Nanomaterial-Mediated Theranostics for Vascular Diseases
Abstract
:1. Introduction
2. Vascular Disease and Nanomedicine
3. Therapeutic Role in Vascular Diseases
4. Diagnostic Role in Vascular Diseases
5. Advantages and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.A.; Norredin, A. The Potential Contribution of Nanoparticles in the Treatment of Inflammatory Diseases. In Translational Studies on Inflammation; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Flores, A.M.; Ye, J.; Jarr, K.-U.; Hosseini-Nassab, N.; Smith, B.R.; Leeper, N.J. Nanoparticle Therapy for Vascular Diseases. Arter. Thromb. Vasc. Biol. 2019, 39, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.-Z.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, V.; Agrawal, D.K. The role of damage- and pathogen-associated molecular patterns in inflammation-mediated vulnerability of atherosclerotic plaques. Can. J. Physiol. Pharmacol. 2017, 95, 1245–1253. [Google Scholar] [CrossRef]
- Rai, V.; Agrawal, D.K. Pathogenesis of the plaque vulnerability in diabetes mellitus. In Mechanisms of Vascular Defects in Diabetes Mellitus; Springer: Berlin/Heidelberg, Germany, 2017; pp. 95–107. [Google Scholar]
- Rai, V.; Agrawal, D.K. Role of Vitamin D in Cardiovascular Diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef]
- Rao, V.H.; Rai, V.; Stoupa, S.; Subramanian, S.; Agrawal, D.K. Tumor necrosis factor-α regulates triggering receptor expressed on myeloid cells-1-dependent matrix metalloproteinases in the carotid plaques of symptomatic patients with carotid stenosis. Atherosclerosis 2016, 248, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Rao, V.H.; Shao, Z.; Agrawal, D.K. Dendritic Cells Expressing Triggering Receptor Expressed on Myeloid Cells-1 Correlate with Plaque Stability in Symptomatic and Asymptomatic Patients with Carotid Stenosis. PLoS ONE 2016, 11, e0154802. [Google Scholar] [CrossRef]
- Rao, V.H.; Rai, V.; Stoupa, S.; Subramanian, S.; Agrawal, D.K. Data on TREM-1 activation destabilizing carotid plaques. Data Brief 2016, 8, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J. Prevention and treatment of atherosclerosis: A practitioner’s guide for 2008. Am. J. Med. 2009, 122, S38–S50. [Google Scholar] [CrossRef]
- Rao, V.H.; Rai, V.; Stoupa, S.; Agrawal, D.K. Blockade of Ets-1 attenuates epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Am. J. Physiol. Circ. Physiol. 2015, 309, H1075–H1086. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.K.; Agrawal, T.; Rai, V.; Del Core, M.G.; Hunter, W.J., III; Agrawal, D.K.; Vitamin, D. Supplementation reduces intimal hyperplasia and restenosis following coronary intervention in atherosclerotic swine. PLoS ONE 2016, 11, e0156857. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J. West of Scotland Coronary Prevention Study Group: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Eng. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Transition at Technology in Cancer Research & Treatment. Technol. Cancer Res. Treat. 2015, 14, 147.
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, D. Peripheral vascular disease: Diagnosis and treatment. Am. Fam. Physician 2006, 73, 1971–1976. [Google Scholar] [PubMed]
- Forster, R.; Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Gene therapy for peripheral arterial disease. Cochrane Database Syst. Rev. 2018, 2018, CD012058. [Google Scholar] [CrossRef]
- Shimamura, M.; Nakagami, H.; Koriyama, H.; Morishita, R. Gene Therapy and Cell-Based Therapies for Therapeutic Angiogenesis in Peripheral Artery Disease. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Noukeu, L.C.; Wolf, J.; Yuan, B.; Banerjee, S.; Nguyen, K.T. Nanoparticles for Detection and Treatment of Peripheral Arterial Disease. Small 2018, 14, e1800644. [Google Scholar]
- Tu, C.; Das, S.; Baker, A.B.; Zoldan, J.; Suggs, L.J. Nanoscale Strategies: Treatment for Peripheral Vascular Disease and Critical Limb Ischemia. ACS Nano 2015, 9, 3436–3452. [Google Scholar] [CrossRef] [Green Version]
- Ambesh, P.; Campia, U.; Obiagwu, C.; Bansal, R.; Shetty, V.; Hollander, G.; Shani, J. Nanomedicine in coronary artery disease. Indian Heart J. 2017, 69, 244–251. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.-K.; Orekhov, A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, C.; Fayad, Z.A. Intraplaque and cellular distribution of dextran-coated iron oxide fluorescently labeled nanoparticles: Insights into atherothrombosis and plaque rupture. Circ. Cardiovasc. Imaging 2017, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Howarth, S.P.S.; Miller, S.R.; Trivedi, R.; Graves, M.; King-Im, J.U.; Li, Z.; Brown, A.P.; Kirkpatrick, P.J.; Gaunt, M.E.; et al. Assessment of Inflammatory Burden Contralateral to the Symptomatic Carotid Stenosis Using High-Resolution Ultrasmall, Superparamagnetic Iron Oxide–Enhanced MRI. Stroke 2006, 37, 2266–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.Y.; Howarth, S.P.S.; Miller, S.R.; Graves, M.J.; Jean-Marie, U.; Trivedi, R.A.; Li, Z.Y.; Walsh, S.R.; Brown, A.P.; Kirkpatrick, P.J.; et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: An ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.; Lipinski, M.J. Faculty Opinions recommendation of The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2009, 53, 2039–2050. [Google Scholar]
- Tang, T.Y.; Howarth, S.P.S.; Miller, S.R.; Graves, M.J.; Jean-Marie, U.; Li, Z.-Y.; Walsh, S.R.; Patterson, A.J.; Kirkpatrick, P.J.; Warburton, E.A.; et al. Correlation of Carotid Atheromatous Plaque Inflammation Using USPIO-Enhanced MR Imaging With Degree of Luminal Stenosis. Stroke 2008, 39, 2144–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egashira, K.; Nakano, K. Formulation of nanoparticle-eluting stents by a cationic electrodeposit coating technology: Efficient and safe nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. Hosokawa Powder Technol. Found. Annu. Rep. 2009, 17, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Tsukie, N.; Nakano, K.; Matoba, T.; Masuda, S.; Iwata, E.; Miyagawa, M.; Zhao, G.; Meng, W.; Kishimoto, J.; Sunagawa, K.; et al. Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J. Atheroscler. Thromb. 2012, 20, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Lee, Y.; Boire, T.C.; Lee, J.-B.; Kim, W.S.; Sung, H.-J. Recent strategies to design vascular theranostic nanoparticles. Nanotheranostics 2017, 1, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Gitsioudis, G.; Chatzizisis, Y.S.; Wolf, P.; Missiou, A.; Antoniadis, A.P.; Mitsouras, D.; Bartling, S.; Arica, Z.; Stuber, M.; Rybicki, F.J.; et al. Combined non-invasive assessment of endothelial shear stress and molecular imaging of inflammation for the prediction of inflamed plaque in hyperlipidaemic rabbit aortas. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishige, K.; Kacher, D.F.; Libby, P.; Josephson, L.; Ganz, P.; Weissleder, R.; Aikawa, M. High-Resolution Magnetic Resonance Imaging Enhanced With Superparamagnetic Nanoparticles Measures Macrophage Burden in Atherosclerosis. Circulation 2010, 122, 1707–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein-Merlob, A.F.; Hara, T.; McCarthy, J.R.; Mauskapf, A.; Hamilton, J.A.; Ntziachristos, V.; Libby, P.; Jaffer, F.A. Atheroma Susceptible to Thrombosis Exhibit Impaired Endothelial Permeability In Vivo as Assessed by Nanoparticle-Based Fluorescence Molecular Imaging. Circ. Cardiovasc. Imaging 2017, 10, e005813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.B.; Olzinski, A.R.; Bernard, R.E.; Aravindhan, K.; Mirabile, R.C.; Boyce, R.; Willette, R.N.; Jucker, B.M. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Segers, F.M.E.; Adel, B.D.; Bot, I.; Van Der Graaf, L.M.; Van Der Veer, E.P.; Gonzalez, W.; Raynal, I.; De Winther, M.; Wodzig, W.K.; Poelmann, R.; et al. Scavenger Receptor-AI–Targeted Iron Oxide Nanoparticles for In Vivo MRI Detection of Atherosclerotic Lesions. Arter. Thromb. Vasc. Biol. 2013, 33, 1812–1819. [Google Scholar] [CrossRef] [Green Version]
- Briley-Saebo, K.C.; Mani, V.; Hyafil, F.; Cornily, J.-C.; Fayad, Z.A. Fractionated feridex and positive contrast: In vivo MR imaging of atherosclerosis. Magn. Reson. Med. 2008, 59, 721–730. [Google Scholar] [CrossRef]
- Chen, W.; Cormode, D.P.; Vengrenyuk, Y.; Herranz, B.; Feig, J.E.; Klink, A.; Mulder, W.J.M.; Fisher, E.A.; Fayad, Z. Collagen-Specific Peptide Conjugated HDL Nanoparticles as MRI Contrast Agent to Evaluate Compositional Changes in Atherosclerotic Plaque Regression. JACC Cardiovasc. Imaging 2013, 6, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Ortega, C.A.; Si, K.; Molinaro, R.; Schoen, F.J.; Leitao, R.F.C.; Xu, X.; Mahmoudi, M.; Ahn, S.; Liu, J.; et al. Nanoparticles targeting extra domain B of fibronectin-specific to the atherosclerotic lesion types III, IV, and V-enhance plaque detection and cargo delivery. Theranostics 2018, 8, 6008–6024. [Google Scholar] [CrossRef]
- Shon, S.-M.; Choi, Y.; Kim, J.-Y.; Lee, N.K.; Park, J.-Y.; Schellingerhout, D.; Kim, N.-E. Photodynamic Therapy Using a Protease-Mediated Theranostic Agent Reduces Cathepsin-B Activity in Mouse Atheromata In Vivo. Arter. Thromb. Vasc. Biol. 2013, 33, 1360–1365. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Peng, Z.; Li, B.; Ye, K.; Zhang, Y.; Yuan, F.; Yang, X.; Huang, L.; Hu, J.; Lu, X. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale 2015, 7, 13991–14001. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Chen, C.; Zhang, X.; Tian, X.; Zhang, Y.; Shi, Z.; Liu, Q. Polymer-Lipid Hybrid Theranostic Nanoparticles Co-Delivering Ultrasmall Superparamagnetic Iron Oxide and Paclitaxel for Targeted Magnetic Resonance Imaging and Therapy in Atherosclerotic Plaque. J. Biomed. Nanotechnol. 2016, 12, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; Van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 1–12. [Google Scholar]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial ανβ3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, L.H.; Lee, S.H.; Norlander, A.E.; Montaniel, K.R.C.; Chen, W.; Harrison, D.G.; Sung, H.-J. Phage-Display-Guided Nanocarrier Targeting to Atheroprone Vasculature. ACS Nano 2015, 9, 4435–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Cao, L.; Shvartsman, D.; Silva, E.A.; Mooney, D.J. Targeted Delivery of Nanoparticles to Ischemic Muscle for Imaging and Therapeutic Angiogenesis. Nano Lett. 2010, 11, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wang, H.; Wang, Y.; Ren, F.; Yi, W.; Zhao, K.; Li, Z.; Zhao, Q.; Liu, Z.; Wu, H.; et al. Induction of Angiogenesis by Controlled Delivery of Vascular Endothelial Growth Factor Using Nanoparticles. Cardiovasc. Ther. 2013, 31, e12–e18. [Google Scholar] [CrossRef]
- Zhang, J.; Postovit, L.-M.; Wang, D.; Gardiner, R.B.; Harris, R.; Abdul, M.M.; Thomas, A.A. In Situ Loading of Basic Fibroblast Growth Factor Within Porous Silica Nanoparticles for a Prolonged Release. Nanoscale Res. Lett. 2009, 4, 1297–1302. [Google Scholar] [CrossRef] [Green Version]
- Golub, J.S.; Kim, Y.-T.; Duvall, C.L.; Bellamkonda, R.V.; Gupta, D.; Lin, A.S.; Weiss, D.; Taylor, W.R.; Guldberg, R.E. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am. J. Physiol. Circ. Physiol. 2010, 298, H1959–H1965. [Google Scholar] [CrossRef] [Green Version]
- Modery, C.L.; Ravikumar, M.; Wong, T.L.; Dzuricky, M.J.; Durongkaveroj, N.; Gupta, A.S. Heteromultivalent liposomal nanoconstructs for enhanced targeting and shear-stable binding to active platelets for site-selective vascular drug delivery. Biomaterials 2011, 32, 9504–9514. [Google Scholar] [CrossRef]
- Albrecht-Schgoer, K.; Barthelmes, J.; Schgoer, W.; Theurl, M.; Nardin, I.; Lener, D.; Gutmann, C.; Dünnhaupt, S.; Bernkop-Schnürch, A.; Kirchmair, R. Nanoparticular delivery system for a secretoneurin derivative induces angiogenesis in a hind limb ischemia model. J. Control. Release 2017, 250, 1–8. [Google Scholar] [CrossRef]
- Nagahama, R.; Matoba, T.; Nakano, K.; Kim-Mitsuyama, S.; Sunagawa, K.; Egashira, K. Nanoparticle-Mediated Delivery of Pioglitazone Enhances Therapeutic Neovascularization in a Murine Model of Hindlimb Ischemia. Arter. Thromb. Vasc. Biol. 2012, 32, 2427–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, B.; Kang, C.; Kim, J.; Yoo, D.; Cho, B.-R.; Kang, P.M.; Lee, D. H2O2 -responsive antioxidant polymeric nanoparticles as therapeutic agents for peripheral arterial disease. Int. J. Pharm. 2016, 511, 1022–1032. [Google Scholar] [PubMed]
- Cho, B.-R.; Ryu, D.R.; Lee, K.-S.; Lee, D.-K.; Bae, S.; Kang, D.G.; Ke, Q.; Singh, S.S.; Ha, K.-S.; Kwon, Y.-G.; et al. p-Hydroxybenzyl alcohol-containing biodegradable nanoparticle improves functional blood flow through angiogenesis in a mouse model of hindlimb ischemia. Biomaterials 2015, 53, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J. Nanotechnology in medical imaging: Probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigovan, M.; Kaye, E.; Lancelot, E.; Corot, C.; Provost, N.; Majd, Z.; Breisse, M.; Canet-Soulas, E. Anti-inflammatory drug evaluation in ApoE−/− mice by ultrasmall superparamagnetic iron oxide–enhanced magnetic resonance imaging. Investig. Radiol. 2012, 47, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Herborn, C.U.; Vogt, F.M.; Lauenstein, T.C.; Dirsch, O.; Corot, C.; Robert, P.; Ruehm, S.G. Magnetic resonance imaging of experimental atherosclerotic plaque: Comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 2006, 24, 388–393. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Jeong, L.; Park, S.; Lee, M.; Song, C.; Lee, D. Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Biomaterials 2019, 192, 282–291. [Google Scholar] [CrossRef]
- Jung, E.; Noh, J.; Kang, C.; Yoo, D.; Song, C.; Lee, D. Ultrasound imaging and on-demand therapy of peripheral arterial diseases using H2O2-Activated bubble generating anti-inflammatory polymer particles. Biomaterials 2018, 179, 175–185. [Google Scholar] [CrossRef]
- England, C.G.; Im, H.-J.; Feng, L.; Chen, F.; Graves, S.A.; Hernandez, R.; Orbay, H.; Xu, C.; Cho, S.Y.; Nickles, R.J.; et al. Re-assessing the enhanced permeability and retention effect in peripheral arterial disease using radiolabeled long circulating nanoparticles. Biomaterials 2016, 100, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Im, H.-J.; England, C.G.; Feng, L.; Graves, S.A.; Hernandez, R.; Nickles, R.J.; Liu, Z.; Lee, D.S.; Cho, S.Y.; Cai, W. Accelerated Blood Clearance Phenomenon Reduces the Passive Targeting of PEGylated Nanoparticles in Peripheral Arterial Disease. ACS Appl. Mater. Interfaces 2016, 8, 17955–17963. [Google Scholar] [CrossRef] [Green Version]
- Konopka, C.J.; Woźniak, M.; Hedhli, J.; Płoska, A.; Schwartz-Duval, A.S.; Siekierzycka, A.; Pan, D.; Munirathinam, G.; Dobrucki, I.T.; Kalinowski, L.; et al. Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles. Theranostics 2018, 8, 5012–5024. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.; Choi, J.; Lee, J.; Huang, N.; Cooke, J.; Yang, F. Polymer-DNA Nanoparticle-Induced CXCR4 Overexpression Improves Stem Cell Engraftment and Tissue Regeneration in a Mouse Hindlimb Ischemia Model. Theranostics 2016, 6, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.H.; Schoormans, J.; Stiekema, L.C.; Calcagno, C.; Cicha, I.; Alexiou, C.; Strijkers, G.J.; Nederveen, A.J.; Stroes, E.S.; Coolen, B.F. Plaque Permeability Assessed With DCE-MRI Associates With USPIO Uptake in Patients With Peripheral Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Nachuraju, P.; Friedman, A.; Friedman, J.M.; Cabrales, P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation 2011, 82, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.M.; Caruthers, S.D.; Allen, J.S.; Cai, K.; Williams, T.A.; Lanza, G.M.; Wickline, S.A. Molecular imaging of angiogenic therapy in peripheral vascular disease with ανβ3-integrin-targeted nanoparticles. Magn. Reson. Med. 2010, 64, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-Y.; Han, P.; Zhang, W.-H.; Liu, B.; Li, H.-L.; Wang, H.-J.; Huang, R.-P. Serum bilirubin level is negatively correlated with disease progression of peripheral arterial disease: An observational cohort study. Angiology 2012, 63, 248–253. [Google Scholar] [CrossRef]
- Fan, J.; Jouni, H.; Khaleghi, M.; Bailey, K.R.; Kullo, I.J. Serum N-Terminal Pro-B-Type Natriuretic Peptide Levels Are Associated With Functional Capacity in Patients With Peripheral Arterial Disease. Angiology 2011, 63, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Khandanpour, N.; Jennings, B.; Armon, M.P.; Wright, A.; Clark, A.B.; Meyer, F.J.; Willis, G. Do Novel Risk Biomarkers Reflect the Severity of Peripheral Arterial Disease? Angiology 2010, 62, 126–133. [Google Scholar] [CrossRef]
- Amirbekian, V.; Lipinski, M.J.; Briley-Saebo, K.C.; Amirbekian, S.; Aguinaldo, J.G.S.; Weinreb, D.B.; Vucic, E.; Frias, J.C.; Hyafil, F.; Mani, V.; et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl. Acad. Sci. USA 2007, 104, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Briley-Saebo, K.C.; Cho, Y.S.; Shaw, P.X.; Ryu, S.K.; Mani, V.; Dickson, S.; Izadmehr, E.; Green, S.; Fayad, Z.A.; Tsimikas, S. Targeted Iron Oxide Particles for In Vivo Magnetic Resonance Detection of Atherosclerotic Lesions With Antibodies Directed to Oxidation-Specific Epitopes. J. Am. Coll. Cardiol. 2011, 57, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Woodside, D.G. Nanoparticle Imaging of Vascular Inflammation and Remodeling in Atherosclerotic Disease. Curr. Cardiovasc. Imaging Rep. 2019, 12, 28. [Google Scholar] [CrossRef]
- Li, T.; Liang, W.; Xiao, X.; Qian, Y. Nanotechnology, an alternative with promising prospects and advantages for the treatment of cardiovascular diseases. Int. J. Nanomed. 2018, 13, 7349–7362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.-C.; Yoon, H.-J.; Kim, K.-H.; Kim, H.-T.; Yoon, Y.-H.; Kim, J.-K. Fluorescence Kinetics of Protoporphyrin-IX Induced from 5-ALA Compounds in Rabbit Postballoon Injury Model for ALA-Photoangioplasty. Photochem. Photobiol. 2008, 84, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, K.D.O.; Da Silva, M.N.; Sicchieri, L.B.; Silva, F.R.D.O.; De Matos, R.A.; Courrol, L.C. Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 2015, 140, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, X. Nanoplatforms for Targeted Molecular Imaging in Living Subjects. Small 2007, 3, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-Q.; Kang, L.; Yin, X.-Z.; Jin, Z.-H.; Gao, Z.-G. Synthesis of PEGylated hyaluronic acid for loading dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt) in nanoparticles for cancer treatment. Chin. Chem. Lett. 2015, 26, 695–699. [Google Scholar] [CrossRef]
- Gupta, P.; Garcia, E.; Sarkar, A.; Kapoor, S.; Rafiq, K.; Chand, H.S.; Jayant, R.D. Nanoparticle Based Treatment for Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Targets 2019, 19, 33–44. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Kharlamov, M.A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica–gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef]
- Kharlamov, M.A.N.; Feinstein, J.A.; Cramer, J.A.; Boothroyd, J.A.; Shishkina, E.; Shur, V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Futur. Cardiol. 2017, 13, 345–363. [Google Scholar] [CrossRef]
- Ambesh, P.; Angeli, D.G. Nanotechnology in neurology: Genesis, current status, and future prospects. Ann. Indian Acad. Neurol. 2015, 18, 382–386. [Google Scholar] [PubMed]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chang, D.W.; Dai, L.; Hong, Y. DNA Damage Induced by Multiwalled Carbon Nanotubes in Mouse Embryonic Stem Cells. Nano Lett. 2007, 7, 3592–3597. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef] [Green Version]
- Hoet, P.; Legiest, B.; Geys, J.; Nemery, B. Do Nanomedicines Require Novel Safety Assessments to Ensure their Safety for Long-Term Human Use? Drug Saf. 2009, 32, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.; Popov, A.; Shcherbakov, A.; Ivanov, V. Layer-by-layer capsules as smart delivery systems of CeO2 nanoparticle-based theranostic agents. Nanosyst. Phys. Chem. Math. 2017, 62, 1064–1079. [Google Scholar] [CrossRef]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
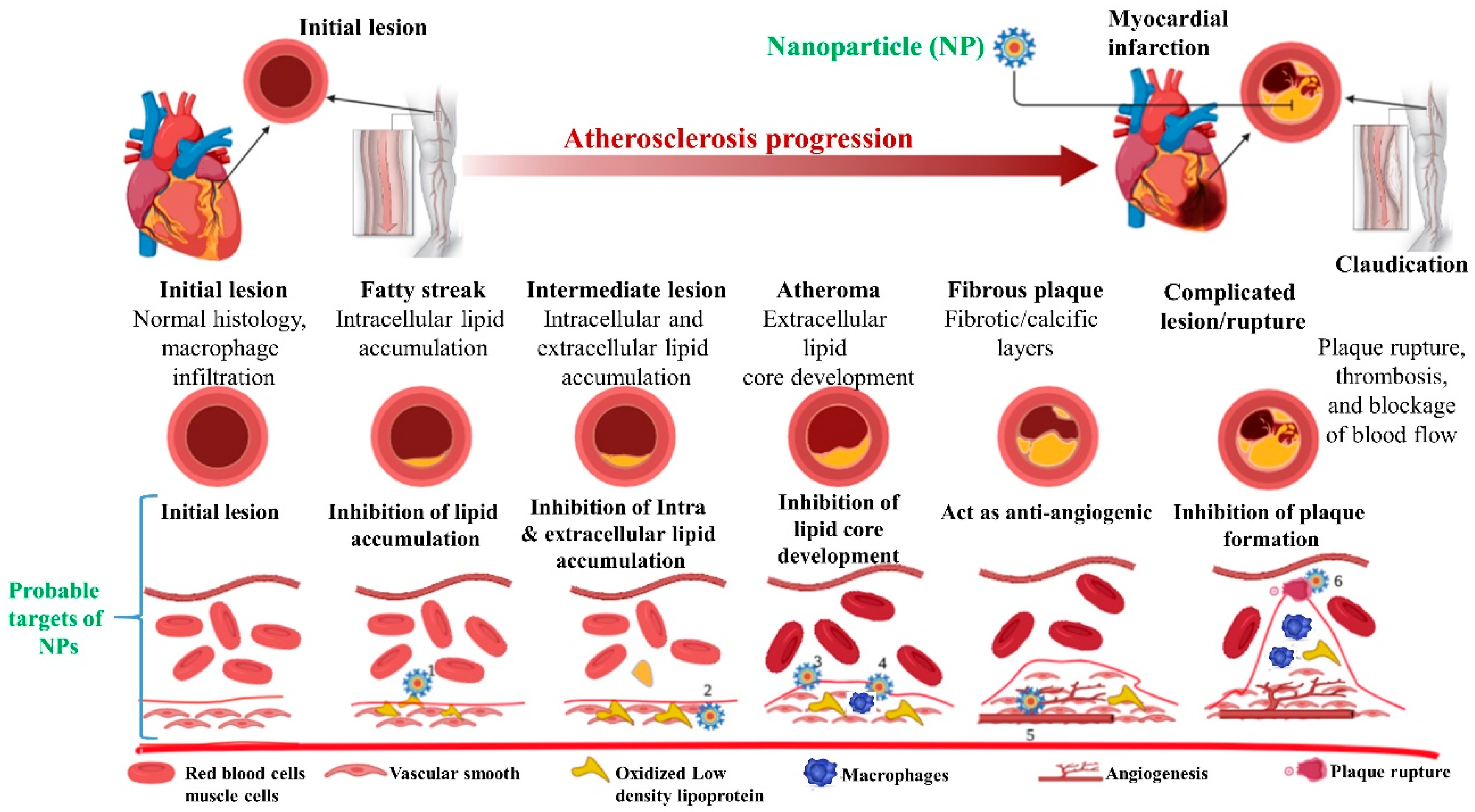
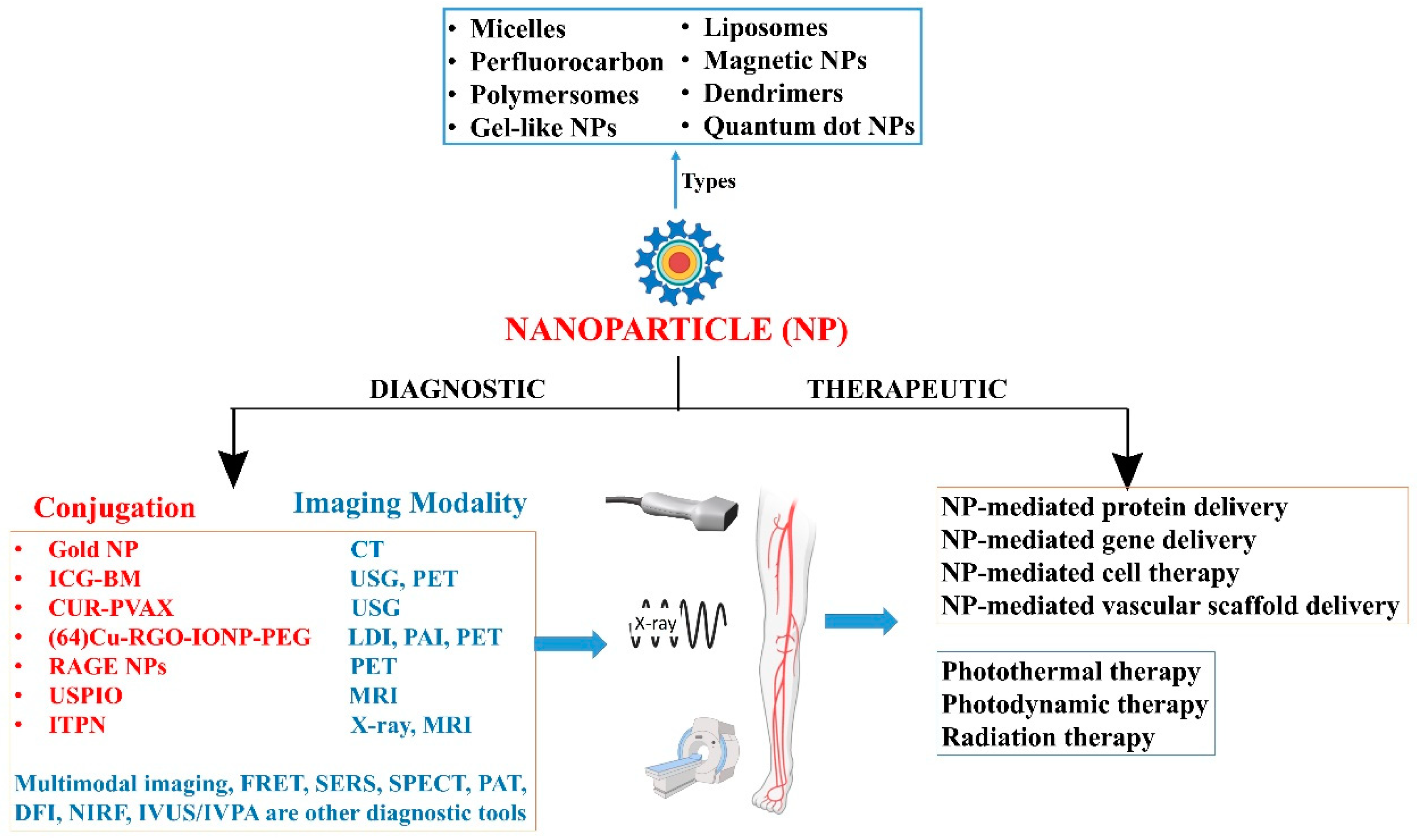
| Study | Nanoparticles | Animal Model | Outcome |
|---|---|---|---|
| [33] | P904 | Hereditary hyperlipidemic rabbit | P904 accumulation and low endothelial shear stress are independent predictors of plaque progression |
| [34] | Dextran-coated monocrystalline iron oxide | Hereditary hyperlipidemic rabbit | Accumulation in vessel wall correlating with plaque macrophages and the accumulation reduced with rosuvastatin |
| [35] | CLIO-CyAm7 | Rabbit with atherosclerosis induced by aortic balloon injury and high-cholesterol diet | Significantly higher CLIO-CyAm7 accumulation in thrombosed than in nonthrombosed plaques |
| [36] | USPIO | ApoE−/− mice model of atherosclerosis | Noninvasive assessment of USPIO uptake is a marker for inflammation in murine atherosclerotic plaque Reduction in ferumoxtran-10 uptake after treatment with p38-MAPK inhibitor |
| [37] | USPIO conjugated to SR-AI ligand | ApoE−/− mice model of atherosclerosis | SR-AI–targeted USPIO displayed accelerated plasma decay and a 3.5-fold increase accumulation in atherosclerotic plaque SR-AI–targeted molecular imaging of USPIO-based contrast may be used to detect inflammatory plaques |
| [38] | Feridex-a dextran-based USPIO | High cholesterol fed rabbits | 15 nm fractionated, but not nonfractionated feridex gets deposited in atherosclerotic plaques and thus size should be further investigated |
| [39] | HDL NPs collagen-specific EP3533 peptides (EP3533-HDL) | Reversa mouse model of atherosclerosis regression | HDL NPs may be used to monitor and evaluate compositional changes in atherosclerotic plaque regression |
| [40] | FN-EDB-specific Gd NPs (APTFN-EDB-[Gd]NP) | Murine ApoE−/− model of atherosclerosis | Augmented FN-EDB expression in Type III, IV, and V atheroma and these NPs may be used to identify and/or deliver agents locally to a subset of atherosclerotic plaques. |
| [41] | Cathepsin-B activatable L-SR15 | Apolipoprotein E knock-out atheromatous mouse model | Selective apoptotic attenuation of macrophages Reduction in cathepsin-B protease activity |
| [42] | Gold nanorods (Au NRs) | Apolipoprotein E knockout mice model with femoral artery restenosis | Ablation of inflammatory macrophage layer in Au NRs group compared to the controls Au NRs are effective for in-vivo imaging and photothermal therapy of inflammatory macrophages |
| [43] | UP-NP-C11 | Rabbit | Significantly higher accumulation of NPs atherosclerotic plaques compared to the control condition |
| [44] | Statin-rHDL NPs | ApoE-knockout mouse model of atherosclerosis | 3-month low-dose statin-rHDL treatment inhibits plaque inflammation progression 1-week of high-dose regimen markedly decreases inflammation in advanced atherosclerotic plaques |
| [45] | Paramagnetic NPs loaded with anti-angiogenic drug fumagillin | Cholesterol-fed rabbit | Targets ανβ3 integrin Showed an anti-angiogenic response compared to controls Molecular imaging combined with drug delivery with NPs noninvasively define atherosclerotic burden and response to treatment |
| [46] | PREY-nanocarriers loaded with BH4 | Fat-fed atheroprone mice (ApoE−/−) | Reduced plaque burden in partially ligated left carotid artery A potential strategy to prevent atherosclerotic plaque formation |
| Nanoparticle | Mechanism | Imaging Method | Outcome |
|---|---|---|---|
| Indocyanine green-loaded boronated maltodextrin (ICG-BM) nanoparticles [59] | Real time detection of ROS through H2O2-activatable CO2 bubble generation by ICG-BM | Photoacoustic imaging with an ultrasound machine and a pulsed laser system | ICG-BM nanoparticles could be used as multiple contrast agents for enhanced fluorescence, ultrasound, and photoacoustic imaging. |
| Curcumin (CUR) loaded vanillyl alcohol-incorporated copolyoxalate (PVAX) nanoparticles (CUR-PVAX) [60] | Generation of echogenic CO2 bubbles through H2O2 | Ultrasound | CUR-PVAX enhances the ultrasound signal and also act therapeutically to suppress expression of pro-inflammatory cytokines. |
| (64)Cu-labeled PEGylated reduced graphene oxide—iron oxide nanoparticles ((64)Cu-RGO-IONP-PEG) [61] | Passive accumulation in ischemic tissues through enhanced permeability and retention (EPR) effect. | Laser Doppler imaging, Photoacoustic imaging, and PET | Quantitative confirmation of accumulation of PEGylated nanoparticles in Ischemic tissues of hindlimb. |
| PEGylated long circulating organic-inorganic hybrid nanoparticles [62] | Passive accumulation in ischemic tissues with reduced ABC (accelerated blood clearance) effect. | Positron emission tomography (PET) | Validation of ABC phenomenon |
| Receptor for advanced glycation end products (RAGE) multimodal nanoparticle [63] | Specific molecular targeting of RAGE expressed in hindlimb ischemia murine model | PET | Non-invasive examination of cellular, tissue and whole body RAGE levels is feasible |
| biodegradable poly(β-amino ester) (PBAE)-based CXCR4 pDNA nanoparticles [64] | Polyester nanoparticles enhance transfection efficiency of adipose-derived stem cells (ADSCs) in vivo. | Bioluminescence imaging of the GFP labeled cells and laser Doppler system for blood flow. | Complete limb salvage in a mouse ischemia limb model. |
| ultrasmall superparamagnetic iron-oxide (USPIO) (ferumoxytol) nanoparticles [65] | Long circulating nanoparticles such as USPIOs are taken up by tissue macrophages that can be imaged in plaques using MRI | Dynamic contrast-enhanced (DCE)-MRI using gadolinium-based contrast agents | Confirmation of accumulation of USPIOs in atherosclerotic plaques, assessed by quantitative DCE-MRI in PAD patients. |
| Nitric Oxide releasing nanoparticles (NO-nps) [66] | Delivery of NO with nanoparticles | NA | Stabilization of hemodynamics preservation of micro vascular perfusion in acute hemorrhage |
| alpha(nu)beta(3)-integrin-targeted perfluorocarbon nanoparticles. [67] | Alpha(nu)beta(3)-integrin is a biomarker for neovascular proliferation in angiogenesis. | MRI and X-ray angiography | In vivo (rabbit femoral artery) demonstration of non-invasive molecular imaging of angiogenesis. |
| Advantages | Disadvantages/Limitations |
|---|---|
| ➢ Site-specific targeted delivery ➢ Faster and accurate delivery ➢ Feasibility of therapy at cellular level ➢ Refined drug production ➢ Drug tailoring at molecular level ➢ Diagnostic, therapeutic and theranostic ➢ Increased vascular permeability ➢ Drug delivery related • ↓systemic effects • ↓Drug-drug interaction • ↓Off target effects • ↓Drug resistance • ↓drug degradation • ↑Drug bioavailability • ↑Biological efficacy • ↑Safety and effectiveness • ↑Water solubility • ↑Drug stability • ↑Drug uptake • ↑Therapeutic index | ➢ Possibilities of contamination ➢ Nanoparticle-mediated infection/sepsis ➢ Nanomaterial-related toxicity ➢ Issues with biodegradability of nanomaterials ➢ Complex synthesis ➢ Low sensitivity ➢ Limited targeted ability ➢ Expensive ➢ Difficult to assess the blood distribution ➢ Discontinuation of therapy is not possible ➢ Autonomic imbalance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, S.; Nooti, S.K.; Singh, H.; Rai, V. Nanomaterial-Mediated Theranostics for Vascular Diseases. J. Nanotheranostics 2021, 2, 1-15. https://doi.org/10.3390/jnt2010001
Agrawal S, Nooti SK, Singh H, Rai V. Nanomaterial-Mediated Theranostics for Vascular Diseases. Journal of Nanotheranostics. 2021; 2(1):1-15. https://doi.org/10.3390/jnt2010001
Chicago/Turabian StyleAgrawal, Swati, Sunil K. Nooti, Harbinder Singh, and Vikrant Rai. 2021. "Nanomaterial-Mediated Theranostics for Vascular Diseases" Journal of Nanotheranostics 2, no. 1: 1-15. https://doi.org/10.3390/jnt2010001
APA StyleAgrawal, S., Nooti, S. K., Singh, H., & Rai, V. (2021). Nanomaterial-Mediated Theranostics for Vascular Diseases. Journal of Nanotheranostics, 2(1), 1-15. https://doi.org/10.3390/jnt2010001






