Therapeutics: A Focus on Nanoparticles as a Drug Delivery System. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Naz, S.; Shahzad, H.; Ali, A.; Zia, M. Nanomaterials as Nanocarriers: A Critical Assessment Why These Are Multi-Chore Vanquisher in Breast Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 899–916. [Google Scholar] [CrossRef] [Green Version]
- Grodzinski, P.; Silver, M.; Molnar, L.K. Nanotechnology for Cancer Diagnostics: Promises and Challenges. Expert Rev. Mol. Diagn. 2006, 6, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local Triple-Combination Therapy Results in Tumour Regression and Prevents Recurrence in a Colon Cancer Model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef]
- Haley, B.; Frenkel, E. Nanoparticles for Drug Delivery in Cancer Treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Artzi, N. Implantable Hydrogel Embedded Dark-Gold Nanoswitch as a Theranostic Probe to Sense and Overcome Cancer Multidrug Resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E1278–E1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-Responsive Polymers and Their Applications in Drug Delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely Triggerable Drug Delivery Systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.C.; Tao Leonard, S.; Stevenson, C.L.; Beck, J.C.; Chen, G.; Jao, R.M.; Johnson, P.A.; Leonard, J.; Skowronski, R.J. An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J. Control. Release 2001, 75, 1–10. [Google Scholar] [CrossRef]
- Dong, Y.; Chin, S.F.; Blanco, E.; Bey, E.A.; Kabbani, W.; Xie, X.J.; Bornmann, W.G.; Boothman, D.A.; Gao, J. Intratumoral Delivery of β-Lapachone via Polymer Implants for Prostate Cancer Therapy. Clin. Cancer Res. 2009, 15, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirmoradi, F.N.; Jackson, J.K.; Burt, H.M.; Chiao, M. On-Demand Controlled Release of Docetaxel from a Battery-Less MEMS Drug Delivery Device. Lab Chip 2011, 11, 2744–2752. [Google Scholar] [CrossRef]
- Liu, R.; Wolinsky, J.B.; Catalano, P.J.; Chirieac, L.R.; Wagner, A.J.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Paclitaxel-Eluting Polymer Film Reduces Locoregional Recurrence and Improves Survival in a Recurrent Sarcoma Model: A Novel Investigational Therapy. Ann. Surg. Oncol. 2012, 19, 199–206. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Liu, R.; Walpole, J.; Chirieac, L.R.; Colson, Y.L.; Grinstaff Mark, W. Prevention of in Vivo Lung Tumor Growth by Prolonged Local Delivery of Hydroxycamptothecin Using Poly(Ester-Carbonate)-Collagen Composites. J. Control. Release 2010, 144, 280–287. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.K.; Panikar, D.; Nair, S.; et al. Theranostic 3-Dimensional Nano Brain-Implant for Prolonged and Localized Treatment of Recurrent Glioma. Sci. Rep. 2017, 7, 43271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. Neuro-Oncology A Phase 3 Trial of Local Chemotherapy with Biodegradable Carmustine (BCNU) Wafers (Gliadel Wafers) in Patients with Primary Malignant Glioma. Neuro Oncol. 1992, 10, 79–88. [Google Scholar]
- Crook, J.M.; Zhang, P.; Pisansky, T.M.; Trabulsi, E.J.; Amin, M.B.; Bice, W.; Morton, G.; Pervez, N.; Vigneault, E.; Catton, C.; et al. A Prospective Phase 2 Trial of Transperineal Ultrasound-Guided Brachytherapy for Locally Recurrent Prostate Cancer After External Beam Radiation Therapy (NRG Oncology/RTOG-0526). Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, S.; Babazadeh, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y.; Mousazadeh, H. An Implantable Smart Hyperthermia Nanofiber with Switchable, Controlled and Sustained Drug Release: Possible Application in Prevention of Cancer Local Recurrence. Mater. Sci. Eng. C 2021, 118, 111384. [Google Scholar] [CrossRef]
- Franco, Y.L.; Vaidya, T.R.; Ait-Oudhia, S. Anticancer and Cardio-Protective Effects of Liposomal Doxorubicin in the Treatment of Breast Cancer. Breast Cancer Targets Ther. 2018, 10, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, G.R.; Tulumello, D.V.; Kelley, S.O. Targeted Delivery of Doxorubicin to Mitochondria. ACS Chem. Biol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; van der Hage, J.A.; van de Velde, C.J.H. Neoadjuvant Chemotherapy for Operable Breast Cancer. Br. J. Surg. 2007, 94, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in Adjuvant Chemotherapy for Breast Cancer: An Overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef] [Green Version]
- Dhankhar, R.; Vyas, S.P.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K. Advances in Novel Drug Delivery Strategies for Breast Cancer Therapy. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 230–249. [Google Scholar] [CrossRef]
- Drǎgǎnescu, M.; Carmocan, C. Hormone Therapy in Breast Cancer. Chirurgia 2017, 112, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Puhalla, S.; Bhattacharya, S.; Davidson, N.E. Hormonal Therapy in Breast Cancer: A Model Disease for the Personalization of Cancer Care. Mol. Oncol. 2012, 6, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Nounou, M.I.; Elamrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies Supplementary Issue: Targeted Therapies in Breast Cancer Treatment. Breast Cancer Basic Clin. Res. 2015, 9, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Balaji, K.; Subramanian, B.; Yadav, P.; Anu Radha, C.; Ramasubramanian, V. Radiation Therapy for Breast Cancer: Literature Review. Med. Dosim. 2016, 41, 253–257. [Google Scholar] [CrossRef]
- Exner, A.A.; Saidel, G.M. Drug-Eluting Polymer Implants in Cancer Therapy. Expert Opin. Drug Deliv. 2008, 5, 775–788. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Dong, K.; Luo, J.; Zhang, Q.; Cheng, Y. Injectable and Responsively Degradable Hydrogel for Personalized Photothermal Therapy. Biomaterials 2016, 104, 129–137. [Google Scholar] [CrossRef]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour Targeted Delivery of Encapsulated Dextran-Doxorubicin Conjugate Using Chitosan Nanoparticles as Carrier. J. Control. R. 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-Based Nanoparticles for Tumor-Targeted Drug Delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. PH-Responsive Drug Delivery of Chitosan Nanoparticles as Tamoxifen Carriers for Effective Anti-Tumor Activity in Breast Cancer Cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Kefayat, A.; Vaezifar, S. Biodegradable PLGA Implants Containing Doxorubicin-Loaded Chitosan Nanoparticles for Treatment of Breast Tumor-Bearing Mice. Int. J. Biol. Macromol. 2019, 136, 48–56. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Y.; Wang, J.; Chen, H.; Wang, X.; Li, X.; Tan, W.; Tan, Z. 3D Printed Intelligent Scaffold Prevents Recurrence and Distal Metastasis of Breast Cancer. Theranostics 2020, 10, 10652–10664. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-Jet 3D Printed Drug Delivery Implants to Inhibit Growth and Metastasis of Orthotopic Breast Cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef]
- Seib, F.P.; Kaplan, D.L. Doxorubicin-Loaded Silk Films: Drug-Silk Interactions and in Vivo Performance in Human Orthotopic Breast Cancer. Biomaterials 2012, 33, 8442–8450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Q.; Li, Z.; Yang, Y.; Guo, G.; Luo, F.; Chen, Z.; Yang, Y.; Qian, Z.Y.; Shi, S. Preparation and Therapeutic Application of Docetaxel-Loaded Poly(D,L-Lactide) Nanofibers in Preventing Breast Cancer Recurrence. Drug Deliv. 2016, 23, 2677–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gligorov, J.; Lotz, J.P. Preclinical Pharmacology of the Taxanes: Implications of the Differences. Oncologist 2004, 9, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, Y.; Hou, J.; Yang, G.; Zhou, S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small 2020, 16, 1902262. [Google Scholar] [CrossRef]
- Li, X.; Xu, F.; He, Y.; Li, Y.; Hou, J.; Yang, G.; Zhou, S. A Hierarchical Structured Ultrafine Fiber Device for Preventing Postoperative Recurrence and Metastasis of Breast Cancer. Adv. Funct. Mater. 2020, 30, 2004851. [Google Scholar] [CrossRef]
- Lei, N.; Gong, C.; Qian, Z.; Luo, F.; Wang, C.; Wang, H.; Wei, Y. Therapeutic Application of Injectable Thermosensitive Hydrogel in Preventing Local Breast Cancer Recurrence and Improving Incision Wound Healing in a Mouse Model. Nanoscale 2012, 4, 5686–5693. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A.; Ricci, K.; Malkawi, A.; Jahed, K.; Vedantham, K.; Wyan, H.; Allen, L.D.; Dréau, D. A Ceramic-Based Anticancer Drug Delivery System to Treat Breast Cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2701–2710. [Google Scholar] [CrossRef]
- Sasikala, A.R.; Unnithan, A.R.; Thomas, R.G.; Ko, S.W.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifaceted implantable anticancer device for potential postsurgical breast cancer treatment: A single platform for synergistic inhibition of local regional breast cancer recurrence, surveillance, and Healthy Breast Reconstruction. Adv. Funct. Mater. 2017, 28, 1704793. [Google Scholar] [CrossRef]
- Ramachandra Kurup Sasikala, A.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional nanocarpets for cancer theranostics: Remotely controlled graphene nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef]
- Xie, J.; Peng, S.; Brower, N.; Pourmand, N.; Wang, S.X.; Sun, S. One-Pot Synthesis of Monodisperse Iron Oxide Nanoparticles for Potential Biomedical Applications. Proc. Pure Appl. Chem. 2006, 78, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An injectable supramolecular polymer nanocomposite hydrogel for prevention of breast cancer recurrence with Theranostic and mammoplastic functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Scherer, S.D.; Riggio, A.I.; Haroun, F.; DeRose, Y.S.; Ekiz, H.A.; Fujita, M.; Toner, J.; Zhao, L.; Li, Z.; Oesterreich, S.; et al. An Immune-Humanized Patient-Derived Xenograft Model of Estrogen-Independent, Hormone Receptor Positive Metastatic Breast Cancer. Breast Cancer Res. 2021, 23, 100. [Google Scholar] [CrossRef]
- Wilson, B.E.; Gorrini, C.; Cescon, D.W. Breast cancer immune microenvironment: From pre-clinical models to clinical therapies. Breast Cancer Res. Treat. 2021, 191, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, R.; Najafiaghdam, H.; Ghanbari, M.M.; Papageorgiou, E.P.; Zhao, B.; Roschelle, M.; Stojanovic, V.; Muller, R.; Anwar, M. Towards an Implantable Fluorescence Image Sensor for Real-Time Monitoring of Immune Response in Cancer Therapy. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) IEEE, Guadalajara, Mexico, 31 October–4 November 2021; pp. 7399–7403. [Google Scholar]
- Damian, C.; Ghuman, H.; Mauney, C.; Azar, R.; Reinartz, J.; Badylak, S.F.; Modo, M. Post-stroke timing of ECM hydrogel implantation affects biodegradation and tissue restoration. Int. J. Mol. Sci. 2021, 22, 1372. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Carlos-Oliveira, M.; Liu, H.; Mano, J.F.; Bouvy, N.; Moroni, L. 3D printed dual-porosity scaffolds: The combined effect of stiffness and porosity in the modulation of macrophage polarization. Adv. Healthc. Mater. 2021, 11, 2101415. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Obreja, K.; Khoury, C.; Begic, A.; Ramanauskaite, A.; Sculean, A.; Schwarz, F. Influence of Macrophage Polarization on the Effectiveness of Surgical Therapy of Peri-Implantitis. Int. J. Implant. Dent. 2021, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Molina-Peña, R.; Haji Mansor, M.; Najberg, M.; Thomassin, J.-M.; Gueza, B.; Alvarez-Lorenzo, C.; Garcion, E.; Jérôme, C.; Boury, F. Nanoparticle-containing electrospun nanofibrous scaffolds for sustained release of sdf-1α. Int. J. Pharm. 2021, 610, 121205. [Google Scholar] [CrossRef]
- Jeganathan, S.; Budziszewski, E.; Bielecki, P.; Kolios, M.C.; Exner, A.A. In Situ Forming Implants Exposed to Ultrasound Enhance Therapeutic Efficacy in Subcutaneous Murine Tumors. J. Control. Release 2020, 324, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-Y.; Hsieh, C.-H.; Huang, Y.-T.; Chu, S.-Y.; Chen, C.-M.; Lee, W.-J.; Liu, S.-J. Enhanced paclitaxel efficacy to suppress triple-negative breast cancer progression using metronomic chemotherapy with a controlled release system of Electrospun Poly-D-L-Lactide-co-glycolide (PLGA) nanofibers. Cancers 2021, 13, 3350. [Google Scholar] [CrossRef]
- Ménoret, S.; Ouisse, L.H.; Tesson, L.; Remy, S.; Usal, C.; Guiffes, A.; Chenouard, V.; Royer, P.J.; Evanno, G.; Vanhove, B.; et al. In Vivo Analysis of Human Immune Responses in Immunodeficient Rats. Transplantation 2020, 104, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.L.; Jiang, S.; Jang, E.; Niu, L.; Li, L.; Jia, X.; Tong, R. Implantable Optical Fibers for Immunotherapeutics Delivery and Tumor Impedance Measurement. Nat. Comm. 2021, 12, 5138. [Google Scholar] [CrossRef]
- Shafiee, A.; McGovern, J.A.; Lahr, C.A.; Meinert, C.; Moi, D.; Wagner, F.; Landgraf, M.; De-Juan-Pardo, E.; Mazzieri, R.; Hutmacher, D.W. Immune System Augmentation via Humanization Using Stem/Progenitor Cells and Bioengineering in a Breast Cancer Model Study. Int. J. Cancer 2018, 143, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Thibaudeau, L.; Taubenberger, A.V.; Holzapfel, B.M.; Quent, V.M.; Fuehrmann, T.; Hesami, P.; Brown, T.D.; Dalton, P.D.; Power, C.A.; Hollier, B.G.; et al. A Tissue-Engineered Humanized Xenograft Model of Human Breast Cancer Metastasis to Bone. DMM Dis. Models Mech. 2014, 7, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Martine, L.C.; Holzapfel, B.M.; McGovern, J.A.; Wagner, F.; Quent, V.M.; Hesami, P.; Wunner, F.M.; Vaquette, C.; De-Juan-Pardo, E.M.; Brown, T.D.; et al. Engineering a Humanized Bone Organ Model in Mice to Study Bone Metastases. Nat. Protoc. 2017, 12, 639–663. [Google Scholar] [CrossRef]
- Balachander, G.M.; Kotcherlakota, R.; Nayak, B.; Kedaria, D.; Rangarajan, A.; Chatterjee, K. 3D Tumor Models for Breast Cancer: Whither We Are and What We Need. ACS Biomater. Sci. Eng. 2021, 7, 3470–3486. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.J.; Brown, M.E.; Capitini, C.M. GVHD Pathogenesis, Prevention and Treatment: Lessons From Humanized Mouse Transplant Models. Front. Immunol. 2021, 12, 3082. [Google Scholar] [CrossRef] [PubMed]
- Altrock, E.; Sens-Albert, C.; Jann, J.-C.; Flach, J.; Riabov, V.; Schmitt, N.; Xu, Q.; Mehralivand, A.; Hecht, A.; Steiner, L.; et al. Humanized Three-Dimensional Scaffold Xenotransplantation Models for Myelodysplastic Syndromes. Exp. Hematol. 2021, in press. [Google Scholar] [CrossRef]
- Sun, B.L.; Tang, L.; Sun, X.; Garcia, A.N.; Camp, S.M.; Posadas, E.; Cress, A.E.; Garcia, J.G.N. A Humanized Monoclonal Antibody Targeting Extracellular Nicotinamide Phosphoribosyltransferase Prevents Aggressive Prostate Cancer Progression. Pharmaceuticals 2021, 14, 1322. [Google Scholar] [CrossRef]
- Rosato, R.R.; Dávila-González, D.; Choi, D.S.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; Dave, B.; Chang, J.C. Evaluation of anti-pd-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.; Alsharedi, M. Immunotherapy in Triple-Negative Breast Cancer. Med. Oncol. 2018, 35, 13. [Google Scholar] [CrossRef]
- Ohno, Y.; Ohshima, S.; Miyamoto, A.; Kametani, F.; Ito, R.; Tsuda, B.; Kasama, Y.; Nakada, S.; Kashiwagi, H.; Seki, T.; et al. HER2-Antigen-Specific Humoral Immune Response in Breast Cancer Lymphocytes Transplanted in Hu-PBL HIL-4 NOG Mice. Sci. Rep. 2021, 11, 12798. [Google Scholar] [CrossRef]
- Jin, K.T.; Du, W.L.; Lan, H.R.; Liu, Y.Y.; Mao, C.S.; Du, J.L.; Mou, X.Z. Development of Humanized Mouse with Patient-Derived Xenografts for Cancer Immunotherapy Studies: A Comprehensive Review. Cancer Sci. 2021, 112, 2592–2606. [Google Scholar] [CrossRef]
- Wu, G.; Li, L.; Qiu, Y.; Sun, W.; Ren, T.; Lv, Y.; Liu, M.; Wang, X.; Tao, H.; Zhao, L.; et al. A Novel Humanized MUC1 Antibody–Drug Conjugate for the Treatment of Trastuzumab-Resistant Breast Cancer. Acta Biochim. Biophys. Sin. 2021, 53, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Khalighfard, S.; Alizadeh, A.M.; Poorkhani, A.; Motahari, M.; Tahmasebifar, A.; Omranipour, R.; Keshavarz, P.; Haddad, P. Evaluation of the treatment strategies on patient-derived xenograft mice of human breast tumor. Eur. J. Pharmacol. 2020, 889, 173605. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, D.; Cao, B.; Carvajal, R.; Kim, M. PDXGEM: Patient-Derived Tumor Xenograft-Based Gene Expression Model for Predicting Clinical Response to Anticancer Therapy in Cancer Patients. BMC Bioinform. 2020, 21, 288. [Google Scholar] [CrossRef]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of Immune Responses to Anti-PD-1 Mono and Combination Immunotherapy in Hematopoietic Humanized Mice Implanted with Tumor Xenografts. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, T.E.; Suominen, M.I.; Mäki-Jouppila, J.H.; Halleen, J.M.; Tanaka, A.; Seiler, M.; Bernoulli, J. Human immune system increases breast cancer-induced osteoblastic bone growth in a humanized mouse model without affecting normal bone. J. Immunol. Res. 2019, 2019, 4260987. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Zhang, X.; Kong, D.; Kong, J.; Zhao, D.; Guo, Y.; Sun, L.; Chu, L.; Liu, S.; et al. The Anti-B7-H4 Checkpoint Synergizes Trastuzumab Treatment to Promote Phagocytosis and Eradicate Breast Cancer. Neoplasia 2020, 22, 539–553. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Capasso, P.; Sharma, M. The Road to Market Implantable Drug Delivery Systems: A Review on US FDA’s Regulatory Framework and Quality Control Requirements. Pharm. Dev. Technol. 2018, 23, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Marchand, H.C.; Rose, B.J.; Fine, A.M.; Kremzner, M.E. The U.S. Food and Drug Administration: Drug Information Resource for Formulary Recommendations. J. Manag. Care Pharm. 2012, 18, 713–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDRH Transparency. Overview of Medical Devices and Their Regulatory Pathways. Available online: https://wayback.archive-it.org/7993/20170404175540/https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm203018.htm (accessed on 30 January 2022).
| Implantable Device | Type of Cancer Treated | Reference |
|---|---|---|
| Osmotic system-based implantable device | Prostate cancer | [30] |
| β-lapachone-containing polymer implants (millirods) | Prostate cancer | [31] |
| Magnetically controlled drug delivery MEMS device | Prostate cancer | [32] |
| Biocompatible poly(glycerol monostearate-co-e-caprolactone) polymer film-loaded PTX | Lewis lung carcinoma | [33] |
| Polymeric delivery platform made up of poly(glycerol monostearate-co-ε-caprolactone) films loaded with HCPT | Lewis lung carcinoma | [34] |
| Polyester nanofibers of the PLGA–PLA–PCL nanofiber implant | Brain gliomas | [35] |
| Smart hyperthermia nanofibrous scaffolds consisting of the N-isopropylacrylamide and N-hydroxymethylacrylamide polymers | Skin cancer | [38] |
| Implantable Devices | Notable Features | Reference |
|---|---|---|
| PLGA incorporated with CS-DOX | Inhibit 4T1 breast tumor growth and metastasis, thus increasing the survival rate from 60 days to 115 days | [55] |
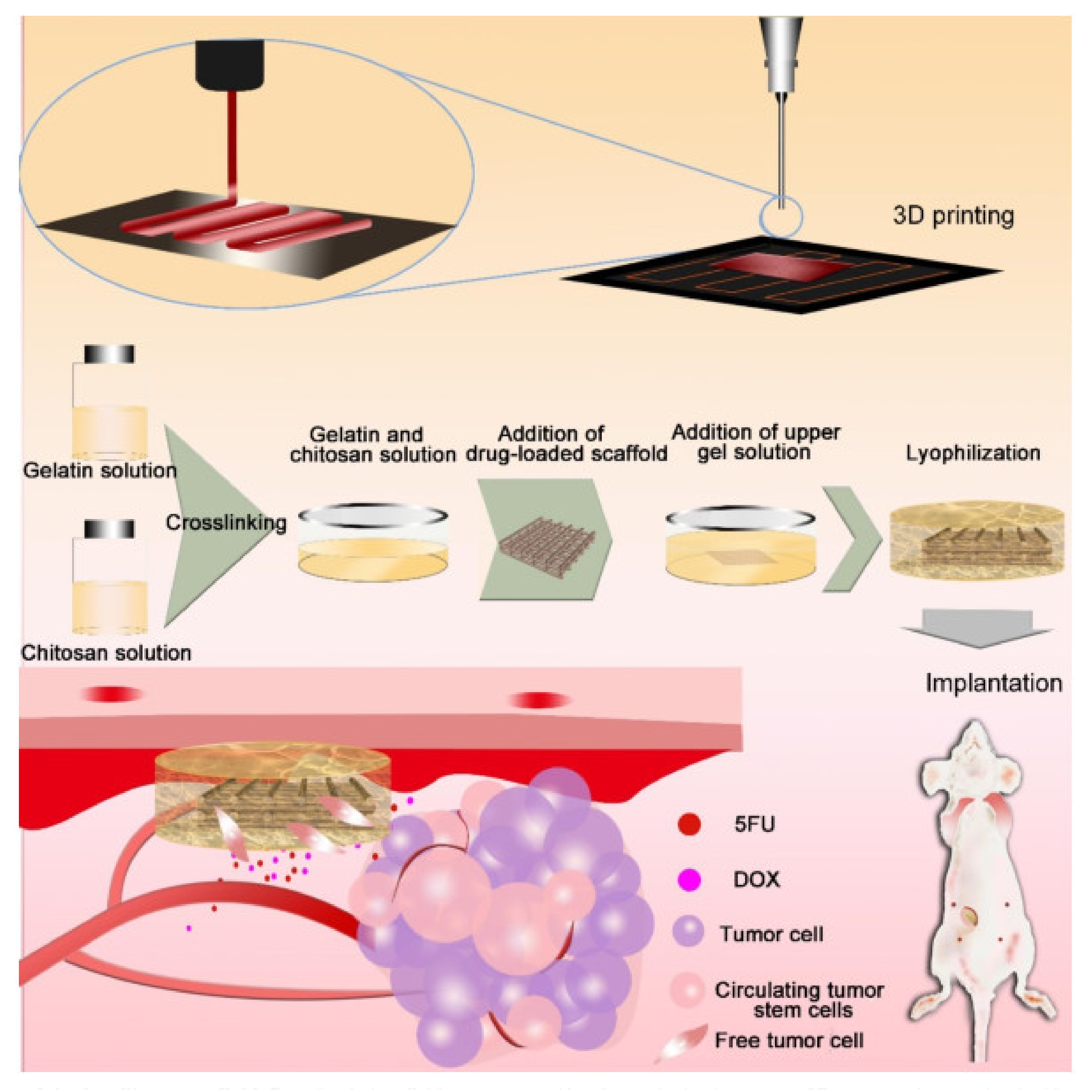
| Intelligent 3D PLGA, gelatin, and chitosan scaffold loaded with 5-FU and DOX | Good blood-clotting ability helps with wound healing, and no damage was observed in the liver, spleen, and kidney tissues | [56] |
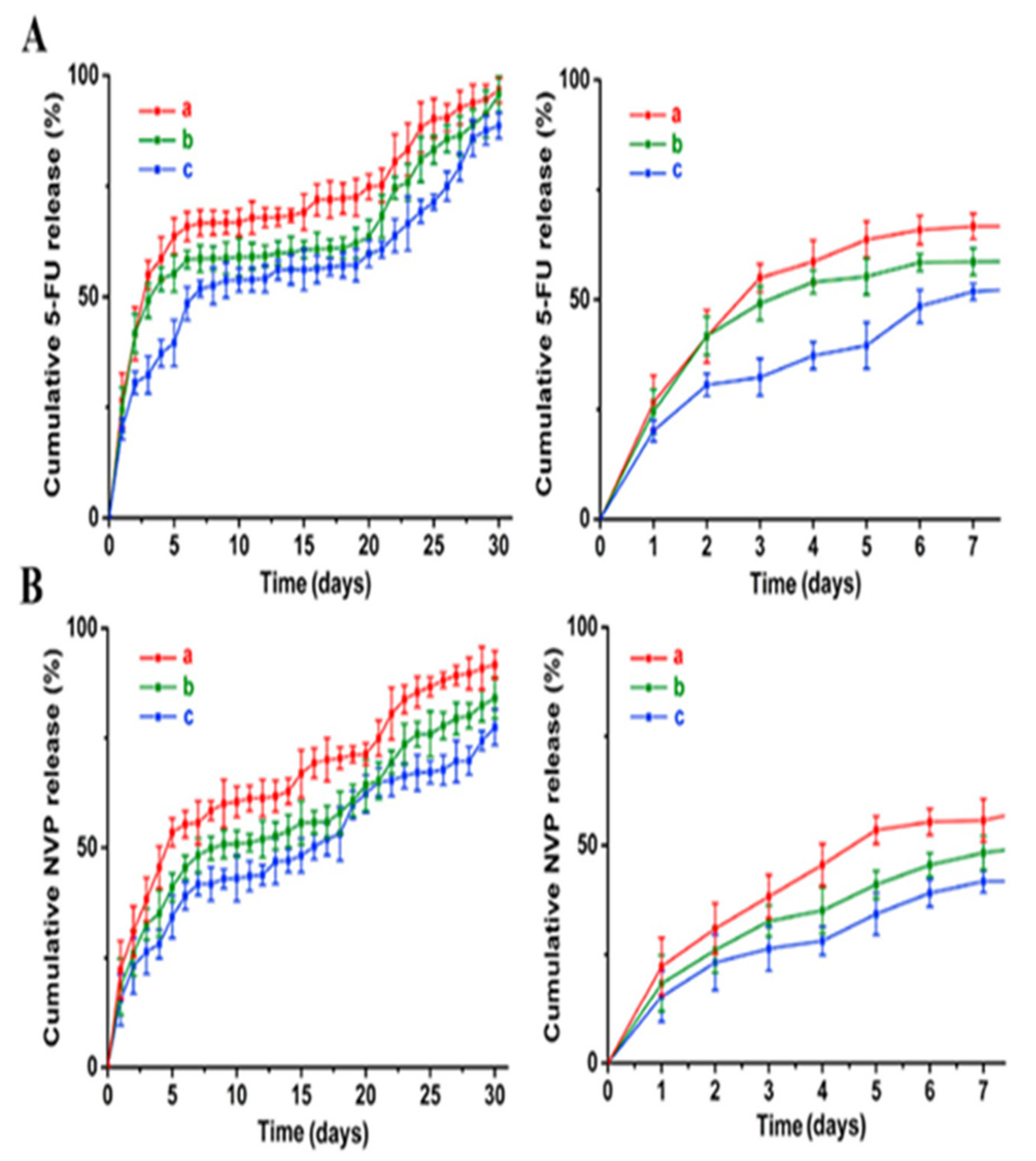
| Scaffolds were made up of PLGA and immobilized with 5-FU and NVP-BEZ235 | Decrease cancer cell viability to less than 30% after 7 days and tumor growth after 4 weeks | [57] |
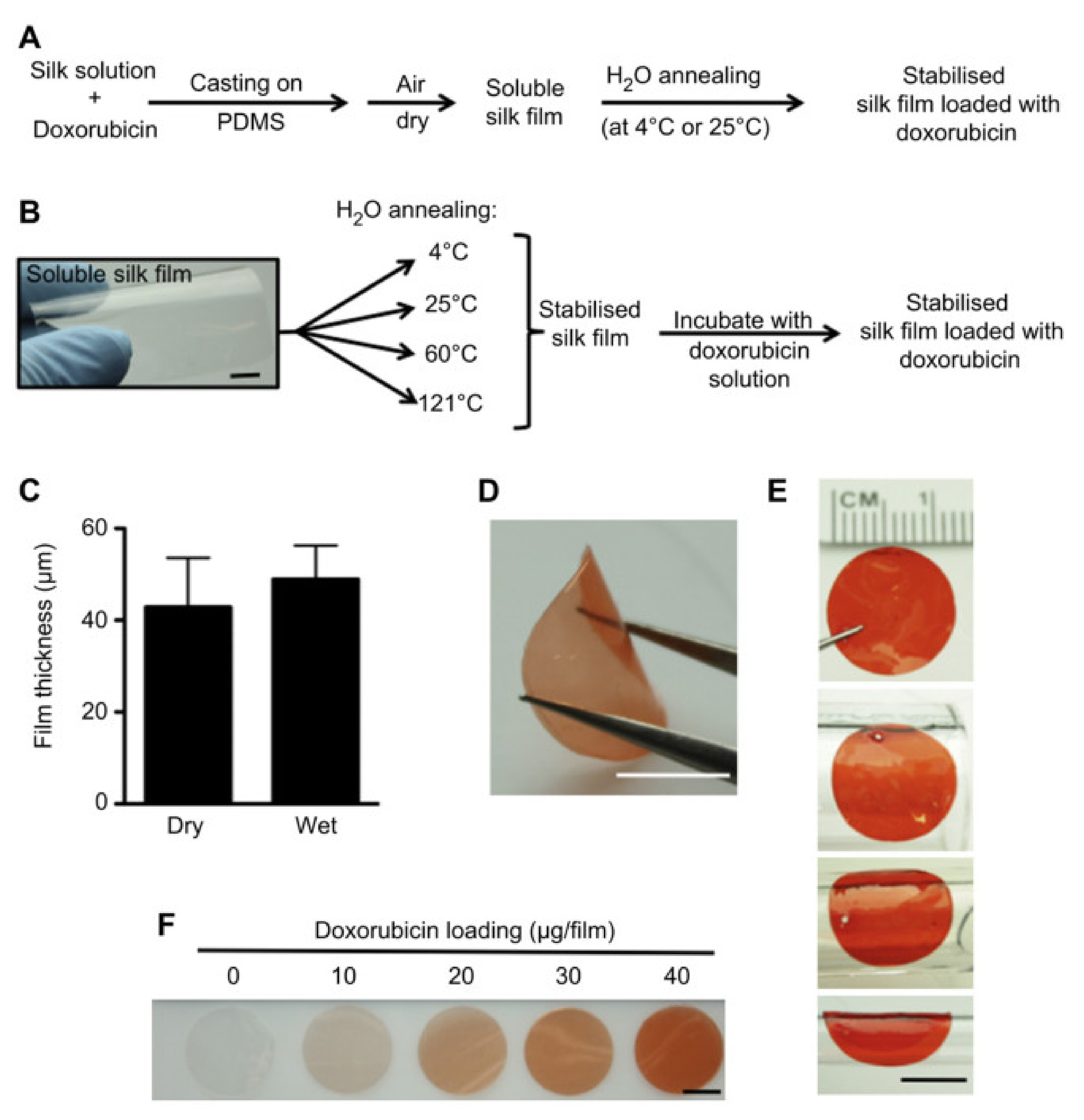
| DOX-loaded silk films | MDA-MB-231 viability and tumor weight significantly decreased | [58] |
| DTX-loaded PDLLA nanofibers | Prolonged delivery and a sufficient local cytotoxic drug preventing local tumor recurrence | [59] |
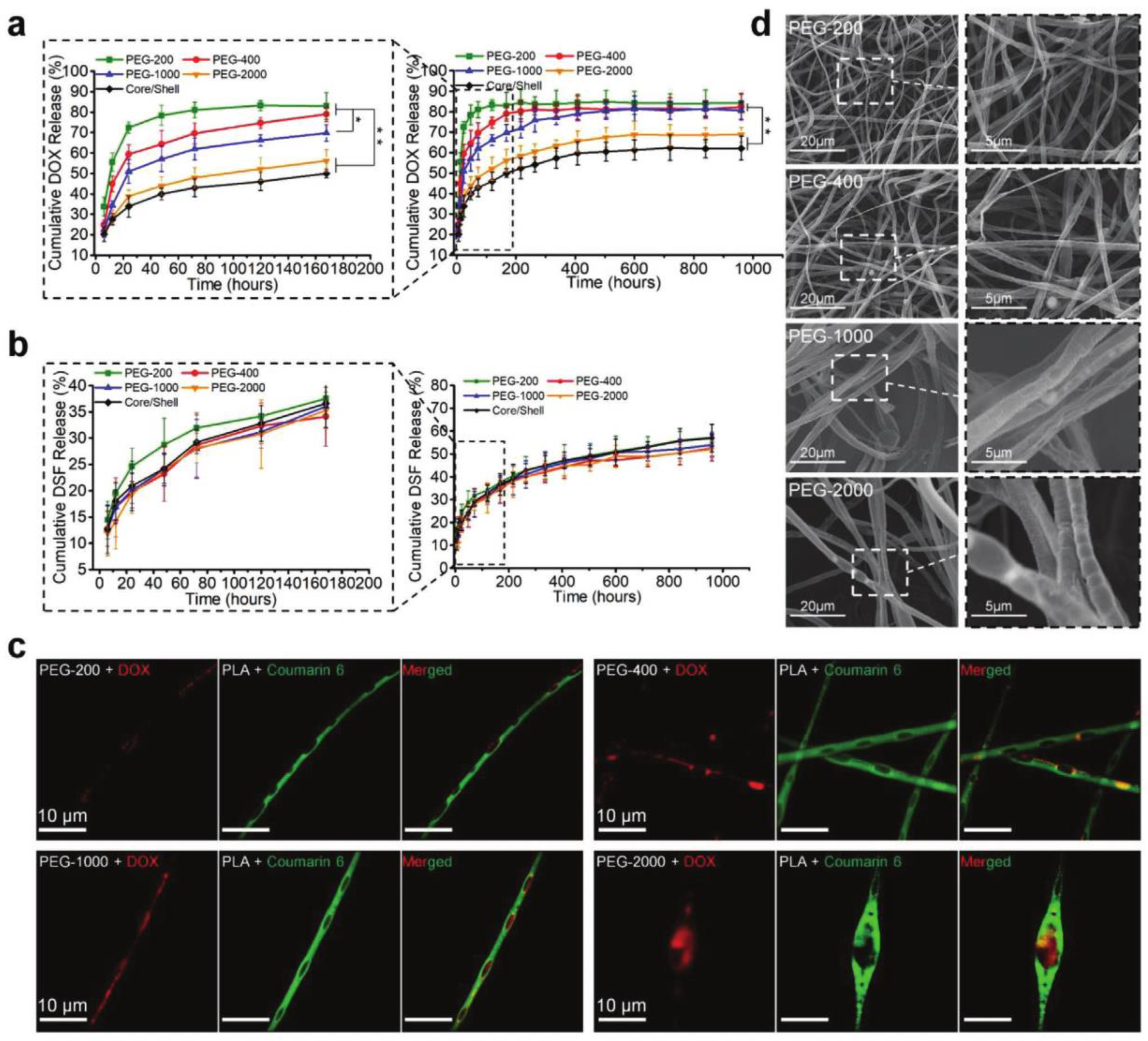
| Hierarchically structured fibers with hydrophilic internal chambers (containing PEG and DOX-HCl) and hydrophobic fiber matrix (containing PLA and DSF) | Tumors in the three mice out of five treated with the implant completely disappeared and in the other mice, they decreased by 80% | [62] |
| Injectable hydrogel based on PEG–PCL–PEG, PECE loaded with PTX | Recurrence rate in vivo was significantly decreased and fast wound healing was observed | [63] |
| SCPC nanocomposite | No significant growth of 4T1 breast tumors was seen after 40 days | [64] |
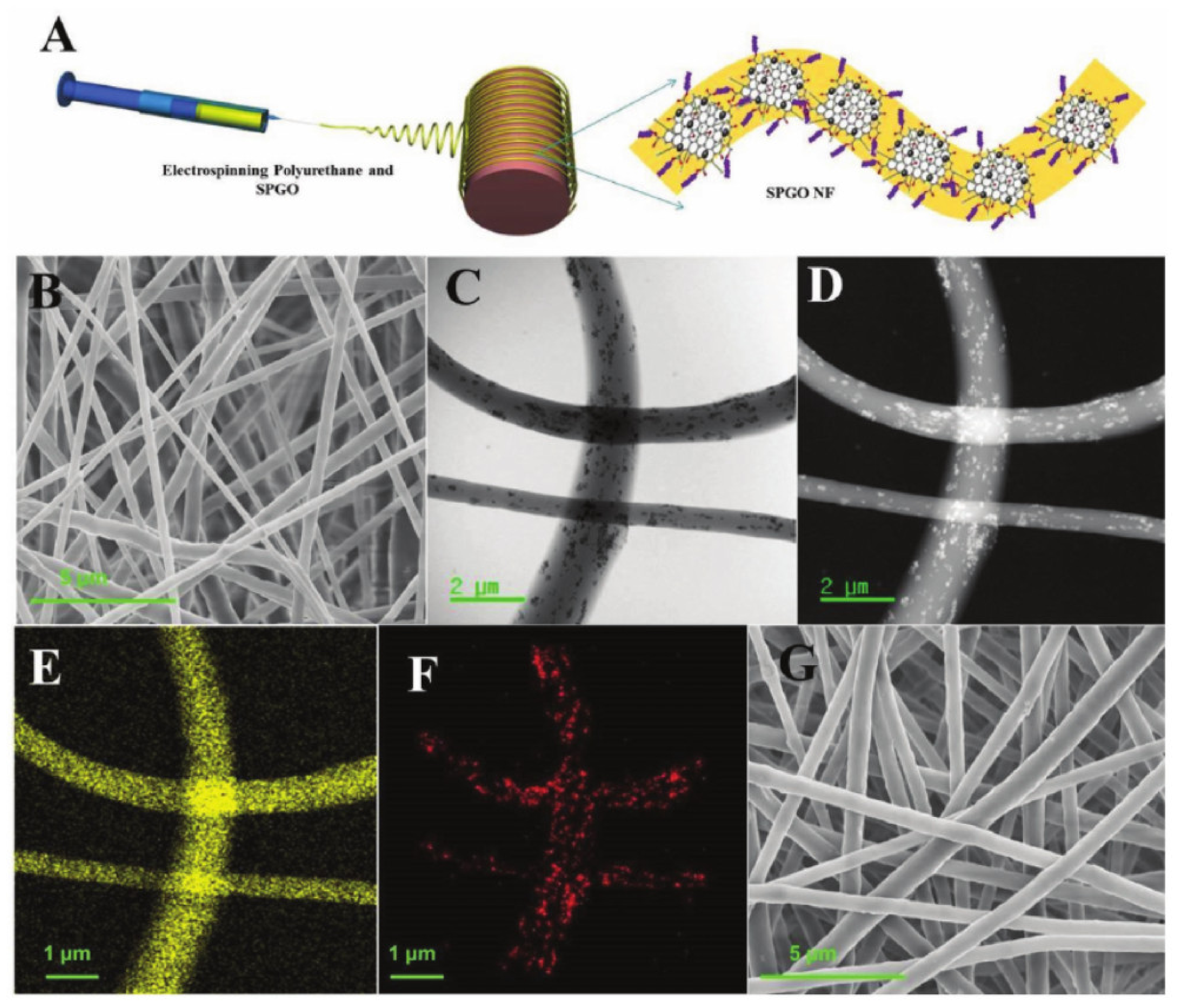
| SPGO nanocomposite incorporated with nanofiber matrix PU and drug DOX | Proliferation percentage of 3T3L1 cell lines decreased from around 60% on day 1 to less than 20% on day 4 | [65] |
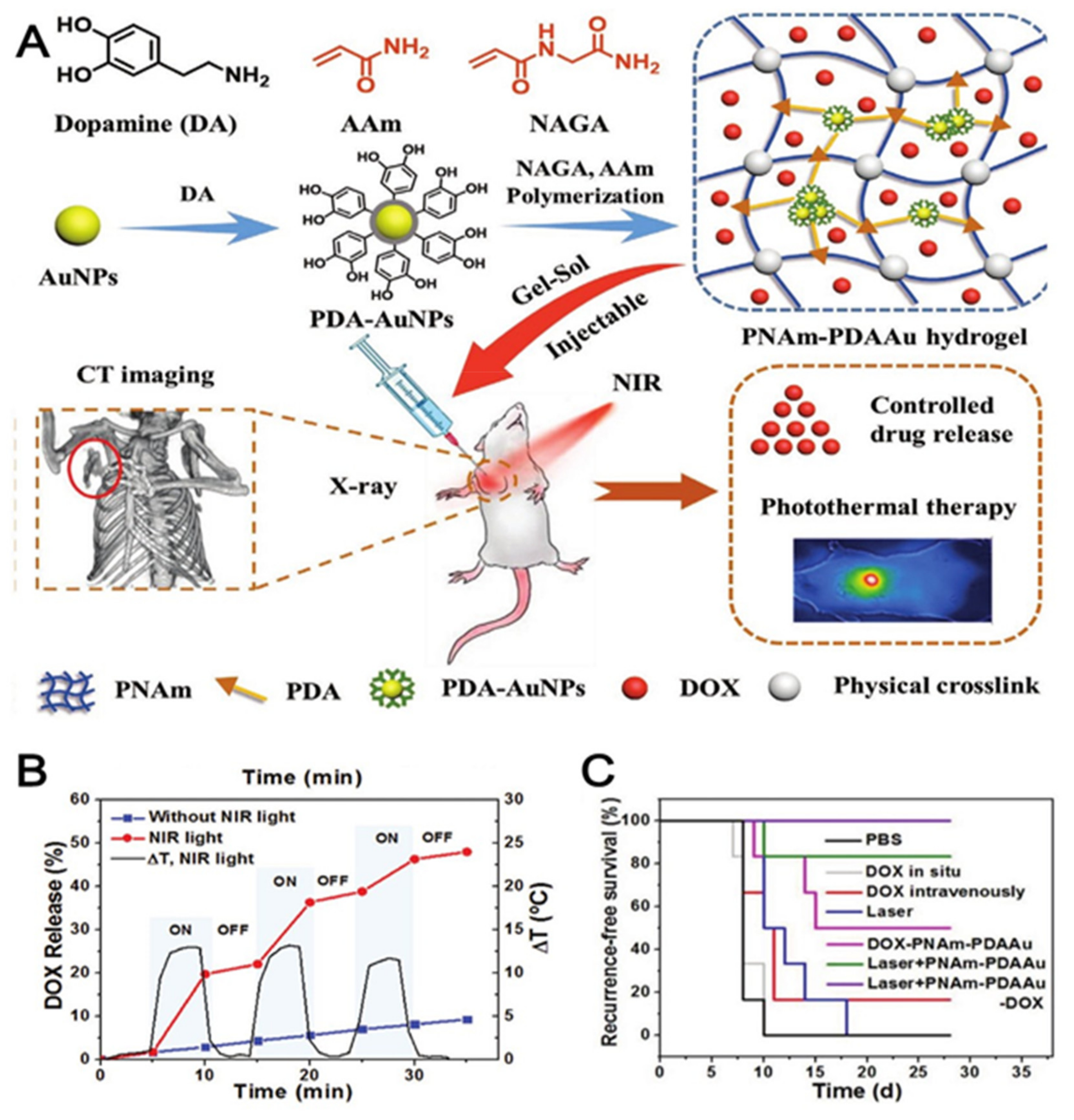
| PNAm hydrogel bearing PDA-coated AuNPs and DOX | No locoregional tumor recurrence was observed and overall survivability rate increased | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pial, M.M.H.; Tomitaka, A.; Pala, N.; Roy, U. Implantable Devices for the Treatment of Breast Cancer. J. Nanotheranostics 2022, 3, 19-38. https://doi.org/10.3390/jnt3010003
Pial MMH, Tomitaka A, Pala N, Roy U. Implantable Devices for the Treatment of Breast Cancer. Journal of Nanotheranostics. 2022; 3(1):19-38. https://doi.org/10.3390/jnt3010003
Chicago/Turabian StylePial, Mohammad Mohtasim Hamid, Asahi Tomitaka, Nezih Pala, and Upal Roy. 2022. "Implantable Devices for the Treatment of Breast Cancer" Journal of Nanotheranostics 3, no. 1: 19-38. https://doi.org/10.3390/jnt3010003
APA StylePial, M. M. H., Tomitaka, A., Pala, N., & Roy, U. (2022). Implantable Devices for the Treatment of Breast Cancer. Journal of Nanotheranostics, 3(1), 19-38. https://doi.org/10.3390/jnt3010003








