Abstract
Glycerol is a biogenic waste that is generated in both the biodiesel and oleo-chemical industries. The value addition of surplus glycerol is of utmost importance for making these industries economically profitable. In line with this, glycerol is converted into glycerol carbonate, a potential candidate for the industrial production of polymers and biobased non-isocyanate polyurethanes. In addition, glycerol can also be converted into solketal, which is the protected form of glycerol with a primary hydroxyl functional group. In this contribution, we developed a microwave-assisted solvent and catalyst-free method for converting solketal into solketal carbonate. Under conventional heating conditions, the reaction of solketal with dimethyl carbonate resulted in 70% solketal carbonate in 48 h. However, under microwave heating, 90% solketal carbonate was obtained in just 30 min. From the perspective of sustainability and green chemistry, biomass-derived heterogeneous catalysts are gaining importance. Therefore, in this project, several green catalysts, such as molecular sieves (MS, 4Å), Hβ-Zeolite, Montmorillonite K-10 clay, activated carbon from groundnut shell (Arachis hypogaea), biochar prepared from the pyrolysis of sawdust, and silica gel, were successfully used for the carbonyl transfer reaction. The obtained solketal carbonate was thoroughly characterized by 1H NMR, 13C NMR, IR, and MS. The method presented here is facile, clean, and environmentally benign, as it eliminates the use of complicated procedures, toxic solvents, and toxic catalysts.
1. Introduction
The glycerol moiety is the backbone of glycerides (tri-, di- and mono-glycerides), which is present in fats and oils. According to estimates, around 10 wt.% of glycerol is associated with oils and fats; itis generated as a by-product during biodiesel and oleo-chemical syntheses [1,2,3]. Quantitatively, the production of 9 tons of biodiesel leads to the simultaneous formation of 1 ton of glycerol as the by-product. From an environmental sustainability perspective, demand for the production of biodiesel is increasing steadily, and therefore, by-product (glycerol) production will continue to rise. The global glycerol market was valued at approximately USD 2.6 billion in 2019. Between 2020 and 2027, the glycerol market is expected to grow further at a compound annual growth rate of 4% [4].
It is reported that the accumulation of large amounts of crude glycerol is creating an additional burden on the oleo-chemical industries [5,6]. Also, in the longer term, it will hamper the market potential of both pure glycerol and biodiesel [5,6]. Overall, the piling up of unutilized crude glycerol can negatively impact the economic viability of the biodiesel sector [5,6]. Given this background, valorization of glycerol is necessary from the perspective of the circular economy, environmental sustainability, and the economics of oleo-chemical industries.
At present, extensive research is being carried out in academia and industries with regard to the utilization of glycerol to synthesize a wide range of value-added products [7,8,9,10,11,12]. In line with this, glycerol carbonate preparation is being explored as an important candidate for the synthesis of various polymers and bio-NIPUs (biobased non-isocyanate polyurethanes) [7]. Moreover, glycerol can also be converted into solketal (isopropylidene glycerol)—a commercially available chemical compound. Solketal has various applications as a solvent, fuel additive, and starting material for many chemical compounds, such as mono-glycerides (Figure 1) [13,14,15,16,17,18,19,20,21,22]. Mono-glycerides are widely used in the food, nutraceutical, cosmetic, and life sciences industries.
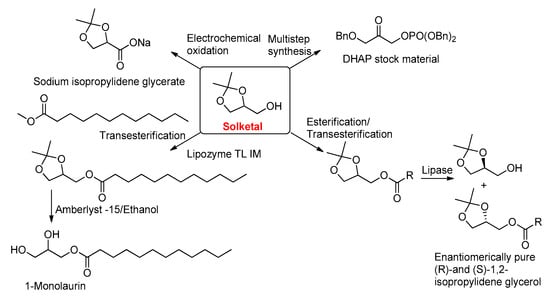
Figure 1.
Utilization of solketal for the synthesis of glycerate, protected dihydroxyacetone phosphate (DHAP), mono-glycerides, and optically pure isopropylidene. (Bn—Benzyl group).
To further expand the scope of glycerol utilization via the solketal route, it can be converted into solketalcarbonate, which has several industrial applications (Figure 2) (Scheme 1) [23]. Recent research shows that solketal carbonate-based liquid electrolyte is of immense interest and is being investigated for application in potassium batteries [24]. The experimental results indicate that solketal carbonate-based liquid electrolyte displayed stable electrochemical behavior and performance close to those of commercially available oil-derived alternatives [24].
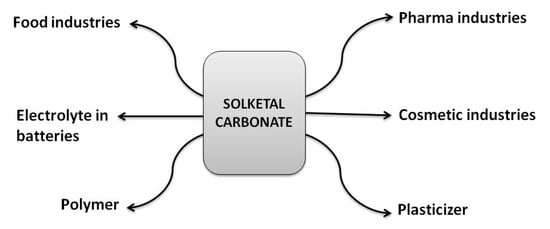
Figure 2.
Potential applications of solketal carbonate in different areas.
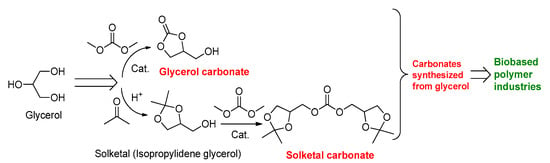
Scheme 1.
Preparations of glycerol carbonate and solketal carbonate from glycerol.
However, currently, there is limited research on the synthesis of solketal carbonate. The reported synthesis of solketal carbonate requires a long reaction time and employs a highly corrosive base catalyst (sodium methoxide) [23,25]. Furthermore, the reported method uses 60 mL of chlorinated solvent (CH2Cl2, dichloromethane) and 75 mL of water for the work-up procedure to yield 11.3 g of solketal carbonate [23]. Dichloromethane is a well-known carcinogen, and therefore, its use in teaching labs and industry is restricted. Additionally, the use of homogeneous base catalysts, such as NaOH and KOH, can cause burns to skin, eyes, and mucous membranes. Such catalysts are harmful to human health and the environment.
The syntheses of glycerol carbonate from glycerol under microwave heating conditions have been reported [26,27]. Microwave-assisted synthesis has various advantages, such as (i) reducing reaction times, allowing faster synthesis of compounds compared to conventional heating methods; (ii) microwaves are known to heat up materials directly, leading to low energy consumption and cost minimization; (iii) microwaves generally provide uniform heating of the reaction mixture, resulting in consistency with regard to the yield, selectivity, and purity of products; and (iv) microwave-assisted methods often need less solvent and reagent, aligning with the principles of green chemistry [26,27,28,29,30].Therefore, the main objective of this project was to develop a catalyst-free and solvent-free method for the production of solketal carbonate, which is significant from the point of view of environmental sustainability and circular bioeconomy.
In the above context, solketal carbonate was synthesized via microwave-assisted reaction of solketal with dimethyl carbonate under catalyst- and solvent-free reaction conditions. The method presented in this contribution is green and less harmful to the environment compared to the previously described conventional alkali-catalyzed method.
2. Experimental Section
2.1. Materials and Instruments
Glycerol (purity ≥ 99.5%), dimethyl carbonate (purity ≥ 99.5%) and all the necessary chemicals and solvents required for the experiments were purchased from local suppliers. The required chemicals and solvents were used without any further purification. Also, during the experiments, no attempts were made to remove atmospheric moisture unless otherwise stated in the experimental procedures. The glassware was dried in an oven (90 °C) for at least 2 h before use. Microwave-assisted reactions were performed in a microwave reactor (Milestones, Milan, Italy) equipped with various essential components (an infrared temperature sensor, a temperature probe, a reaction chamber, a magnetic stirrer, and a condenser). Various catalysts, such as silica gel, molecular sieves (MS, 4Å), Hβ-Zeolite, and Montmorillonite K-10 clay were obtained from local chemical suppliers. Activated carbon from groundnut shell (prepared via chemical activation) and biochar from pyrolysis of sawdust, which were used in the experiments, were prepared in-house [31,32,33]. The progress of all reactions was monitored using thin-layer chromatography (TLC) on silica gel plates. The 1H NMR and 13C NMR spectra were recorded using Bruker (400 MHz and 100 MHz) instruments (Bruker, Ettlingen, Germany). The structure of the product was confirmed by 1H and 13C NMR, FTIR, and MS.
2.2. Synthesis of Solketal from Glycerol
Glycerol (30 mmol, 3 g), 2,2-DMP (2,2-dimethoxypropane) (45 mmol, 4.6 g), acetone (30 mL), and a catalytic amount of p-TSA (Para-toluene sulfonic acid) (10 mg) were stirred at room temperature for 24 h. Afterwards, residual reactants (acetone and 2,2-dimethoxy propane) were separated under a vacuum. The few remaining impurities in the reaction mixture were removed by silica gel column chromatography using ethyl acetate–n-hexane (5:95) as an eluent. Solketal was isolated in 86% yield (3.72 g) as a colorless oil. Characterization of the product was performed as reported earlier [18].
2.3. Synthesis of Solketal Carbonate Under Conventional Heating Condition
The reaction was carried out using a screw-capped vial (5 mL) and a thermomixer. Solketal carbonate was synthesized by reacting dimethyl carbonate (90.08 mg, 1 mmol) with solketal (330.4 mg, 2.5 mmol) in the presence of catalysts (0.5 g) at a specified temperature. Catalysts were not employed for the autocatalytic reactions, and unless otherwise stated, the amount of reactants and other parameters were kept constant. After completion of the reaction, as observed by TLC, catalyst was filtered from the reaction mixture. Then, the resulting mixture was concentrated in vacuo using a rotary evaporator. The yield of solketal carbonate was determined after performing silica gel column chromatography using a mixture of ethyl acetate and n-hexane as an eluent.
2.4. Microwave-Assisted Synthesis of Solketal Carbonate
Dimethyl carbonate (90.08 mg, 1 mmol) and solketal (330.4 mg, 2.5 mmol) were added into a reactor specifically designed to meet the needs of microwave-assisted synthesis. Whenever necessary, catalyst (0.5 g) was added into the reaction mixture. Subsequently, the reaction mixture was stirred at 700 rpm under microwaves for a fixed time. The reaction was performed with an operating temperature of 140◦C and power output of 175 W. The catalyst was separated through filtration during work-up. The excess reactants and methanol present in the reaction mixture were separated under a vacuum. Solketal carbonate was purified using a silica gel column chromatography and it was obtained as a colorless oil.
2.5. Characterization of Solketal Carbonate
Solketal carbonate was characterized by 1H NMR, 13C NMR, IR, MS, and density measurements. Detailed structural data of the chemical compound is presented below.
1H NMR (400 MHz, CDCl3):δ 4.31 (m, 2H), 4.13 (d, 4H), 4.06 (dd, 2H), 3.76 (dd, 2H), 1.41(s, 6H), 1.35(s, 6H). 13C NMR (100 MHz, CDCl3): δ 154.81, 109.92, 73.21, 68.12, 68.08, 66.27, 26.66, 25.31.
IR (NaCl) ν (cm−1): 2988, 2938, 2887, 1753, 1457, 1283, 1374, 1252, 1219, 1158, 1085, 1058, 976, 842, 788.
MS (EI): m/z 291 (MH+, 1%).
Density (g/cm3): 1.1491.
3. Results and Discussion
In the present work, as a first step, glycerol was protected using 2,2-dimethoxypropane/acetone in the presence of p-TSA as a catalyst to yield solketal (86%) (Scheme 2). Afterwards, the primary hydroxyl group of solketal was reacted with dimethyl carbonate under conventional heating conditions to yield the desired product (solketal carbonate) (Scheme 3). In a typical experiment, solketal and dimethyl carbonate were added into a vial and mixed thoroughly. The obtained mixture was then heated conventionally to 120 °C under vigorous stirring. The reaction was carried out for 48 h at 120 °C, yielding 70% solketal carbonate. The reaction was conducted without using any catalyst or solvent. It is worth mentioning that under conventional heating conditions, when the reaction was performed using different catalysts, only 77% (highest) yield of solketal carbonate was obtained in 48 h. An earlier report employed a highly corrosive basic catalyst (sodium methoxide) under conventional heating conditions for this reaction and used dichloromethane (a carcinogen) for downstream processing to prepare solketal carbonate at 65% yield [23].
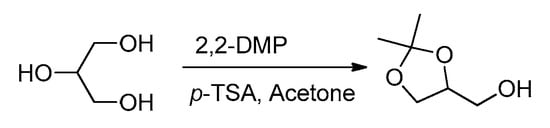
Scheme 2.
Synthesis of solketal from glycerol.
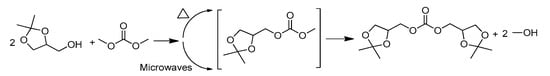
Scheme 3.
Synthesis of solketal carbonate under conventional and microwave heating condition.
For comparative purposes and to evaluate the effect of microwaves, after performing the reaction under conventional heating, it was carried out under microwave irradiation in the absence of any catalyst or solvent (Scheme 3). Under microwaves, a 90% yield of solketal carbonate was observed in 30 min, whereas an 81% yield was obtained in 15 min (Table 1, Entry 3 and 4). Increasing the reaction time from 30 to 45 min had minimal effect on the final product yield (from 90% to 93%) (Table 1, Entries 1–2).

Table 1.
Microwave-assisted synthesis of solketal carbonate.
Heterogeneous catalysts are promising because they are easily accessible and can display high activity towards esterification and transesterification reactions. In this regard, several catalysts which were available in-house, such as molecular sieves (MS, 4Å), Hβ-Zeolite, Montmorillonite K-10 clay, activated carbon from groundnut shell, biochar from sawdust, and silica gel were employed for transesterification of solketal with dimethyl carbonate (formula OC(OCH3)2) under microwaves to investigate their catalytic effect (Table 1, Entries 5–10). As can be seen from Table 1, in a short reaction time (15 min), molecular sieves (MS, 4Å), Hβ-Zeolite, Montmorillonite K-10 clay, activated carbon from groundnut shell, and biochar from sawdust provided92%, 96%, 94%, 97% and 90% solketalcarbonate, respectively (Table 1, Entries 5–9). Additionally, using silica gel as a catalyst provided86% of solketal carbonate in 15 min (Table 1, Entry 10).
It is important to note that heterogeneous catalysts derived from groundnut shell (Arachis hypogaea) and sawdust are renewable in nature. As mentioned above, a wide range of catalysts were used for the reaction. We assume that they were acting both as a catalyst and as a form of support. The exact nature of their catalytic role is still unclear and requires further investigation. However, it is believed that they absorb methanol formed during the reaction, thereby shifting the equilibrium towards product formation [34]. In line with this, several research groups utilized molecular sieves, LTA zeolites, K-zeolite from coal fly ash, and biochar for the valorization of glycerol to glycerol carbonate [34,35,36,37].
The autocatalytic synthesis of solketal carbonate was successfully performed under microwave irradiation. Additionally, various catalysts were employed to shorten the reaction time from 30 min to 15 min, which also resulted in a higher yield. As can be observed from Table 1, high product yields were obtained for most of the cases; thus, an extensive work-up process was not necessary for purification of the product. The obtained solketal carbonate was characterized by 1H and 13C NMR, FT-IR and MS. In the 1H NMR spectra, hydrogens present in the isopropylidene methyl groups were observed as singlets (1.41 and 1.35 ppm). The 1H NMR signals corresponding to methylene hydrogens connected with the carbonate functional group were observed at 4.13 ppm as doublets, showing an integral value of four hydrogens. This was indicative of the presence of solketal carbonate. The 13C NMR of solketal carbonate displayed a chemical shift for the carbonate carbon at 154.8 ppm, which is considered to be the characteristic signal for linear and branched oleo-chemical carbonates [38]. The main characteristic band for solketal carbonate was found at 1753 cm−1 in the IR spectra due to the presence of a C=O functional group. Also, during the electron impact MS analysis, a molecular ion at m/z 291 revealed that the compound was solketal carbonate.
A cost estimation of solketal carbonate prepared using the microwave-assisted method was carried out (Table 2). Furthermore, it was compared with a previously reported method which used conventional heating and utilized sodium methoxide (NaOMe) as a catalyst [23]. For our reaction (microwave-assisted), the cost of solketal carbonate is USD 17.04/kg, whereas, for the method reported by Kenar & Knothe 2008 [23], the cost is USD 24.72/kg (Table 2).

Table 2.
Cost comparison of solketal carbonate synthesis: microwave heating vs. conventional heating, as reported in the literature.
Comparing the cost of solketal carbonate with other reported approaches which deal with valorization of glycerol to value-added products seems inappropriate because each method used for glycerol valorization uses a different set of catalysts and employs diverse reaction conditions and solvents based on the specific needs of the reactions. Additionally, cost calculation of reported products using data presented in the literature encountered difficulties due to the unavailability of complete data pertaining to experimental conditions and the actual cost of some of the raw materials used by the authors.
The method presented in this paper can be integrated with a waste biorefinery, where the lipid fraction of organic wastes is converted into biodiesel (Figure 3). The obtained biodiesel can be used as a liquid fuel, while the glycerol by-product can be converted into glycerol carbonate and solketal carbonate (Figure 3). Such a waste biorefinery would be highly beneficial, combining various aspects: waste management, green chemistry, circularity, and sustainability.
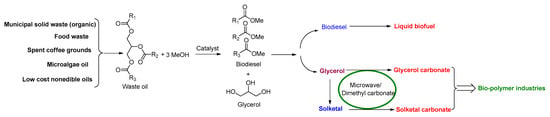
Figure 3.
A conceptual representation of an organic waste-based biorefinery for producing biodiesel, glycerol carbonate, and solketal carbonate. (Me—methyl group).
4. Conclusions
Adding value to glycerol not only addresses waste management issues but also promotes sustainability and economic profitability in the oleo-chemical industries. Microwave heating is regarded as an important tool to expedite organic reactions. It provides fast heating and an enhanced reaction rate, which promotes the formation of cleaner products with high yields. In organic chemistry, microwave-assisted reactions are popular due to their shorter reaction times compared to conventionally heated reactions. In this context, solketal carbonate was synthesized by the reaction of solketal with dimethyl carbonate. Under conventional heating, around 70% solketal carbonate was obtained in 48 h, whereas, under microwave heating, 90% product was obtained in 30 min without using any catalyst or solvent. The use of different catalysts such as silica gel, molecular sieves (MS, 4Å), Hβ-Zeolite, Montmorillonite K-10 clay, activated carbon from groundnut shell, and biochar from sawdust yielded 86%, 92%, 96%, 94%, 97%, and 90% solketalcarbonate, respectively, in a short reaction time (15 min). The presented methodology is green, as it eliminates the use of toxic solvents and catalysts. Furthermore, no hazardous substances were generated during the reaction.
Author Contributions
Conceptualization, S.K.K.; Methodology, S.K.K., S.G. and L.C.M.; Validation, A.H.; Investigation, S.K.K., S.G., L.C.M. and A.H.; Resources, S.K.K.; Data curation, S.K.K. and L.C.M.; Writing—original draft, S.K.K.; Writing—review & editing, S.K.K., S.G., L.C.M. and A.H.; Visualization, S.K.K.; Supervision, S.K.K.; Project administration, S.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to The Odisha Renewable Energy Research Institute (ORERI), India, for its constant support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol-a by-product of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Sandid, A.; Spallina, V.; Esteban, J. Glycerol to value-added chemicals: State of the art and advances in reaction engineering and kinetic modelling. Fuel Process. Technol. 2024, 253, 108008. [Google Scholar] [CrossRef]
- Sahani, S.; Upadhyay, S.N.; Sharma, Y.C. Critical review on production of glycerol carbonate from byproduct glycerol through transesterification. Ind. Eng. Chem. Res. 2021, 60, 67–88. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Conversion of glycerol as by-product from biodiesel production to value-added glycerol carbonate. In Zero-Carbon Energy Kyoto 2011; Green Energy and Technology; Yao, T., Ed.; Springer: Tokyo, Japan, 2012. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Esterification of glycerol from biodiesel production to glycerol carbonate in non-catalytic supercritical dimethyl carbonate. Springer Plus 2016, 5, 923. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: A review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Pina, C.D. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Kenar, J.A. Glycerol as a platform chemical: Sweet opportunities on the horizon? Lipid Technol. 2007, 19, 249–253. [Google Scholar] [CrossRef]
- Budavari, S. Merck Index, 11th ed.; Merck & Co. Inc.: Rahway, NJ, USA, 1989. [Google Scholar]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energ. Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Nazir, M.H.; Ameen, M.; Zulqarnain; Yusoff, M.H.M.; Inayat, A.; Danish, M. Production of fuel additive solketal via catalytic conversion of biodiesel-derived glycerol. Ind. Eng. Chem. Res. 2020, 59, 20961–20978. [Google Scholar] [CrossRef]
- Cychy, S.; Lechler, S.; Muhler, M. Selective anodic oxidation of solketal as acetal-protected glycerol over nickel boride in alkaline media to glyceric acid. ChemElectroChem 2022, 9, e202101214. [Google Scholar] [CrossRef]
- Schwarz, K.-H.; Kleiner, K.; Ludwig, R.; Schrötter, E.; Schick, H. Synthesis of methyl (±)-2,3-O-isopropylideneglycerate by electrochemical oxidation of (±)-1,2-O-isopropylideneglycerol. Liebigs Ann. Chem. 1991, 1991, 503–504. [Google Scholar] [CrossRef]
- Karmee, S.K. Chemo-enzymatic reaction sequence for the synthesis of dihydroxyacetone phosphate (DHAP) stock material. Synth. Commun. 2013, 43, 450–455. [Google Scholar] [CrossRef]
- Machado, A.C.O.; Da Silva, A.A.T.; Borges, C.P.; Simas, A.B.C.; Freire, D.M.G. Kinetic resolution of (R,S)-1,2-isopropylidene glycerol (solketal) ester derivatives by lipases. J. Mol. Catal. B Enzym. 2011, 69, 42–46. [Google Scholar] [CrossRef][Green Version]
- Romano, D.; Ferrario, V.; Molinari, F.; Gardossi, L.; Montero, J.M.S.; Torre, P.; Converti, A. Kinetic resolution of (R, S)-1,2-O-isopropylideneglycerol by esterification with dry mycelia of moulds. J. Mol. Catal. B Enzym. 2006, 41, 71–74. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Angwarmasse, L.S.; Bessy, E.Y.; Wogo, H.E.; Detha, A.I.R.; Tjitda, P.J.P. Improved synthesis of α-glycerol monolaurate using Lipozyme TL IM. J. Oleo Sci. 2022, 71, 1013–1020. [Google Scholar] [CrossRef]
- Kowalska-Kus, J.; Malaika, A.; Held, A.; Jankowska, A.; Janiszewska, E.; Zielinski, M.; Nowinska, K.; Kowalak, S.; Konska, K.; Wróblewski, K. Synthesis of solketal catalyzed by acid-modified pyrolytic carbon black from waste tires. Molecules 2024, 29, 4102. [Google Scholar] [CrossRef]
- Kenar, J.A.; Knothe, G. 1,2-Isopropylidene glycerol carbonate: Preparation, characterization, and hydrolysis. J. Am. Oil. Chem. Soc. 2008, 85, 365–372. [Google Scholar] [CrossRef]
- Prete, P.; Trano, S.; Zaccagnini, P.; Fagiolari, L.; Amici, J.; Lamberti, A.; Proto, A.; Bella, F.; Cucciniello, R. Glycerolcarbonate and solketalcarbonate as circular economy bricks for supercapacitors and potassium batteries. ChemSusChem 2024, 16, e202401636. [Google Scholar] [CrossRef] [PubMed]
- Selva, M.; Benedet, V.; Fabris, M. Selective catalytic etherification of glycerol formal and solketal with dialkyl carbonates and K2CO3. Green Chem. 2012, 14, 188–200. [Google Scholar] [CrossRef]
- Karmee, S.K. Can glycerol carbonate be synthesized without a catalyst? Lett. Org. Chem. 2024, 21, 563–567. [Google Scholar] [CrossRef]
- Das, A.; Shi, D.; Halder, G.; Rokhum, S.L. Microwave-assisted synthesis of glycerol carbonate by transesterification ofglycerol using Mangifera indica peel calcined ash as catalyst. Fuel 2022, 330, 125511. [Google Scholar] [CrossRef]
- Sarkar, A.; Santra, S.; Kundu, S.K.; Hajra, A.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N.; Majee, A. A decade update on solvent and catalyst-free neat organic reactions: A step forward towards sustainability. Green Chem. 2016, 18, 4475–4525. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, J.; Gu, A.; Nong, X.-M.; Zhai, S.; Yue, Z.-Y.; Feng, C.-G.; Liu, Y.; Lin, G.-Q. Solvent-free and catalyst-free direct alkylation of alkenes. Green Chem. 2023, 25, 7073–7078. [Google Scholar] [CrossRef]
- Goswami, U.J.; Xalxo, A.; Khan, A.T. Catalyst- and solvent-free synthesis of pentacyclic-dionederivatives from 4-hydroxythiocoumarin and aldehyde using pseudo-three-component reaction. ChemistrySelect 2023, 8, e202302520. [Google Scholar] [CrossRef]
- Kumari, G.; Soni, B.; Karmee, S.K. Synthesis of activated carbon from groundnut shell via chemical activation. J. Inst. Eng. India Ser. E 2022, 103, 15–22. [Google Scholar] [CrossRef]
- Soni, B.; Karmee, S.K. Towards a continuous pilot scale pyrolysis based biorefinery for production of biooil and biochar from sawdust. Fuel 2020, 271, 117570. [Google Scholar] [CrossRef]
- Karmee, S.K.; Kumari, G.; Soni, B. Pilot scale oxidative fast pyrolysis of sawdust in a fluidized bed reactor: A biorefinery approach. Bioresour. Technol. 2020, 318, 124071. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.-H.; Hatti-Kaul, R. Chlorine-free synthesis of organic alkyl carbonates and five-and six-membered cyclic carbonates. Adv. Synth. Catal. 2016, 385, 834–839. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K.; Góra-Marek, K. LTA zeolites as catalysts for transesterification of glycerol with dimethyl carbonate. Fuel 2024, 362, 130757. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Hameed, B.H. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over K-zeolite derived from coal fly ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Ribeiro, A.P.C.; Orišková, S.; Martins, L.M.D.R.S.; Cristino, A.F.; Dos Santos, R.G. Glycerol valorization-The role of biochar catalysts. Molecules 2022, 27, 5634. [Google Scholar] [CrossRef]
- Kenar, J.A.; Knothe, G.; Copes, A.L. Synthesis and characterization of dialkyl carbonates prepared from mid-, long-chain, and guerbet alcohols. J. Am. Oil. Chem. Soc. 2004, 81, 285–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).