- Review
A Comprehensive Review of Engineered Bone Marrow Mesenchymal Stem Cell-Derived Exosomes as Nanotheranostic Platforms for Acute and Chronic Kidney Diseases
- Marcia Bastos Convento and
- Fernanda Teixeira Borges
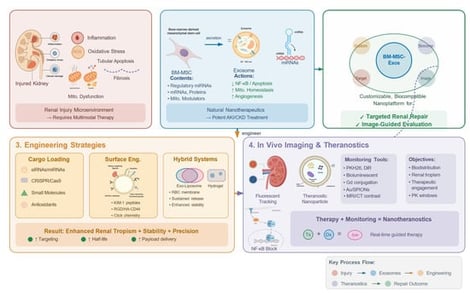
Acute and chronic kidney diseases remain significant challenges in regenerative medicine, with few therapies capable of reversing tissue injury or preventing progression. Bone marrow mesenchymal stem cell-derived exosomes (BM-MSC-Exos) are nanosized vesicles (30–150 nm) that have emerged as multifunctional nanotheranostic platforms, combining targeted therapeutic activity with imaging-enabled monitoring. In renal pathophysiology, BM-MSC-Exos exert anti-inflammatory, anti-fibrotic, angiogenic, and pro-regenerative effects. These actions are mediated by microRNAs, messenger RNAs, mitochondrial regulators, and bioactive proteins that modulate epithelial repair and immune responses. Recent bioengineering advances enable more precise BM-MSC-Exos design, including enrichment with synthetic RNAs or gene-editing components and membrane functionalization to enhance kidney tropism. In parallel, fluorescence, bioluminescence, and nanoparticle-based approaches support in vivo tracking. These tools allow real-time assessment of biodistribution and tubular uptake, strengthening evidence for target engagement. This review synthesizes current knowledge on BM-MSC-Exos in renal repair. We summarize contemporary strategies for cargo and surface engineering, outline imaging methodologies for in vivo tracking, and discuss how administration routes influence renal targeting. We also provide an updated overview of clinical trials evaluating exosomes as therapeutic agents or biomarkers in nephrology. Collectively, engineered BM-MSC-Exos represent a promising and increasingly sophisticated platform for precision-guided kidney therapy, supported by monitoring tools that facilitate preclinical evaluation of biodistribution and efficacy.
13 February 2026