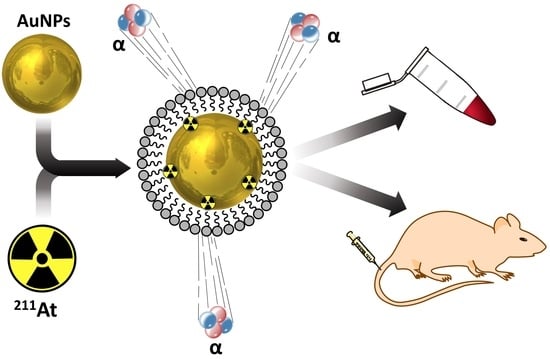
Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
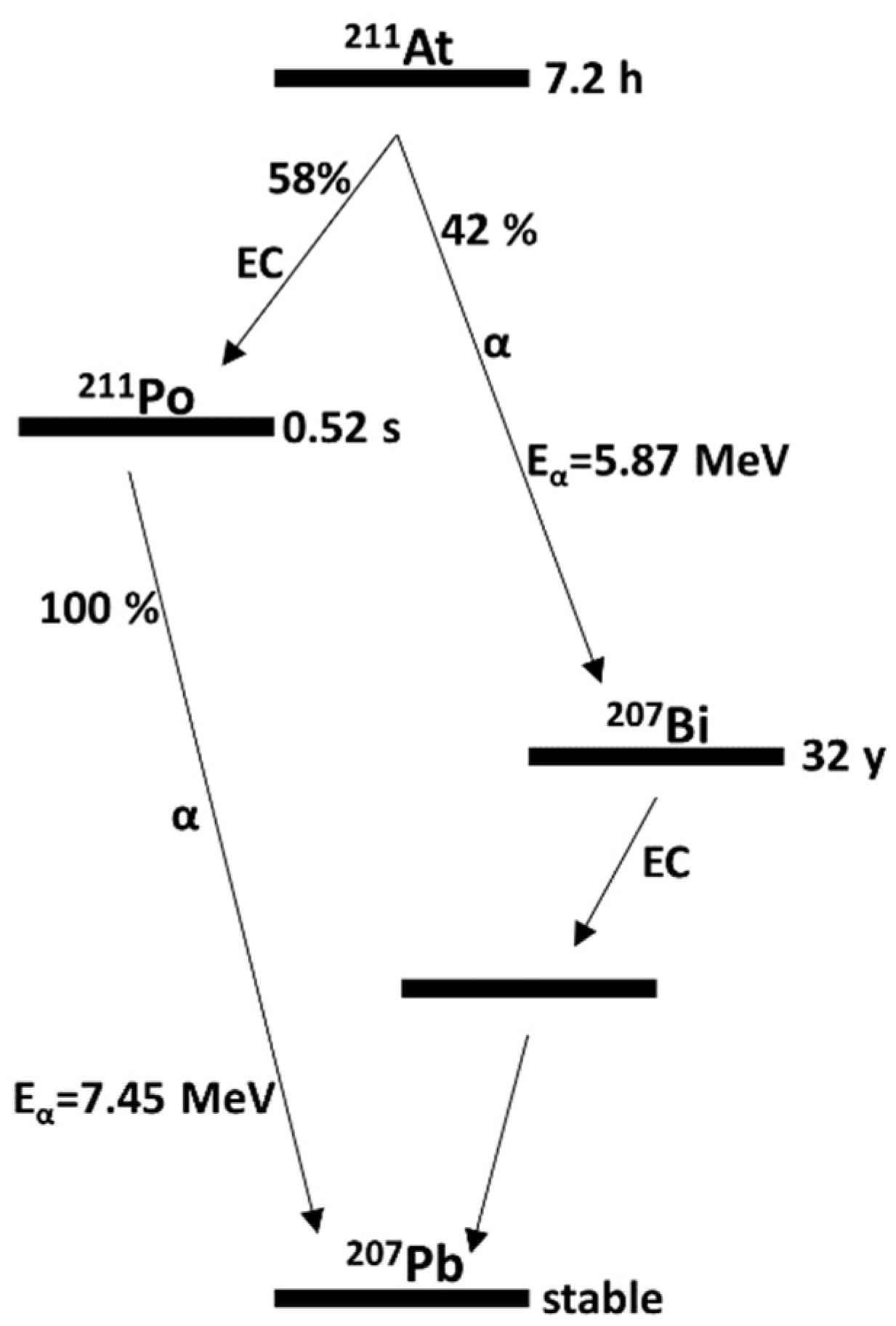
2.2. Production of 211At
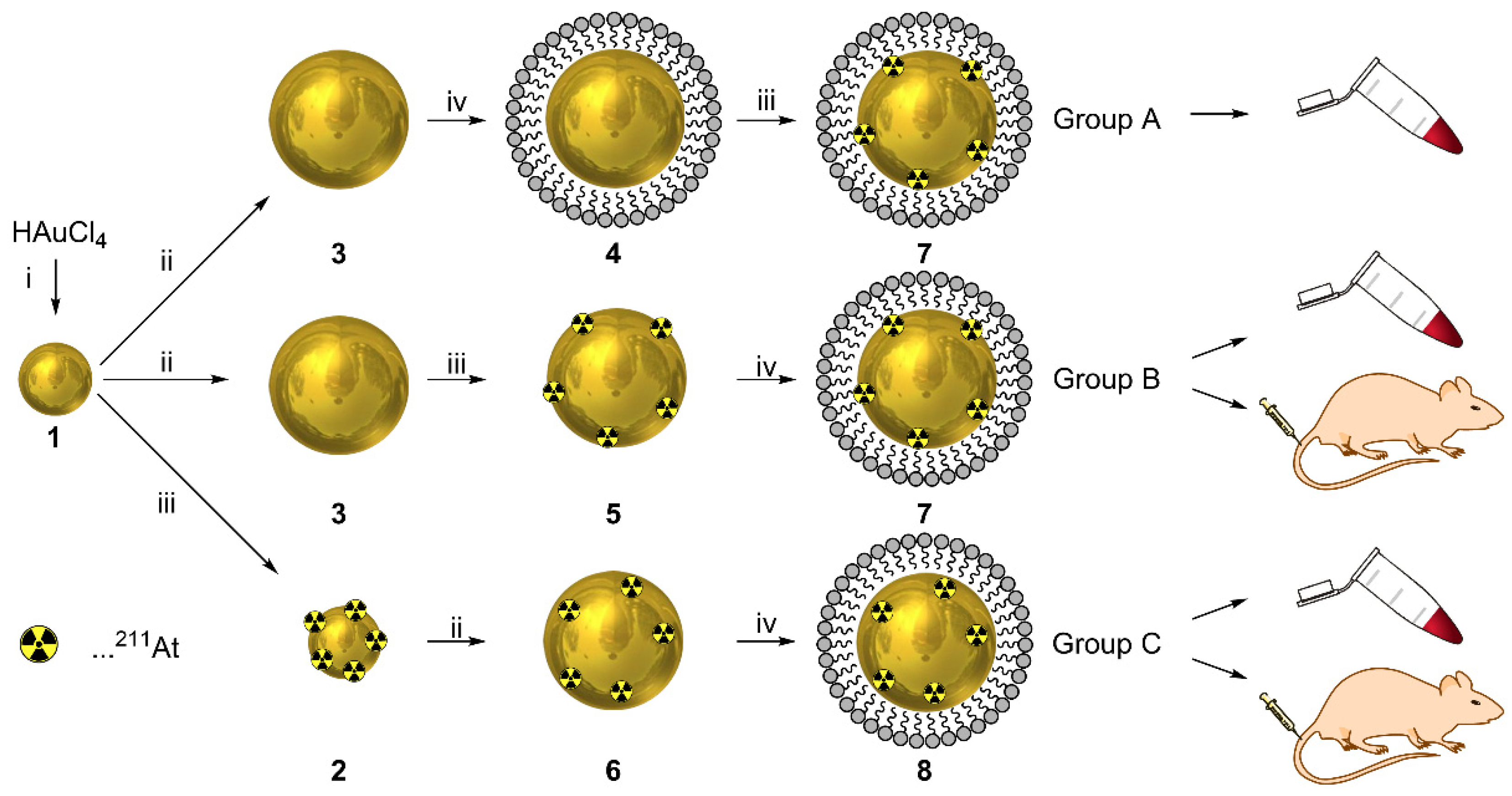
2.3. Preparation of Citrate-Stabilized 1st Generation AuNPs (1)
2.4. Preparation of Citrate-Stabilized 2nd Generation AuNPs (3)
2.5. Surface Adsorption of 211At to 2nd Generation AuNPs (5, 7, Group A, Group B)
2.6. Embedding of 211At in 2nd Generation AuNPs (6, Group C)
2.7. PEG Coating of 2nd Generation AuNPs (4, 7, 8)
2.8. Oxidative Stability Test
2.9. Serum Stability Test
2.10. ICP Determination of Gold Content for the Serum Stability Test
2.11. In Vivo Stability Study
3. Results and Discussion
3.1. Gold Nanoparticle Preparation
3.2. Radiolabeling with 211At
3.3. Stability Evaluation by Oxidative Challenge
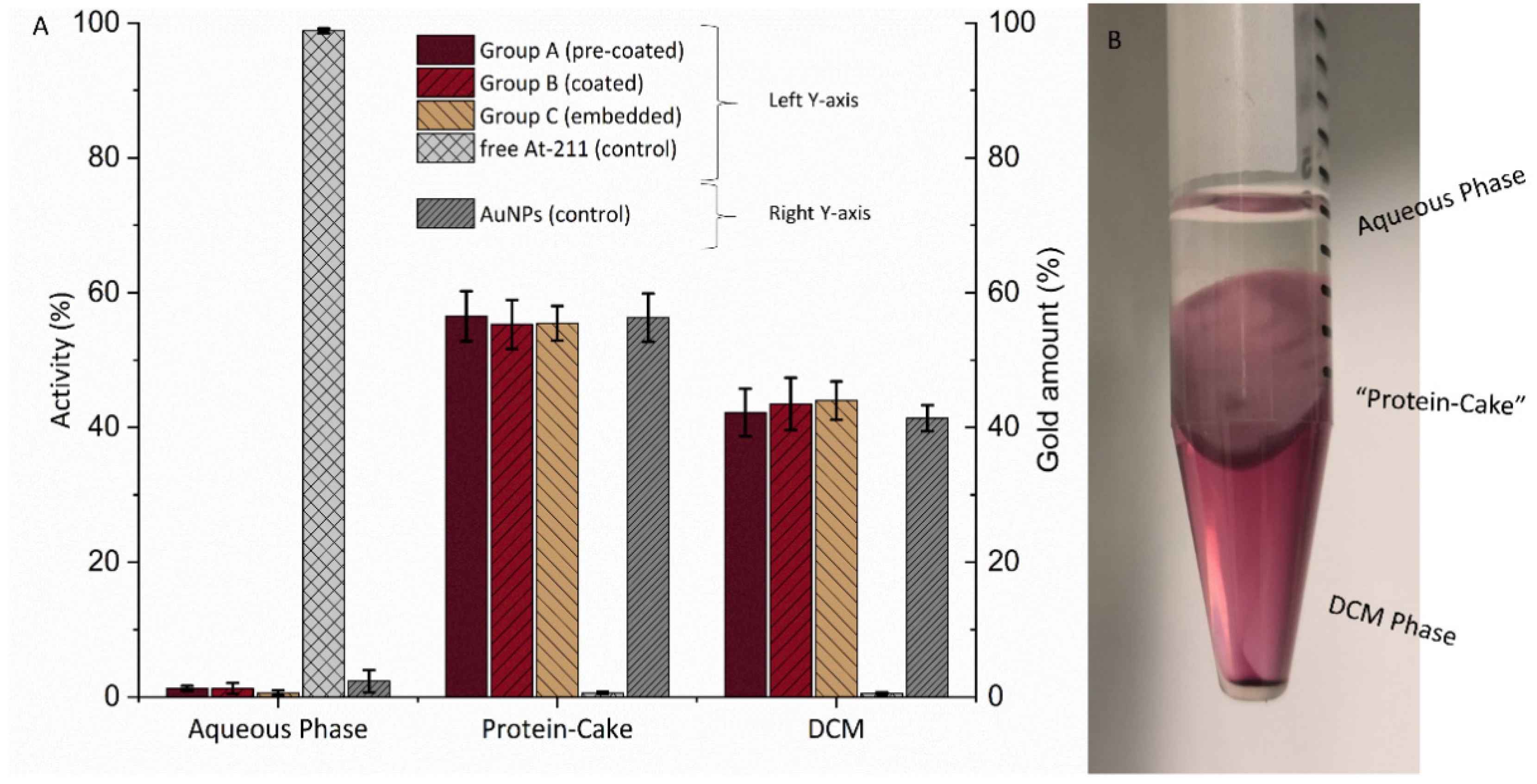
3.4. Stability Evaluation in Serum
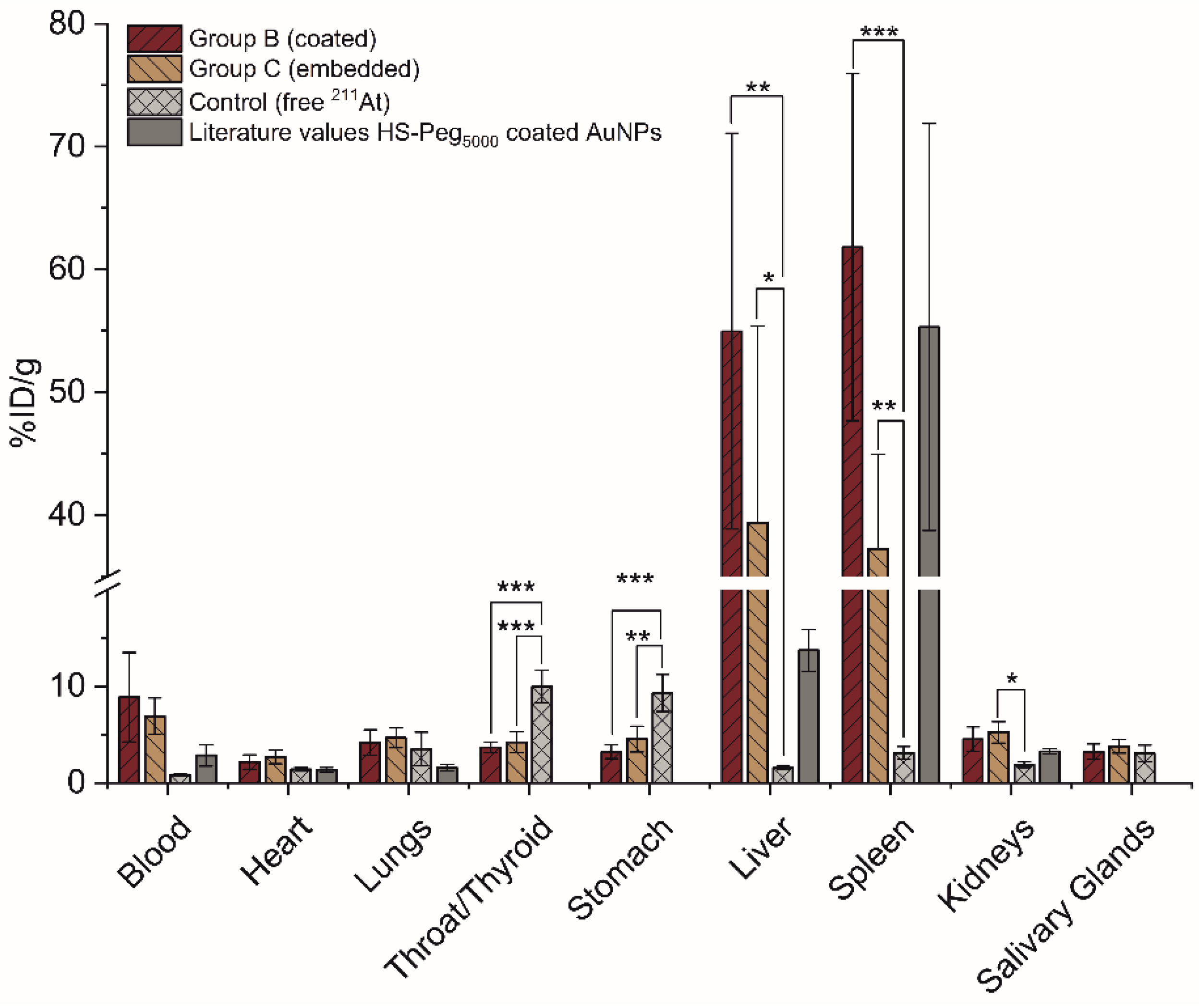
3.5. In Vivo Stability Evaluation in Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jadvar, H. Targeted α-Therapy in Cancer Management: Synopsis of Preclinical and Clinical Studies. Cancer Biother. Radiopharm. 2020, 35, 475–484. [Google Scholar] [CrossRef]
- Denk, C.; Wilkovitsch, M.; Aneheim, E.; Herth, M.M.; Jensen, H.; Lindegren, S.; Mikula, H. Multifunctional Clickable Reagents for Rapid Bioorthogonal Astatination and Radio-Crosslinking. ChemPlusChem 2019, 84, 775–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizdarevic, S.; Jessop, M.; Begley, P.; Main, S.; Robinson, A. (223)Ra-Dichloride in castration-resistant metastatic prostate cancer: Improving outcomes and identifying predictors of survival in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2264–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Lindén, O.; Noordzij, W.; Park, J.; Saad, F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [PubMed]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Pantel, K.; Cote, R.J.; Fodstad, Ø. Detection and Clinical Importance of Micrometastatic Disease. JNCI J. Natl. Cancer Inst. 1999, 91, 1113–1124. [Google Scholar] [CrossRef]
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing Clinical Trials with Astatine-211: The Chemistry Infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Berei, K.; Vasaros, L. Organic Chemistry of Astatine, Hungarian Academy of Sciences Report, 1981, KFKI-1981-10. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/12/609/12609573.pdf?r=1 (accessed on 29 September 2021).
- Milius, R.A.; McLaughlin, W.H.; Lambrecht, A.P.; Carroll, J.J.; Adelstein, S.J. Organoastatine chemistry. Astatination via electrophilic destannylation. Appl. Radiat. Isot. 1986, 37, 799–802. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Garg, P.K.; Friedman, H.S.; Bigner, D.D. Labeling of monoclonal antibodies and F(ab’)2 fragments with the α-particle-emitting nuclide At-211:Preservation of immunoreactivity and in vivo localizing capacity. Proc. Natl. Acad. Sci. USA 1989, 86, 7149–7715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, H.; Elgqvist, J.; Horvath, G.; Hultborn, R.; Jacobsson, L.; Jensen, H.; Karlsson, B.; Lindegren, S.; Palm, S. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: An overview of results in an ovarian tumor model. Clin. Cancer Res. 2003, 9, 3914S–3921S. [Google Scholar] [PubMed]
- Hadley, W.S.; Wilbur, D.S.; Gray, M.A.; Atcher, W.R. Astatine-211 labeling of an antimelanoma antibody and its Fab fragment using N-succinimidyl p-[211At] astatobenzoate: Comparisons in vivo with the p-[125I] iodobenzoyl conjugate. Bioconjug. Chem. 1991, 2, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Teze, D.; Sergentu, D.C.; Kalichuk, V.; Barbet, J.; Deniaud, D.; Galland, N.; Maurice, R. Targeted radionuclide therapy with astatine-211: Oxidative dehalogenation of astatobenzoate conjugates. Sci. Rep. 2017, 7, 2579. [Google Scholar] [CrossRef] [Green Version]
- Kucka, J.; Hruby, M.; Konak, C.; Kozempel, J.; Lebeda, O. Astatination of nanoparticles containing silver as possible carriers of 211At. Appl. Radiat. Isot. 2006, 64, 201–206. [Google Scholar] [CrossRef]
- Cedrowska, E.; Łyczko, M.; Piotrowska, A.; Bilewicz, A.; Stolarz, A.; Trzcińska, A.; Szkliniarz, K.; Wąs, B. Silver impregnated nanoparticles of titanium dioxide as carriers for 211At. Radiochim. Acta 2016, 104, 267–275. [Google Scholar] [CrossRef]
- Jeong, E.H.; Jung, G.; Hong, C.A.; Lee, H. Gold nanoparticle (AuNP)-based drug delivery and molecular imaging for biomedical applications. Arch. Pharm. Res. 2014, 37, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, L.; Kozminski, P.; Meczynska-Wielgosz, S.; Pruszynski, M.; Łyczko, M.; Was, B.; Celichowski, G.; Grobelny, J.; Jastrzebski, J.; Bilewicz, A. Gold nanoparticle bioconjugates labelled with 211At for targeted alpha therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, S.; Majkowska-Pilip, A.; Bilewicz, A.; Dobrowolski, J.C. On AunAt clusters as potential astatine carriers. RSC Adv. 2017, 7, 35854–35857. [Google Scholar] [CrossRef] [Green Version]
- Lindegren, S.; Bäck, T.; Jensen, H.J. Dry-distillation of astatine-211 from irradiated bismuth targets: A time-saving procedure with high recovery yields. Appl. Radiat. Isot. 2001, 55, 157–160. [Google Scholar] [CrossRef]
- Frellsen, A.F.; Hansen, A.E.; Jølck, R.I.; Kempen, P.J.; Severin, G.W.; Rasmussen, P.H.; Kjær, A.; Jensen, A.T.; Andresen, T.L. Mouse Positron Emission Tomography Study of the Biodistribution of Gold Nanoparticles with Different Surface Coatings Using Embedded Copper-64. ACS Nano 2016, 10, 9887–9898. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, K.; Engel, T.B.; Stensballe, A.; Simonsen, J.B.; Andresen, L.T. The hard protein corona of stealth liposomes is sparse. J. Control. Release 2019, 307, 1–15. [Google Scholar] [CrossRef]
- Fach, M.; Fliedner, F.P.; Kempen, P.J.; Melander, F.; Hansen, A.E.; Bruun, L.M.; Köster, U.; Sporer, E.; Kjær, A.; Andresen, T.L.; et al. Effective Intratumoral Retention of [103Pd] AuPd Alloy Nanoparticles Embedded in Gel-Forming Liquids Paves the Way for New Nanobrachytherapy. Adv. Healthc. Mater. 2021, 10, 2002009. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Bani Yaseen, A.I.; Park, J.; Eller, J.R.; Murphy, C.J. Facile phase transfer of gold nanoparticles from aqueous solution to organic solvents with thiolated poly(ethylene glycol). RSC Adv. 2014, 4, 52676–52679. [Google Scholar] [CrossRef]
- Spetz, J.; Rudqvist, N.; Forssell-Aronsson, E. Biodistribution and dosimetry of free 211At, 125I- and 131I- in rats. Cancer Biother. Radiopharm. 2013, 28, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Steffen, A.C.; Almqvist, Y.; Chyan, M.K.; Lundqvist, H.; Tolmachev, V.; Wilbur, D.S.; Carlsson, J. Biodistribution of 211At labeled HER-2 binding affibody molecules in mice. Oncol. Rep. 2007, 17, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sporer, E.; Poulie, C.B.M.; Lindegren, S.; Aneheim, E.; Jensen, H.; Bäck, T.; Kempen, P.J.; Kjaer, A.; Herth, M.M.; Jensen, A.I. Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy. J. Nanotheranostics 2021, 2, 196-207. https://doi.org/10.3390/jnt2040012
Sporer E, Poulie CBM, Lindegren S, Aneheim E, Jensen H, Bäck T, Kempen PJ, Kjaer A, Herth MM, Jensen AI. Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy. Journal of Nanotheranostics. 2021; 2(4):196-207. https://doi.org/10.3390/jnt2040012
Chicago/Turabian StyleSporer, Emanuel, Christian B. M. Poulie, Sture Lindegren, Emma Aneheim, Holger Jensen, Tom Bäck, Paul J. Kempen, Andreas Kjaer, Matthias M. Herth, and Andreas I. Jensen. 2021. "Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy" Journal of Nanotheranostics 2, no. 4: 196-207. https://doi.org/10.3390/jnt2040012
APA StyleSporer, E., Poulie, C. B. M., Lindegren, S., Aneheim, E., Jensen, H., Bäck, T., Kempen, P. J., Kjaer, A., Herth, M. M., & Jensen, A. I. (2021). Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy. Journal of Nanotheranostics, 2(4), 196-207. https://doi.org/10.3390/jnt2040012