Abstract
This work aimed to extract nanolignin from green coconut husk and fiber using the acetosolv method, with the aim of transforming waste into high-value-added products and promoting sustainability and bioeconomy. The acetosolv pulping was carried out in two stages, varying temperature conditions and the presence or absence of extractives. Lignin was obtained by precipitation and subsequently characterized through chemical and morphological analyses. The analyses of the primary components of the coconut husk and fiber demonstrated lignin, cellulose, and hemicellulose contents of 40%, 15.90%, and 15.86%, respectively. Then, nanolignin was produced through ultrasonication (850 W for 10 and 20 min). The characteristics of the obtained products were analyzed, considering the influence of two temperatures (100 °C and 120 °C) and the need for a pretreatment step (removal of extractives). The temperature variation between 100 °C and 120 °C, as well as the presence of extractives, did not significantly influence the lignin quality or extraction efficiency. The nanolignin produced under this condition was subjected to the DLS technique to determine the hydrodynamic diameter and polydispersity of the nanoparticles obtained, with an average diameter of 533.75 ± 15.12 nm after 20 min of ultrasonication. The purity of the lignin was confirmed by analyses such as the Klason lignin and ash content, which presented values of 78.82 ± 0.81% and 0.55 ± 0.26%, respectively. FTIR analyses revealed typical lignin characteristics, such as the presence of ketone groups, aromatic structures, and methoxylation, while thermograms confirmed the thermal stability of the lignin. Acetosolv pulping proved to be particularly interesting, preserving good quality lignin and allowing for partial recovery of the solvents used, promoting the sustainability and energy efficiency of the process.
1. Introduction
The growing concern about replacing fossil materials with renewable resources that have less environmental impact has been a central theme in numerous scientific articles over the years. Within this context, lignocellulosic biomass has gained prominence due to its renewability and wide availability. This type of biomass can be used as a raw material for the development of the bioeconomy, leading to the synthesis of a wide range of chemical products [1].
Among the various sources of lignocellulosic biomass, coconut cultivation (Cocos nucifera) stands out for generating large quantities of waste that are often not properly discarded or used [2]. Brazil, being the world’s fifth largest producer of coconut, focuses mainly on removing and drying the pulp to obtain copra. The main byproducts of copra are coconut oil and coconut flour [3], resulting in approximately 15% of the fruit being used. This generates around 850 kg of waste for each ton of fruit produced [4,5].
Despite being an organic waste, the large amount of lignocellulosic material in coconuts means that it takes much longer to decompose in the environment than other organic materials. Given the high consumption of fruit and the consequent generation of waste, it is important to develop reuse techniques that not only reduce the environmental impact but also add economic value to by-products and waste, fostering a sustainable bioeconomy [6].
Lignin is the second most abundant biopolymer in nature. The proportion of lignin present in lignocellulosic biomass can vary between 15 and 40%, which corresponds to a significant percentage of the lignocellulosic material, thus justifying the intensification of research aiming at its reuse [7]. Lignin is currently used in a variety of products, including cosmetics as a UV-protective agent, nutraceuticals, and biocides. Its benefits extend to biostabilizers and coating materials, as well as flame retardants in the paper industry. Lignin is also valued for its antimicrobial and antioxidant properties and is used in the preparation of biofertilizers, epoxy resins, and various fine chemicals [8].
Traditional methods for extracting lignin from lignocellulosic biomass are acid or alkaline hydrolysis, enzymatic processes, cleaning with ionic liquids, the use of organic solvents, or combinations of these techniques [9]. The acetosolv pulping technique is an organosolv method for lignin extraction, where a combination of organic solvents is used, such as acetic acid (in higher concentration), hydrochloric acid as a catalyst, and water, with the solution being subjected to a temperature ranging from 125 to 170 °C and kept under reflux for a period of 60 to 180 min [10]. This process selectively dissolves lignin from plant biomass, which is of high purity, has little degradation, low levels of inorganic solids, and is free of sulfur compounds. These characteristics make acetosolv lignin an attractive option for several industrial applications, such as in the manufacture of chemicals, advanced materials, and bioplastics. Additionally, acetosolv pulping is considered environmentally friendly, as it allows for the recovery of approximately 70% of the solvent used and its reuse, reducing the environmental impact and improving the economic viability of the process [11].
Although the removal of extractives is commonly considered an essential step to improve the yield and quality of lignin, this research is distinguished by its systematic investigation of the impact of this pretreatment step on the characteristics and yield of the extracted lignin. By comparing the results with and without the removal of extractives, it was possible to identify that eliminating this step did not compromise the extraction process performance, presenting significant operational advantages. Among these advantages, the reduction in energy consumption and chemical products, making the process more efficient and sustainable, simplifying extraction, and lowering operational costs can be mentioned.
Despite its promise, lignin, as a macromolecule, is a difficult material to apply due to some characteristics, such as its resistance to water, which makes it difficult to incorporate into aqueous mixtures. However, when reduced to a nanometric size, this resistance decreases, facilitating the incorporation process, and for this reason nanolignin has been standing out and gaining increasingly more space in scientific research and in the industrial sector [12].
Nanolignin is widely recognized as one of the most effective strategies for improving the properties of lignin. Its reduced size facilitates interactions with polymeric matrices, while its spherical shape contributes to greater stability and dispersion in water, ensuring homogeneous and stable dispersion.
Moreover, the increased surface area of the nanoparticles enhances their chemical modification due to the presence of various functional groups, such as thiols, aliphatic hydroxyls, and phenolics, making these functional groups more accessible. This increased accessibility facilitates chemical reactions. Nanolignin also exhibits antimicrobial activity, further expanding its applications [13]. These characteristics make lignin nanoparticles multifunctional, enabling their use as surfactants, plasticizers, emulsifiers, or additives in polymer matrices, such as epoxy and thermoplastics. Furthermore, they may contribute to sustainability by serving as nanocarriers for environmentally sustainable pesticides [14,15].
There are several approaches to reducing lignin to the nanometric scale, including chemical methods, such as hydrolysis and acid precipitation, and mechanical methods, such as homogenization and ultrasonication [16]. In ultrasonic treatment, high-frequency sound waves generate cavitation in the liquid, fragmenting the lignin into nanostructures. This method is useful for maintaining desirable properties of lignin, such as its interaction with materials and its antioxidant characteristics [17].
This work aimed to obtain nanolignin from the shell and fiber of green coconut, promoting more effective use of these residues using the acetosolv method (applying different temperatures and with or without extractives in the biomass) and ultrasonic treatment (with different contact times).
2. Methodology
2.1. Materials
Green coconut residues (Cocos sp.) were obtained from small traders in Maceió (Alagoas, Brazil) in September 2022. Initially, the outer part of the coconuts was washed with running water. The almonds were removed, and the shells and fibers were cut to reduce the size of the biomass. Then, the residue was subjected to drying in an oven with forced air circulation (7Lab digital stainless steel 1152 L 220v) for a period of 72 h. Subsequently, the fibers were crushed in a micro knife mill (Mod. MA680 from Marconi, Piracicaba, Brazil) and sieved using a 40-mesh sieve to uniformize the particles. This flour was then stored in airtight plastic containers and kept in a dry, dark place. The flour composition was determined in terms of % (w/w): lignin 40.0 ± 0.5%. The sample was digested with 72% (v/v) H2SO4 in a thermostatic bath at 45 °C for 7 min. The reaction was stopped by adding 50 mL of distilled water, and the solution was transferred to an Erlenmeyer flask, where 225 mL of distilled water was added. The solution was then autoclaved for 30 min at 121 °C [18]. Cellulose was 15.9 ± 1.5% and hemicellulose 15.86 ± 0.33% [19]. Extractives (8.17 ± 1.43%) were determined by weighing 2 to 5 g of the biomass sample into filter paper cartridges and subjecting it to extraction in a Soxhlet apparatus, using a hexane/ethanol solvent mixture in a 2:1 ratio for 5 h [20]. Ash content (3.28 ± 0.13%) was determined by transferring the sample to porcelain crucibles and heating it in a muffle furnace at 550 °C for 5 h [21]. Moisture content (9.59 ± 0.13%) was determined by the gravimetric method after drying the material at 105 °C [22].
2.2. Lignin Extraction by Acetosolv Pulping
Acetosolv pulping was performed using a methodology adapted from Benar et al. [23] and Nascimento et al. [24], in which the experiment was carried out using in natura coconut flour (FCIN) and coconut flour without extractives (FCSE), to which 8 g of biomass was added to a flat-bottomed reaction flask, followed by 120 mL of acetosolv solution (acetic acid 93% w/w, hydrochloric acid 0.3% w/w, distilled water 6.7% w/w), in a biomass–solvent ratio of 1:15 w:v. After the liquor reached the boiling point, the system was kept at reflux for 3 h, conducted under two temperature conditions: 100 °C and 120 °C. Due to the volatility of the solution components (acetic acid, water, and HCl), it is likely that an increase in internal pressure occurred during the process but this is characteristic of the apparatus and temperature applied and can easily be reproduced, although the pressure was not measured directly. The temperature variation was applied to the process to verify significant differences in the lignin yield obtained.
The resulting pulp was then filtered through filter paper using a vacuum pump to separate the cellulose fibers and the acidic black liquor containing lignin. The resulting black liquor was subjected to rotary evaporation at 60 °C to concentrate the liquor volume up to 10 times and recover the acetosolv solution. The lignin generated during the process was precipitated in hot distilled water (80 °C) in a ratio of 1:10 to the volume of rotary evaporated lignin. The mixture was left to stand at room temperature for 24 h.
After this period, vacuum filtration was performed, and the material was washed with distilled water until the pH of the wash water was close to the pH of the water used. The residue was dried in an oven at 60 °C until a constant mass was obtained (Nogueira, 2016) [25]. The percentage yields of all extracted lignin were calculated from Equation (1) [26], where YL is the yield of extracted lignin on a dry basis (%), ML is the recovered lignin obtained after precipitation on a dry basis (g), and MIL is the initial mass (dry basis) of lignin in the fibers before the process (g) (it is important to highlight that MIL was determined based on the initial characterization of the coconut flour, considering the correction for the sample’s moisture content and the lignin percentage present in the biomass):
2.3. Lignin Characterization
The lignin obtained was characterized by moisture, ash, and Klason lignin analysis according to the methodologies mentioned for flour characterization in Section 2.1: Scanning Electron Microscopy (SEM), thermogravimetric analysis (TG), and Fourier-transform infrared spectroscopy (FTIR).
For Scanning Electron Microscopy (SEM) (Vegan3 from Tescan, Brno, Czech Republic), the samples were subjected to metallization using a platinum target for 3 min, followed by a gold target for 5 min at a current of 10 mA. The SEM was operated at 5 kV, with a focus magnification between 457 and 1800 times. Fields of view of 454 μm to 115 μm were used, with scales of 100 μm and 20 μm for the photomicrographs. The material was scanned at several points, with magnifications of 300 to 4000 times.
The thermogravimetric (TG) analyses of the lignin samples were evaluated using a TGA-51/51H from Shimadzu, Kyoto, Japan. The analysis was performed at a continuous heating rate of 10 °C per minute until reaching 900 °C in a nitrogen (N2) atmosphere to prevent undesired reactions. Thermal decomposition curves were obtained by means of a microcomputer connected to the instrument, using TA-60 WS Shimadzu software (version 2.21), which captured the data at a rate of one point every 0.5 s, ensuring high resolution in the results. Fourier-transform infrared spectroscopy (FTIR) spectra were obtained using a spectrophotometer (FTIR IRAffinity-1, Shimadzu) and were used to analyze the isolated samples of the raw biomass, without extractives, and the extracted lignin. The spectroscope operated with a wavelength range of 4000 to 600 cm−1 and performed 64 scans.
2.4. Process of Obtaining Nanolignin
The lignin extracted through the acetosolv pulping process at 100 °C, derived from raw coconut flour, was initially suspended in water and then subjected to intensive ultrasonic treatment for 10 and 20 min using the 850 W Ultrasonic Sonicator from Ecosonics (Hwaseong-si, South Korea), based on the method by Rani and Venkatachalam [27]. This ultrasonication process was carried out to reduce particle size, ensure homogeneous dispersion of the lignin, and improve the efficiency of subsequent analyses.
2.5. Dynamic Light Scattering (DLS)
The dynamic light scattering (DLS) technique was used to determine the hydrodynamic diameter and polydispersity of the obtained nanoparticles. The samples were diluted in a proportion of 500 μL to 25 mL and treated with tip ultrasound for 3 min at 200 W. The size of the nanolignins was measured at 25 °C using a dynamic light scattering particle size distribution analyzer, model Zetasizer Nano (ZS Malvern Instruments Ltd., Worcestershire, UK).
3. Results and Discussion
3.1. Yield and Characterization of Acetosolv Lignin
The acetosolv lignin extracted from raw coconut flour showed an average yield of 13.86%, as shown in Table 1. The lignin yields at two temperatures (100 and 120 °C) were investigated for raw coconut flour (FCIN) and for the one without extractives (FCSE). In general, the removal of extractives (FCSE) resulted in higher yields compared to FCIN. The temperature of 120 °C increased the yields for FCSE but reduced them for FCIN. Although the results revealed greater process efficiency for FCSE and for the process carried out applying higher temperatures (120 °C), the analysis of variance (ANOVA) revealed that these variations did not result in statistically significant differences in the yields obtained (95% of confidence level, experiments performed in duplicate). This suggests that, within the range studied, the pretreatment step for extractive removal can be eliminated, and the process can be carried out at milder temperatures, making it more economically and environmentally viable.

Table 1.
Extraction yield and characterization of lignin after acetosolv method.
Previous work on lignin extraction has shown a wide variation in the yields obtained, which can be attributed to significant differences in the experimental conditions and the nature of the biomass used. Nascimento et al. [24] reported a 30% yield using coconut fiber as the raw material, obtained under constant agitation, which suggests that agitation may play an important role in the efficiency of the process. However, Verçosa et al. [28] achieved a 98% yield from mango tegument, a considerably higher value, which can be explained by the lower structural resistance of the biomass used, facilitating the release of lignin. In addition, Marques et al. [29] observed a 13.88% yield while applying coconut fiber, a result that corroborates the present research, suggesting that temperatures below 200 °C are more effective in the acetosolv process. These results highlight the importance of considering different operational conditions to optimize lignin extraction in industrial and environmental applications.
Lignin purity was assessed using Klason lignin analysis, in addition to determining the ash content present in the sample. In this methodology, the sample is subjected to strong acidic treatment, which removes soluble fractions such as hemicellulose and sugars, leaving the lignin insoluble [30]. Additionally, the ash content of the sample was determined, reflecting the mineral impurities. Together, these methodologies provide a more accurate estimate of lignin purity as they consider critical elements of the biomass that could interfere with the Klason analysis results if not properly adjusted for.
Table 1 shows the results of lignin extraction from raw coconut (LCIN) and without extractives (LCSE) using the acetosolv method at two different temperatures (100 and 120 °C). The data are presented in terms of ash content (%) and Klason lignin content (%). This temperature comparison aims to identify the temperature influence for lignin extraction, reflecting the quality and purity of the final product.
The amount of ash obtained in this work varied between 0.45 and 0.58%, significantly low values that indicate the high purity of the extracted lignin, reflecting the minimal presence of inorganic contaminants such as metals and minerals. This characteristic is important because the amount of ash is directly related to the quality of the lignin, with significant impacts on its possible industrial applications [31].
While the Kraft method involves the use of aggressive chemicals, which often result in the formation of impurities, the acetosolv process—based on the use of acetic acid—is less severe and, consequently, more selective, favoring the production of lignin with lower ash contents. These data reinforce that the methodology applied in this work not only allows for the extraction of lignin but also guarantees a superior quality of the final product [32]. The purity levels of the lignin obtained varied between 78.2% and 80.94%. In terms of lignin purity, these results are close to those obtained by Verçosa et al. [28], who achieved 88% purity in the lignin from mango tegument extracted using the acetosolv method, and 86% found by Marques et al. [29] from green coconut fiber using the ethanosolv method.
However, the values obtained in this work are lower than the 97.3% purity obtained by Marques et al. [29] from sugarcane bagasse, also obtained by the ethanosolv method. Although the ethanosolv process, as demonstrated by Marques et al. [29], achieved higher purity than the present work, this method has significant disadvantages. The ethanol used in this method is more expensive than the acetic acid applied in the acetosolv method, and the temperatures used in the process are very high (170 to 224 °C) compared to those used in this study; furthermore, it was not mentioned if recovery of the solvent was possible or not. Additionally, mango tegument and sugarcane bagasse are biomasses with structures that are more accessible for breaking, resulting in more efficient extraction. However, green coconut fiber, with a more resistant structure, challenges the extraction process, which may justify the slightly lower purity compared to other biomasses. Therefore, the variation in the purity percentages found in different studies reflects the complexity of lignocellulosic materials and the significant influence of experimental conditions on the efficiency of lignin extraction. The consistency of the results obtained in this work reinforces the suitability of the acetosolv method for the extraction of lignin from green coconut flour since this method was demonstrated to have processes with a high percentage of recovery of the solvents used, and the purity level obtained is very close to values found in the literature.
3.2. Thermogravimetric Analysis (TG) of Lignin
Thermogravimetry (TG) is a technique used to analyze the thermal stability of lignins, which allows for the measurement of mass loss over time. This technique is particularly relevant for industrial applications involving high temperatures. The observed mass loss is associated with the degradation of compounds in lignin, including phenols, phenolic derivatives, carboxylic acids, aldehydes, and ketones [33].
In Figure 1, it is possible to observe the mass variations in the samples in response to the increases in temperature, providing a detailed view of the thermal stability of lignin. The thermogram reveals different mass loss events that correspond to distinct thermal processes in the sample. Initially, a mass reduction is observed around 50 °C in the precursor flour, indicating the release of moisture or residual solvents [34]. This phenomenon is present in both the raw material and the material without extractives. For lignin samples, the first significant event occurs only around 200 °C, suggesting the decomposition of lighter compounds. A second mass loss event close to 400 °C points to the decomposition of heavier components or the burning of organic residues [35]. The thermal profiles of lignin show similarities, and, after this temperature, the TG curve stabilizes, indicating the absence of additional mass loss events and suggesting minimal or no further decomposition.
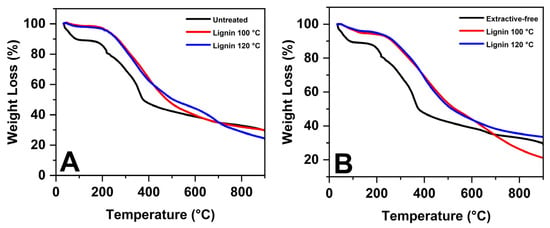
Figure 1.
Thermogravimetric analysis of (A) LCIN, and (B) LCSE.
3.3. Fourier-Transform Infrared (FTIR) Analysis of Lignin
Fourier-transform infrared (FTIR) spectroscopy was used to analyze potential structural differences in the peak positions of vibrational bands between the raw and extractive-free biomass, as well as for the lignins extracted by the acetosolv method.
Figure 2 shows the peaks obtained by infrared spectroscopy analysis for both fresh biomass and the biomass without extractives, where Figure 2A shows significant peaks in the range of 3700 to 3000 cm−1, corresponding to the vibrations of the aliphatic and phenolic -OH groups, indicating the formation of hydrogen bonds [26]. This analysis confirms the integrity of the lignin chemical bonds and helps identify impurities, enabling optimizations in the extraction process.
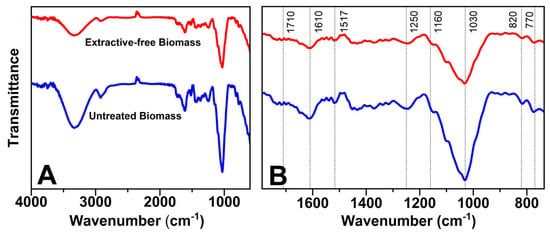
Figure 2.
Infrared spectra of biomass in natura (FCIN) and without extractives (FCSE). (A) Peaks in the range of 3700 to 3000 cm−1. (B) Expansion of the reading between 1750 and 700 cm−1.
The comparative evaluation between fresh biomass and that subjected to pretreatment aimed to evaluate the changes caused by the removal of extractives, essential for the preparation of acetosolv pulping, as described in the literature [36]. FTIR analysis of the precursor showed a reduction in peak intensity at 1030 cm−1, indicating effective removal of waxes, resins, and other constituents during pretreatment (Figure 2B) [37]. Despite these changes, the characteristic peaks of the sample remained virtually unchanged, as seen in Figure 2, suggesting that pretreatment was able to eliminate unwanted components without compromising the essential properties of the biomass [38].
The results suggest that the pretreatment step can be omitted since acetosolv pulping did not show significant changes in the absence of this process. Eliminating pretreatment could reduce chemical and energy costs, allowing the raw biomass to be used directly, optimizing the production process.
The untreated biomass was compared with the variations in the analyzed parameters (100 and 120 °C), focusing on the expanded absorption band (700 to 1800 cm−1) in the FTIR spectra of the lignin samples (Figure 3). The samples maintained their characteristic peaks, evidencing stability even with changes in the process conditions, without presenting significant modifications.
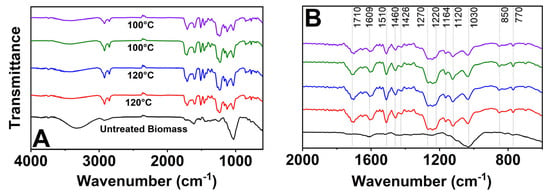
Figure 3.
Infrared spectra of fresh biomass and lignin obtained at different LCIN (red and green) and LCSE (blue and purple) parameters. (A) Peaks in the range of 4000 to 900 cm−1. (B) Expansion of the reading between 700 and 1800 cm−1.
The FTIR spectroscopy identified significant peaks that provide a detailed analysis of the composition and structure of lignin. The peak around 1710 cm−1, indicative of C=O stretching in unconjugated ketones, carbonyls, and esters, suggests the existence of lignin–carbohydrate complexes, as reported by Abd Latif et al. [39]. These complexes involve interactions between lignin and carbohydrates such as cellulose, hemicellulose, and pectin. Peaks at 1609 cm−1 and 1510 cm−1, associated with C=C bond stretching, confirm the presence of aromatic structures typical of lignin [11]. Furthermore, peaks at 1460 cm−1 and 1426 cm−1 indicate methylation at CH3 and CH2 groups, while the peak at 850 cm−1 is associated with out-of-plane C-H deformation of lignin [40].
The peaks at 1270 cm−1, 1220 cm−1, and 1164 cm−1 indicate the presence of guaiacylic and syringyl units in lignin, associated with C=O stretching, characteristic of HGS and GS lignins [39]. The peak at 1120 cm−1 reflects C-O stretching in aliphatic ethers and alcohols, suggesting the presence of these functional groups in the lignin structure [41]. Furthermore, the peak at 770 cm−1 is related to out-of-plane C-H deformation, complementing the structural analysis of the lignocellulosic structure [42].
The analysis of the FTIR spectra of lignin extracted by the acetosolv method indicates the presence of ketone groups, aromatic structures, guaiacylic and syringic units, methylations, ethers, and alcohols [43]. These data provide us with important information about the structure and functionality of lignin, which helps us understand the best applications for the obtained lignin.
3.4. Scanning Electron Microscopy (SEM) of Lignin
Scanning Electron Microscopy (SEM) analyses were performed to study the morphology and surface appearance of LCIN and LCSE after acetosolv pulping at different temperatures. The micrographs in Figure 4, obtained at 100 and 120 °C, show that LCSE (Figure 4B) has a smoother and more uniform surface due to the removal of extractives, while LCIN (Figure 4A) presents a rougher morphology. In addition, in Figure 4C,D, the LCIN and LCSE samples obtained at 120 °C were analyzed at a resolution of 200 µm and an average magnification of 205.5×, which revealed the amorphous structure of lignin.
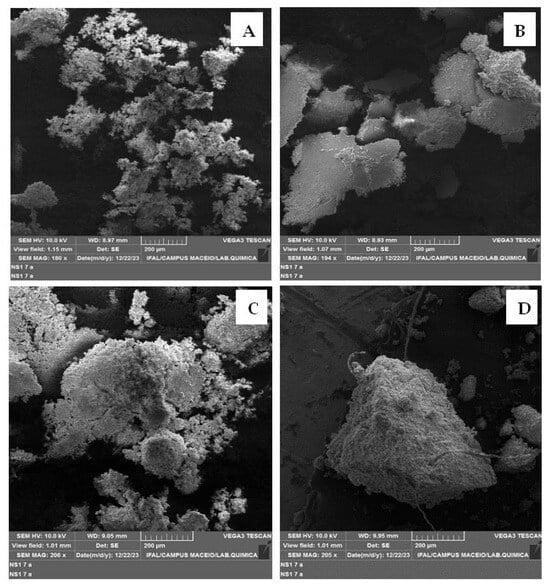
Figure 4.
Microscopy of lignin in natura (LCIN) (A,C), and lignin pretreated with ultrasonication at 100 °C (B) and 120 °C (D).
The lignin obtained by the acetosolv method revealed the formation of spherical agglomerates (Figure 5). These agglomerates suggest that the acetosolv process—which involves the dissolution of lignin in acetic acid—promoted changes in the structure and molecular interactions of lignin, leading to the formation of such structures. Previous studies have also observed this phenomenon, highlighting the complexity and behavior of lignin during acetosolv treatment [44,45].
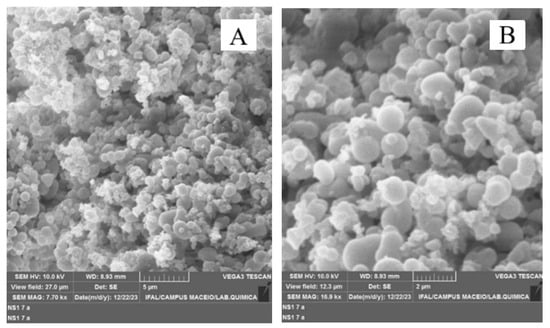
Figure 5.
Microscopy of lignin in natura (LCIN): (A) magnified 7.70 kx and (B) magnified 16.9 kx.
The micrographs revealed that porosities in lignin clusters may arise due to the processes involved in the formation of these structures. During acetosolv pulping, lignin may precipitate, resulting in agglomeration of the molecules and creating pores or empty spaces in the clusters, due to nucleation phenomena [46]. In addition, the formation of small cavities in the clusters may occur due to the release of gases or vapors that escape during the process; these variations in the structure and composition of lignin may also contribute to the emergence of these porosities [47].
No significant morphological variations were detected in lignin when the temperature was changed during the pulping process. This indicates that the morphological structure of lignin remains stable in the face of the temperature variations employed, thus suggesting that lignin is resistant to morphological modifications within the thermal conditions tested.
3.5. Dynamic Light Scattering (DLS) of Nanolignin
The analysis of lignin nanoparticles extracted from raw material by means of DLS (dynamic light scattering) indicated a significant decrease in the average particle size with the increase in the applied processing time. The nanolignin was obtained from lignin obtained through acetosolv pulping carried out at 100 °C from raw coconut flour. Particle sizes between 500 nm and 750 nm, with an average diameter of 656.25 nm, were obtained after 10 min of ultrasonic processing, and sizes between 400 nm and 600 nm, with an average diameter of 533.75 nm, were achieved after 20 min. These results suggest that longer processing times lead to the greater fragmentation of lignin. The choice of 10 and 20 min was based on the higher power employed (850 W), exceeding the 400–600 W range commonly reported in the literature [16,17]. The objective was to determine whether shorter times, combined with higher power, could yield satisfactory results, thereby enhancing process feasibility by reducing energy consumption and improving operational efficiency.
The distribution of nanolignin size is in relation to the percentage of nanoparticles, where the adjustment in the processing time optimized the dispersion and uniformity of the nanolignins (Figure 6).
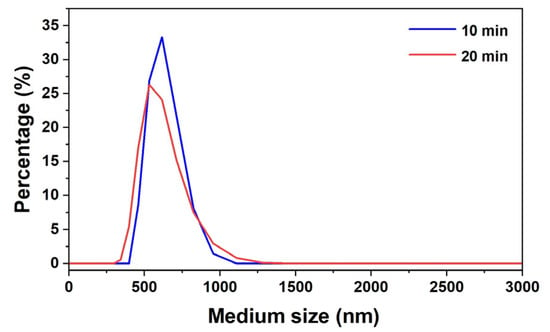
Figure 6.
Distribution of mean particle size of nanolignin.
Juikar and Vigneswaran [40] reported lignin nanoparticles with an average diameter of 340.3 nm determined by DLS. The lignin nanoparticles were obtained through the sonication process, where the material was subjected to cleavage in an ultrasonicator for 1 h. The nanolignin obtained by the authors presented a smaller size compared to that obtained in this work, which can be attributed to the longer exposure time of the precursor lignin to the shear forces generated by the ultrasonicator. Matsakas et al. [48] studied a hybrid fractionation method, combining organosolv pulping and steam explosion, followed by a post-treatment of homogenization of birch biomass to obtain nanolignin. The authors used different solvent compositions for the homogenization process and investigated the influence of this combination of techniques on the lignin particles formed. According to the authors [48], the reported DLS results indicated that the most satisfactory values of lignin nanoparticles obtained with 75% v/v ethanol during homogenization presented diameters between 250 nm and 500 nm. The operational conditions, as well as the type of biomass and solvents used by the authors and/or the combination between them, may have contributed to obtaining smaller lignin nanoparticles.
Although DLS provides reliable data on the hydrodynamic size of the particles, additional analyses are necessary to verify morphological aspects and confirm the size dispersion of lignin nanoparticles. Techniques such as SEM and TEM are essential for understanding the morphology of nanolignins and comparing the influence of the methods of obtaining them applied in the modification of these structures. Furthermore, the analysis of the zeta potential provides information on the colloidal stability of the nanoparticles, determining their behavior in suspension and their dispersion, this information being essential to guarantee more efficient industrial applications for the type of nanolignin obtained.
4. Conclusions
This research investigated the production of nanolignin from the shell and fiber of green coconuts, using acetosolv pulping, with the aim of transforming waste into high-value-added products and promoting sustainability. The results obtained during lignin extraction revealed that the biomass pretreatment step to remove extractives is not necessary since this process does not significantly alter the lignin yields. The percentage yield of lignin obtained in the process without the pretreatment step was 13.89 ± 4.35%, contributing to a reduction in chemical and energy resource consumption during the extraction process. The acetosolv method preserves the chemical structure of lignin and, at the same time, removes cellulose and hemicellulose with high quality. This is essential to ensure purity, as confirmed by methods such as the corrected calculation of Klason lignin and ash, presenting high purity values and indicative of the quality of the product obtained, with FTIR and thermograms also identifying characteristic peaks of lignin and thermal stability, important for its applications. Furthermore, the ultrasonic treatment was essential to obtain lignin at a nanometric scale, with the average size of the obtained nanolignin particles being 533.75 nm.
Author Contributions
Conceptualization, L.N.L., R.M.R.G.A. and R.R.d.L.A.; methodology, L.N.L., R.M.R.G.A. and R.R.d.L.A.; formal analysis, L.N.L., R.R.d.L.A., J.M.D.d.F., J.D.d.F. and M.B.d.S.; investigation, L.N.L., R.R.d.L.A. and F.P.d.A.; writing—original draft preparation, L.N.L., R.R.d.L.A. and F.P.d.A.; resources, R.M.R.G.A., C.E.d.F.S. and J.D.d.F.; visualization, R.R.d.L.A., F.P.d.A., R.M.R.G.A. and C.E.d.F.S.; software, C.E.d.F.S. and R.R.d.L.A.; validation, R.M.R.G.A.; data curation, R.M.R.G.A., R.R.d.L.A., F.P.d.A. and C.E.d.F.S.; writing—review and editing, R.M.R.G.A., R.R.d.L.A., F.P.d.A., C.E.d.F.S. and P.H.B.F.; supervision, R.M.R.G.A.; project administration, R.M.R.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from MCTI—Brazil/CNPq/BRICS-ST nº 28/2023—process 440026/2024-5. Coordinator: Carlos Eduardo de Farias Silva. In addition, funding was provided by the Research Support Foundation of Alagoas (Project number: E:60030.0000000463/2020).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors express their sincere gratitude to the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Alagoas State Research Support Foundation (FAPEAL) for their financial support through the scholarships awarded. They also thank the Federal Institute of Alagoas (IFAL) for technical support in the analyses performed, as well as the Federal University of Alagoas and the Food and Beverage Technology (LTBA) and Bioprocesses (LaBio) Laboratories for allowing the use of their facilities and for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsegaye, B.; Ström, A.; Hedenqvist, M.S. Thermoplastic lignocellulose materials: A review on recent advancement and utilities. Carbohydr. Polym. Technol. Appl. 2023, 5, 100319. [Google Scholar] [CrossRef]
- Tripathi, M.; Srivastava, N.; Tripathi, S.C.; Singh, R.; Ahmad, I.; Rai, A.K.; Razik, N.E.A.; Mishra, P.K.; Gupta, V.K. Co-fermentation of acid treated coconut wastes using mixed Bacillus cultures for enhanced production of extracellular enzymes: Application in bioconversion of raw coconut fibers. Food Bioprod. Process. 2024, 146, 177–184. [Google Scholar] [CrossRef]
- Rocha, K.D.C.; Ferreira, M.S.; Garcia, C.E.R. Produção e produtos à base de coco (Cocos nucifera L.): A review. Braz. J. Dev. 2022, 8, 41476–41491. [Google Scholar] [CrossRef]
- Pereira Junior, A.O. Aproveitamento energético de resíduos: Um mercado que não se pode descartar. Instituto de Pesquisa Econômica Aplicada (Ipea). Bol. Reg. Urbano Ambient. 2020, 24, 159–161. [Google Scholar]
- Queiroz, L.P.O.; Albuquerque, F.B.; de Souza, J.C.R. Análise bibliométrica sobre a utilização de resíduos do coco (Cocos nucifera L.) em aplicações para biocombustíveis. Rev. Iberoam. Cienc. Ambient. 2021, 12, 9. [Google Scholar] [CrossRef]
- Singh, P.; Dubey, P.; Younis, K.; Yousuf, O. A review on the valorization of coconut shell waste. Biomass Convers. Biorefinery 2022, 14, 8115–8125. [Google Scholar] [CrossRef]
- Hu, H.; Tan, W.; Xi, B. Lignin-phenol monomers govern the pyrolytic conversion of natural biomass from lignocellulose to products. Environ. Sci. Ecotechnol. 2021, 8, 100131. [Google Scholar] [CrossRef]
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Dafchahi, M.N.; Acharya, B. Transforming lignin into renewable fuels, chemicals, and materials: A review. Bioresour. Technol. Rep. 2023, 22, 101463. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; He, F.; Liao, Y.; Duns, G.J.; Qin, Z. Lignin valorization: A crucial step towards full utilization of biomass, zero waste and circular bioeconomy. Biocatal. Agric. Biotechnol. 2013, 51, 102777. [Google Scholar] [CrossRef]
- Razali, N.; Ong, P.; Ibrahim, M.; Daud, W.R.W.; Zainuddin, Z. Modeling of acetosolv pulping of oil palm fronds using response surface methodology and wavelet neural networks. Cellulose 2019, 26, 4615–4628. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Carmo, K.P.; Freitas, R.R.M.; Silva, J.O.; Lima, V.; Botaro, V.R. Isolamento e caracterização de lignina acetossolve extraída do bagaço de cana-de-açúcar. Rev. Virtual Quím. 2020, 12, 4. [Google Scholar]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.L.; Puglia, D. Multifunctional lignin-based nanocomposites and nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S. Lignin nanoparticles: Eco-friendlyand versatile tool for a new era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Ortega-Sanhueza, I.; Girard, V.; Ziegler-Devin, I.; Chapuis, H.; Brosse, N.; Valenzuela, F.; Banerjee, A.; Fuentealba, C.; Cabrera-Barjas, G.; Torres, C.; et al. Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources. Polymers 2024, 16, 1610. [Google Scholar] [CrossRef] [PubMed]
- Savy, D.; Verrillo, M.; Cangemi, S.; Cozzolino, V. Lignin nanoparticles from hydrotropic fractionation of giant reed and eucalypt: Structural elucidation and antibacterial properties. Int. J. Biol. Macromol. 2024, 262, 129966. [Google Scholar] [CrossRef]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A recent advancement on preparation, characterization and application of nanolignin. J. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef]
- Kim, I.T.; Sinha, T.K.; Lee, J.; Lee, Y.; Oh, J.S. Ultrasonic treatment: An acid-free green approach toward preparing high-performance activated carbon from lignin. Ind. Eng. Chem. Res. 2021, 60, 2439–2446. [Google Scholar] [CrossRef]
- Gouveia, E.R.; Nascimento, R.T.; Souto-Maior, A.M.; Rocha, G.J.d.M. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quím. Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- T 203 cm-99; Alpha-, Beta- and Gamma-Cellulose in Pulp. Tappi: Atlanta, GA, USA, 2009; 7p.
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef]
- Hojo, O.; Ernesto, V.A.R.T.; Ribeiro, C.A.; Fiscarelli, P.; Fertonani, F.L. Comparação metodológica entre mufla convencional e automática para análise de umidade e cinzas em bagaço de cana. In Anais do Congresso da Qualidade em Metrologia; REMESP: São Paulo, Brazil, 2008; pp. 1–6. [Google Scholar]
- T412 om-11; Moisture in Pulp, Paper and Paperboard. Tappi: Atlanta, GA, USA, 2011; 3p.
- Benar, P. Polpação Acetosolv de Bagaço de Cana e Madeira de Eucalipto. Master’s Dissertation, Instituto de Química, Universidade Estadual de Campinas, São Paulo, Brazil, 1992. [Google Scholar]
- Nascimento, D.M.D.; Almeida, J.S.; Do Vale, M.S.; Leitão, R.C.; Muniz, C.R.; Figueirêdo, M.C.B.D.; Morais, J.P.S.; Rosa, M.F. A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: Proposition of technological pathways. Ind. Crops Prod. 2016, 93, 66–67. [Google Scholar] [CrossRef]
- Nogueira, I.M. Obtenção de nanolignina de resíduos fibrosos do dendê. Master’s Dissertation, Universidade Federal do Ceará, Fortaleza, Brazil, 2016. [Google Scholar]
- Marques, F.P.; Soares, A.K.L.; Lomonaco, D.; Alexandre e Silva, L.M.; Santaella, S.T.; de Freitas Rosa, M.; Leitão, R.C. Steam Explosion Pretreatment Improves Acetic Acid Organosolv Delignification of Oil Palm Mesocarp Fibers and Sugarcane Bagasse. Int. J. Biol. Macromol. 2021, 175, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.S.J.; Venkatachalam, S. Cleaner approach for the cascade production of nanocellulose, nanohemicellulose and nanolignin from Prosopis juliflora. Carbohydr. Polym. 2022, 11, 119807. [Google Scholar] [CrossRef] [PubMed]
- Verçosa, F.G.; Filho, M.S.; Pereira, V.A.; Azeredo, H.M.C. Extração de Lignina do Tegumento de Mangas; Embrapa—Comunicado técnico; Embrapa: Brasília, Brazil, 2019; Volume 259. [Google Scholar]
- Marques, F.P.; Colares, A.S.; Cavalcante, M.N.; Almeida, J.S.; Lomonaco, D.; Silva, L.M.A.; Rosa, M.F.; Leitao, R.C. Optimization by Response Surface Methodology of Ethanosolv Lignin Recovery from Coconut Fiber, Oil Palm Mesocarp Fiber, and Sugarcane Bagasse. Ind. Eng. Chem. Res. 2022, 61, 4058–4067. [Google Scholar] [CrossRef]
- Gaudenzi, E.; Cardone, F.; Lu, X.; Canestrari, F. The use of lignin for sustainable asphalt pavements: A literature review. Constr. Build. Mater. 2023, 362, 129773. [Google Scholar] [CrossRef]
- Olgun, Ç.; Ateş, S. Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests 2023, 14, 882. [Google Scholar] [CrossRef]
- Duarte, L.C.; Sampaio, B.; Carvalheiro, F. Organosolv Pretreatment of Lignocellulosic Biomass. In Handbook of Biorefinery Research and Technology; Bisaria, V., Ed.; Springer: Dordrecht, The Netherlands, 2024. [Google Scholar]
- Wang, Q.; Sarkar, J. Pyrolysis behaviors of waste coconut shell and husk biomasses. Int. J. Energy Prod. Manag. 2018, 3, 34–43. [Google Scholar] [CrossRef]
- Freitas, B.R.; Braga, J.O.; Orlandi, M.P.; Silva, B.P.; Aoki, I.V.; Lins, V.F.C.; Cotting, F. Characterization of coir fiber powder (Cocos nucifera L.) as an environmentally friendly inhibitor pigment for organic coatings. J. Mater. Res. Technol. 2022, 19, 1332–1342. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Alias, Y. Lignin extraction from coconut shell using aprotic ionic liquids. BioResources 2017, 12, 5749–5774. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefin. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Ramezani, N.; Sain, M. Thermal and physiochemical characterization of lignin extracted from wheat straw by organosolv process. J. Polym. Environ. 2018, 26, 3109–3116. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef] [PubMed]
- Abd Latif, N.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Hashim, R.; Hussin, M. A comparison of alkaline and organosolv lignin extraction methods from coconut husks as an alternative material for green applications. BioResources 2022, 17, 469–491. [Google Scholar] [CrossRef]
- Juikar, S.J.; Vigneswaran, N. Extraction of nanolignin from coconut fibers by controlled microbial hydrolysis. Ind. Crops Prod. 2017, 109, 420–425. [Google Scholar] [CrossRef]
- Hernández, J.M.G.; Escalante, A.; Vázquez, R.N.M.; Delgado, E.; González, F.J.; Toríz, G. Use of Agave tequilana-lignin and zinc oxide nanoparticles for skin photoprotection. J. Photochem. Photobiol. B Biol. 2016, 163, 156–161. [Google Scholar] [CrossRef]
- Jose, S.; Mishra, L.; Basu, G.; Kumarsamanta, A. Study on Reuse of Coconut Fiber Chemical Retting Bath. Parte II—Recovery and Characterization of Lignin. J. Natural Fibers 2017, 14, 510–518. [Google Scholar]
- Pandharipande, S.L.; Gujrati, M.; Mulkutkar, N.; Pandey, S. Comparative Study of Extraction & Characterization of Lignin from Wet and Dry Coconut Husk. Int. J. Eng. Sci. Res. Technol. 2018, 7, 659–666. [Google Scholar] [CrossRef]
- Pinheiro, F.G.C.; Soares, A.K.L.; Santaella, S.T.; Silva, L.M.A.; Canuto, K.M.; Cáceres, C.A.; Rosa, M.F.; Feitosa, J.P.A.; Leitão, R.C. Optimization of the acetosolv extraction of lignin from sugarcane bagasse for phenolic resin production. Ind. Crops Prod. 2017, 96, 80–90. [Google Scholar] [CrossRef]
- Meng, X.; Yunxuan, W.; Austin, J.C.; Shuyang, Z.; Jiae, R.J.J.W.; Yunqiao, P.; Brian, H.D.; Chang, G.Y.; Arthur, J.R. Applications of biomass-derived solvents in biomass pretreatment—Strategies, challenges, and prospects. Bioresour. Technol. 2023, 368, 128280. [Google Scholar] [CrossRef]
- Soh, L.; Eckelman, M.J. Green solvents in biomass processing. ACS Sustain. Chem. Eng. 2016, 4, 5821–5837. [Google Scholar] [CrossRef]
- Aguiar, D.S.; Silva, B.A.M.; Gonçalves, R.D.; Pereira, A.C.C.; Mota, I.O.; Bandeira, C.F.; Rosa, J.L.; Montoro, S.R. Verificação do potencial da biomassa proveniente da fibra de coco como possível reforço em compósitos poliméricos via caracterização morfológica por MEV após tratamento alcalino na presença de NaBH4. In Proceedings of the Congresso Brasileiro de Ciências e Saberes Multidisciplinares, Volta Redonda, Brazil, 26–28 October 2023. No. 2. [Google Scholar]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).