Abstract
Acetoin biosynthesis by two Bacillus subtilis strains valorising crude glycerol was thoroughly explored within a pre-defined range of culture conditions and systems. B. subtilis ACA-DC 1176 stood out for its higher efficiency in acetoin production, prompting an investigation into the potential for enhanced productivity through the evaluation of diverse culture conditions and media compositions. The primary by-products of the biodiesel and corn industries, namely crude glycerol and corn steep liquor, respectively, were successfully employed as the principal carbon and nitrogen sources of the newly developed low-cost culture medium. Furthermore, the results of the various feeding strategies that were tested indicated that the conversion of 2,3-butanediol (BDO) to acetoin occurred exclusively when the concentration of glycerol was below approximately 5 g/L. This seemed to be necessary for the production of NADH, which is essential for maintaining cellular processes. Following the complete depletion of glycerol, acetic acid increased and became the predominant metabolite, while both acetoin and BDO decreased, presumably resulting in ATP generation. This is likely a mechanism employed by the cell to generate energy in the absence of a carbon source. In the fed-batch bioreactor culture, the kinetics of metabolites differed, as there was no conversion of BDO to acetoin at the final depletion of glycerol. At volumetric mass transfer coefficient (kLa) levels exceeding approximately 70 1/h, the production of acetoin was favoured over that of BDO, with the highest observed acetoin/BDO ratio reaching 4.29 g/g. Conversely, at kLa values below approximately 60 1/h, the titres of acetoin and BDO were found to be nearly equal. The final concentrations of acetoin and BDO reached 36.0 g/L and 25.5 g/L, respectively, resulting in a total yield of both (acetoin + BDO) per glycerol consumption of 0.40 g/g. To the best of our knowledge, this is the first study to focus on acetoin production from crude glycerol fermentative valorisation. The study presents new findings regarding the parameters influencing the level of BDO conversion to acetoin. However, further research is required in order to gain a comprehensive understanding of the underlying phenomena and metabolic pathways involved.
1. Introduction
The development of petrochemical substitutes within the context of sustainable and biobased processes has attracted considerable scientific and industrial interest in recent years, as humanity is on the verge of transitioning from a fossil-recourse to a biobased economy. Residues derived from industrial and agro-industrial activities have been demonstrated to be suitable substrates for biobased products through microbial fermentations, contributing to both industry profitability and environmental sustainability [1,2,3]. The valorisation of crude glycerol, an industrial residue, attracts remarkable and constantly increasing interest, as the global biodiesel market, which supplies 63% of the entire glycerol market, is expected to grow at a CAGR of 10.0% from 2022 to 2030 [4,5,6].
There are two major ways to upcycle crude glycerol: one is via purification methods and its disposal to the industry as a valuable chemical, and the other is via its use as a feedstock for microbial cultures to produce bioproducts. The implementation of crude glycerol as feedstock holds great potential in terms of sustainability and viability of the biodiesel and oleochemical industry, as additional revenues can be generated. From a biorefining point of view, the valorisation of glycerol through microbial conversions presents enormous possibilities [7,8,9,10,11]. This route falls under the category of industrial/white biotechnology, which aims to achieve sustainable production of biochemicals, biomaterials, and biofuels by utilising renewable resources through whole-cell systems or isolated enzymes.
The development of biobased acetoin production through biotechnological means has attracted a lot of interest in the last decades due to its great industrial potential and the environmental concerns related to other alternatives, such as fossil-based chemicals. In 2004, the U.S. Department of Energy classified acetoin as one of the 30 platform chemicals that were given priority for their development and application [12].
Acetoin, also referred to as acetyl methyl alcohol, is a C4 volatile compound and one of the simplest aliphatic ketones. The acetoin molecule contains a single chiral carbon and exists in two optical isomers, as (R)-acetoin or (S)-acetoin. It is naturally presented in food products such as bananas, butter, corn, apples, meat, and many others. It has a wide application in the food industry as a flavour enhancer due to its pleasant yoghurt odour and fatty, creamy butter taste. There is a continuous increase in applications, such as dairy products, ready-to-eat foods, and bakery, which leads to a rapid pace of increasing demand for acetoin production. Furthermore, acetoin is used in cosmetics, detergents, soaps, plant growth promoters, and biological pest controls. In 2022, the acetoin market was valued at USD 177 million and is expected to grow, with a CAGR of 4.94% during the forecasted period of 2022–2028 [13]. The industrial production of acetoin is currently carried out through chemical synthesis. Amongst others, it can be produced by the partial reduction of diacetyl and by the dehydrogenation of 2,3-butanediol (BDO) [14,15]. Acetoin can be synthesised in mammals, plant cells (e.g., tobacco, carrot) or even by yeasts during the fermentation of wine [16,17]. Nevertheless, none of the eucaryotic species are capable of acetoin production at levels that would be of interest to the biotechnology sector. In contrast, bacteria are considered the most efficient acetoin producers [18].
In 1898, Daniel Wilhelm Otto Voges and Bernhard Proskauer, German bacteriologists from the Institute for Infectious Diseases, developed a test, namely the Voges–Proskauer test (VP), to detect acetoin in bacterial culture broth [14,19]. Some VP-positive organisms include Enterobacter, Klebsiella, Serratia, Hafnia, and Vibrio genera. There is a plethora of Gram-negative bacteria, such as Klebsiella, Salmonella, and Enterobacter, and Gram-positive bacteria, such as Bacillus, Leuconostoc, and Lactococcus, reported for acetoin production. The most well-known natural producers of acetoin are the species of Bacillus licheniformis, B. subtilis, B. amyloliquefaciens, and Paenibacillus polymyxa.
The biotechnological synthesis of acetoin can be divided into enzymatic conversion and microbial fermentation. The high cost and low availability of precursor substrates for the enzymatic synthesis of acetoin make the process unsuitable for industrialisation [20]. In contrast, microbial fermentation is the most competitive method for acetoin production. Glucose is the most widely used carbon source, although it would be more advantageous economically and from a sustainability standpoint for the bioprocess to valorise low-cost and renewable biomass or industrial residues. Acetoin is generally produced as a by-product in BDO or diacetyl fermentation by a plethora of bacteria. The maximum theoretical yield (Ymt) of acetoin production per glucose or glycerol consumption is 0.48 g/g and 0.49 g/g, respectively.
The pathway of acetoin synthesis in bacterial cells, such as Bacillus sp., involves the catabolism of a hexose or glycerol through the Embden–Meyerhof–Parnas metabolic pathway. Two moles of pyruvate are condensed to generate α-acetolactate, a reaction catalysed by α-acetolactate synthase (ALS) (Figure 1).
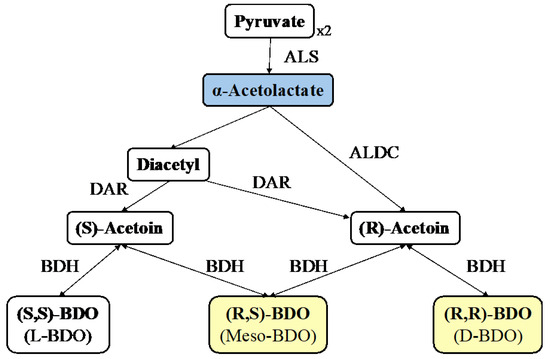
Figure 1.
Metabolic pathway for acetoin and 2,3-butanediol (BDO) synthesis from 2 moles of pyruvate. ALS: a-Acetolactate synthase; ALDC: a-Acetolactate decarboxylase; DAR: diacetyl reductase; BDH: BDO dehydrogenase.
In an anaerobic environment, α-acetolactate is converted directly to acetoin. In the presence of oxygen, α-acetolactate is spontaneously decarboxylated to diacetyl, which is then converted to acetoin by diacetyl reductase (DAR) [1,21,22]. The specificity of DAR is responsible for the stereoisomeric form, (R) or (S), of the generated acetoin. The production of one mole of acetoin from two moles of glycerol generates two moles of ATP and four moles of NADH [23]. Parameters, such as oxygen levels, NAD+/NADH, and available carbon source, affect the activity of BDO dehydrogenase, which controls the reversible reaction of acetoin to BDO [24].
Several studies have reported the mutation of acetoin producers, such as blocking BDH enzyme, or the use of an engineering host for acetoin biosynthesis in order to enhance the ratio of acetoin/BDO synthesis [18,25]. Furthermore, a two-stage agitation speed control strategy was proposed by Zhang et al. in order to first achieve a high BDO concentration, which will be subsequently converted to acetoin in the second stage [25]. On the contrary, Tsigoriyna et al. concluded that the design of the bioprocess cannot be based on the conversion of BDO to acetoin in the later stages of fermentation because, in fed-batch trials, BDO was not completely converted back to acetoin [26]. It is likely that the upper limit of tolerance in acetoin has been reached, with a concentration of approximately 75–80 g/L. This would explain why no further acetoin can be generated from either BDO conversion or glycerol assimilation. To the best of our knowledge, this was the only study to investigate acetoin production as a major product of glycerol valorisation. Nevertheless, the use of high-cost organic nitrogen sources (e.g., 10 g/L of yeast extract and 10 g/L of tryptone) in the substrate resulted in a notable increase in the cost of the bioprocess. Molasses, a by-product of the sugar industry, has been referred to as a by-product that can serve as a carbon source for acetoin synthesis [27]. It has been demonstrated that the synthesis of acetoin requires a significantly higher level of available oxygen than that of BDO [24]. However, a study conducted by Dai et al. indicates 61.15 g/L of acetoin from 200 g/L of molasses under low-air supply conditions, viz., the volume of air sparged per unit volume of growth medium per minute (VVM) of 0.4 L/min/L [28].
B. subtilis is commonly utilised in microbiology research as a model organism for studying biofilms. It possesses the capability to switch from a motile to a sessile state [29]. Biofilms are surface-bound bacterial communities where bacteria co-exist embedded in an extra-cellular matrix [30]. The B. subtilis extra-cellular matrix comprises exopolysaccharides, protein fibres, and a hydrophobin-like protein that imparts hydrophobicity to the community [31,32]. Biofilm formation allows bacteria to survive in extreme pH, nutrient-depleted, or otherwise hostile environments [33]. Biofilm cells can access hard-to-reach nutrients and are protected from fluctuations in environmental conditions [34,35].
The industrial application of B. subtilis is based on its efficient secretion of valuable proteins and metabolites. More specifically, B. subtilis can be used as a cell factory for the production of vitamins (e.g., riboflavin), acetoin, inositol, and enzymes like cellulases and proteases. Additionally, its spore surface display technology can be used as a platform for vaccine preparation [36]. In agriculture, B. subtilis can serve as a feed additive to improve soil quality and promote crop growth [37]. Bacillus species are well known for their production of acetoin and, to a lesser extent, BDO. The ratio of these products may vary depending on the strain and condition [14,25]. They are the most well-known microorganisms in nature for acetoin production.
The aim of this study was to examine the parameters associated with acetoin synthesis in B. subtilis cells and to enhance acetoin productivity in a low-cost substrate medium. Various parameters, including the purity of glycerol, culture temperature, feeding and aeration strategies, and nitrogen composition of the medium, were evaluated and optimised. This study represents the inaugural investigation into the valorisation of crude glycerol for the production of acetoin.
2. Materials and Methods
2.1. Microorganism and Media
The bacterial strains Bacillus subtilis ACA-DC 1225 and Bacillus subtilis ACA-DC 1176, which were used in this study, were kindly provided by the culture collection of The Laboratory of Dairy Research, Department of Food Science and Human Nutrition, Agricultural University of Athens. Long-term storage took place at T = −80 °C in tryptic soy broth, supplemented with 20% w/v glycerol. For short-term storage, strains were stored in glycerol solution (50% v/v) in the freezer (T = −18 °C) and were maintained on YPDA medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, 20 g/L agar) at T = 4 °C. All microorganisms were regenerated in YPDA and were incubated for 24–48 h at T = 30 °C once per month to maintain their viability.
Pure glycerol (99.0% analytical grade, Sigma-Aldrich, Darmstadt, Germany) or crude glycerol at different initial concentrations was used as the main carbon source. Crude glycerol came from the biodiesel plant of Verd S.A. (Velestino, Greece), and its appearance was brownish and turbid with no visible impurities. Analyses conducted by ASG Analytik-Service AG (Neusäss, Germany) revealed that the glycerol content was 85.1% (Cr85), the water content was 7.8%, the ash content was 1.5%, the methanol content was 1.0%, and the non-glycerol organic matter content was 4.6%. Corn steep liquor, which contains high amounts of proteins, free amino acids, and lactic acid, is the main by-product of the corn wet milling industry and was purchased from FeedStimulants (Zoetermeer, The Netherlands). The study also employed the following commercial reactants: glucose, yeast extract, bacterial peptone, meat extract, CH3COONa, K2HPO4, MgSO4·7H2O, MnSO4·H2O, NaOH, and HCl.
2.2. Cultures of B. subtilis
2.2.1. Preculture
The preculture medium is used to set the microorganism to the exponential phase before inoculating the main culture medium. The composition of the preculture medium was based on Karayannis et al. [23]. More precisely, the carbon sources were (per L deionised water) 5 g glucose and 5 g glycerol, while that of nitrogen were (per L deionised water) 10 g yeast extract and 10 g peptone. The preculture medium was transferred into 250 mL Erlenmeyer flasks (50 ± 1 mL working volume) and was inoculated after being sterilised (121 °C for 15 min) in the autoclave. Incubation took place in an orbital shaker (Lab-Line, Chicago, IL, USA) at an incubation temperature of 30 ± 1 °C and an agitation speed of 200 ± 5 RPM for efficient oxygen transfer for 18 ± 1 h.
2.2.2. Shake-Flask and Duran Bottle Cultures
The culture medium used in all experiments contained the following minerals (per L of deionised water): 2.00 g K2HPO4, 5.00 g CH3COONa, 0.40 g MgSO4·7H2O, and 0.05 g MnSO4·H2O. The nitrogen content of the cultures was as follows: 5.00 g peptone, 5.00 g meat extract, and 2.50 g yeast extract unless otherwise stated. Pure glycerol (99.0% analytical grade), crude glycerol, or glucose was used as a carbon source at different initial concentrations. All reactants and their concentrations refer to the culture medium composition prior to any optimisation step.
The initial pH value was set at 7.2 ± 0.1 using a pH/mV meter (model HΙ 8014, Hanna Instruments Hellas, Athens, Greece), and it was adjusted, if necessary, by periodically adding an appropriate volume of NaOH (5 M) or HCl (5 M) under aseptic conditions. The main culture was inoculated after sterilisation (121 °C, 20 min); strains were derived from the preculture when the cell growth was in the exponential phase. Cultures were performed in 250 mL Erlenmeyer shake flasks (50 mL working volume) and in 100 mL airtight borosilicate glass (Duran) bottles (80 mL working volume). These two culture vessels provided different environments in terms of dissolved oxygen levels. The shake-flask cultures were carried out in an orbital shaker (Lab-Line, Chicago, IL, USA) at an incubation temperature of 30 ± 1 or 37 ± 1 °C and a stirring rate of 200 ± 5 RPM. The inoculum size was 1% and 5% in shake flasks and Duran bottles, respectively.
2.2.3. Bioreactor Culture
The bioreactor culture was performed in a 3.6 L benchtop bioreactor (Labfors 4, Infors HT, Bottmingen, Switzerland) with a working volume of 1.65 L at an incubation temperature of 37 ± 1 °C. The inoculum size was 10% (viz., 150 mL). The pH value was adjusted using NaOH (5 M) or HCl (5 M). The stirrer agitation speed and the volume of air sparged per unit volume of growth medium per minute (VVM) varied according to the stage of the culture. Measurements of the volumetric mass transfer coefficient (kLa) were calculated from the dissolved oxygen value in aeration response experiments in a cell-free system [38].
2.3. Analytical Methods
2.3.1. Determination of Physicochemical Characteristics of Raw Materials
Total Kjeldahl Nitrogen was determined through the Kjeldahl method in a KjeltekTM 8100 Distillation Unit (Foss A/S, Hillerød, Denmark) [39].
2.3.2. Growth Determination
Bacterial growth was determined by cell dry weight (DCW). Cells were collected by centrifugation (9000× g, 10 min, 4 °C) in a Hettich Universal 320-R (Darmstadt, Germany) and washed twice with distilled water. The total dry cell weight (DCW) was gravimetrically determined after drying the precipitate at T = 80 °C until a constant weight was reached.
2.3.3. Substrate and Metabolites Quantitative Determination
Samples for quantitative analyses were periodically taken in order to perform kinetics on the microbial fermentations. The cells were harvested as precipitate through centrifugation (9000× g, 10 min, 4 °C) in a Hettich Universal 320-R (Darmstadt, Germany) and washed twice with distilled water. The supernatant was collected and analysed by a High-Pressure Liquid Chromatography (HPLC) (Waters Alliance 2695, Milford, MA, USA) system equipped with an Aminex HPX-87H (Bio-Rad Laboratories, Hercules, CA, USA) column of size 30.0 cm × 7.8 mm coupled to a differential refractometer. The mobile phase used was H2SO4 (0.005 M), with a flow rate of 0.5 mL/min (isocratic elution) and a column temperature of T = 60 °C. The samples were diluted to appropriate concentrations and were filtered through a 0.2 μm membrane before injection (injection volume = 20 μL). Metabolic products (e.g., 2,3-butanediol, acetoin), as well as substrate components (e.g., glycerol and acetic acid), were detected and subsequently quantified based on the corresponding standard curves.
2.3.4. Estimation of kLa Value
The estimation of the volumetric mass transfer coefficient (kLa) value of the bioreactor was based on the measurement of dissolved oxygen (DO) in aeration and step response experiments in a cell-free system [38,40]. The probe step response is obtained by first immersing the DO probe in a vessel into which nitrogen is sparged, while the cell-free fermentation medium is sparged with air so that the liquid is saturated in oxygen. The DO probe is then immediately removed from the oxygen-free medium and immersed in the oxygen-saturated bioreactor, and the DO readings (i(t)|step) of the probe are recorded. The aeration response is obtained by sparging air into an initially oxygen-free bioreactor and recording the probe readings (i(t)|aeration) until oxygen saturation is achieved. The two responses are then normalised with respect to the probe reading at saturation, and then kLa is estimated using Equation (1). The reciprocal of the volumetric mass transfer coefficient is equal to the area between the two normalised responses.
2.4. Data Analysis
Each experimental point of all the kinetics presented in the tables and figures is the mean value of two independent determinations. The relative standard error (SE) for most experimental points was ≤20%
3. Results
3.1. Initial Trials on Acetoin Production Feasibility
Two strains of Bacillus subtilis species, namely B. subtilis ACA-DC 1176 and B. subtilis ACA-DC 1225, were cultivated under different culture conditions. More precisely, cultures took place in aerobic or anaerobic regimes while different types of carbon sources were employed. The aim of the experiments presented in this paragraph was to estimate the feasibility towards acetoin production. Glucose was used as a reference substrate. During the microbial cultures, kinetic and physiological studies were performed. The two strains studied were cultivated in shake-flask (aerobiosis) cultures and in Duran bottles (self-generated anaerobiosis). The carbon sources used were glucose, glycerol, and crude glycerol at an initial concentration of ~20 g/L. Crude glycerol was supplied by Verd S.A. plant and had a purity level of 85% (Cr.). The temperature of the culture medium was set at 30 °C.
B. subtilis ACA-DC 1176 was initially cultured under aerobic conditions in shake flasks, where the consumption of the carbon source (SubC) was 100% in all cases (Table 1). Acetoin was the main product in all trials, whereas the ratio of acetoin to 2,3-butanediol (BDO) was higher when glycerol was used as substrate. The use of crude glycerol (Cr.) resulted in a slightly lower titre of acetoin (2.6 g/L) compared to 3.5 and 3.8 g/L for glucose and pure glycerol, respectively. The ratio of acetoin to BDO (ACTN/BDO) at the end of the carbon source was significantly lower in the glucose culture. The dry cell weight (DCW) reached values higher than 5 g/L in the glycerol cases, while in the glucose case, it was 3.4 g/L. In anaerobic cultures of B. subtilis ACA-DC 1176, both pure and crude glycerol were not assimilated (<15%), while no product formation occurred, and the DCW remained at 1.1 g/L. A biofilm was formed at the surface of the culture broth. Biofilms are surface-bound bacterial communities where bacteria co-exist and are embedded in an extra-cellular matrix [30]. The anaerobic regime is likely to cause stress to the microorganism, corresponding to biofilm formation, which allows the bacteria to survive and be protected from harsh conditions, such as low dissolved oxygen concentration.

Table 1.
Initial trials of B. subtilis ACA-DC 1176 and B. subtilis ACA-DC 1225 cultures under aerobic (aerobiosis) and self-generated anaerobic (anaerobiosis) regimes.
Under aerobic conditions, B. subtilis ACA-DC 1225 was able to completely assimilate all the carbon sources used (Table 1). In the case of glucose, 4.9 g/L and 2.5 g/L of acetoin and BDO, respectively, were secreted. The concentration of DCW was higher when glycerol was the main carbon source, but the time required for complete glycerol assimilation increased to 55 or 70 h. The purity of glycerol did not affect the production of metabolites or microbial growth. The culture of B. subtilis ACA-DC 1225 under anaerobic conditions showed a different profile regarding the purity of the glycerol used. Pure glycerol was assimilated by 50%, and 3.8 g/L of DCW was produced. The biomass produced was mainly in the form of a biofilm, as in the case of B. subtilis ACA-DC 1176. In contrast, the microorganism showed no growth in the crude glycerol culture.
3.2. Investigation of Acetoin Production by B. subtilis ACA-DC 1176 in Shake-Flask Cultures
Further investigation into the synthesis of acetoin was conducted using B. subtilis ACA-DC 1176 in accordance with the results of the previous paragraph. The results of a study in which this wild-type bacterium was identified as a notable producer of acetoin and BDO on glycerol-based media under aerobic conditions were also considered [41].
The sole carbon source employed in the subsequent trials was either pure glycerol or crude glycerol (Cr.). Considering that acetoin production by B. subtilis only occurs under aerobic conditions, all the following experiments were performed in shake flasks under aerobic conditions. The objective of the experiments presented in this section was to screen different culture conditions in order to enhance acetoin production. More specifically, several experiments were carried out regarding the initial concentration and purity of the glycerol used, the type and concentration of the nitrogen source, the temperature of the culture medium, and the feeding strategy.
3.2.1. Culture Medium and Temperature
In this optimisation phase, pure or crude glycerol (Cr.) was used as the main carbon source in the aerobic cultures of B. subtilis ACA-DC 1176. The initial nitrogen (N) source composition was based on previous studies [41,42], where it consisted of a mixture of three commodity organic sources (ComOrg), namely 5 g/L peptone, 5 g/L meat extract, and 2.5 g/L yeast extract. The aim was to reduce the cost of N sources by proposing more economical alternatives (e.g., by-products) while maintaining or even improving the fermentation efficiency of glycerol by B. subtilis for acetoin synthesis. The N sources used were ComOrg and corn steep liquor (CSL). The ComOrg contains 1.45 g/L N, which is equivalent to 100% N (Table 2). The N content of the CSL, which was calculated based on Kjeldahl analysis, was 6.5%. Each N source was evaluated at different concentrations (e.g., 100%, 50%) and as a sole or co-source of N. The temperature was set at 30 °C or 37 °C.

Table 2.
Quantitative data of shake-flask cultures of B. subtilis ACA-DC 1176 under different nitrogen compositions and temperatures.
The results of this set of 14 trials are presented in Table 2, with emphasis on each culture condition, as well as the metabolite titre, rate, and yield. In the first two rows of the table, representing the reference conditions, the highest acetoin concentration and YACTN/Gly are obtained: 17.5 g/L and 0.27 g/g, respectively. In the following rows (3, 4), replacing half of the ComOrg with the CSL results in a higher value of acetoin concentration in one case and a lower value in the other, compared to the first rows (1, 2). More precisely, when initial glycerol concentration (Gly0) was ~40 g/L, acetoin was the main product at 11.2 g/L, and when Gly0 was ~60 g/L, acetoin dropped to 5.6, and BDO was the major product at 11.4 g/L.
Figure 2 depicts the kinetics of trials in rows 3, 4, 11, and 12 of Table 2. In the trials in rows 3 and 11, both with Gly0 ~40 g/L, acetoin was the major product throughout the culture. Acetoin concentration peaked in the last stage when glycerol had been depleted or was about to be depleted. Concurrently, the concentration of BDO dropped as it was converted back to acetoin, presumably in order to regenerate NADH. Based on the metabolic pathway of BDO synthesis, BDO can be converted back to its predominant molecule (acetoin), as there is evidence to suggest that this is a reversible reaction (Figure 1). It is hypothesised that one mole of NAD+ is reduced to NADH. This phenomenon has been observed in circumstances where there is a limited availability of carbon sources under aerobic conditions [24]. At a later stage, the ratio of acetoin to BDO was increased, an observation that coincides with the recent literature [27,43]. This motive is not presented in the trials in rows 4 and 12, in which Gly0 was ~60 g/L, as BDO did not reconvert to acetoin in the later stage of culture.
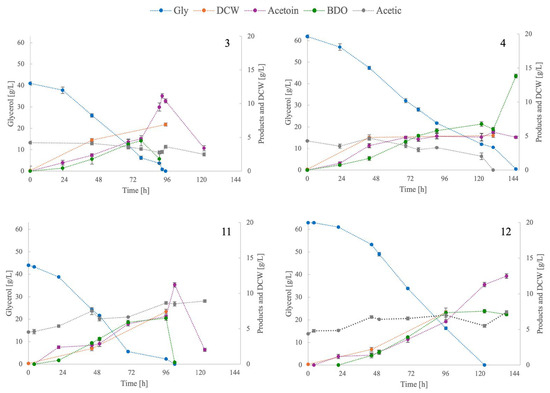
Figure 2.
Kinetics of glycerol (Gly), dry cell weight (DCW), acetoin, 2,3-butanediol (BDO), and acetic acid concentrations during shake-flask batch culture. Numbers 3, 4, 11, and 12 represent the trials in Table 2.
The difference between the conditions of rows 3 and 4 was the level of Gly0 employed. For the Gly0 of 40 g/L, acetoin was the major product throughout the culture and peaked when glycerol was depleted, reaching 11.2 g/L. In this case, the YACTN/Gly (0.28 g/g) was the highest of the values presented in Table 2. Conversely, for Gly0 of 60 g/L, BDO became the major product after ~75 h and peaked at the point of glycerol and acetic acid depletion. This phenomenon has not previously been observed in this study. BDO reached a concentration of 13.8 g/L, which is the highest in Table 2. The ConRate was slightly higher for the Gly0 of 40 g/L (0.57 g/g) compared to that of 60 g/L (0.49 g/g). In rows 5 and 6 of Table 2, only 50% of ComOrg was used, which resulted in very low concentrations of both acetoin and BDO, as both were less than 2 g/L, as well as an extended culture duration. The ConRate of both Gly0 employed was also lower, namely 0.27 g/g and 0.37 g/g in each case.
The objective of the subsequent stage was to ascertain the impact of crude glycerol on microbial growth and acetoin production. In row 7, acetoin production was observed to be unaffected in comparison to the results obtained with pure glycerol trials (e.g., row 1), while acetoin production was 8.5 g/L. The YACTN/Gly was almost identical to that observed in pure glycerol. In row 8 (Gly0 = 60 g/L), both acetoin and BDO values were increased, namely 10.1 and 6.9 g/L, respectively. In this case, ConRate exhibited the highest value of the entire Table 2, reaching 0.56 g/L/h. Consequently, the glycerol uptake rate and selectivity towards acetoin synthesis were not affected by the crude type of glycerol, although the acetoin titre was lower than in row 2.
The valorisation of crude glycerol and corn steep liquor was explored in the following experiments. In row 9, the substitution of half of the ComOrg with CSL resulted in a higher ConRate and DCW, while the acetoin and BDO production decreased by less than 10% compared to row 7. Interestingly, in row 10, when Gly0 was ~60 g/L, BDO was the main product. No conversion of BDO to acetoin was observed at any stage of the culture. It is noteworthy that a similar phenomenon was observed in Figure 2 and row 3, where Gly0 was also approximately 60 g/L and the same N composition was employed.
In the final stage of acetoin optimisation, the culture temperature was altered to 37 °C. Consequently, the conditions of a new culture temperature were established, comprising an N composition of 50% ComOrg and 50% CSL, in addition to the utilisation of crude glycerol. In the case where Gly0 was ~40 g/L (row 11), both YACTN/Gly and ConRate were among the highest values in Table 2. In addition, BDO was converted to acetoin when glycerol was depleted. Acetoin reached a concentration of 11.2 g/L and subsequently decreased after the depletion of glycerol. At a Gly0 of ~65 g/L (row 12), acetoin was the predominant product, reaching 12.5 g/L, and the ConRate was 0.49 g/L/h. In the two cases depicted in Figure 2, acetic acid was not consumed, yet its concentration increased in the final stage, reaching 8.9 and 7.4 g/L, respectively, for Gly0 = 40 g/L and 65 g/L. In rows 13 and 14, there was a reduction in the production of acetoin and BDO, as the N content was decreased by 50%, like in the case of rows 4 and 5.
The results of this paragraph indicate the successful replacement of half of ComOrg with CSL, the use of crude glycerol (with no negative impact on glycerol uptake rate), and setting the culture temperature at 37 °C. All subsequent experiments were conducted under these conditions. The specificity towards acetoin production cannot be easily explained based on the cultures presented in this paragraph. In experiments 3 and 11, shown in Figure 2, the sharp increase in acetoin concentration was enhanced by the low availability of glycerol, which probably triggered the conversion of BDO to acetoin. This increase was followed by a sharp decrease, hypothetically favoured by the direct assimilation of acetoin or through the production of acetic acid to generate energy since no other carbon source was available.
Different profiles were observed regarding BDO-to-acetoin conversion, even when the only parameter changed was the Gly0, as illustrated in Figure 2. Furthermore, BDO was the major product, and the phenomenon of its conversion to acetoin was not observed at 30 °C when the initial crude or pure glycerol concentration was 60 g/L, and the N composition was 50% ComOrg and 50% CSL, as shown in rows 4 and 10 of Table 2. However, this was not the case when the temperature was 37 °C, as shown in row 12. Therefore, the study was focused on the later stages of glycerol assimilation and the feeding strategy in fed-batch-type cultures.
3.2.2. Feeding Strategy
Based on the most efficient conditions outlined in the previous paragraph, three fed-batch cultures were conducted in shake flasks. In all subsequent experiments, crude glycerol was used at an initial concentration (Gly0) of approximately 40 g/L or 60 g/L, while the nitrogen (N) composition was 50% ComOrg and 50% CLS, and the temperature was set at 37 °C [14]. The objective of this study was to determine the effect of the timing of glycerol addition (feeding strategy) on the fermentation broth and the production of acetoin. Parameters, such as the level of available carbon source, exert a significant influence on the activity of BDO dehydrogenase, which controls the reversible reaction of acetoin to BDO [24].
The first two fed-batch cultures both had a Gly0 of approximately 40 g/L, with disparate strategies employed regarding the timing of feeding (addition of ~15 or ~20 g/L of glycerol). Figure 3 illustrates the kinetics of glycerol assimilation and product secretion when feeding occurred after a period of more than 20 h of glycerol depletion. At approximately 70 h, acetoin and BDO concentrations were both at ~6.5 g/L.
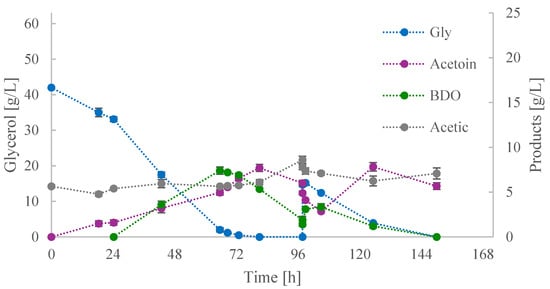
Figure 3.
Kinetics of glycerol (Gly), acetoin, 2,3-butanediol (BDO), and acetic acid concentrations during shake-flask fed-batch culture (i).
Prior to the feeding, BDO concentration dropped at ~2.0 g/L, while acetoin initially reached a peak at 7.7 g/L before dropping back to 6.5 g/L. During this period, acetic acid was increased by ~3 g/L, becoming the major product, with a concentration of 8.6 g/L. It is conceivable that a quantity of BDO was initially converted to acetoin in order to generate NADH.
Subsequently, in the absence of an available carbon source, BDO and acetoin were converted to acetic acid, potentially in order to generate ATP and satisfy the energy requirements of the cell. The precise mechanism by which BDO and acetoin are converted to acetic acid remains to be elucidated. However, it is established that the conversion of pyruvic acid to acetic acid generates one mole of NADH and one mole of ATP [43]. Following the addition of glycerol (~15 g/L), the concentration of BDO instantly increased, and that of acetoin decreased. This was then accompanied by a reversal change in the concentrations of these metabolites. The final titre of acetoin was 7.8 g/L, while acetic acid peaked for the second time at 7.1 g/L and became the major product after the depletion of glycerol. In contrast, the concentration of BDO initially increased before subsequently and consistently decreasing.
In the second fed-batch culture, the feeding occurred earlier, coinciding with the complete assimilation of glycerol (Figure 4). Prior to the feeding, BDO was the predominant product, with a subsequent decline in its concentration reported when glycerol concentration was below 2 g/L or was not further found in the medium. Concurrently, there was a slight increase in the concentration of acetoin, amounting to 1.5 g/L. At this point, both products exhibited values of approximately 6.5 g/L. Following the addition of ~15 g/L of glycerol, acetoin became the predominant product of the culture. Its concentration peaked at 11.7 g/L when glycerol was completely consumed for the second time. According to the kinetic results obtained, it may be assumed that the carbon flux of glycerol consumption was primarily directed towards acetoin production, with minimal conversion of BDO to acetoin. Only after glycerol consumption was reported were both acetoin and BDO subjected to conversion to acetic acid for energy production.
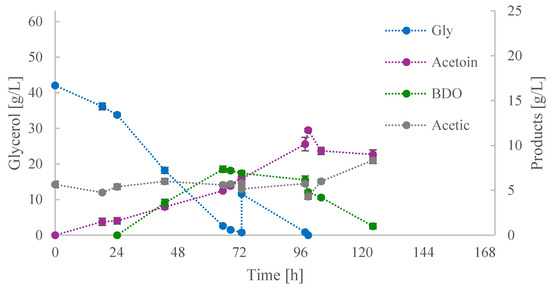
Figure 4.
Kinetics of glycerol (Gly), acetoin, 2,3-butanediol (BDO), and acetic acid concentrations during shake-flask fed-batch culture (ii).
The feeding strategy in the third fed-batch culture employed a higher Gly0. Figure 5 depicts the kinetics of metabolic products and glycerol. At 120 h of culture, approximately 62 g/L of glycerol had been consumed, and 20 g/L of glycerol was fed into the culture broth. Until the feeding, BDO was the major product—its concentration was 11.0 g/L—while that of acetoin reached 7.8 g/L. Concurrently, the concentration of acetic acid was increased to 6.2 g/L from its initial value of 4.3 g/L.
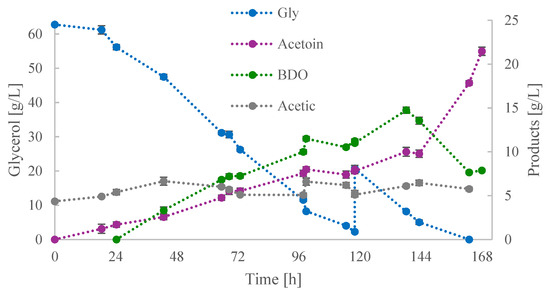
Figure 5.
Kinetics of glycerol (Gly), acetoin, 2,3-butanediol (BDO), and acetic acid concentrations during shake-flask fed-batch culture (iii).
Following the addition of glycerol, BDO and acetoin productivities exhibited a similar profile to that observed during the initial 120 h. Upon the decline in glycerol concentration to 5 g/L, the phenomenon of reversible conversion of BDO to acetoin was observed. This process continued until glycerol was completely assimilated, at which point acetoin reached its maximum value of 21.5 g/L, with a YACTN/Gly of 0.17 g/g. From 144 until 168 h, the concentrations of BDO and glycerol both decreased by 6 g/L, while that of acetic acid decreased by 2 g/L. These reductions resulted in an increase in the concentration of acetoin by ~12 g/L. The BDO concentration started to decrease in both phases of glycerol depletion, and more precisely, when glycerol dropped between 8 and 5 g/L. Its conversion to acetoin occurred at glycerol values of ~4 g/L.
3.3. Scale-Up in Bioreactor Culture
The production of acetoin was investigated in a bioreactor system where different volumetric mass transfer coefficient (kLa) values of oxygen were employed. In a recent study, it was demonstrated that low oxygen supply (kLa = 64 1/h) favoured D-BDO production, while high oxygen supply (kLa = 200 1/h) enhanced acetoin production in B. amyloliquefaciens cultures [44]. The composition of substrate was as described in Section 3.2, and the volume of the culture medium was 1.65 L. Three feedings of crude glycerol occurred, resulting in 151.1 g/L of available glycerol (Figure 6). The added glycerol was completely assimilated at approximately 170 h, with a total glycerol consumption rate (ConRate) of 0.88 g/L/h. The cell dry weight (CDW) reached a value of 10 g/L on the first day and remained at this level until the end of the culture.
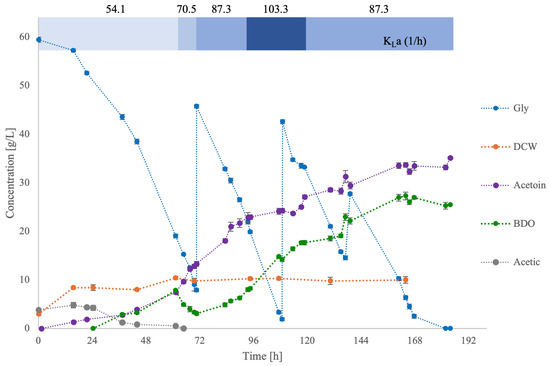
Figure 6.
Kinetics of glycerol (Gly), dry cell weight (DCW), acetoin, 2,3-butanediol (BDO), and acetic acid (acetic) concentrations during bioreactor fed-batch culture. Values of oxygen transfer coefficient (kLa) in the top phase.
In the first stages of the culture (kLa = 54.1 1/h), the acetoin-to-BDO ratio (ACTN/BDO) was ~1 g/g. These results are in contrast to those obtained in shake flasks, in which the BDO concentration surpassed that of acetoin at approximately 40 h and became the predominant product. This phenomenon may be attributed to the elevated oxygen regime in the bioreactor culture relative to that of shake flasks. Following approximately the first 65 h, during which the glycerol concentration dropped to ~15 g/L and the kLa value increased to 70.5 1/h, acetoin emerged as the dominant product of the culture.
Between 65 and 71 h, before the first feeding, the concentration of BDO and glycerol decreased by 4.8 and 7 g/L, respectively, while at the same time, acetoin increased by 6 g/L. This was the only instance during the culture where a pronounced decline in BDO was observed in conjunction with an increase in acetoin concentration. In subsequent feedings, particularly the second in which glycerol concentration was left to decline to ~2 g/L, BDO does not convert back to acetoin. Furthermore, no conversion of BDO to acetoin was observed during the final depletion of glycerol. The reason behind the lack of further conversions remains unclear. One hypothesis is that when acetoin concentration reaches a maximum value (e.g., >20 g/L), further acetoin accumulation may predominately occur due to glycerol catabolism and not due to BDO’s reversible reaction. With regard to the final glycerol depletion, at approximately 160 h, a product inhibition phenomenon may occur in relation to the concentrations of acetoin and BDO, which together reached approximately 60 g/L. As a consequence, there is no substantial further production of either metabolite or conversion between them in the last 30 h.
The final titres of acetoin and BDO were 36.0 and 25.5 g/L, respectively, resulting in ACTN/BDO of 1.41 g/g. The maximum ACTN/BDO was 4.29 g/g and occurred at 71 h. The total yield of acetoin and BDO production per glycerol consumption was 0.40 g/g. Table 3 illustrates the distinct phases of the fed-batch culture depicted in Figure 6. It can be stated that at kLa values exceeding 70 1/h, acetoin was the predominant metabolite, irrespective of the glycerol concentration. Conversely, at a kLa of 54.1, the acetoin and BDO titres were found to be almost equal.

Table 3.
Quantitative data of fed-batch bioreactor culture under different aeration regimes.
During the cultivation of B. subtilis ACA-DC 1176, both biofilm and intensive foam formation were observed. Biofilm formation occurred on the cells of the bioreactor, with some cells moving out of the liquid phase due to the foam and detaching from the wall surface (Figure 7). In the absence of nutrients outside of the culture broth (viz., the wall of the bioreactor), it can be postulated that cells formed a biofilm in order to safeguard themselves from such environmental conditions [35]. The Bacillus subtilis species is renowned for its capacity to produce proteases [45]. Consequently, the foam formation observed during the culture process was attributable to protein secretion and the elevated agitation rate.

Figure 7.
Bioreactor culture of B. subtilis ACA-DC 1176.
4. Concluding Remarks
To the best of our knowledge, this is the first study to examine the biotechnological production of acetoin from crude glycerol valorisation. It was established that the glycerol uptake rate of B. subtilis was not affected by the impurities of the crude glycerol employed. Furthermore, the main by-product of the corn industry, viz., corn steep liquor, successfully replaced half the high-cost commodity organic nitrogen sources.
New insights into the navigation of carbon in the cell during glycerol depletion are presented. In the later stages of culture (in most cases), when the glycerol concentration was below c. 5 g/L, an amount of BDO was first converted to acetoin, presumably to produce NADH. Subsequently, in the absence of a carbon source available, BDO and acetoin were converted to acetic acid, potentially for ATP generation. Nevertheless, further research is required at the enzymatic level to fully understand the factors that regulate the equilibrium of the reversible conversion of BDO to acetoin in glycerol assimilation.
Author Contributions
Conceptualization, D.K.; Methodology, D.K. and E.M.; Validation, O.K. and S.P.; Formal analysis, O.K.; Investigation, D.K. and E.M.; Data curation, S.P.; Writing—original draft, D.K.; Writing—review & editing, D.K. and S.P.; Visualization, D.K.; Supervision, D.K. and S.P.; Project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 8, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Kachrimanidou, V.; Koutinas, A.A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Vasilakis, G.; Karayannis, D.; Massouras, T.; Politis, I.; Papanikolaou, S. Biotechnological conversions of Mizithra second cheese whey by wild-type non-conventional yeast strains: Production of yeast cell biomass, single-cell oil and polysaccharides. Appl. Sci. 2022, 12, 11471. [Google Scholar] [CrossRef]
- The Brainy Insights. Biodiesel Market Size, Share & Trends Analysis Report by Feedstock (Vegetable Oils, Animal Fats), by Application (Fuel, Power Generation), by Region (Europe, APAC), and Segment Forecasts, 2022–2030. Available online: https://www.researchandmarkets.com/reports/4375442/biodiesel-market-size-share-and-trends-analysis?srsltid=AfmBOoqb4ZMkLeq4ksLwxTvW2m4iEQ94cng7BwLI3VMNg_4tevNoaxKH (accessed on 10 July 2024).
- Papanikolaou, S.; Aggelis, G. Microbial products from wastes and residues. FEMS Microbiol. Lett. 2020, 367, fnaa156. [Google Scholar] [CrossRef]
- Zhang, C.; Sharma, S.; Ma, C.; Zeng, A.P. Strain evolution and novel downstream processing with integrated catalysis enable highly efficient coproduction of 1,3-propanediol and organic acid esters from crude glycerol. Biotechnol. Bioeng. 2022, 119, 1450–1466. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2016, 17, 262–281. [Google Scholar] [CrossRef]
- Karayannis, D.; Papanikolaou, S.; Vatistas, C.; Paris, C.; Chevalot, I. Yeast lipid produced through glycerol conversions and its use for enzymatic synthesis of amino acid-based biosurfactants. Int. J. Mol. Sci. 2023, 24, 714. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Vastaroucha, E.S.; Maina, S.; Michou, S.; Kalantzi, O.; Pateraki, C.; Koutinas, A.A.; Papanikolaou, S. Bioconversions of biodiesel-derived glycerol into sugar alcohols by newly isolated wild-type Yarrowia lipolytica strains. Reaction. 2021, 2, 499–513. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004. [Google Scholar]
- Market Research. Global Acetoin Market Research Report 2023. Available online: https://www.marketresearch.com/Maia-Research-v4212/Global-Acetoin-Research-Competitive-Status-34497228/ (accessed on 10 July 2024).
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Al-Auda, Z.; Li, X.; Hohn, K.L. Dehydrogenation of 2,3-butanediol to acetoin using copper catalysts. Ind. Eng. Chem. Res. 2022, 61, 3530–3538. [Google Scholar] [CrossRef]
- Forlani, G.; Mantelli, M.; Nielsen, E. Biochemical evidence for multiple acetoin-forming enzymes in cultured plant cells. Phytochemistry 1999, 50, 255–262. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria; Williams and Wilkins: Baltimore, MD, USA, 1980. [Google Scholar]
- Wang, Q.; Zhang, X.; Ren, K.; Han, R.; Lu, R.; Bao, T.; Pan, X.; Yang, T.; Xu, M.; Rao, Z. Acetoin production from lignocellulosic biomass hydrolysates with a modular metabolic engineering system in Bacillus subtilis. Biotechnol. Biofuels Bioprod. 2022, 15, 87. [Google Scholar] [CrossRef]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol--current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Chen, C.; Wei, D.; Shi, J.; Wang, M.; Hao, J. Mechanism of 2,3-butanediol stereoisomer formation in Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2014, 98, 4603–4613. [Google Scholar] [CrossRef]
- Karayannis, D.; Aggelou, N.; Vasilakis, G.; Charisteidis, I.; Litinas, A.; Papanikolaou, S. A non-aseptic bioprocess for production and recovery of 2,3-butanediol via conversion of crude glycerol and corn steep liquor at pilot-scale. Carbon Resour. Convers. 2024, in press. [Google Scholar] [CrossRef]
- Maina, S.; Mallouchos, A.; Nychas, G.J.E.; Freire, D.M.; de Castro, A.M.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess development for (2R, 3R)-butanediol and acetoin production using very high polarity cane sugar and sugarcane molasses by a Bacillus amyloliquefaciens strain. J. Chem. Technol. Biotechnol. 2019, 94, 2167–2177. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Yang, T.; Zhang, J.; Xu, M.; Li, H.; Xu, Z.; Rao, Z. Mutation breeding of acetoin high producing Bacillus subtilis blocked in 2,3-butanediol dehydrogenase. World J. Microbiol. Biotechnol. 2013, 29, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Petrova, P.; Petrov, K. High production of acetoin from glycerol by Bacillus subtilis 35. Appl. Microbiol. Biotechnol. 2022, 107, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.J.; Liu, P.H.; Qin, J.Y.; Xu, P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007, 75, 61–68. [Google Scholar] [CrossRef]
- Dai, J.Y.; Cheng, L.; He, Q.F.; Xiu, Z.L. High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Process Biochem. 2015, 50, 1730–1734. [Google Scholar] [CrossRef]
- Gingichashvili, S.; Duanis-Assaf, D.; Shemesh, M.; Featherstone, J.D.B.; Feuerstein, O.; Steinberg, D. Bacillus subtilis Biofilm Development—A Computerized Study of Morphology and Kinetics. Front. Microbiol. 2017, 8, 2072. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Hobley, L.; Ostrowski, A.; Rao, F.V.; Bromley, K.M.; Porter, M.; Prescott, A.R.; MacPhee, C.E.; van Aalten, D.M.; Stanley-Wall, N.R. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2013, 110, 13600–13605. [Google Scholar] [CrossRef]
- Kobayashi, K.; Iwano, M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012, 85, 51–66. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, A.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure-straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Aroniada, M.; Maina, S.; Koutinas, A.; Kookos, I.K. Estimation of volumetric mass transfer coefficient (kLa)—Review of classical approaches and contribution of a novel methodology. Biochem. Eng. J. 2020, 155, 107458. [Google Scholar] [CrossRef]
- ISO 8968-1; Milk Determination of Nitrogen Content–Part 1: Kjeldahl Method. First Edition IDF 20-1; ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/35120.html (accessed on 10 June 2024).
- Lee, Y.H.; Tsao, G.T. Dissolved oxygen electrodes. Adv. Biochem. Eng. 1979, 13, 35–86. [Google Scholar]
- Karayannis, D.; Vasilakis, G.; Charisteidis, I.; Litinas, A.; Manolopoulou, E.; Tsakalidou, E.; Papanikolaou, S. Screening of mew industrially important bacterial strains for 1,3-propanediol, 2,3-butanediol and ethanol production through biodiesel-derived glycerol fermentations. Microorganisms 2023, 11, 1424. [Google Scholar] [CrossRef]
- Metsoviti, M.; Zeng, A.P.; Koutinas, A.A.; Papanikolaou, S. Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J. Biotechnol. 2013, 163, 408–418. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D-2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef]
- Danilova, I.; Sharipova, M. The practical potential of Bacilli and their enzymes for industrial production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).