Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review
Abstract
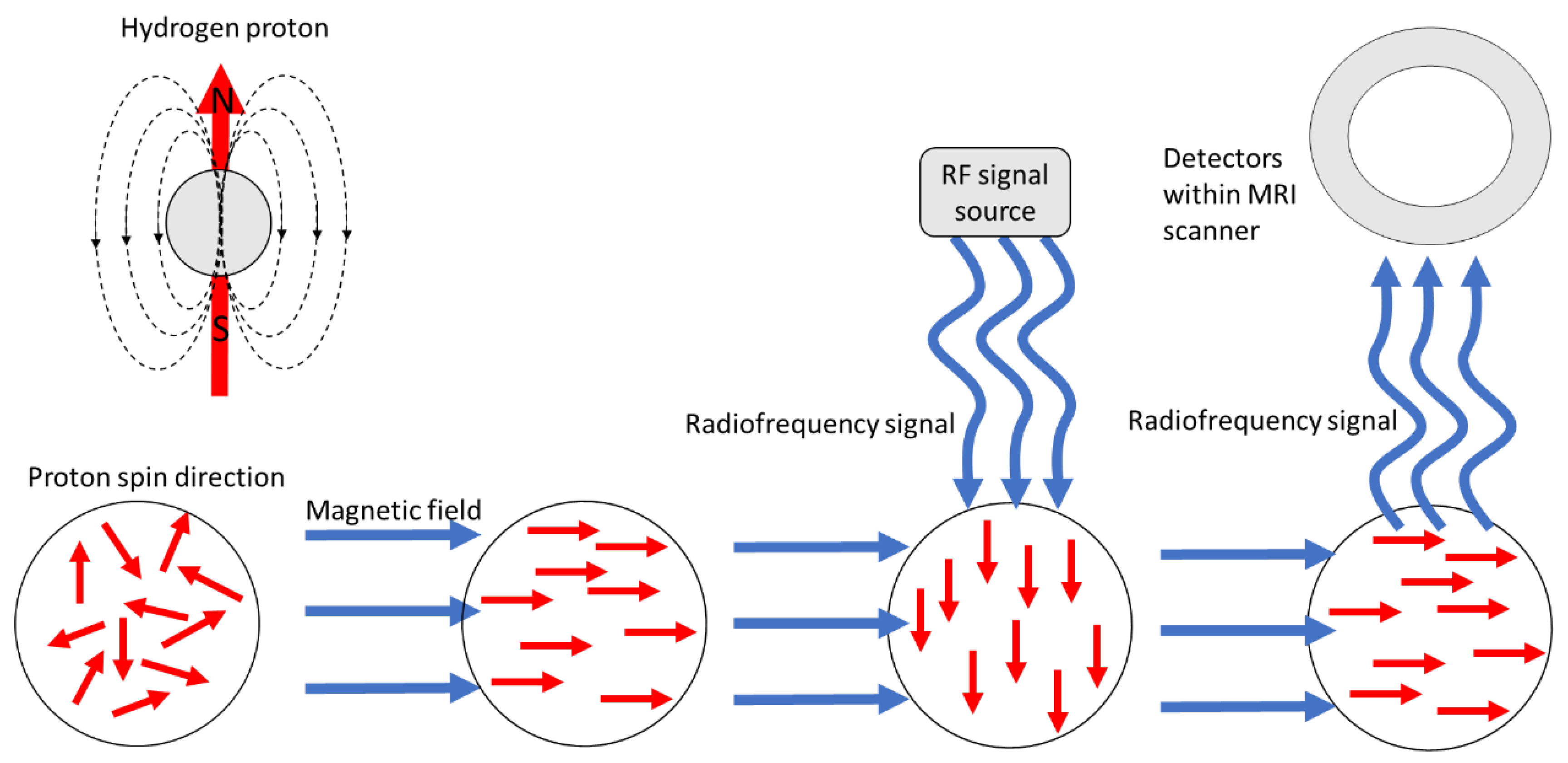
:1. Overview of Magnetic Resonance Imaging
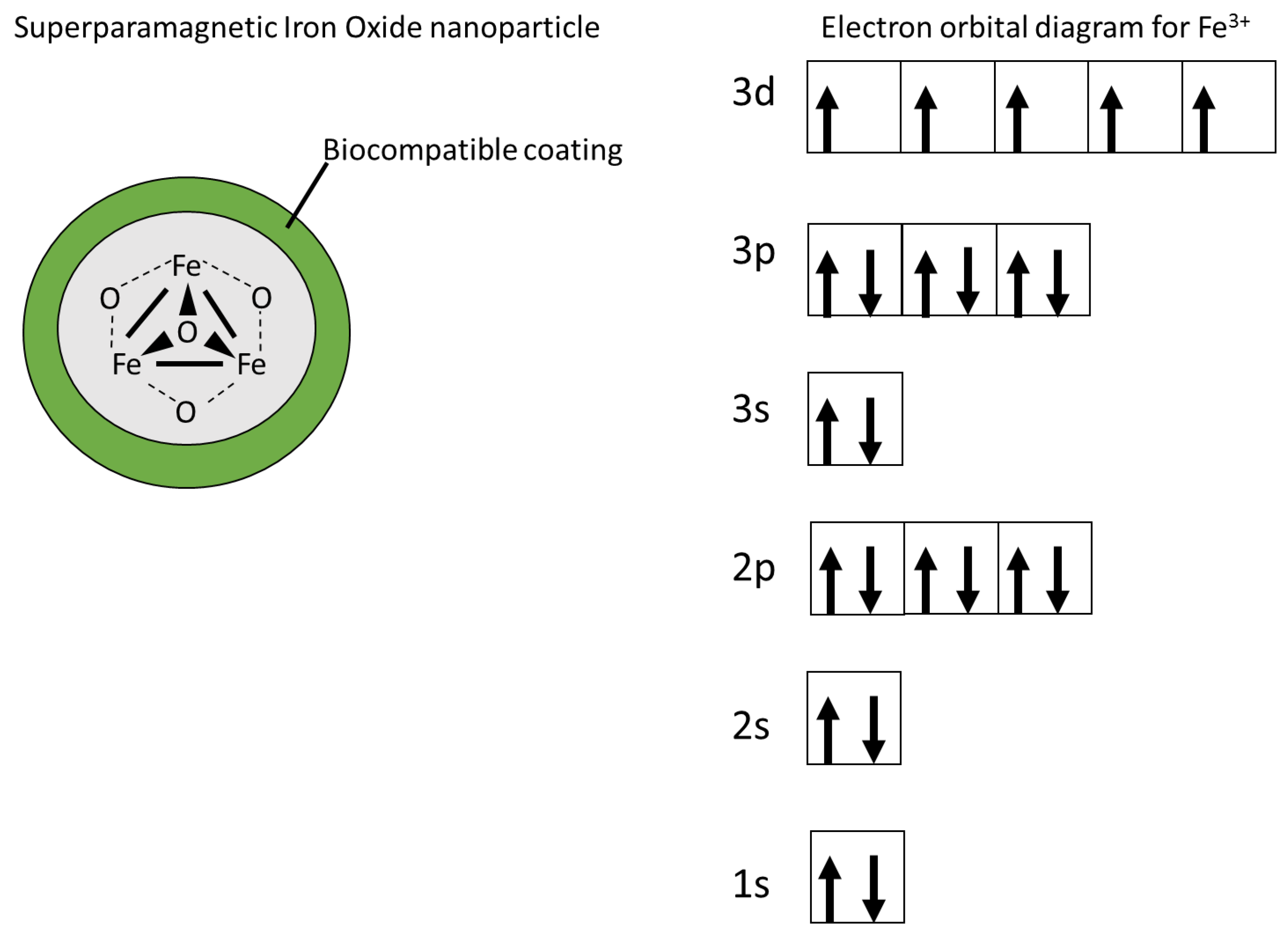
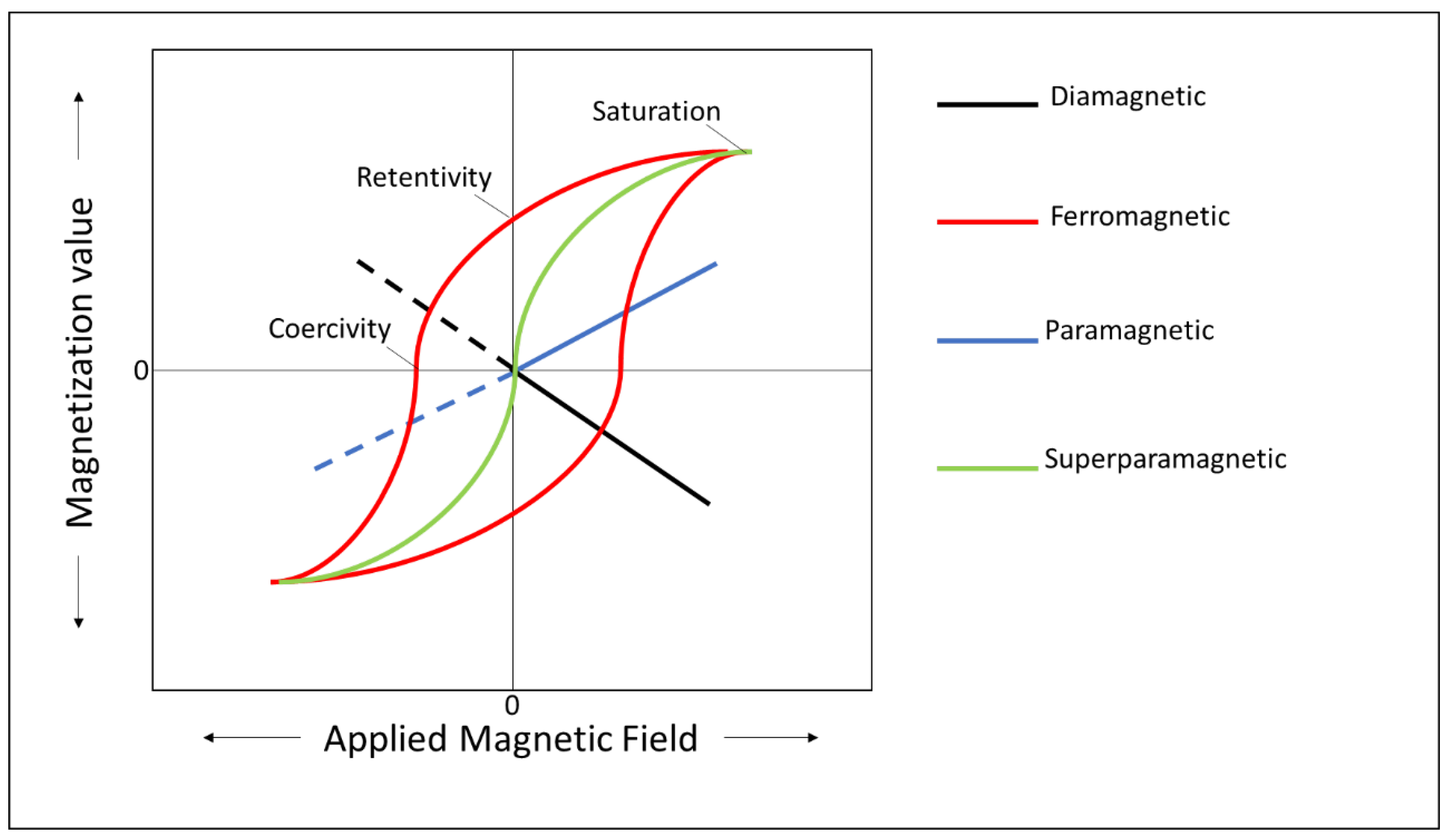
2. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) in Magnetic Resonance Imaging (MRI)
3. Toxicity and Modification of SPIONs
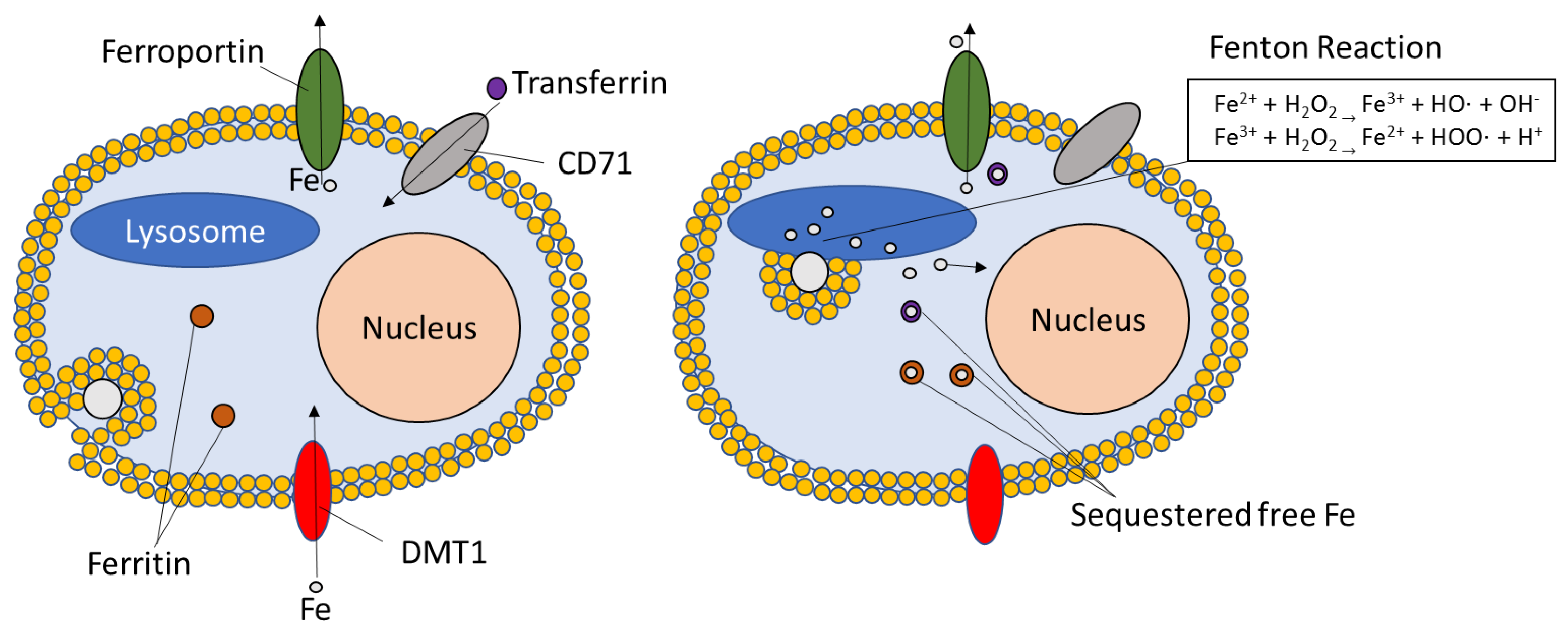
3.1. Oxidative Damage
3.2. Unique Size/Shape Toxicity
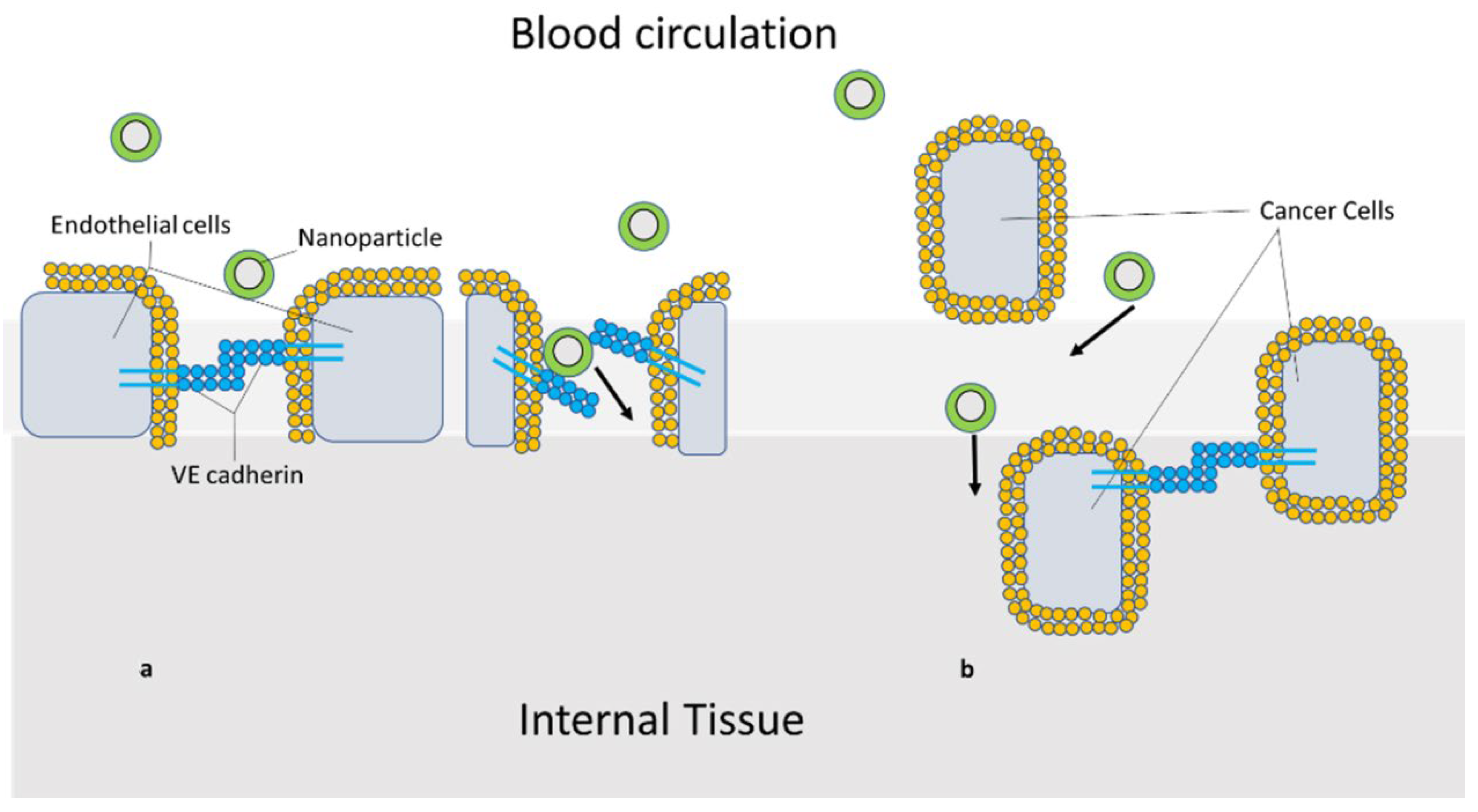
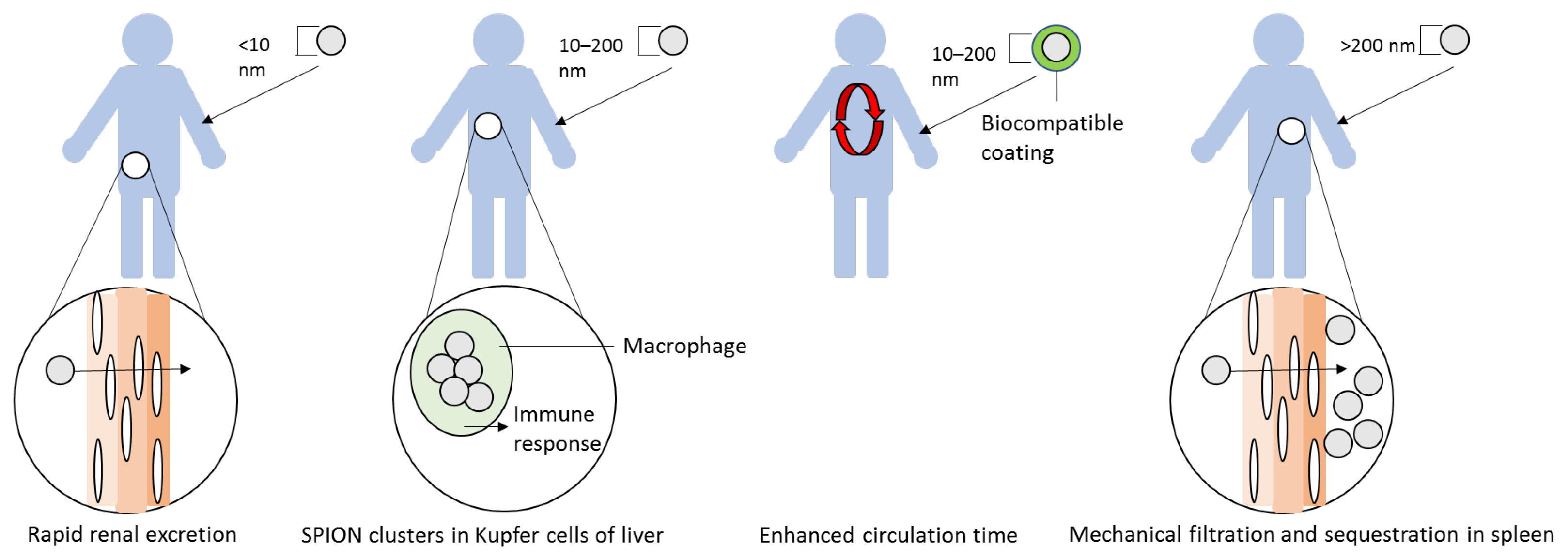
3.3. Site-Specific Accumulation
3.4. Pulmonary Exposure Results
3.4.1. Mechanisms
3.4.2. Animal Studies
3.4.3. Genotoxicity
3.5. Human Studies
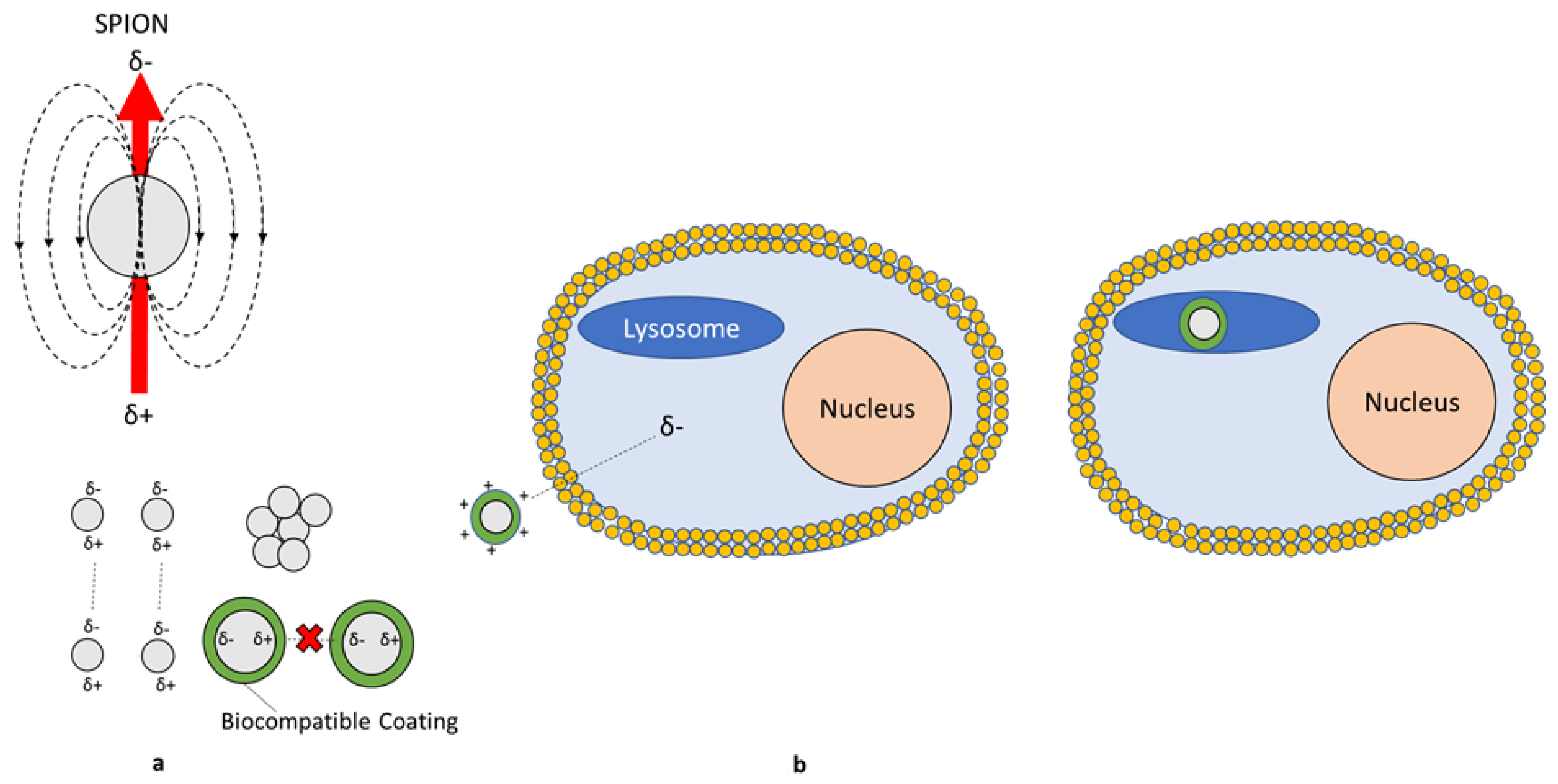
4. Coatings
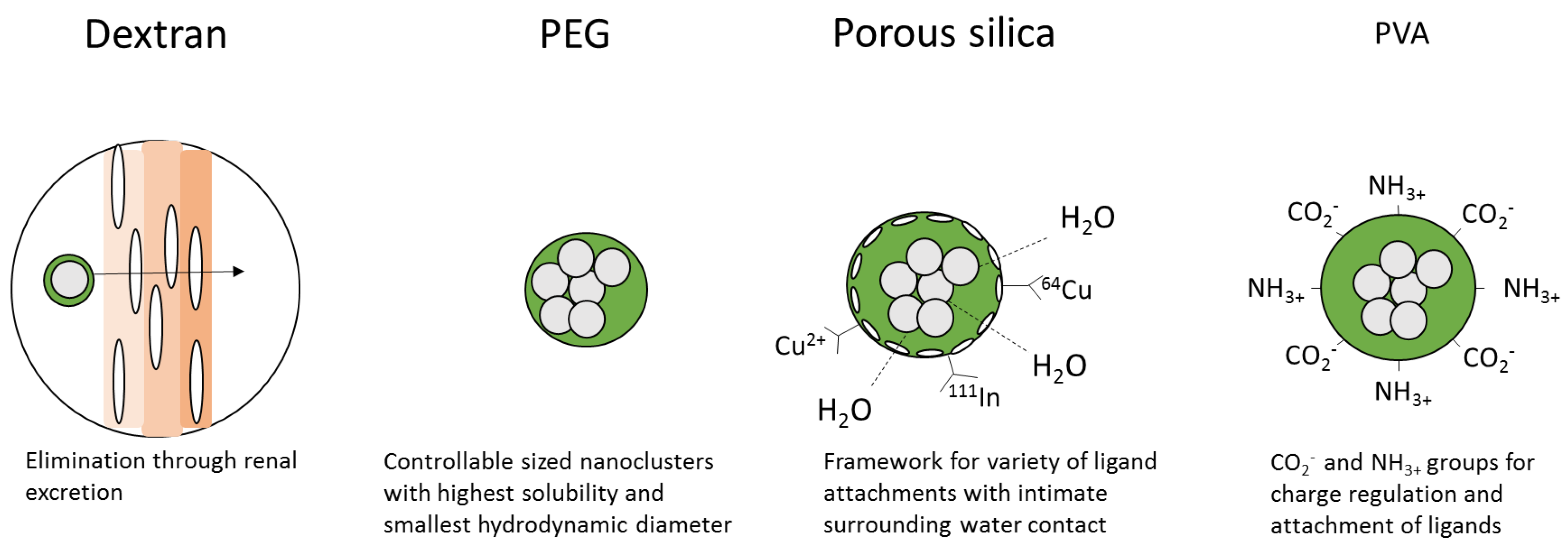
4.1. Dextran
4.2. Poly(ethylene)glycol (PEG)
4.3. Silica
4.4. Polyvinyl Alcohol (PVA)
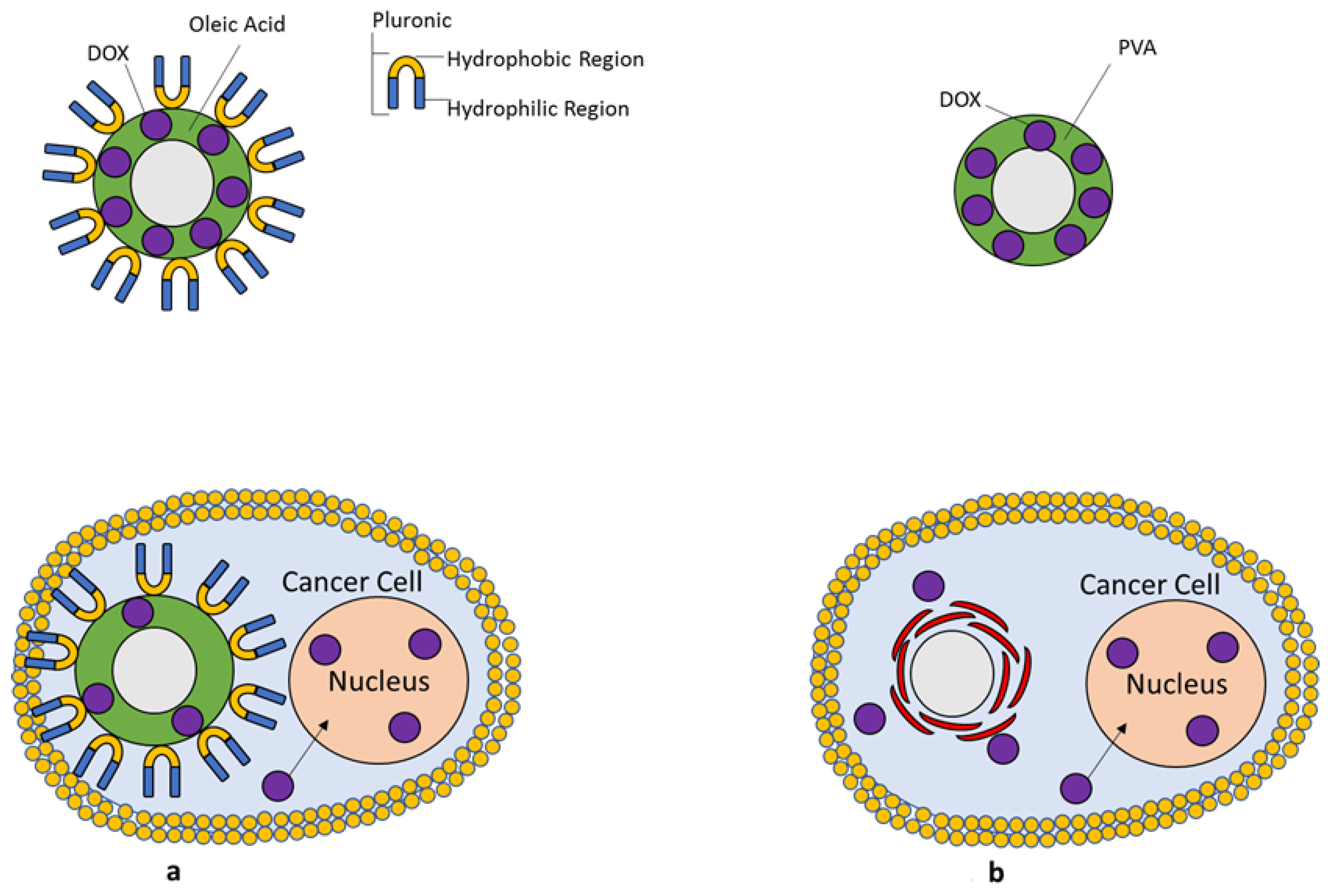
4.5. Lipids
4.6. Other Coatings
5. Hybrid Use of SPIONs
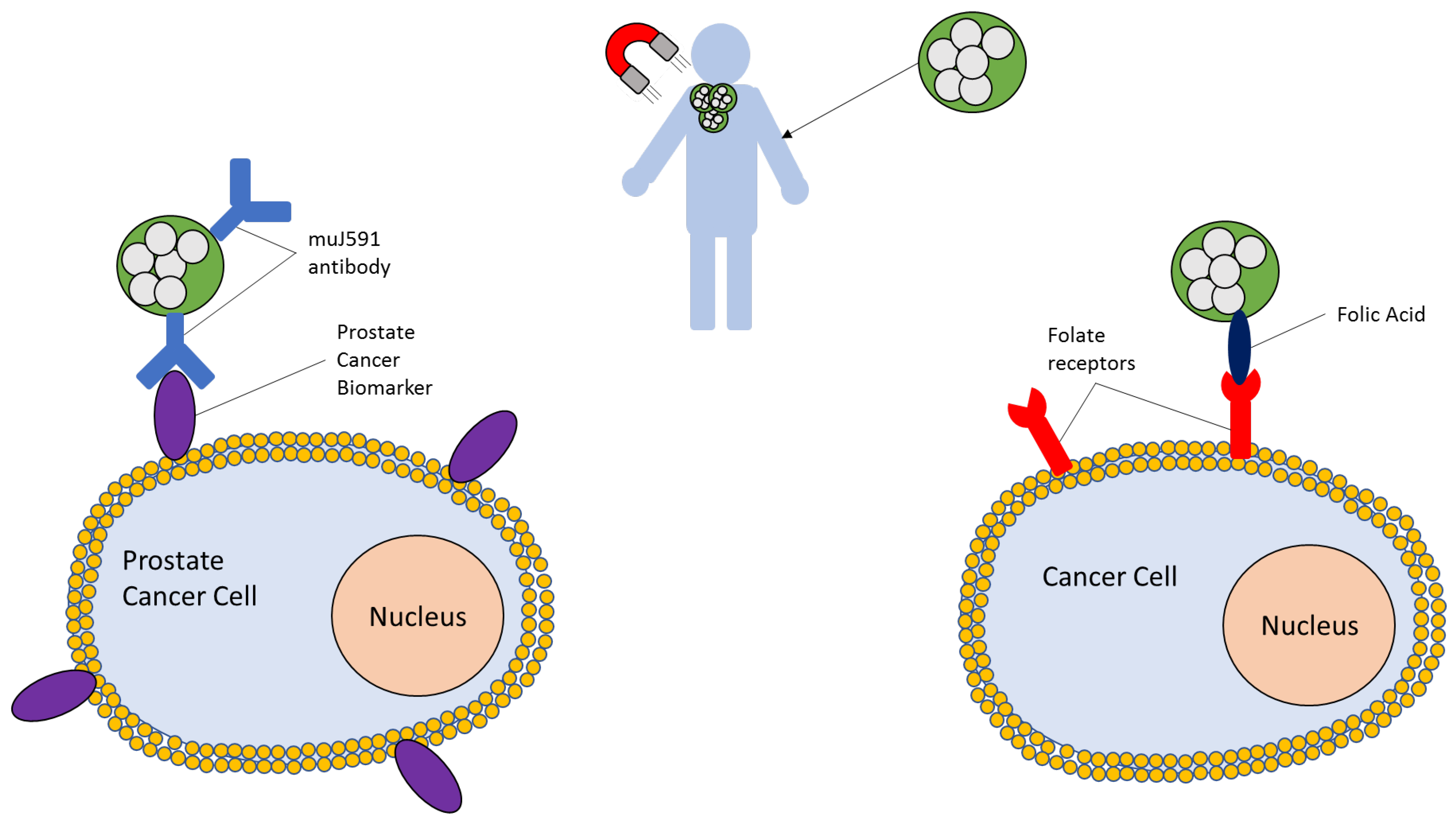
5.1. Targeting of SPIONs
5.2. Magnetic Hyperthermia Treatment
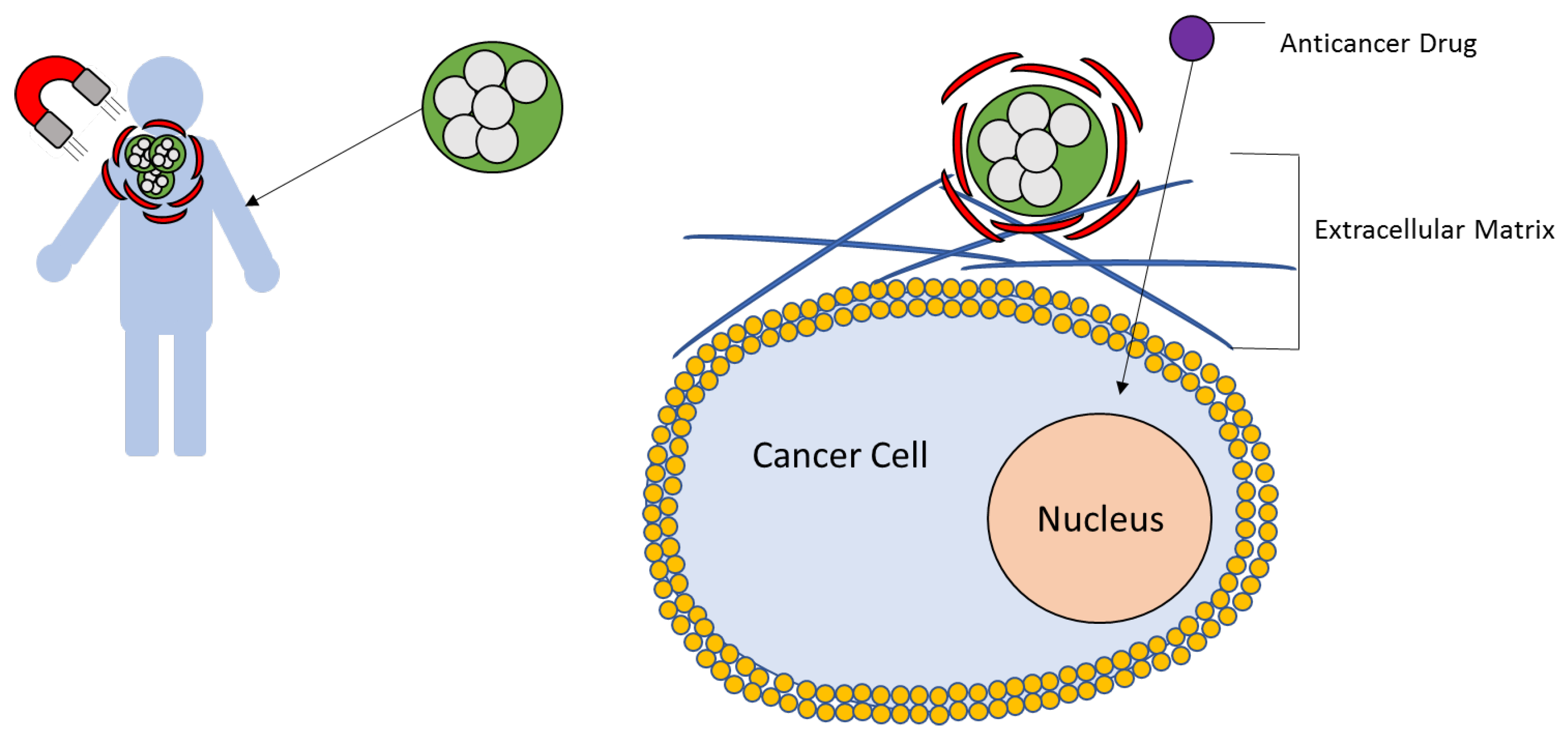
5.3. Drug Loaded SPIONs
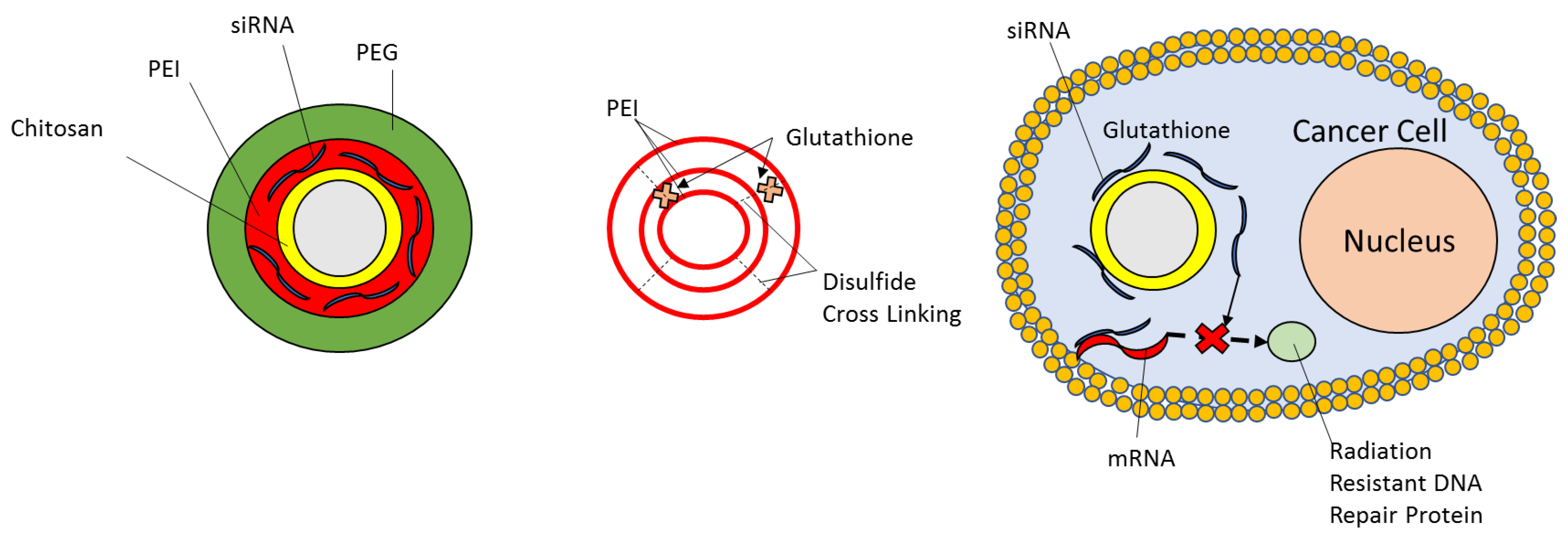
5.4. Biotherapeutics
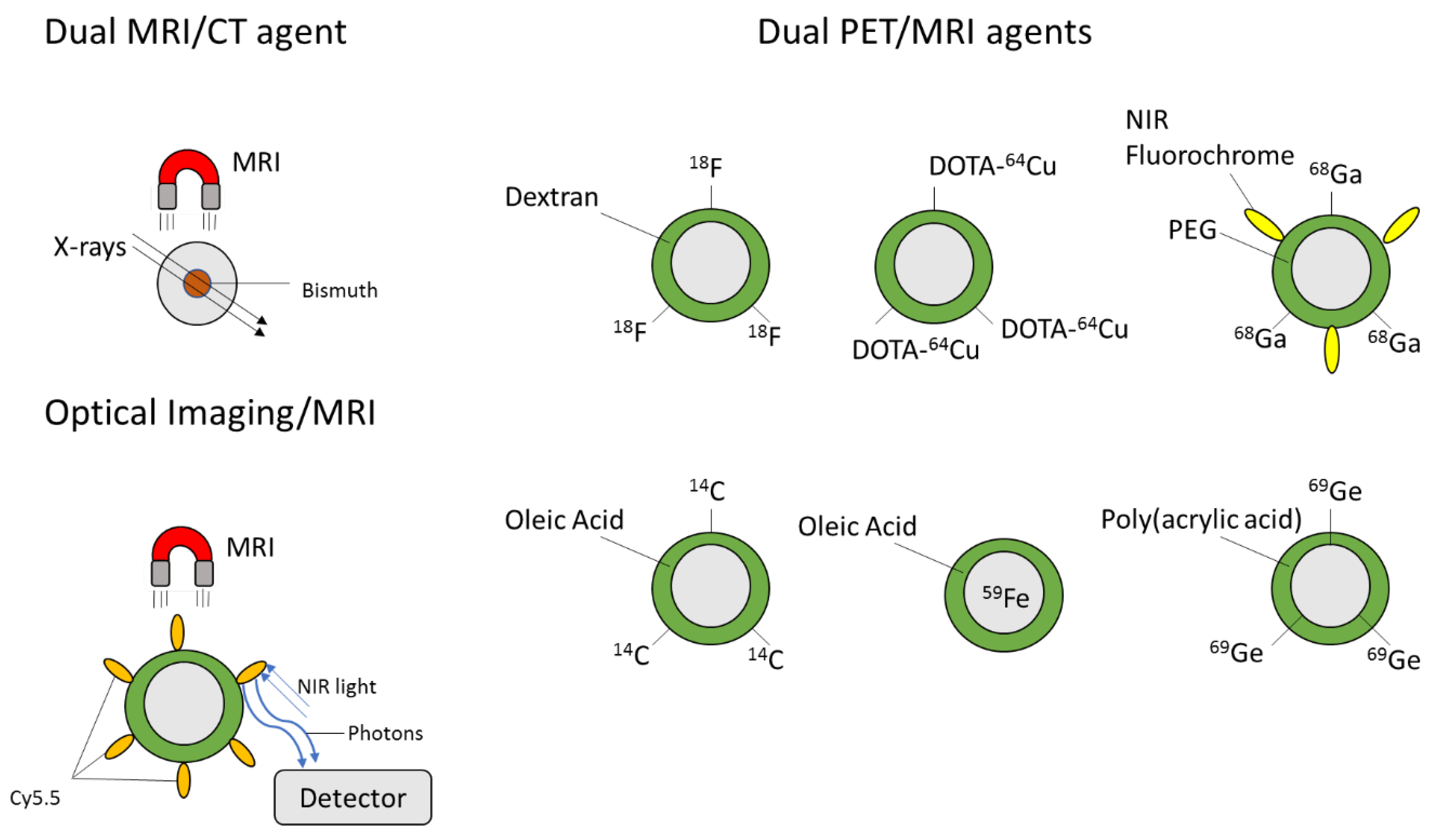
5.5. Multi-Modal Imaging Probes
6. Summary
Funding
Conflicts of Interest
References
- Eryaman, Y.; Zhang, P.; Utecht, L.; Kose, K.; Lagore, R.L.; DelaBarre, L.; Kulesa, J.; Eberly, L.E.; Adriany, G.; Iles, T.L.; et al. Investigating the physiological effects of 10.5 Tesla static field exposure on anesthetized swine. Magn. Reson. Med. 2018, 79, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Runge, V.M.; Narayanan, M.; Greene, J.F.; Nickel, A.E. Nephrogenic systemic fibrosis: A review of 6 cases temporally related to gadodiamide injection (Omniscan). Investig. Radiol. 2007, 42, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Edward, M.; Quinn, J.A.; Mukherjee, S.; Jensen, M.B.V.; Jardine, A.G.; Mark, P.B.; Burden, A.D. Gadodiamide contrast agent “activates” fibroblasts: A possible cause of nephrogenic systemic fibrosis. J. Pathol. 2008, 214, 584–593. [Google Scholar] [CrossRef]
- Runge, V.M. Dechelation (Transmetalation): Consequences and Safety Concerns with the Linear Gadolinium-Based Contrast Agents, in View of Recent Health Care Rulings by the EMA (Europe), FDA (United States), and PMDA (Japan). Investig. Radiol. 2018, 53, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Radbruch, A.; Weberling, L.D.; Kieslich, P.J.; Eidel, O.; Burth, S.; Kickingereder, P.; Heiland, S.; Wick, W.; Schlemmer, H.P.; Bendszus, M. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015, 275, 783–791. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadoliniumbased contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Kanda, T.; Osawa, M.; Oba, H.; Toyoda, K.; Kotoku, J.; Haruyama, T.; Takeshita, K.; Furui, S. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: Association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015, 275, 803–809. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.J.; McDonald, J.S.; Dai, D.; Schroeder, D.; Jentoft, M.E.; Murray, D.L.; Kadirvel, R.; Eckel, L.J.; Kallmes, D.F. Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 2017, 285, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Lord, M.L.; Chettle, D.R.; Gräfe, J.L.; Noseworthy, M.D.; McNeill, F.E. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: Results of a small pilot feasibility study. Radiology 2018, 287, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kartamihardja, A.A.P.; Nakajima, T.; Kameo, S.; Koyama, H.; Tsushima, Y. Impact of Impaired Renal Function on Gadolinium Retention after Administration of Gadolinium-Based Contrast Agents in a Mouse Model. Investig. Radiol. 2016, 51, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, H.B.; Gowda, V.; Cheng, G. Gadolinium Deposition Disease: A New Risk Management Threat. J. Am. Coll. Radiol. 2020, 17, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef]
- Kehagias, D.T.; Gouliamos, A.D.; Smyrniotis, V.; Vlahos, L.J. Diagnostic efficacy and safety of MRI of the liver with superparamagnetic iron oxide particles (SH U 555 A). J. Magn. Reson. Imaging 2001, 14, 595–601. [Google Scholar] [CrossRef]
- Madru, R.; Tran, T.A.; Axelsson, J.; Ingvar, C.; Bibic, A.; Ståhlberg, F.; Knutsson, L.; Strand, S.-E. (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 60–69. [Google Scholar]
- Gupta, A.K.; Wells, S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. IEEE Trans. Nanobiosci. 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Kuchma, E.A.; Zolotukhin, P.V.; Belanova, A.A.; Soldatov, M.A.; Lastovina, T.A.; Kubrin, S.P.; Nikolsky, A.V.; Mirmikova, L.I.; Soldatov, A.V. Low toxic maghemite nanoparticles for theranostic applications. Int. J. Nanomed. 2017, 12, 6365–6371. [Google Scholar] [CrossRef] [Green Version]
- Smolensky, E.D.; Park, H.Y.E.; Zhou, Y.; Rolla, G.A.; Marjańska, M.; Botta, M.; Pierre, V.C. Scaling laws at the nanosize: The effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J. Mater. Chem. B 2013, 1, 2818–2828. [Google Scholar] [CrossRef] [Green Version]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Falk, R.; Zurbrügg, S.; Dawson, J.; Engelhardt, P. Macrophage infiltration into the rat knee detected by MRI in a model of antigen-induced arthritis. Magn. Reson. Med. 2003, 49, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, E.A.; Koenders, M.I.; Bennink, M.B.; Crowe, L.A.; Maurizi, L.; Vallée, J.P.; Hofmann, H.; Van Den Berg, W.B.; Van Lent, P.L.E.M.; Van De Loo, F.A.J. The in-vivo use of superparamagnetic iron oxide nanoparticles to detect inflammation elicits a cytokine response but does not aggravate experimental arthritis. PLoS ONE 2015, 10, e0126687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, X.; Jin, L.; Zhou, R.; Liu, S.; Xu, N.; Liao, D.J. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: Clearance of a few misconceptions. Oncoscience 2014, 1, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Corot, C.; Robert, P.; Idée, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Donaldson, K.; Schinwald, A.; Murphy, F.; Cho, W.S.; Duffin, R.; Tran, L.; Poland, C. The biologically effective dose in inhalation nanotoxicology. Acc. Chem. Res. 2013, 46, 723–732. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Oxford University Press: New York, NY, USA, 1991; Volume 10, ISBN 0198500440. [Google Scholar]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collard, K.J. Iron homeostasis in the neonate. Pediatrics 2009, 123, 1208–1216. [Google Scholar] [CrossRef]
- Kornberg, T.G.; Stueckle, T.A.; Antonini, J.M.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Rojanasakul, L.W. Potential toxicity and underlying mechanisms associated with pulmonary exposure to iron oxide nanoparticles: Conflicting literature and unclear risk. Nanomaterials 2017, 7, 307. [Google Scholar] [CrossRef] [Green Version]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef]
- Prabhakar, P.V.; Reddy, U.A.; Singh, S.P.; Balasubramanyam, A.; Rahman, M.F.; Indu Kumari, S.; Agawane, S.B.; Murty, U.S.N.; Grover, P.; Mahboob, M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J. Appl. Toxicol. 2012, 32, 436–445. [Google Scholar] [CrossRef]
- Reddy, U.A.; Prabhakar, P.V.; Mahboob, M. Biomarkers of oxidative stress for in vivo assessment of toxicological effects of iron oxide nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e8583. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Umh, H.N.; Choi, D.H.; Cho, M.H.; Choi, W.; Kim, S.W.; Kim, Y.; Kim, J.H. Magnetite- and maghemite-induced different toxicity in murine alveolar macrophage cells. Arch. Toxicol. 2014, 88, 1607–1618. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Naya, M.; Endoh, S.; Maru, J.; Yamamoto, K.; Nakanishi, J. Comparative pulmonary toxicity study of nano-TiO2 particles of different sizes and agglomerations in rats: Different short- and long-term post-instillation results. Toxicology 2009, 264, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yin, J.J.; Zhou, Y.T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.F.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.; Kell, A.; Simard, B.; Deng, J.; Xiang, B.; Lin, H.Y.; Gruwel, M.; Tian, G. Cu2+-labeled, SPION loaded porous silica nanoparticles for cell labeling and multifunctional imaging probes. Biomaterials 2010, 31, 2866–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using cometchip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef] [Green Version]
- Freyria, F.S.; Bonelli, B.; Tomatis, M.; Ghiazza, M.; Gazzano, E.; Ghigo, D.; Garrone, E.; Fubini, B. Hematite nanoparticles larger than 90 nm show no sign of toxicity in terms of lactate dehydrogenase release, nitric oxide generation, apoptosis, and comet assay in murine alveolar macrophages and human lung epithelial cells. Chem. Res. Toxicol. 2012, 25, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.H.; De Cuyper, M. Assessing cytotoxicity of (iron oxide-based) nanoparticles: An overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol. Imaging 2009, 4, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre Toxicol. 2007, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, T.; Choyke, P.L.; Kobayashi, H. Imaging of the lymphatic system: New horizons. Contrast Media Mol. Imaging 2006, 1, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Deen, W.M.; Lazzara, M.J.; Myers, B.D. Structural determinants of glomerular permeability. Am. J. Physiol. Ren. Physiol. 2001, 281, F579–F596. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, M.; Sörensson, J.; Haraldsson, B. A gel-membrane model of glomerular charge and size selectivity in series. Am. J. Physiol. Ren. Physiol. 2001, 280, F396–F405. [Google Scholar] [CrossRef] [Green Version]
- Stolnik, S.; Illum, L.; Davis, S.S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 2012, 64, 290–301. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.T.; Feng, W.Y.; Wang, Y.; Wang, B.; Wang, M.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol. Sci. 2009, 107, 342–351. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.; Inman, A.; Young, S.-H.; Muhammed, M.; Burks, T.; Uheida, A.; Tkach, A.; Waltz, M.; Castranova, V.; et al. Oxidative Stress and Dermal Toxicity of Iron Oxide Nanoparticles In Vitro. Cell Biochem. Biophys. 2013, 67, 461–476. [Google Scholar] [CrossRef]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Kodali, V.; Littke, M.H.; Tilton, S.C.; Teeguarden, J.G.; Shi, L.; Frevert, C.W.; Wang, W.; Pounds, J.G.; Thrall, B.D. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano 2013, 7, 6997–7010. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Oh, S.Y.; Lee, S.J.; Lee, K.; Kim, Y.; Lee, B.S.; Kim, J.S. Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environ. Res. 2015, 143, 138–147. [Google Scholar] [CrossRef]
- Beaver, L.M.; Stemmy, E.J.; Schwartz, A.M.; Damsker, J.M.; Constant, S.L.; Ceryak, S.M.; Patierno, S.R. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ. Health Perspect. 2009, 117, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Langonné, I.; Huguet, N.; Guichard, Y.; Goutet, M. Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicol. Lett. 2013, 216, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Å.; Bergström, U.; Ågren, L.; Österlund, L.; Sandström, T.; Bucht, A. Differential cellular responses in healthy mice and in mice with established airway inflammation when exposed to hematite nanoparticles. Toxicol. Appl. Pharmacol. 2015, 288, 1–11. [Google Scholar] [CrossRef]
- Ma, J.Y.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Scabilloni, J.; Ma, J.K.; Castranova, V. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol. Appl. Pharmacol. 2012, 262, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Kim, H.; Kim, Y.; Yi, J.; Choi, K.; Park, K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 2010, 275, 65–71. [Google Scholar] [CrossRef]
- Szalay, B.; Tátrai, E.; Nyíro, G.; Vezér, T.; Dura, G. Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments. J. Appl. Toxicol. 2012, 32, 446–453. [Google Scholar] [CrossRef]
- Sadeghi, L.; Yousefi Babadi, V.; Espanani, H.R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Med. J. 2015, 116, 373–378. [Google Scholar] [CrossRef]
- Srinivas, A.; Rao, P.J.; Selvam, G.; Goparaju, A.; Murthy, B.P.; Reddy, N.P. Oxidative stress and inflammatory responses of rat following acute inhalation exposure to iron oxide nanoparticles. Hum. Exp. Toxicol. 2012, 31, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Ishino, K.; Kato, T.; Goto, S.; Tada, Y.; Nakae, D.; Watanabe, M.; Wakabayashi, K. Magnetite Nanoparticles Induce Genotoxicity in the Lungs of Mice via Inflammatory Response. Nanomaterials 2014, 4, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbeli, J.A. Effects of precipitated silica and of iron oxide on the incidence of primary lung tumours in mice. Br. Med. J. 1940, 2, 275. [Google Scholar] [CrossRef] [PubMed]
- Villacis, R.A.R.; Filho, J.S.; Piña, B.; Azevedo, R.B.; Pic-Taylor, A.; Mazzeu, J.F.; Grisolia, C.K. Integrated assessment of toxic effects of maghemite (Γ-Fe2O3) nanoparticles in zebrafish. Aquat. Toxicol. 2017, 191, 219–225. [Google Scholar] [CrossRef]
- De Oliveira, G.M.T.; Kist, L.W.; Pereira, T.C.B.; Bortolotto, J.W.; Paquete, F.L.; De Oliveira, E.M.N.; Leite, C.E.; Bonan, C.D.; De Souza Basso, N.R.; Papaleo, R.M.; et al. Transient modulation of acetylcholinesterase activity caused by exposure to dextran-coated iron oxide nanoparticles in brain of adult zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 162, 77–84. [Google Scholar] [CrossRef]
- Boyd, J.T.; Doll, R.; Faulds, J.S.; Leiper, J. Cancer of the lung in iron ore (haematite) miners. Br. J. Ind. Med. 1970, 27, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Andujar, P.; Simon-Deckers, A.; Galateau-Sallé, F.; Fayard, B.; Beaune, G.; Clin, B.; Billon-Galland, M.A.; Durupthy, O.; Pairon, J.C.; Doucet, J.; et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part. Fibre Toxicol. 2014, 11. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.J.; Eaton, J.W.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Shiba, E.; Lindon, J.N.; Kushner, L.; Matsueda, G.R.; Hawiger, J.; Kloczewiak, M.; Kudryk, B.; Salzman, E.W. Antibody-detectable changes in fibrinogen adsorption affecting platelet activation on polymer surfaces. Am. J. Physiol. Cell Physiol. 1991. [Google Scholar] [CrossRef]
- Simak, J.; De Paoli, S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Simberg, D.; Zhang, W.M.; Merkulov, S.; McCrae, K.; Park, J.H.; Sailor, M.J.; Ruoslahti, E. Contact activation of kallikrein-kinin system by superparamagnetic iron oxide nanoparticles in vitro and in vivo. J. Control. Release 2009, 140, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simberg, D.; Park, J.H.; Karmali, P.P.; Zhang, W.M.; Merkulov, S.; McCrae, K.; Bhatia, S.N.; Sailor, M.; Ruoslahti, E. Differential proteomics analysis of the surface heterogeneity of dextran iron oxide nanoparticles and the implications for their in vivo clearance. Biomaterials 2009, 30, 3926–3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strehl, C.; Maurizi, L.; Gaber, T.; Hoff, P.; Broschard, T.; Poole, A.R.; Hofmann, H.; Buttgereit, F. Modification of the surface of superparamagnetic iron oxide nanoparticles to enable their safe application in humans. Int. J. Nanomed. 2016, 11, 5883. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Bai, R.; Zhou, H.; Wang, R.; Liu, J.; Zhao, Y.; Chen, C. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 2020, 10. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Solorio-Rodríguez, A.; Uribe-Ramírez, M.; Lozano, O.; Lucas, S.; Chagolla-López, A.; Winkler, R.; De Vizcaya-Ruiz, A. Plasma protein adsorption on Fe3O4-PEG nanoparticles activates the complement system and induces an inflammatory response. Int. J. Nanomed. 2019. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Sanjai, C.; Kothan, S.; Gonil, P.; Saesoo, S.; Sajomsang, W. Chitosan-triphosphate nanoparticles for encapsulation of super-paramagnetic iron oxide as an MRI contrast agent. Carbohydr. Polym. 2014, 104, 231–237. [Google Scholar] [CrossRef]
- Naha, P.C.; Al Zaki, A.; Hecht, E.; Chorny, M.; Chhour, P.; Blankemeyer, E.; Yates, D.M.; Witschey, W.R.T.; Litt, H.I.; Tsourkas, A.; et al. Dextran coated bismuth-iron oxide nanohybrid contrast agents for computed tomography and magnetic resonance imaging. J. Mater. Chem. B 2014, 2, 8239–8248. [Google Scholar] [CrossRef]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Zaitsev, V.S.; Filimonov, D.S.; Presnyakov, I.A.; Gambino, R.J.; Chu, B. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. J. Colloid Interface Sci. 1999, 212, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hajesmaeelzadeh, F.; Shanehsazzadeh, S.; Grüttner, C.; Daha, F.J.; Oghabian, M.A. Effect of coating thickness of iron oxide nanoparticles on their relaxivity in the MRI. Iran. J. Basic Med. Sci. 2016, 19, 166–171. [Google Scholar] [PubMed]
- Tian, F.; Chen, G.; Yi, P.; Zhang, J.; Li, A.; Zhang, J.; Zheng, L.; Deng, Z.; Shi, Q.; Peng, R.; et al. Fates of Fe3O4 and Fe3O4 at SiO2 nanoparticles in human mesenchymal stem cells assessed by synchrotron radiation-based techniques. Biomaterials 2014, 35, 6412–6421. [Google Scholar] [CrossRef] [PubMed]
- Lévy, M.; Lagarde, F.; Maraloiu, V.A.; Blanchin, M.G.; Gendron, F.; Wilhelm, C.; Gazeau, F. Degradability of superparamagnetic nanoparticles in a model of intracellular environment: Follow-up of magnetic, structural and chemical properties. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Cengelli, F.; Maysinger, D.; Tschudi-Monnet, F.; Montet, X.; Corot, C.; Petri-Fink, A.; Hofmann, H.; Juillerat-Jeanneret, L. Interaction of functionalized superparamagnetic iron oxide nanoparticles with brain structures. J. Pharmacol. Exp. Ther. 2006, 318, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Investig. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Hoekstra, Y.; Kamman, R.L.; Magin, R.L.; Webb, A.G.; Briggs, R.W.; Gwan Go, K.; Hulstaert, C.E.; Miltenyi, S.; Hauw The, T.; et al. Specific MR imaging of human lymphocytes by monoclonal antibody-guided dextran-magnetite particles. Magn. Reson. Med. 1992, 25, 148–157. [Google Scholar] [CrossRef]
- Weissleder, R.; Lee, A.S.; Khaw, B.A.; Shen, T.; Brady, T.J. Antimyosin-labeled monocrystalline iron oxide allows detection of myocardial infarct: MR antibody imaging. Radiology 1992, 182, 381–385. [Google Scholar] [CrossRef]
- Yeh, T.-C.; Zhang, W.; Ildstad, S.T.; Ho, C. In Vivo Dynamic MRI Tracking of Rat T-Cells Labeled with Superparamagnetic Iron-Oxide Particles. Magn. Reson. Med. 1995, 33, 200–208. [Google Scholar] [CrossRef]
- Beckmann, N.; Cannet, C.; Fringeli-Tanner, M.; Baumann, D.; Pally, C.; Bruns, C.; Zerwes, H.G.; Andriambeloson, E.; Bigaud, M. Macrophage labeling by SPIO as an early marker of allograft chronic rejection in a rat model of kidney transplantation. Magn. Reson. Med. 2003, 49, 459–467. [Google Scholar] [CrossRef]
- Madru, R.; Kjellman, P.; Olsson, F.; Wingårdh, K.; Ingvar, C.; Ståhlberg, F.; Olsrud, J.; Lätt, J.; Fredriksson, S.; Knutsson, L.; et al. 99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes. J. Nucl. Med. 2012, 53, 459–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Mark Haacke, E. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, S.E.; Brindley, A.; Davis, S.S.; Davies, M.C.; Illum, L. Polystyrene-Poly (Ethylene Glycol) (PS-PEG2000) Particles as Model Systems for Site Specific Drug Delivery. 2. The Effect of PEG Surface Density on the in Vitro Cell Interaction and in VivoBiodistribution. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1994, 11, 1016–1022. [Google Scholar] [CrossRef]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res. 2004, 316, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically germanium-69-labeled iron oxide nanoparticles: Synthesis and in-vivo dual-modality PET/MR zimaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef]
- Al Faraj, A.; Shaik, A.P.; Shaik, A.S. Effect of surface coating on the biocompatibility and in vivo MRI detection of iron oxide nanoparticles after intrapulmonary administration. Nanotoxicology 2015, 9, 825–834. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Alcalá, M.D.; Real, C. Synthesis based on the wet impregnation method and characterization of iron and iron oxide-silica nanocomposites. Solid State Ion. 2006, 177, 955–960. [Google Scholar] [CrossRef]
- Ma, D.; Guan, J.; Normandin, F.; Dénommée, S.; Enright, G.; Veres, T.; Simard, B. Multifunctional nano-architecture for biomedical applications. Chem. Mater. 2006, 18, 1920–1927. [Google Scholar] [CrossRef]
- Tsai, C.P.; Hung, Y.; Chou, Y.H.; Huang, D.M.; Hsiao, J.K.; Chang, C.; Chen, Y.C.; Mou, C.Y. High-contrast paramagnetic fluorescent mesoporous silica nanorods as a multifunctional cell-imaging probe. Small 2008, 4, 186–191. [Google Scholar] [CrossRef]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): Colloidal stability, cytotoxicity, and cellular uptake studies. Eur. J. Pharm. Biopharm. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chastellain, M.; Petri, A.; Hofmann, H. Particle size investigations of a multistep synthesis of PVA coated superparamagnetic nanoparticles. J. Colloid Interface Sci. 2004, 278, 353–360. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Understanding the nano-bio interfaces: Lipid-coatings for inorganic nanoparticles as promising strategy for biomedical applications. Front. Chem. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Gill, R.L.; Kim, E.Y.; Briley, N.E.; Tyndall, E.R.; Xu, J.; Li, C.; Ramamurthi, K.S.; Flanagan, J.M.; Tian, F. Spherical Nanoparticle Supported Lipid Bilayers for the Structural Study of Membrane Geometry-Sensitive Molecules. J. Am. Chem. Soc. 2015, 137, 14031–14034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.R.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Durfee, P.N.; Lin, Y.S.; Dunphy, D.R.; Muñiz, A.J.; Butler, K.S.; Humphrey, K.R.; Lokke, A.J.; Agola, J.O.; Chou, S.S.; Chen, I.M.; et al. Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells. ACS Nano 2016, 10, 8325–8345. [Google Scholar] [CrossRef]
- Liu, J.; Stace-Naughton, A.; Jiang, X.; Brinker, C.J. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J. Am. Chem. Soc. 2009, 131, 1354–1355. [Google Scholar] [CrossRef] [Green Version]
- Savarala, S.; Ahmed, S.; Ilies, M.A.; Wunder, S.L. Formation and colloidal stability of dmpc supported lipid bilayers on SiO2 nanobeads. Langmuir 2010, 26, 12081–12088. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Ramakrishna Matte, H.S.S.; Voggu, R.; Govindaraj, A. Recent progress in the synthesis of inorganic nanoparticles. Dalt. Trans. 2012, 41, 5089–5120. [Google Scholar] [CrossRef]
- Luchini, A.; Irace, C.; Santamaria, R.; Montesarchio, D.; Heenan, R.K.; Szekely, N.; Flori, A.; Menichetti, L.; Paduano, L. Phosphocholine-decorated superparamagnetic iron oxide nanoparticles: Defining the structure and probing: In vivo applications. Nanoscale 2016, 8, 10078–10086. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.; Gerelli, Y.; Fragneto, G.; Nylander, T.; Pálsson, G.K.; Appavou, M.S.; Paduano, L. Neutron Reflectometry reveals the interaction between functionalized SPIONs and the surface of lipid bilayers. Colloids Surf. B Biointerfaces 2017, 151, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Pavel, I.A.; Girardon, M.; El Hajj, S.; Parant, S.; Amadei, F.; Kaufmann, S.; Tanaka, M.; Fierro, V.; Celzard, A.; Canilho, N.; et al. Lipid-coated mesoporous silica microparticles for the controlled delivery of β-galactosidase into intestines. J. Mater. Chem. B 2018, 6, 5633–5639. [Google Scholar] [CrossRef] [PubMed]
- Van Schooneveld, M.M.; Vucic, E.; Koole, R.; Zhou, Y.; Stocks, J.; Cormode, D.P.; Tang, C.Y.; Gordon, R.E.; Nicolay, K.; Meijerink, A.; et al. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: A multimodality investigation. Nano Lett. 2008, 8, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Simeone, L.; Mangiapia, G.; Vitiello, G.; Irace, C.; Colonna, A.; Ortona, O.; Montesarchio, D.; Paduano, L. Cholesterol-based nucleolipid-ruthenium complex stabilized by lipid aggregates for antineoplastic therapy. Bioconjug. Chem. 2012, 23, 758–770. [Google Scholar] [CrossRef]
- Patil-Sen, Y.; Torino, E.; De Sarno, F.; Ponsiglione, A.M.; Chhabria, V.; Ahmed, W.; Mercer, T. Biocompatible superparamagnetic core-shell nanoparticles for potential use in hyperthermia-enabled drug release and as an enhanced contrast agent. Nanotechnology 2020, 31. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm. 2005, 2, 194–205. [Google Scholar] [CrossRef] [Green Version]
- Sitharaman, B.; Tran, L.A.; Pham, Q.P.; Bolskar, R.D.; Muthupillai, R.; Flamm, S.D.; Mikos, A.G.; Wilson, L.J. Gadofullerenes as nanoscale magnetic labels for cellular MRI. Contrast Media Mol. Imaging 2007, 2, 139–146. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef]
- Cochran, D.B.; Wattamwar, P.P.; Wydra, R.; Hilt, J.Z.; Anderson, K.W.; Eitel, R.E.; Dziubla, T.D. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomaterials 2013, 34, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, P.; Chakraborty, S.; Qiang, Q.; Deo, P.; Wang, J.; Zhang, R. Surfactants, polymers and their nanoparticles for personal care applications. J. Cosmet. Sci. 2004, 55, 135–136. [Google Scholar] [CrossRef]
- Wang, H.; Kumar, R.; Nagesha, D.; Duclos, R.I.; Sridhar, S.; Gatley, S.J. Integrity of 111In-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse. Nucl. Med. Biol. 2015, 42, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Abraham, S.; Campbell, M.; Zehbe, I.; Curiel, L. Development and characterization of an antibody-labeled super-paramagnetic iron oxide contrast agent targeting prostate cancer cells for magnetic resonance imaging. PLoS ONE 2014, 9, e97220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, T.A.; Bankson, J.; Aaron, J.; Sokolov, K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; Van Der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Moroz, P.; Jones, S.K.; Gray, B.N. Magnetically mediated hyperthermia: Current status and future directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef]
- Steeves, R.A. Hyperthermia in cancer therapy: Where are we today and where are we going? Bull. N. Y. Acad. Med. J. Urban Heal. 1992, 68, 341–350. [Google Scholar]
- Christophi, C.; Winkworth, A.; Muralihdaran, V.; Evans, P. The treatment of malignancy by hyperthermia. Surg. Oncol. 1998, 7, 83–90. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Di Corato, R.; Lartigue, L.; Marangon, I.; Guardia, P.; Silva, A.K.A.; Luciani, N.; Clément, O.; Flaud, P.; Singh, J.V.; et al. Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano 2014, 8, 4268–4283. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Destouches, D.; Page, N.; Hamma-Kourbali, Y.; Machi, V.; Chaloin, O.; Frechault, S.; Birmpas, C.; Katsoris, P.; Beyrath, J.; Albanese, P.; et al. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 2011, 71, 3296–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Matsumine, A.; Takegami, K.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Uchida, A.; Sudo, A. A novel hyperthermia treatment for bone metastases using magnetic materials. Int. J. Clin. Oncol. 2011, 16, 101–108. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Gawęda, W.; Osial, M.; Żuk, M.; Pękała, M.; Bilewicz, A.; Krysinski, P. Lanthanide-doped SPIONs bioconjugation with trastuzumab for potential multimodal anticancer activity and magnetic hyperthermia. Nanomaterials 2020, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Piazza, R.D.; Viali, W.R.; Dos Santos, C.C.; Nunes, E.S.; Marques, R.F.C.; Morais, P.C.; Da Silva, S.W.; Coaquira, J.A.H.; Jafelicci, M. PEGlatyon-SPION surface functionalization with folic acid for magnetic hyperthermia applications. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Weiss, R.B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Van Zomeren, D.M.; Buijs, D.; Ouwens, L.; Nooter, K.; Stoter, G.; Sparreboom, A. Influence of cremophor EL on the bioavailability of intraperitoneal paclitaxel. Clin. Cancer Res. 2002, 8, 1237–1241. [Google Scholar] [PubMed]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lubbe, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000, 60, 6641–6648. [Google Scholar] [PubMed]
- Hu, S.H.; Liao, B.J.; Chiang, C.S.; Chen, P.J.; Chen, I.W.; Chen, S.Y. Core-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugs. Adv. Mater. 2012, 24, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Basuki, J.S.; Duong, H.T.T.; Macmillan, A.; Erlich, R.B.; Esser, L.; Akerfeldt, M.C.; Whan, R.M.; Kavallaris, M.; Boyer, C.; Davis, T.P. Using fluorescence lifetime imaging microscopy to monitor theranostic nanoparticle uptake and intracellular doxorubicin release. ACS Nano 2013, 7, 10175–10189. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B.; et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, K.S.; Durfee, P.N.; Theron, C.; Ashley, C.E.; Carnes, E.C.; Brinker, C.J. Protocells: Modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery. Small 2016, 12, 2173–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zhang, X.; Miao, Y.; Li, J.; Gan, Y. Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 2033–2044. [Google Scholar] [CrossRef] [Green Version]
- Traini, G.; Ruiz-de-Angulo, A.; Blanco-Canosa, J.B.; Zamacola Bascarán, K.; Molinaro, A.; Silipo, A.; Escors, D.; Mareque-Rivas, J.C. Cancer Immunotherapy of TLR4 Agonist–Antigen Constructs Enhanced with Pathogen-Mimicking Magnetite Nanoparticles and Checkpoint Blockade of PD-L1. Small 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Ko, Y.T. Lipid-coated gold nanocomposites for enhanced cancer therapy. Int. J. Nanomed. 2015, 10, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, D.J.; Coffman, M.D.; Knight, J.D.; Reed, S.M. Lipid-Coated Gold Nanoparticles and FRET Allow Sensitive Monitoring of Liposome Clustering Mediated by the Synaptotagmin-7 C2A Domain. Langmuir 2017, 33, 9222–9230. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.A.; Sadat, M.E.; Potter, S.J.; Mast, D.B.; Mohamed, D.F.; Habib, F.S.; Pauletti, G.M. Stability and magnetically induced heating behavior of lipid-coated Fe3O4 nanoparticles. Nanoscale Res. Lett. 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA J. Am. Med. Assoc. 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Liu, G.; Zhu, J.; Hou, Y.; Chen, X. Functional magnetic nanoparticles for non-viral gene delivery and MR imaging. Pharm. Res. 2014, 31, 1377–1389. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Kievit, F.M.; Stephen, Z.R.; Wang, K.; Dayringer, C.J.; Sham, J.G.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Nanoparticle mediated silencing of DNA repair sensitizes pediatric brain tumor cells to γ-irradiation. Mol. Oncol. 2015, 9, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Berg, K. The Photodegradation of Porphyrins in Cells Can Be Used To Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging: Progress continues in the development of smaller, more penetrable probes for biological imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutry, S.; Mahieu, I.; Elst, L.; Muller, R. Iron Oxide Based MR Contrast Agents: From Chemistry to Cell Labeling. Curr. Med. Chem. 2009, 16, 4712–4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wu, B.; Xing, D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Medarova, Z.; Pham, W.; Kim, Y.; Dai, G.; Moore, A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int. J. Cancer 2006, 118, 2796–2802. [Google Scholar] [CrossRef]
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, N.K.; Keliher, E.J.; Thurber, G.M.; Nahrendorf, M.; Weissleder, R. 18F labeled nanoparticles for in Vivo PET-CT imaging. Bioconjug. Chem. 2009, 20, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, P.; Alauddin, M.; Bankson, J.A.; Kirui, D.; Seifi, P.; Huls, H.; Lee, D.A.; Babakhani, A.; Ferrari, M.; Li, K.C.; et al. Tumor lysing genetically engineered t cells loaded with multi-modal imaging agents. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Freund, B.; Tromsdorf, U.I.; Bruns, O.T.; Heine, M.; Giemsa, A.; Bartelt, A.; Salmen, S.C.; Raabe, N.; Heeren, J.; Ittrich, H.; et al. A simple and widely applicable method to 59Fe-radiolabel monodisperse superparamagnetic iron oxide nanoparticles for in vivo quantification studies. ACS Nano 2012, 6, 7318–7325. [Google Scholar] [CrossRef]
- Yip, S.; Christofides, A.; Banerji, S.; Downes, M.R.; Izevbaye, I.; Lo, B.; MacMillan, A.; McCuaig, J.; Stockley, T.; Yousef, G.M.; et al. A Canadian guideline on the use of next-generation sequencing in oncology. Curr. Oncol. 2019, 26, e241–e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| SPION Coating | Size/Concentration | Additional Properties |
|---|---|---|
| Dextran | 150 nm-Endorem® 30 nm-Sinerem® at 11.3 μg/mL | 89% renal excretion by 56 days, can add amine to dextran for attachment of 18F for PET imaging |
| Poly(ethylene)glycol (PEG) | 50nm SPIONs at 1 mg/mL | Highest solubility and smallest hydrodynamic diameter, can attach folic acid to target upregulated receptors in cancerous tissue, can attach 68Ga for PET imaging by mixing with [68Ga]GaCl3 |
| Polyvinyl alcohol (PVA) | 45 nm SPIONs at 6 μg of Fe per mouse knee | Available amino or carboxyl groups to both regulate the charge of the coating as well as provide a point of attachment for peptides, proteins, antibodies, fluorescent dyes, and drugs. Can apply magnetic hyperthermia treatment to release attachments from heat-labile PVA coating. Specific uptake in arthritic joints |
| Silica | 4–33 nm | Provides a framework for the attachment of a variety of ligands such as 64Cu and 111In for PET and SPECT imaging respectively, Cu2+ to enhance endocytosis. Low cost and tolerant of a broad range of pH |
| Oleic acid | 10 nm | Rapidly removed from IONP core |
| Oleic acid-Pluronic | 10 nm SPIONs at 100 μg/mL | Prevents removal of oleic acid and allows for loading of water-insoluble drugs onto IONP surface |
| Tween 80 | 30 nm SPIONs at 100 μg/mL for 6 h | Hydrophilic, avoids uptake by RES to give prolonged half-life |
| Gold nanocages | 40 nm | Inert nature, extreme resistance to oxidation, absorbs external near-infrared light |
| Chitosan | 50 nm | Biocompatible and biodegradable surface, allows conjugation with TMZ and chlorotoxin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, N.R.; Port, J.D.; Pandey, M.K. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranostics 2020, 1, 105-135. https://doi.org/10.3390/jnt1010008
Nelson NR, Port JD, Pandey MK. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. Journal of Nanotheranostics. 2020; 1(1):105-135. https://doi.org/10.3390/jnt1010008
Chicago/Turabian StyleNelson, Nicholas R., John D. Port, and Mukesh K. Pandey. 2020. "Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review" Journal of Nanotheranostics 1, no. 1: 105-135. https://doi.org/10.3390/jnt1010008
APA StyleNelson, N. R., Port, J. D., & Pandey, M. K. (2020). Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. Journal of Nanotheranostics, 1(1), 105-135. https://doi.org/10.3390/jnt1010008







