Photophysical Process of Hypocrellin-Based Photodynamic Therapy: An Efficient Antimicrobial Strategy for Overcoming Multidrug Resistance
Abstract
1. Introduction
Conventional Treatments for AMR and Their Limitations
2. Photodynamic Therapy as an Alternative Antimicrobial Strategy
3. Biophysical Mechanism of PDT in AMR
3.1. Photosensitizer Activation
3.2. Reactive Oxygen Species Generation
3.2.1. Type I Mechanism: Electron Transfer-Based Reactions
3.2.2. Type II Mechanism: Energy Transfer Reactions
3.3. ROS Pathway Divergence Between Antimicrobial and Antitumor PDT
4. Advantages of PDT over Conventional Antimicrobial Treatments and Limitations
5. Natural Sensitizers over Commercial Sensitizers in PDT
6. Hypocrellin as a Natural Photosensitizer for PDT
6.1. Photophysical and Photochemical Properties
6.2. Photodynamic Activity of Hypocrellin
7. Comparative Analysis of Hypocrellin with Other Sensitizers
7.1. Biofilm Penetration and Antimicrobial Effectiveness
7.2. Pharmacokinetics and Systemic Safety
Plasma Half-Life and Clearance Rate
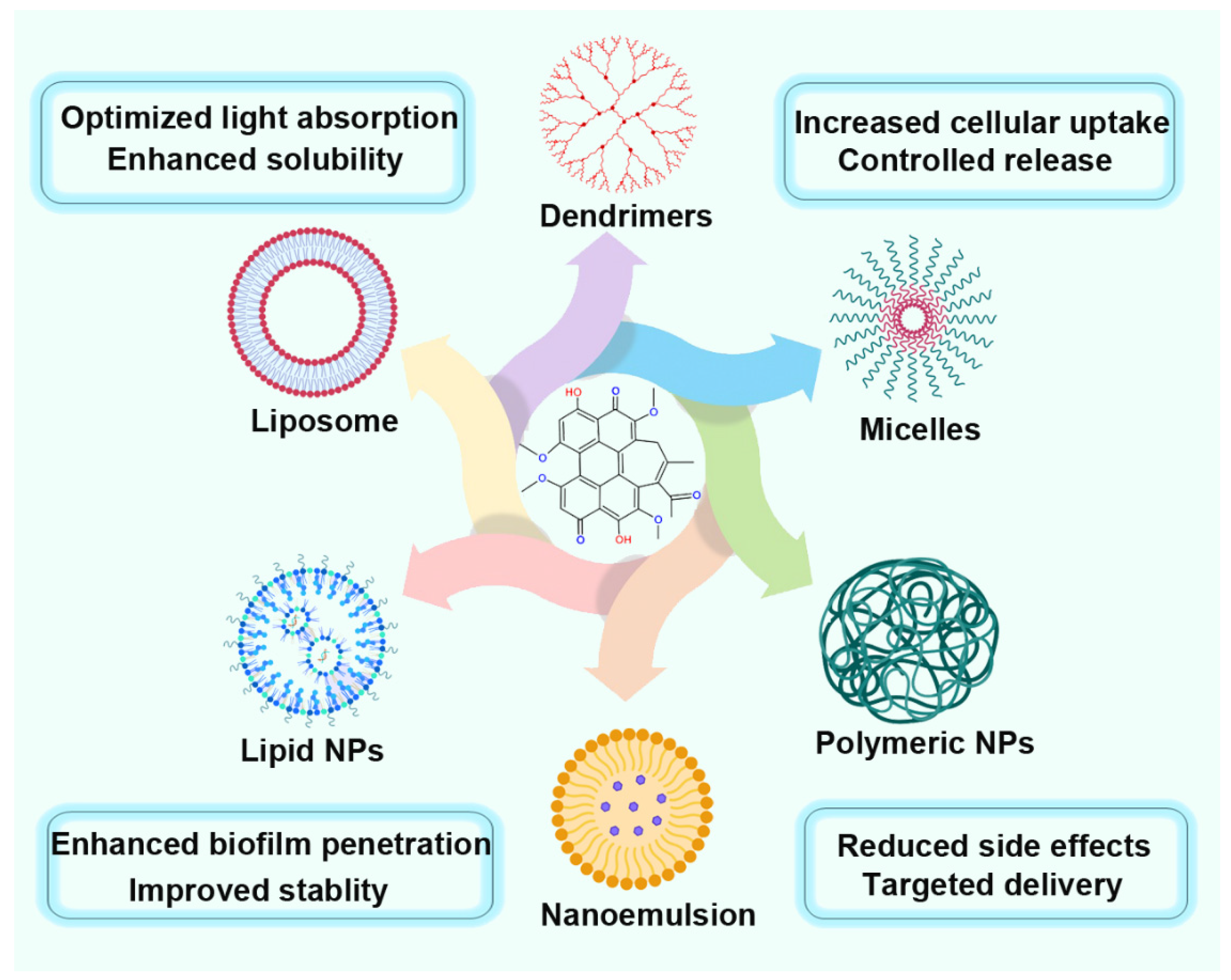
8. Nanoformulations of Hypocrellin for Enhanced PDT
| Nano Carrier | Nanoformulation | Hypocrellin Type | Application | Key Observations | Ref. |
|---|---|---|---|---|---|
| Mixed polymer NPs | Transferrin-modified Poly(D, L-Lactide-co-glycolide) and corboxymethyl chitosan NPs | A | PDT and targeted therapy | Strong ROS, significant photo-cytotoxicity, and 63% tumor inhibition rate | [126] |
| Copolymeric NPs | Nano silver-loaded Poly(lactide-co-glycolide)- d-α-tocopheryl polyethylene glycol 1000 succinate NPs | B | PDT | Higher encapsulation efficiency 84.06 ± 11.43%, ROS production 90.62 ± 20.12, significant antiangiogenic effect 89.9%, and superior phototoxic effect 85.5% | [127] |
| Polymer NPs | Poly(D,L-loactic-co-glycolic NPs | A | PDT and cancer therapy | Enhanced photostability, reduced dark cytotoxicity, exceptional antitumor property, and ROS production ability | [128] |
| Polymer NPs | Hyp-B and Paclitaxeel-encapsulated hyaluronic acid–ceramide NPs | B | PDT and chemotherapy | Higher phototoxicity and encapsulation efficiency of 70%, and exceptional antitumor properties | [129] |
| Composite NPs (polymer and metal NPs) | Poly(D,L-loactic-co-glycolic NPs incorporated nano silver | B | PDT | Enhanced singlet oxygen production, higher ROS production 138.02 ± 13.23, superior phototoxic effect 82.2%, and significant anti-angiogenic effect | [130] |
| Polymer NPs | Poly(D,L-loactic-co-glycolic Nps | A | Drug delivery, PDT | pH-dependent delivery, higher solubility, superior stability, and enhanced bioavailability | [131] |
| Polymer NPs | Neutrophil membrane coated Poly(D,L-loactic-co-glycolic NPs | B | NIR fluorescence imaging, targeted therapy, and PDT | Inhibit the expression of JUNB and promote ROS production, exceptional antitumor efficacy, and better anti-inflammation effect | [132] |
| Polymer NPs | Transferrin-modified HepG2 cell membrane-coated hypocrellin bionic NPs | B | Targeted therapy and fluorescence imaging | Long-term stability, exceptional biocompatibility, lower toxicity, and higher ROS production | [133] |
| Composite | Hypocrellin–cisplatin- intercalated hectorite nanoformulation | A | Chemo therapy and PDT | Exceptional biocompatibility, high payload, controlled release, and higher photostability and photobleachability | [134] |
| Mixed polymer | Self-assembled 1,2-diamino-2-methyl- propane and PEG-PLGA nanovesicles | B | PDT, PTT, fluorescence imaging, and photoacoustic imaging | Exceptional photothermal stability, higher singlet oxygen production, and photothermal conversion efficiency. | [135] |
| Nanofiber | Poly(L-lactic acid)–silk fibroin nanofiber | A | Chemotherapy and drug delivery | Exceptional pH stability, strong inhibitory effects, and controlled drug release | [136] |
| Micelles | Folate-conjugated poly(ethylene glycol)-poly (lactic acid) micelle | B | Targeted therapy and PDT | High drug loading capacity, quite good biocompatibility, controlled release, and enhanced targeting and antitumor effect | [137] |
| Nanorods | Dopamine-modified hypocrellin derivative-loaded calcium phosphate nanorods | B | Fluorescence imaging and PDT | Lower cytotoxicity, good enough biocompatibility, efficient singlet oxygen production, and enhanced antitumor activity | [138] |
8.1. Liposomal Hypocrellin
8.2. Polymeric Nanoparticles
8.3. Inorganic Nanoparticles for Hypocrellin-Based PDT
8.4. Micelle-Based Hypocrellin Nanoformulations
9. Future Perspectives and Challenges
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | absorption, distribution, metabolism, and excretion |
| AMR | antimicrobial resistance |
| ATCC | American Type Culture Collection |
| aPDI | antimicrobial PDI |
| aPDT | antimicrobial PDT |
| BCP | bromocresol purple |
| C. | Candida |
| CA | community-associated |
| CF | Cystic Fibrosys |
| CFU | colony-formated unit |
| CNV | choroidal neovascularization |
| D | dyxtro-tartaric acid (isomer form) |
| DNA | deoxyribonucleic acid |
| DNase | deoxyribonuclease |
| EPR | enhanced permeability and retention |
| EPS | extracellular polymeric substance |
| ESBL | extended-spectrum beta-lactamase |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter (species) |
| FA | folate |
| FICI | fractional inhibitory concentration index |
| FLC | fluconazole |
| FR | folate receptor |
| GAP | Global Action Plan |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| GSH | glutathione |
| HA | Hyp-A |
| HAI | hospital-acquired infection |
| HB | Hyp-B |
| HNK | honokiol |
| HepG2 | human liver cancer cell line, specifically derived from a hepatoblastoma |
| Hyp | hypocrellin |
| ICD | immunogenic cell death |
| IC50 | half-maximum inhibitory concentration |
| ISC | intersystem crossing |
| JUNB | proto-oncogene and a member of the AP-1 (Activator Protein-1) transcription factor family |
| KP | Klebsiella pneumoniae |
| L | levo-tartaric acid (isomer form) |
| L. | Leishmania |
| LED | Light Emitting Diode |
| LHB | liposomal Hyp-B |
| LL-37 | human antimicrobial peptide |
| log p | partition coefficient |
| log10 | log10CFU (inhibition efficiency) |
| MB | methylene blue |
| MBC | minimum bactericidal concentratio |
| MDR | multidrug-resistant |
| MFC | minimum fungicidal concentration |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-resistant Staphylococcus aureus |
| MTT | 3-(4,5-di Methyl Thiazol-2-yl)-2,5-diphenylTetrazolium (bromide) |
| m | methoxy (for example, mPEG–PCL) |
| NBD | 7-nitro-2,1,3-benzoxadiazole |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIR | near-infrared |
| NP | nanoparticle |
| OMKO1 | opportunistic pathogene that infects and kills bacteria, particularly P. aeruginosa |
| OXPHOS | oxidative phosphorylation |
| P. | Pseudomonas |
| Pa | pheophorbide a |
| PCL | poly(ε-caprolactone) |
| PDMS | poly(dimethylsiloxane) |
| PDT | photodynamic therapy |
| PDI | photodynamic inactivation |
| PEG | poly(ethylene glycol) |
| PET | Photoinduced electron transfer |
| PLGA | poly(lactic co-glycilic acid) |
| PS | photosensitizer |
| PTT | phototermal therapy |
| pH | potential of hydrogen (density of hydrogen (alkalinity or acidity) in a substance) |
| Q (band) | 33–50 GHz electromagnetic spectrum frequency region |
| ROS | reactive oxygen species |
| S. | Staphylococcus |
| SEM | scanning electron microscopy |
| SKOV3 | Human ovarian cancel cell line |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SNU 80 | Seoul National University cell line |
| SOD | superoxide dismutase |
| S0 | ground singlet state |
| S1 | Electronically excited singlet state |
| TEM | transmission electron microscopy |
| TIVP-H | Type IV pili-mediated adherence |
| TMPyP | meso-tetrakis(N-methyl-4-pyridyl)porphine tetrakis(p-toluenesulfonate) |
| T1 | triplet state |
| UV | ultra-violet |
| VRSA | vancomycin-resistant Staphylococcus aureus |
| vis | visible |
| 4-log (reduction) | 99.9% reductionin the number of microorganisms |
| π-conjugated system | system of connected p-orbitals with delocalized electrons |
References
- van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, A.; Girigoswami, K.; Harini, K.; Pallavi, P.; Gowtham, P.; Girigoswami, A. A review of the current state of probiotic nanoencapsulation and its future prospects in biomedical applications. Biocatal. Agric. Biotechnol. 2024, 57, 103101. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, cmr.00181-19. [Google Scholar] [CrossRef]
- Venkatesan, L.S.; Sathishkumar, P. Combination therapy of natural products for the treatment of ESKAPE pathogens. Nat. Prod. Res. 2024. in print. [Google Scholar] [CrossRef]
- Dey, N.; Kamatchi, C.; Vickram, A.S.; Anbarasu, K.; Thanigaivel, S.; Palanivelu, J.; Pugazhendhi, A.; Ponnusamy, V.K. Role of nanomaterials in deactivating multiple drug resistance efflux pumps—A review. Environ. Res. 2022, 204, 111968. [Google Scholar] [CrossRef]
- Antinate Shilpa, S.; Subbulakshmi, M.S.; Hikku, G.S. Nanoparticles of metal/metal oxide embedded fabrics to impart antibacterial activity to counteract hospital acquired infections. Engin. Res. Express 2022, 4, 032002. [Google Scholar] [CrossRef]
- Lebreton, F.; van Schaik, W.; McGuire, A.M.; Godfrey, P.; Griggs, A.; Mazumdar, V.; Corander, J.; Cheng, L.; Saif, S.; Young, S.; et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 2013, 4, mbio.00534-13. [Google Scholar] [CrossRef]
- Alduhaidhawi, A.H.M.; AlHuchaimi, S.N.; Al-Mayah, T.A.; Al-Ouqaili, M.T.S.; Alkafaas, S.S.; Muthupandian, S.; Saki, M. Prevalence of CRISPR-Cas systems and their possible association with antibiotic resistance in Enterococcus faecalis and Enterococcus faecium collected from hospital Wastewater. Infect. Drug Resist. 2022, 15, 1143–1154. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter Infection. N. Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Pereira, M.O. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—A review. Pathogens 2014, 3, 680–703. [Google Scholar] [CrossRef]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef]
- Jolivet-Gougeon, A.; Bonnaure-Mallet, M. Biofilms as a mechanism of bacterial resistance. Drug Discov. Today Technol. 2014, 11, 49–56. [Google Scholar] [CrossRef]
- Bano, S.; Hassan, N.; Rafiq, M.; Hassan, F.; Rehman, M.; Iqbal, N.; Ali, H.; Hasan, F.; Kang, Y.-Q. Biofilms as battlefield armor for bacteria against antibiotics: Challenges and combating strategies. Microorganisms 2023, 11, 2595. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Azmy, H.; Sofy, A.; Aboseidah, A.; El-Morsi, E.-S.; Elmorshedy, H.; Hmed, A. Combating multidrug resistance: The potential of antimicrobial peptides and biofilm challenges. Int. J. Innov. Sci. Res. Technol. 2024, 9, 29. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.; Baquero, F.; Martínez, J. Defining and combating antibiotic resistance from One Health and Global-Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Comeau, P.; Manso, A. A systematic evaluation of curcumin concentrations and blue light parameters towards antimicrobial photodynamic therapy against cariogenic microorganisms. Pharmaceutics 2023, 15, 2707. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Vollmer, A.; Al-Ahmad, A.; Argyropoulou, A.; Thurnheer, T.; Hellwig, E.; Attin, T.; Vach, K.; Wittmer, A.; Ferguson, K.; Skaltsounis, A.L.; et al. Antimicrobial photoinactivation using visible light plus water-filtered infrared-A (VIS + wIRA) and Hypericum perforatum modifies In Situ oral biofilms. Sci. Rep. 2019, 9, 20325. [Google Scholar] [CrossRef]
- Gugu Nkosi, P.W.; Chandran, R.; Abrahamse, H. Hypocrellin: A natural hotosensitizer and nano-formulation for enhanced molecular targeting of PDT of melanoma. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1997. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Vega, N.M.; Gore, J. Collective antibiotic resistance: Mechanisms and implications. Curr. Opin. Microbiol. 2014, 21, 28–34. [Google Scholar] [CrossRef]
- Jin, J.; Guo, N.; Zhang, J.; Ding, Y.; Tang, X.; Liang, J.; Li, L.; Deng, X.; Yu, L. The synergy of honokiol and fluconazole against clinical isolates of azole-resistant Candida albicans. Lett. Appl. Microbiol. 2010, 51, 351–357. [Google Scholar] [CrossRef]
- Sullivan, G.J.; Delgado, N.N.; Maharjan, R.; Cain, A.K. How antibiotics work together: Molecular mechanisms behind combination therapy. Curr. Opin. Microbiol. 2020, 57, 31–40. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Stanley, G.L.; Kortright, K.E.; Modak, M.; Ott, I.M.; Sun, Y.; Würstle, S.; Grun, C.; Kazmierczak, B.; Rajagopalan, G.; et al. Personalized inhaled bacteriophage therapy decreases multidrug-resistant Pseudomonas aeruginosa. medRxiv 2023. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial peptides therapy: An emerging alternative for treating drug-resistant bacteria. Yale J. Biol. Med. (YJBM) 2022, 95, 445–463. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9765339/ (accessed on 11 June 2025). [PubMed]
- Fadaka, A.O.; Sibuyi, N.R.; Madiehe, A.M.; Meyer, M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Seixas, A.M.M.; Sousa, S.A.; Leitão, J.H. Antibody-based immunotherapies as a tool for tackling multidrug-resistant bacterial infections. Vaccines 2022, 10, 1789. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting biofilms therapy: Current research strategies and development hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage therapy: A targeted approach to overcoming antibiotic resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Pallavi, P.; Sharmiladevi, P.; Haribabu, V.; Girigoswami, K.; Girigoswami, A. A nano approach to formulate photosensitizers for photodynamic therapy. Curr. Nanosci. 2022, 18, 675–689. [Google Scholar] [CrossRef]
- Hansda, S.; Girigoswami, K. Photodynamic therapy in cancer: An overview. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer Nature Singapore Pte Ltd: Singapore, 2022; Volume 1, pp. 1285–1308. [Google Scholar] [CrossRef]
- Malik, R.; Manocha, A.; Suresh, D.K. Photodynamic therapy—A strategic review. Indian J. Dent. Res. 2010, 21, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, P.; Harini, K.; Mahata, A.; Thirumalai, A.; Girigoswami, K.; Girigoswami, A. Revolutionizing cancer treatment through nanoengineered photosensitizer formulations for advanced photodynamic therapy. Int. J. Nano Dimens. 2024, 15, 152410. [Google Scholar] [CrossRef]
- Ion, R.-M. Revisiting tetra-p-sulphonated porphyrin as antimicrobial photodynamic Therapy agent. Coatings 2021, 11, 393. [Google Scholar] [CrossRef]
- Sun, Y.; Xing, D.; Shen, L.; Sun, M.; Fang, M.; Bi, L.; Sui, Y.; Zhang, Z.; Cao, W. Bactericidal effects of hematoporphyrin monomethyl ether-mediated photosensitization against pathogenic communities from supragingival plaque. Appl. Microbiol. Biotechnol. 2013, 97, 5079–5087. [Google Scholar] [CrossRef]
- Lambrechts, S.A.; Aalders, M.C.; Langeveld-Klerks, D.H.; Khayali, Y.; Lagerberg, J.W. Effect of monovalent and divalent cations on the photoinactivation of bacteria with meso-substituted cationic porphyrins. Photochem. Photobiol. 2004, 79, 297–302. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Therapy 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Wainwright, M.; Byrne, M.N.; Gattrell, M.A. Phenothiazinium-based photobactericidal materials. J. Photochem. Photobiol. B Bio. 2006, 84, 227–230. [Google Scholar] [CrossRef]
- Turbay, M.B.; Rey, V.; Argañaraz, N.M.; Morán Vieyra, F.E.; Aspée, A.; Lissi, E.A.; Borsarelli, C.D. Effect of dye localization and self-interactions on the photosensitized generation of singlet oxygen by rose bengal bound to bovine serum albumin. J. Photochem. Photobiol. B 2014, 141, 275–282. [Google Scholar] [CrossRef]
- Ragàs, X.; Dai, T.; Tegos, G.P.; Agut, M.; Nonell, S.; Hamblin, M.R. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: In vitro and in vivo studies. Lasers Surg. Med. 2010, 42, 384–390. [Google Scholar] [CrossRef]
- Vecchio, D.; Bhayana, B.; Huang, L.; Carrasco, E.; Evans, C.L.; Hamblin, M.R. Structure-function relationships of Nile blue (EtNBS) derivatives as antimicrobial photosensitizers. Eur. J. Med. Chem. 2014, 75, 479–491. [Google Scholar] [CrossRef][Green Version]
- Kasimova, K.R.; Sadasivam, M.; Landi, G.; Sarna, T.; Hamblin, M.R. Potentiation of photoinactivation of Gram-positive and Gram-negative bacteria mediated by six phenothiazinium dyes by addition of azide ion. Photochem. Photobiol. Sci. 2014, 13, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Berezin, D.B.; Zorin, V.P.; Morshnev, P.K.; Kukushkina, N.y.V.; Krestyaninov, M.A.; Kustova, T.V.; Strelnikov, A.I.; Lyalyakina, E.V.; Zorina, T.E.; et al. Monocationic chlorin as a promising photosensitizer for antitumor and antimicrobial Photodynamic therapy. Pharmaceutics 2023, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Hackbarth, S.; Röder, B.; Faustino, M.A.F. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorg. Med. Chem. Lett. 2014, 24, 808–812. [Google Scholar] [CrossRef]
- Felifel, N.T.; Sliem, M.A.; Kamel, Z.; Bojarska, J.; Seadawy, M.G.; Amin, R.M.; Elnagdy, S.M. Antimicrobial photodynamic therapy against Escherichia coli and Staphylococcus aureus using nanoemulsion-encapsulated zinc phthalocyanine. Microorganisms 2023, 11, 1143. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Vimaladevi, M.; Divya, K.C.; Girigoswami, A. Liposomal nanoformulations of rhodamine for targeted photodynamic inactivation of multidrug resistant gram negative bacteria in sewage treatment plant. J. Photochem. Photobiol. B Bio. 2016, 162, 146–152. [Google Scholar] [CrossRef]
- Pallavi, P.; Girigoswami, K.; Harini, K.; Gowtham, P.; Thirumalai, A.; Girigoswami, A. Theranostic dye entrapped in an optimized blended-polymer matrix for effective photodynamic inactivation of diseased cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 867–880. [Google Scholar] [CrossRef]
- Pallavi, P.; Harini, K.; Crowder, S.; Ghosh, D.; Gowtham, P.; Girigoswami, K.; Girigoswami, A. Rhodamine-conjugated anti-Stokes gold nanoparticles with higher ROS quantum yield as theranostic probe to arrest cancer and MDR bacteria. Appl. Biochem. Biotechnol. 2023, 195, 6979–6993. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Therapy 2005, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Therapy 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, L.; Zhang, M.; Liu, Z.; Wu, C.; Pan, X.; Huang, Z.; Lu, C.; Quan, G. Photodynamic therapy for cancer: Mechanisms, photosensitizers, nanocarriers, and clinical studies. MedComm 2024, 5, e603. [Google Scholar] [CrossRef]
- Alvarez, N.; Sevilla, A. Current advances in photodynamic therapy (PDT) and the future potential of PDT-combinatorial cancer therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Therapy. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Fujii, J.; Soma, Y.; Matsuda, Y. Biological action of singlet molecular oxygen from the standpoint of cell signaling, injury and death. Molecules 2023, 28, 4085. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive oxygen species (ROS)-mediated antibacterial oxidative therapies: Available methods to generate ROS and a novel option proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Malá, Z.; Žárská, L.; Bajgar, R.; Bogdanová, K.; Kolář, M.; Panáček, A.; Binder, S.; Kolářová, H. The application of antimicrobial photodynamic inactivation on methicillin-resistant S. aureus and ESBL-producing K. pneumoniae using porphyrin photosensitizer in combination with silver nanoparticles. Photodiagn. Photodyn. Therapy 2021, 33, 102140. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic dispersion of biofilms: An emerging biocatalytic avenue to combat biofilm-mediated microbial infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef] [PubMed]
- Demidova, T.N.; Hamblin, M.R. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 2004, 17, 245–254. [Google Scholar] [CrossRef]
- Akhtar, F.; Khan, A.U. Antimicrobial photodynamic therapy (aPDT) against vancomycin resistant Staphylococcus aureus (VRSA) biofilm disruption: A putative role of phagocytosis in infection control. Photodiagn. Photodyn. Therapy 2021, 36, 102552. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Galiardi-Campoy, A.E.B.; Machado, F.C.; Carvalho, T.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effects of photodynamic therapy mediated by emodin in cervical carcinoma cells. Photodiagn. Photodyn. Therapy 2021, 35, 102394. [Google Scholar] [CrossRef]
- Ahn, J.-C.; Biswas, R.; Chung, P.-S. Combination with genistein enhances the efficacy of photodynamic therapy against human anaplastic thyroid cancer cells. Lasers Surg. Med. 2012, 44, 840–849. [Google Scholar] [CrossRef]
- Freitas, M.A.A.; Pereira, A.H.C.; Pinto, J.G.; Casas, A.; Ferreira-Strixino, J. Bacterial viability after antimicrobial photodynamic therapy with curcumin on multiresistant Staphylococcus aureus. Future Microbiol. 2019, 14, 739–748. [Google Scholar] [CrossRef]
- Ribeiro, I.d.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm. Photodiagn. Photodyn. Therapy 2022, 37, 102729. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.A.; Navarro, R.; Tim, C.R.; de Paula Ramos, L.; de Oliveira, L.D.; Araki, Â.T.; Fernandes, K.G.C.; Macedo, D.; Assis, L. Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: In vitro study. Lasers Med. Sci. 2021, 36, 1235–1240. [Google Scholar] [CrossRef]
- Chan, B.C.; Dharmaratne, P.; Wang, B.; Lau, K.M.; Lee, C.C.; Cheung, D.W.; Chan, J.Y.; Yue, G.G.; Lau, C.B.; Wong, C.K.; et al. Hypericin and pheophorbide a mediated photodynamic therapy fighting MRSA wound infections: A translational study from In vitro to in vivo. Pharmaceutics 2021, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Christina Pires Gonçalves, L. Photophysical properties and therapeutic use of natural photosensitizers. J. Photochem. Photobiol. 2021, 7, 100052. [Google Scholar] [CrossRef]
- Wu, Y.a.; Yin, X.; Li, M. Mini-review of developments in the chemical modification of plant-derived photosensitizing drug hypocrellin and its biomedical applications. Interdiscip. Med. 2024, 2, e20240027. [Google Scholar] [CrossRef]
- Al Subeh, Z.Y.; Waldbusser, A.L.; Raja, H.A.; Pearce, C.J.; Ho, K.L.; Hall, M.J.; Probert, M.R.; Oberlies, N.H.; Hematian, S. Structural diversity of perylenequinones is driven by their redox behavior. J. Org. Chem. 2022, 87, 2697–2710. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Q.; Tian, L.; Huang, Z.; Tang, Y.; Wen, Y.; Yu, F.; Yan, X.; Zhao, Y.; Wu, Z.; et al. Advancements and future prospects in hypocrellins production and modification for photodynamic therapy. Fermentation 2024, 10, 559. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakata, H.; Takahashi, D.; Toshima, K. Hypocrellin B-based activatable photosensitizers for specific photodynamic effects against high H2O2-expressing cancer cells. Chem. Commun. 2022, 58, 242–245. [Google Scholar] [CrossRef]
- Zhou, L.; Ge, X.; Zhou, J.; Wei, S.; Shen, J. Modulating the photo-exciting process of photosensitizer to improve in vitro phototoxicity by preparing its self-assembly nanostructures. RSC Adv. 2015, 5, 2794–2805. [Google Scholar] [CrossRef]
- Lan, J.; Chen, S.; Chen, Z.; Luo, D.; Yu, C.; Zeng, L.; Sun, W.; Zhang, X.; Yao, X.; Wu, F.; et al. Chemo-photodynamic antitumour therapy based on Er-doped upconversion nanoparticles coated with hypocrellin B and MnO2. Biomater. Advan. 2024, 161, 213891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Z.; Zhang, X.; Zhang, Z. Photophysical and photochemical events during the photosensitization of Hypocrellin A on a colloidal CdS semiconductor. Dye. Pigment. 2001, 51, 9–14. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, Y.H.; Liao, P.Y.; Chen, D.Y.; Sun, N.N.; Nguyen Thi, P.A.; Yan, Y.J.; Wu, X.F.; Chen, Z.L. Comparison between porphin, chlorin and bacteriochlorin derivatives for photodynamic therapy: Synthesis, photophysical properties, and biological activity. Eur. J. Med. Chem. 2018, 160, 146–156. [Google Scholar] [CrossRef]
- Deng, H.; Liu, X.; Xie, J.; Yin, R.; Huang, N.; Gu, Y.; Zhao, J. Quantitative and site-directed chemical modification of hypocrellins toward direct drug delivery and effective photodynamic activity. J. Med. Chem. 2012, 55, 1910–1919. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, L. Photogeneration of singlet oxygen (1O2) and free radicals (Sen•−, O2•−) O.−2) by tetra-brominated hypocrellin B derivative. Free Radic. Res. 2001, 35, 767–777. [Google Scholar] [CrossRef]
- Diwu, Z. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem. Photobiol. 1995, 61, 529–539. [Google Scholar] [CrossRef]
- Su, Y.; Sun, J.; Rao, S.; Cai, Y.; Yang, Y. Photodynamic antimicrobial activity of hypocrellin A. J. Photochem. Photobiol. B Biol. 2011, 103, 29–34. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic antifungal activity of hypocrellin A against Candida albicans. Front. Microbiol. 2019, 10, 01810. [Google Scholar] [CrossRef]
- Su, Y.; Yin, X.; Rao, S.; Cai, Y.; Reuhs, B.; Yang, Y. Natural colourant from Shiraia bambusicola: Stability and antimicrobial activity of hypocrellin extract. Int. J. Food Sci. Technol. 2009, 44, 2531–2537. [Google Scholar] [CrossRef]
- Song, S.; Sun, X.; Meng, L.; Wu, Q.; Wang, K.; Deng, Y. Antifungal activity of hypocrellin compounds and their synergistic effects with antimicrobial agents against Candida albicans. Microb. Biotechnol. 2021, 14, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Ran, X.; Gao, R.; Sun, J.; Zhuang, K.; You, Z.; Zhang, Z.; Ran, Y. Hypocrellin A-mediated photodynamic antibacterial activity against Cutibacterium acnes: An in vitro study. Photodiagn. Photodyn. Therapy 2025, 51, 104467. [Google Scholar] [CrossRef] [PubMed]
- Otieno, W.; Liu, C.; Deng, H.; Li, J.; Zeng, X.; Ji, Y. Hypocrellin B-ediated photodynamic inactivation of Gram-positive antibiotic-resistant bacteria: An in vitro study. Photobiomodulation Photomed. Laser Surg. 2019, 38, 36–42. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liao, Q.; Xie, M.; Tao, H.; Wang, H.-L. Synergistic effect of hypocrellin B and curcumin on photodynamic inactivation of Staphylococcus aureus. Microb. Biotechnol. 2021, 14, 692–707. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B. Photodiagn. Photodyn. Therapy 2013, 10, 600–606. [Google Scholar] [CrossRef]
- Ma, G.; Khan Shabana, I.; Jacob Melissa, R.; Tekwani Babu, L.; Li, Z.; Pasco David, S.; Walker Larry, A.; Khan Ikhlas, A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, T.; Zheng, X.; Wang, Y.; Sha, J.; Shan, L.; Mu, T.; Zhang, W.; Lee, C.S.; Liu, W.; et al. A glutathione responsive photosensitizer based on hypocrellin B for photodynamic therapy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 325, 125052. [Google Scholar] [CrossRef]
- Martins Antunes de Melo, W.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Zhang, L.; Chen, H.; Gu, Y.; Zhao, J. A novel hypocrellin B derivative designed and synthesized by taking consideration to both drug delivery and biological photodynamic activity. J. Photochem. Photobiol. B Biol. 2009, 94, 171–178. [Google Scholar] [CrossRef]
- Toffoli, D.; Gomes, L.; Vieira, N.; Courrol, L. Photodynamic potentiality of hypocrellin B and its lanthanide complexes. J. Opt. A Pure Appl. Opt. 2008, 10, 104026. [Google Scholar] [CrossRef]
- Tanielian, C.; Schweitzer, C.; Mechin, R.; Wolff, C. Quantum yield of singlet oxygen production by monomeric and aggregated forms of hematoporphyrin derivative. Free Radic. Biol. Med. 2001, 30, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ding, R. Enhanced Singlet Oxygen Production from Metal Nanoparticle Based Hybrid Photosensitizers. Ph.D. Thesis, University of Cincinnati, Cincinnati, OH, USA, 2016. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=ucin1447690607 (accessed on 11 June 2025).
- Chan-Bacab, M.J.; Reyes-Estebanez, M.M.; Camacho-Chab, J.C.; Ortega-Morales, B.O. Microorganisms as a potential source of molecules to control trypanosomatid diseases. Molecules 2021, 26, 1388. [Google Scholar] [CrossRef]
- Najm, M.; Pourhajibagher, M.; Badirzadeh, A.; Razmjou, E.; Alipour, M.; Khoshmirsafa, M.; Bahador, A.; Hadighi, R. Photodynamic therapy using Toluidine Blue O (TBO) dye as a photosensitizer against leishmania major. Iran. J. Public Health 2021, 50, 2111–2120. [Google Scholar] [CrossRef]
- Lin, J.; Sahakian, D.C.; de Morais, S.M.; Xu, J.J.; Polzer, R.J.; Winter, S.M. The Role of Absorption, Distribution, Metabolism, Excretion and Toxicity in Drug Discovery. Curr. Top. Med. Chem. 2003, 3, 1125–1154. [Google Scholar] [CrossRef]
- Davis, A.E.; Kennelley, G.E.; Amaye-Obu, T.; Jowdy, P.F.; Ghadersohi, S.; Nasir-Moin, M.; Paragh, G.; Berman, H.A.; Huss, W.J. The phenomenon of phototoxicity and long-term risks of commonly prescribed and structurally diverse drugs. J. Photochem. Photobiol. 2024, 19, 100221. [Google Scholar] [CrossRef]
- Li, T.; Hou, X.; Deng, H.; Zhao, J.; Huang, N.; Zeng, J.; Chen, H.; Gu, Y. Liposomal hypocrellin B as a potential photosensitizer for age-related macular degeneration: Pharmacokinetics, photodynamic efficacy, and skin phototoxicity in vivo. Photochem. Photobiol. Sci. 2015, 14, 972–981. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Khan, S.; Singh, A.D.; Muheem, A.; Soni, A.; Taleuzzaman, M. Nanophytomedicine in clinical management: An introductory evidence-based review. J. Pharm. Res. Sci. Technol. 2022, 6, 26–37. [Google Scholar] [CrossRef]
- Mehrdadi, S. Lipid-based nanoparticles as oral drug delivery systems: Overcoming poor gastrointestinal absorption and enhancing bioavailability of peptide and protein therapeutics. Adv. Pharm. Bull. 2024, 14, 48–66. [Google Scholar] [CrossRef]
- Thirumalai, A.; Girigoswami, K.; Prabhu, A.D.; Durgadevi, P.; Kiran, V.; Girigoswami, A. 8-anilino-1-naphthalenesulfonate-conjugated carbon-coated ferrite nanodots for fluoromagnetic imaging, smart drug delivery, and biomolecular sensing. Pharmaceutics 2024, 16, 1378. [Google Scholar] [CrossRef]
- Prabakaran, L.; Sathyaraj, W.V.; Yesudhason, B.V.; Subbaraj, G.K.; Atchudan, R. Green synthesis of multifunctional silver nanoparticles using Plectranthus amboinicus for sensitive detection of triethylamine, with potential in vitro antibacterial and anticancer activities. Chemosensors 2023, 11, 373. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Vajagathali, M.; Bose, D.; Thirumalai, A.; Kiran, V.; Durgadevi, P.; Girigoswami, A. Enhanced behavioral impact of optimized bupropion-encapsulated bilosomes over traditional niosomes treating depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 4373–4392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, J.-H.; Dong, C.; Ma, F.; Wei, S.-H.; Shen, J. Water-soluble hypocrellin A nanoparticles as a photodynamic therapy delivery system. Dyes Pigment. 2009, 82, 90–94. [Google Scholar] [CrossRef]
- Lin, X.; Yan, S.-Z.; Qi, S.-S.; Xu, Q.; Han, S.-S.; Guo, L.-Y.; Zhao, N.; Chen, S.-L.; Yu, S.-Q. Transferrin-modified nanoparticles for photodynamic therapy enhance the antitumor efficacy of hypocrellin A. Front. Pharmacol. 2017, 8, 00815. [Google Scholar] [CrossRef]
- Krishnaswami, V.; Ponnusamy, C.; Sankareswaran, S.; Paulsamy, M.; Madiyalakan, R.; Palanichamy, R.; Kandasamy, R.; Natesan, S. Development of copolymeric nanoparticles of hypocrellin B: Enhanced phototoxic effect and ocular distribution. Eur. J. Pharm. Sci. 2018, 116, 26–36. [Google Scholar] [CrossRef]
- Qi, S.-S.; Lin, X.; Zhang, M.-M.; Yan, S.-Z.; Yu, S.-Q.; Chen, S.-L. Preparation and evaluation of hypocrellin A loaded poly(lactic-co-glycolic acid) nanoparticles for photodynamic therapy. RSC Adv. 2014, 4, 40085–40094. [Google Scholar] [CrossRef]
- Chang, J.-E.; Cho, H.-J.; Yi, E.; Kim, D.-D.; Jheon, S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid–ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B Biol. 2016, 158, 113–121. [Google Scholar] [CrossRef]
- Natesan, S.; Krishnaswami, V.; Ponnusamy, C.; Madiyalakan, M.; Woo, T.; Palanisamy, R. Hypocrellin B and nano silver loaded polymeric nanoparticles: Enhanced generation of singlet oxygen for improved photodynamic therapy. Mater. Sci. Engin. C 2017, 77, 935–946. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Yan, S.-Z.; Li, Q.; Xu, Q.; Lin, X.; Qi, S.-S.; Yu, S.-Q.; Chen, S.-L. Poly(lactic-co-glycolic) acid nanoparticles improve oral bioavailability of hypocrellin A in rat. RSC Adv. 2017, 7, 42073–42082. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Cao, Y.; Wang, Y.; Wang, F.; Zhang, F.; Zheng, S. Biodegradable Hypocrellin B nanoparticles coated with neutrophil membranes for hepatocellular carcinoma photodynamics therapy effectively via JUNB/ROS signaling. Int. Immunopharmacol. 2021, 99, 107624. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhao, K.; Guo, Z.; Liu, Y.; Zeng, D.; Ban, X.; Zhao, L.; Ma, X.; Zheng, S. Transferrin-modified cancer cell member coating hypocrellin B-derived nanomaterials for enhanced photodynamic therapy efficacy in hepatocellular carcinoma. Med. Drug Discov. 2021, 11, 100088. [Google Scholar] [CrossRef]
- Khatoon, N.; Afthab, J.; Zhang, Z.; Chu, M.Q.; Huang, Y.; Li, J.; Wang, B.; Pu, G.; Zhou, C.H. Hypocrellin A-cisplatin-intercalated hectorite nano formulation for chemo-photodynamic tumor-targeted synergistic therapy. J. Mater. Sci. 2024, 59, 2087–2103. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, J.; Wu, J.; Liu, W.; Guo, L.; Jia, Q.; Ding, Y.; Zhang, H.; Wang, P. Biodegradable hypocrellin derivative nanovesicle as a near-infrared light-driven theranostic for dually photoactive cancer imaging and therapy. Biomaterials 2018, 185, 133–141. [Google Scholar] [CrossRef]
- Lin, P.; Gu, H.; Zhuang, X.; Wang, F.; Hu, X. Controlled release of curcumin and hypocrellin A from electrospun poly(l-lactic acid)/silk fibroin nanofibers for enhanced cancer cell inhibition. ACS Appl. Bio Mater. 2024, 7, 5423–5436. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.; Wang, K.; Lu, Z.; Su, X.; Li, L.; Yuan, C.; Feng, J.; Yan, S.; Kong, B.; et al. Hypocrellin B-loaded, folate-conjugated polymeric micelle for intraperitoneal targeting of ovarian cancer in vitro and in vivo. Cancer Sci. 2018, 109, 1958–1969. [Google Scholar] [CrossRef]
- Wang, H.; Jia, Q.; Liu, W.; Nan, F.; Zheng, X.; Ding, Y.; Ren, H.; Wu, J.; Ge, J. Hypocrellin derivative-loaded calcium phosphate nanorods as NIR light-triggered phototheranostic Agents with enhanced tumor accumulation for cancer therapy. ChemMedChem 2020, 15, 177–181. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Thirumalai, A.; Girigoswami, A. Polymer-based antimicrobial peptide mimetics for treating multi-drug resistant infections: Therapy and toxicity evaluation. Int. J. Pept. Res. Therapy 2024, 30, 64. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Yan, S.-Z.; Tao, X.; Yang, Q.; Li, Q.; Wang, T.-S.; Yu, S.-Q.; Chen, S.-L. Evaluation of hypocrellin A-loaded lipase sensitive polymer micelles for intervening methicillin-resistant Staphylococcus aureus antibiotic-resistant bacterial infection. Mater. Sci. Engin. C 2020, 106, 110230. [Google Scholar] [CrossRef]
- Thirumalai, A.; Elboughdiri, N.; Karthick, H.; Girigoswami, K.; Girigoswami, A. Phosphorus-carrying cascade molecules: Inner architecture to biomedical applications. Turk. J. Chem. 2023, 47, 667–688. [Google Scholar] [CrossRef]
- Chai, S.; Kan, S.; Sun, R.; Zhou, R.; Sun, Y.; Chen, W.; Yu, B. Fabricating polydopamine-coated MoSe2-wrapped hollow mesoporous silica nanoplatform for controlled drug release and chemo-photothermal therapy. Int. J. Nanomed. 2018, 13, 7607–7621. [Google Scholar] [CrossRef]
- Gao, L.; Fei, J.; Zhao, J.; Li, H.; Cui, Y.; Li, J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012, 6, 8030–8040. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Chen, J.; Lei, W.; Wang, X.; Zhang, B. Hypocrellin B doped and pH-responsive silica nanoparticles for photodynamic therapy. Sci. China Chem. 2010, 53, 1994–1999. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, J.; Zhang, Y.; Qiao, R.; Xia, S.; Chen, J.; Wang, X.; Zhang, B. Photodynamic properties of hypocrellin A, complexes with rare earth trivalent ions: Role of the excited state energies of the metal ions. J. Phys. Chem. B 2007, 111, 2688–2696. [Google Scholar] [CrossRef] [PubMed]




| Class | Photosensitizer | Chemical Structure | Charge of the Photosensitizer | Bacterial Species Tested | Soret (S)/ Q-Band (Q) (nm) | Quantum Yield of Singlet Oxygen | Ref. |
|---|---|---|---|---|---|---|---|
| Porphyrin | Hematoporphyrin monomethyl ether |  | Anion | Staphylococcus aureus | S = 400 Q = 505, 536, or 630 | 0.13 | [45] |
| 5,10,15,20-(tetra-N-methyl-4-pyridyl) porphyrin tetraiodide | 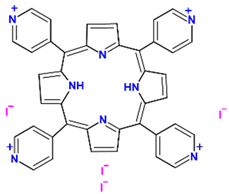 | Cation | Escherichia coli | S = 416 Q = 515, 555, 586, or 640 | 0.74 | [46] | |
| Phenothiazinium | Methylene blue |  | Cation | Staphylococcus aureus | Q = 665 or 613 | 0.49 | [47] |
| New methylene blue |  | Cation | Staphylococcus epidermidis | Q = 588 or 632 | 1.35 | [48] | |
| Rose Bengal | 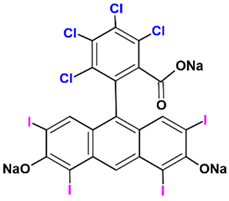 | Anion | Streptococcus mutans | Q = 549 | 0.74 | [49] | |
| Dimethyl methylene blue |  | Cation | Acinetobacter baumannii | Q = 542 or 647 | 1.22 | [50] | |
| 5-(Ethylamino)-9-diethylaminobenzophenothiazinium chloride |  | Cation | Staphylococcus aureus | Q = 654 | 0.025 | [51] | |
| 5-(Ethylamino)-9-diethylaminobenzophenothiazinium chloride-COOH |  | Cation | Methicillin- resistant Staphylococcus aureus | Q = 655 | 0.023 | [51] | |
| Toluidine blue O |  | Cation | Staphylococcus aureus, Escherichia coli | Q = 628 | 0.86 | [52] | |
| Chlorin | Chlorin e6 |  | Cation | Pseudomonas aeruginosa, Escherichia coli | S = 403 Q = 505, 540, 595, or 667 | 0.63 | [53] |
| Isobacteriochlorin | 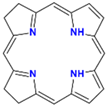 | Neutral | Escherichia coli | S = 386 Q = 506, 540, 580, or 660 | 0.49 | [54] | |
| Phthalocyanine | Zinc phthalocyanine |  | Neutral | Staphylococcus aureus, Escherichia coli | S = 350 Q = 672 | 0.56 | [55] |
| Classification of Bacteria | Bacterial Species | Type of Hypocrellin | Origin of the Compound | Concentration | Excitation Wavelength (nm) | Inhibition Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Gram-positive | Bacillus subtilis | Hyp-A | Plant pathogen | 1 μm | 650 | above 99.9% | [99] |
| Gram-positive and negative bacteria | Staphylococcus aureus, Bacillus subtillis, Escherichia coli, and Salmonella typhimurium | Hyp-A | Human pathogen | 1 μM | 650 | 99.8% | [99] |
| Fungus | Candida albicans | Hyp-A | Human pathogen | 1.0 µg/mL | 400 | 70.19 ± 4.87% | [100] |
| Gram-positive | Methicillin-resistant S. aureus | Hypocrellin | Human pathogen | 4 mg/mL | 464 | Zone of inhibition 18.5 ± 0.5 µg/mL, MIC 0.75 µg/mL, and MBC 1.5 µg/mL | [101] |
| Fungus | Candida albicans | Hyp-A, -B, and -C | Human pathogen | 10 μM | 590 | 85% | [102] |
| Gram-positive bacteria | Cutibacterium acnes | Hyp-A | Human pathogen | 8 µg/mL | 470 | MIC 1 µg/mL, and MBC 4 µg/mL | [103] |
| Gram-positive bacteria | Staphylococcus aureus, Enterococcus faecalis, and Streptococcus pneumonis | Hyp-B | Human pathogen | 100 µM | 492 | 7 log10 | [104] |
| Gram-positive bacteria | Staphylococcus aureus | Hyp-B | Human pathogen | 500 nM | 460 | 5–6 log10 | [105] |
| Gram-positive bacteria | Staphylococcus aureus | Hyp-B | Human pathogen | 2.5 µM | 470 | - | [106] |
| Fungus, Gram-positive and negative bacteria | Candida albicans, Staphylococcus aureus, Staphylococcus aureus, Pseudomonas aeruginosa, and Mycobacterium intracellular | Hyp-A and -B | Human pathogen | 3–10 µg/mL | 544 | - | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durgadevi, P.; Girigoswami, K.; Girigoswami, A. Photophysical Process of Hypocrellin-Based Photodynamic Therapy: An Efficient Antimicrobial Strategy for Overcoming Multidrug Resistance. Physics 2025, 7, 28. https://doi.org/10.3390/physics7030028
Durgadevi P, Girigoswami K, Girigoswami A. Photophysical Process of Hypocrellin-Based Photodynamic Therapy: An Efficient Antimicrobial Strategy for Overcoming Multidrug Resistance. Physics. 2025; 7(3):28. https://doi.org/10.3390/physics7030028
Chicago/Turabian StyleDurgadevi, Pazhani, Koyeli Girigoswami, and Agnishwar Girigoswami. 2025. "Photophysical Process of Hypocrellin-Based Photodynamic Therapy: An Efficient Antimicrobial Strategy for Overcoming Multidrug Resistance" Physics 7, no. 3: 28. https://doi.org/10.3390/physics7030028
APA StyleDurgadevi, P., Girigoswami, K., & Girigoswami, A. (2025). Photophysical Process of Hypocrellin-Based Photodynamic Therapy: An Efficient Antimicrobial Strategy for Overcoming Multidrug Resistance. Physics, 7(3), 28. https://doi.org/10.3390/physics7030028






