Impact of Inverse Manganese Promotion on Silica-Supported Cobalt Catalysts for Long-Chain Hydrocarbons via Fischer–Tropsch Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
- Non-promoted silica-supported Co catalyst (basis).
- 2.
- Inverse promotion: promoting the support before loading the metal catalyst.
2.2. Catalyst Characterization
2.3. Catalyst Testing
3. Results and Discussions
3.1. Catalysts Textural Properties
3.2. Structure and Crystallite Size of Cobalt Species
3.3. X-ray Photoelectron Spectroscopy: Calcined Catalysts
3.4. Reducibility of Co Species
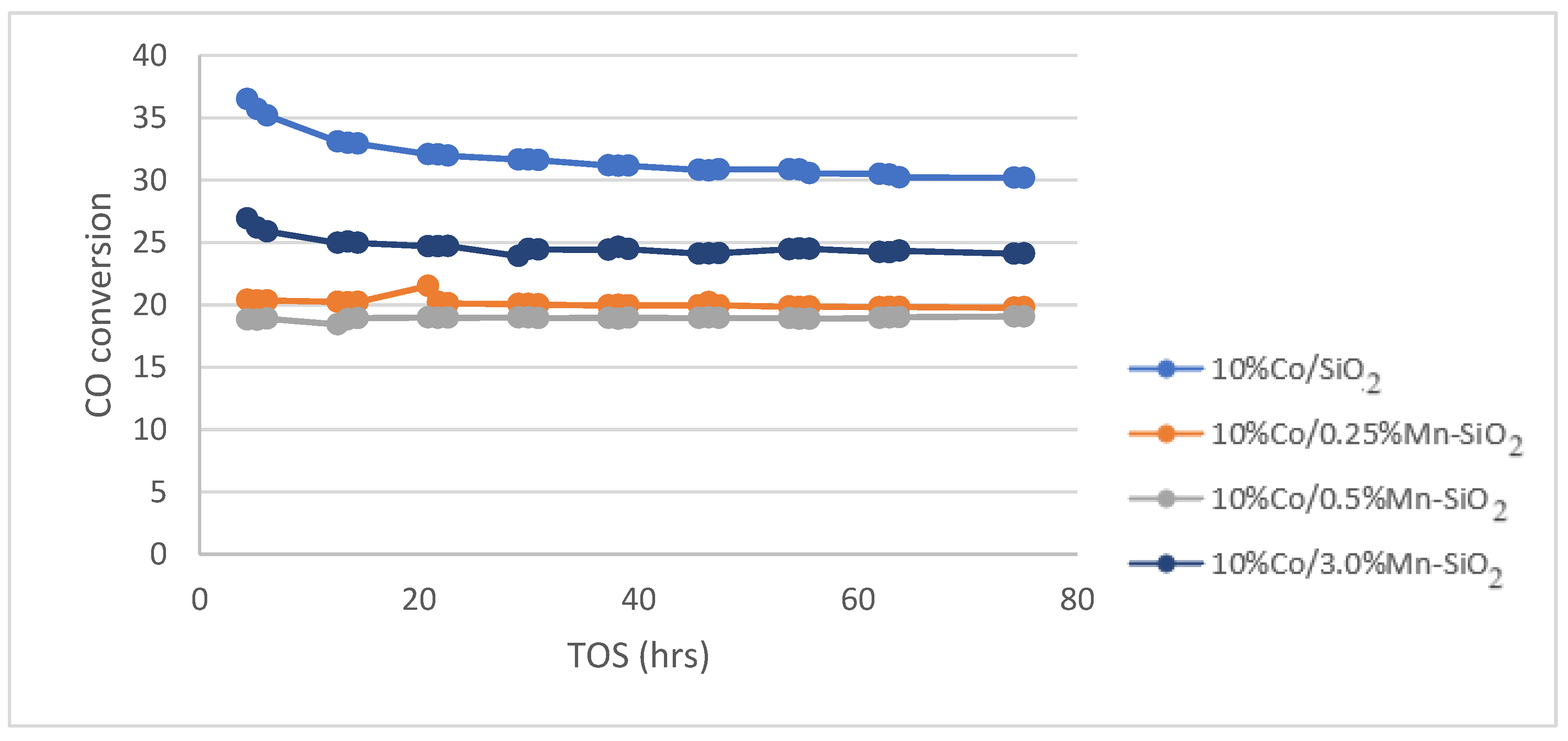
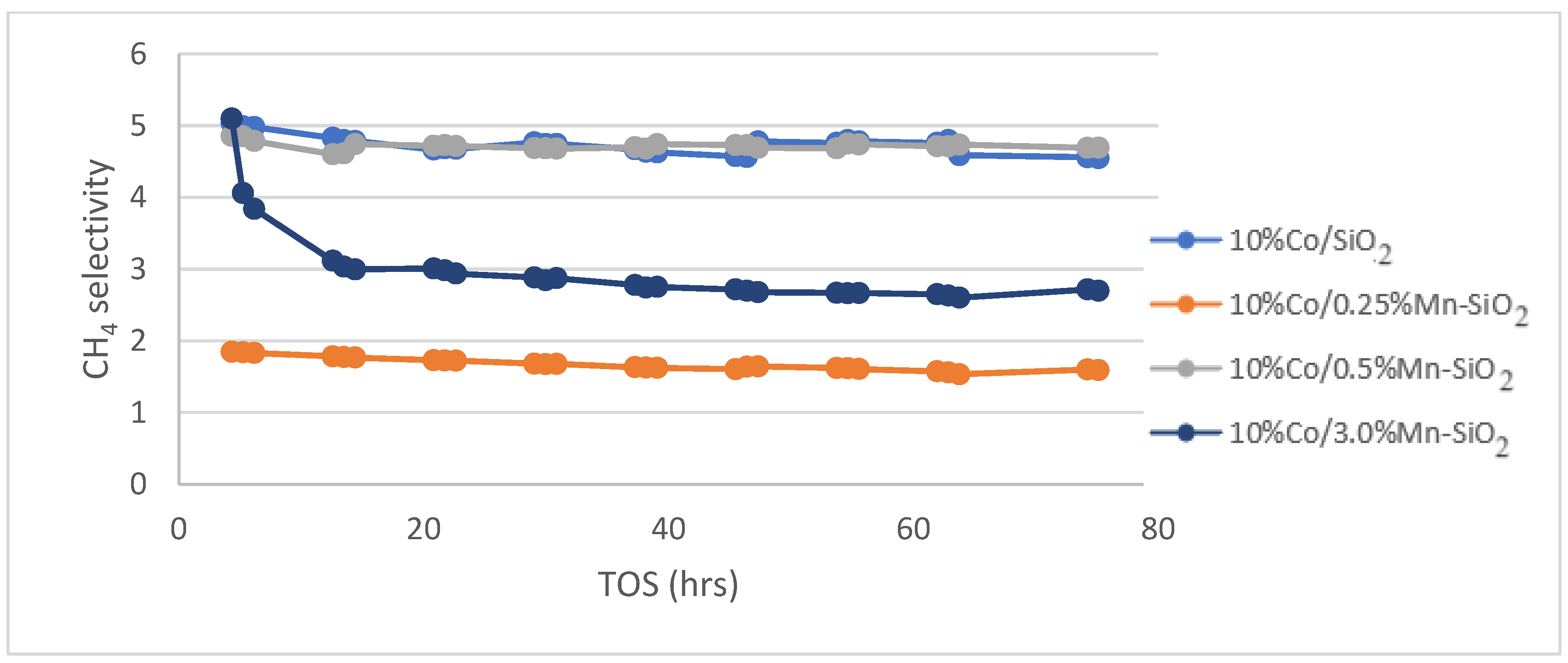
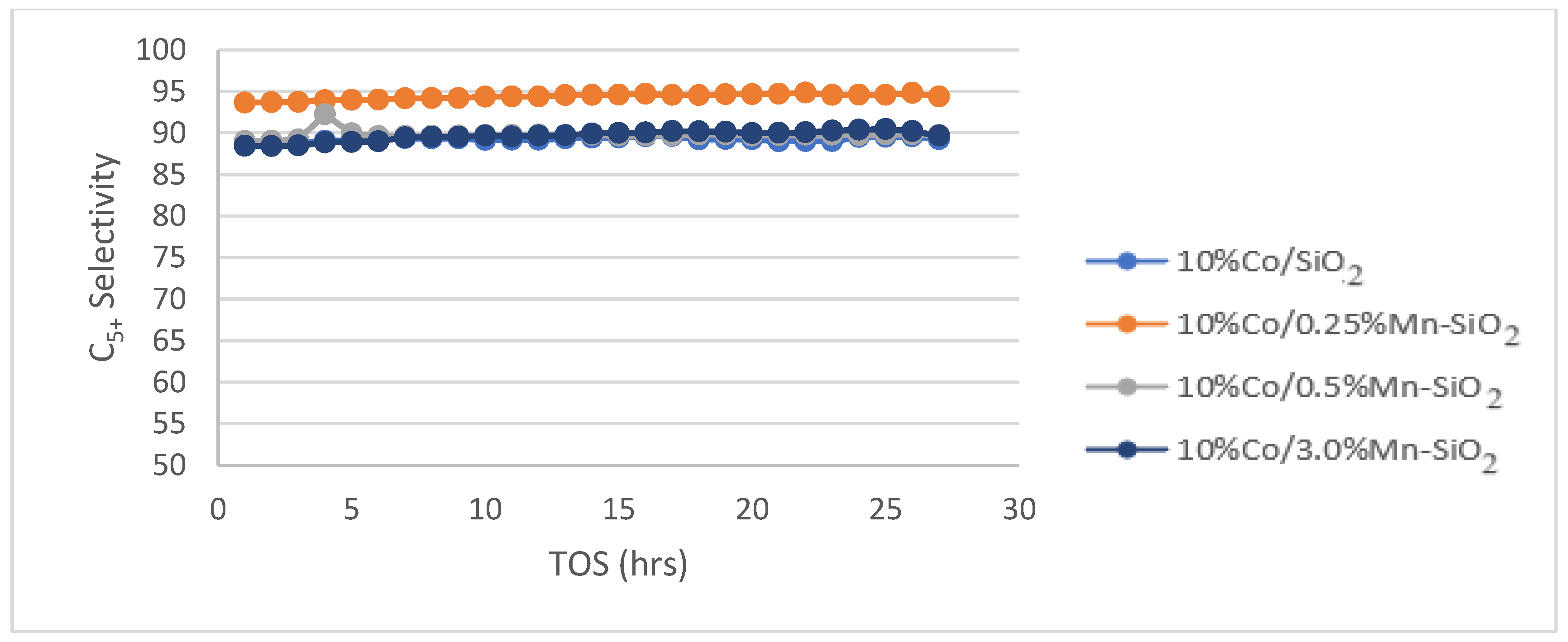
3.5. Fischer Tropsch Tests
- Fout = outlet total molar flow rate in mol/min.
- XN2, in = inlet molar fraction of N2.
- XN2,out = outlet molar fraction of N2.
- Xco2,in = CO molar fraction in the gas feed.
- Xco2,out = molar fraction of CO in the gas stream leaving the reactor.
- mcat = mass of the catalyst (in grams).
- = moles of carbons in the product stream.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Potgieter, J.H.; Moodley, D.; Botha, T.; Visagie, J.; Manong, T.; Frank, M.; Claeys, M.; van Steen, E.; Böltken, T.; Pfeifer, P. Development of promoted cobalt/alumina Fischer-Tropsch catalysts for increased activity and selectivity: Micro-reactor to piloting scale. Catal. Today 2024, 432, 114554. [Google Scholar] [CrossRef]
- Moodley, D.J. On the Deactivation of Cobalt-based Fischer-Tropsch Synthesis Catalysts. Ph.D. Thesis, Chemical Engineering and Chemistry, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2008. [Google Scholar]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Shiba, N.C.; Liu, X.; Yao, Y. Insight into the Physicochemical Properties of Co-Based Catalysts in Fischer—Tropsch Synthesis. Reactions 2023, 4, 420–431. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Moyo, M.; Hildebrandt, D. The effect of hydrophobicity on SiO2–supported Co catalysts in Fischer-Tropsch synthesis. Fuel 2021, 296, 120667. [Google Scholar] [CrossRef]
- Ma, W.; Dalai, A.K. Effects of Structure and Particle Size of Iron, Cobalt and Ruthenium Catalysts on Fischer–Tropsch Synthesis. Reactions 2021, 2, 62–77. [Google Scholar] [CrossRef]
- Li, Z.; Si, M.; Xin, L.; Liu, R.; Liu, R.; Lü, J. Cobalt catalysts for Fischer-Tropsch synthesis: The effect of support, precipitant and pH value. Chin. J. Chem. Eng. 2018, 26, 747–752. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Review Formation of metal-support compounds in cobalt-based Fischer-Tropsch synthesis: A review. Chem. Catal. 2021, 1, 1014–1041. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Liu, X.; Lu, X.; Moyo, M.; Hildebrandt, D. Cobalt hybrid catalysts in Fischer-Tropsch synthesis. Rev. Chem. Eng. 2020, 36, 437–457. [Google Scholar] [CrossRef]
- Maximov, A.L.; Kulikova, M.V.; Dementyeva, O.S.; Ponomareva, A.K. Cobalt-Containing Dispersion Catalysts for Three-Phase Fischer—Tropsch Synthesis. Front. Chem. 2020, 8, 567848. [Google Scholar] [CrossRef]
- Wentrup, J.; Pesch, G.R.; Thöming, J. Dynamic operation of Fischer-Tropsch reactors for power-to-liquid concepts: A review. Renew. Sustain. Energy Rev 2022, 162, 112454. [Google Scholar]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors Recent advances in Fischer-Tropsch synthesis using. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Smith, D.F.; Hawk, C.O.; Golden, P.L. The mechanism of the formation of higher hydrocarbons from water gas. J. Am. Chem. Soc. 1930, 52, 3221–3232. [Google Scholar] [CrossRef]
- Niemel, M.K.; Krause, A.O.I.; Vaara, T.; Lahtinen, J. Preparation and characterization of Co/SiO2. Top. Catal. 1995, 2, 45–57. [Google Scholar]
- Bartholomew, C.H. Effects of Support and Dispersion on the CO Hydrogenation Activity/Selectivity Properties of Cobalt. J. Catal. 1984, 88, 78–88. [Google Scholar]
- Bartholomew, C.H. The Stoichiometries of H2 and CO Adsorptions Support and Preparation on Cobalt: Effects of support and preparation. J. Catal. 1984, 77, 63–77. [Google Scholar]
- Peng, X.; Cheng, K.; Kang, J.; Gu, B.; Yu, X.; Zhang, Q.; Wang, Y. Impact of Hydrogenolysis on the Selectivity of the Fischer—Tropsch Synthesis: Diesel Fuel Production over Mesoporous Zeolite-Y-supported cobalt nanoparticles. Angew. Comun. 2015, 54, 4553–4556. [Google Scholar]
- Pei, Y.; Ding, Y.; Zhu, H.; Zang, J.; Song, X.; Dong, W. Effect of Al2O3 Promoter on a Performance of C1—C 14 a -Alcohols Direct Synthesis over Co/AC Catalysts via Fischer—Tropsch Synthesis. Catal. Lett. 2014, 144, 1433–1442. [Google Scholar] [CrossRef]
- Okoye-chine, C.G.; Mbuya, C.O.L.; Ntelane, T.S.; Moyo, M.; Hildebrandt, D. The effect of silanol groups on the metal-support interactions in silica- supported cobalt Fischer-Tropsch catalysts. A temperature programmed surface reaction. J. Catal. 2020, 381, 121–129. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Yu, F.; An, Y.; Dai, Y.; Yang, Y.; Lin, T.; Li, S.; Wang, H.; Gao, P.; et al. Effects of Sodium on the Catalytic Performance of CoMn Catalysts for Fischer-Tropsch to Olefin Reactions. ACS Catal. 2017, 7, 3622–3631. [Google Scholar] [CrossRef]
- Colley, S.; Copperthwaite, R.G.; Hutchings, G.J.; Van der Riet, M. Carbon Monoxide Hydrogenation Using Cobalt Manganese Oxide Catalysts: Initial Catalyst Optimization Studies. Ind. Eng. Chem. Res. 1988, 27, 1339–1344. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Radstake, P.B.; Falke, U.; Oosterbeek, H.; Kuipers, H.P.C.E.; Van Dillen, A.J. Investigation of promoter effects of manganese oxide on carbon nanofiber-supported cobalt catalysts for Fischer—Tropsch synthesis. J. Catal. 2006, 237, 152–161. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Dinse, A.; Aigner, M.; Ulbrich, M.; Johnson, G.R.; Bell, A.T. Effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer—Tropsch Synthesis. J. Mol. Catal. A Chem. 2012, 288, 104–114. [Google Scholar] [CrossRef]
- Morales, F.; Desmit, E.; Degroot, F.; Visser, T.; Weckhuysen, B.M. Effects of manganese oxide promoter on the CO and H 2 adsorption properties of titania-supported cobalt Fischer—Tropsch catalysts. J. Catal. 2007, 246, 91–99. [Google Scholar] [CrossRef]
- Morales, F.; De Groot, F.M.F.; Gijzeman, O.L.J.; Mens, A.; Stephan, O. Mn promotion effects in Co/TiO2 Fischer—Tropsch catalysts as investigated by XPS and STEM-EELS. J. Catal. 2005, 230, 301–308. [Google Scholar] [CrossRef]
- Cano, F.M.; Gijzeman, O.L.J.; De Groot, F.M.F.; Weckhuysen, B.M. Manganese promotion in cobalt-based Fischer-Tropsch catalysis. Stud. Surf. Sci. Catal. 2004, 147, 271–276. [Google Scholar]
- Feltes, T.E.; Espinosa-Alonso, L.; de Smit, E.; D’Souza, L.; Meyer, R.J.; Weckhuysen, B.M.; Regalbuto, J.R. Selective adsorption of manganese onto cobalt for optimized Mn/Co/TiO2 Fischer—Tropsch catalysts. J. Catal. 2010, 270, 95–102. [Google Scholar] [CrossRef]
- Thiessen, J.; Rose, A.; Meyer, J.; Jess, A.; Curulla-ferré, D. Microporous and Mesoporous Materials Effects of manganese and reduction promoters on carbon nanotube supported cobalt catalysts in Fischer—Tropsch synthesis. Microporous Mesoporous Mater. 2012, 164, 199–206. [Google Scholar] [CrossRef]
- Werner, S.; Johnson, G.R.; Bell, A.T. Synthesis and Characterization of Supported Cobalt—Manganese Nanoparticles as Model Catalysts for Fischer—Tropsch Synthesis. J. ChemCatChem 2014, 6, 2881–2888. [Google Scholar] [CrossRef]
- Johnson, G.R.; Werner, S.; Bell, A.T. An Investigation into the Effects of Mn Promotion on the Activity and Selectivity of Co/SiO2 for Fischer-Tropsch Synthesis: Evidence for Enhanced CO Adsorption and Dissociation. ACS Catal. 2015, 5, 5888–5903. [Google Scholar] [CrossRef]
- Tucker, C.L.; Ragoo, Y.; Mathe, S.; Macheli, L.; Bordoloi, A.; Rocha, T.C.; Govender, S.; Kooyman, P.J.; van Steen, E. Manganese promotion of a cobalt Fischer-Tropsch catalyst to improve operation at high conversion. J. Catal. 2022, 411, 97–108. [Google Scholar] [CrossRef]
- Zhai, P.; Sun, G.; Zhu, Q.; Ma, D. Fischer-tropsch synthesis nanostructured catalysts: Understanding structural characteristics and catalytic reaction. Nanotechnol. Rev. 2013, 2, 547–576. [Google Scholar] [CrossRef]
- Panpranot, J.; Goodwin, J.G.; Sayari, A. CO hydrogenation on Ru-promoted Co/MCM-41 catalysts. J. Catal. 2002, 211, 530–539. [Google Scholar] [CrossRef]
- Jean-Marie, A.; Griboval-Constant, A.; Khodakov, A.Y.; Diehl, F. Cobalt supported on alumina and silica-doped alumina: Catalyst structure and catalytic performance in Fischer-Tropsch synthesis. Comptes Rendus Chim. 2009, 12, 660–667. [Google Scholar] [CrossRef]
- Oukaci, R.; Singleton, A.H.; Goodwin, J.G. Comparison of patented Co F–T catalysts using fixed-bed and slurry bubble column reactors. Appl. Catal. A Gen. 1999, 186, 129–144. [Google Scholar] [CrossRef]
- Barradas, S.; Caricato, E.A.; Van Berge, P.J.; Van de Loosdrecht, J. Support modification of cobalt based slurry phase Fischer-Tropsch catalyst. Stud. Surf. Sci. Catal. 2000, 143, 55–65. [Google Scholar]
- Feller, A.; Claeys, M.; Van Steen, E. Cobalt Cluster Effects in Zirconium Promoted Co/SiO2 Fischer—Tropsch Catalysts. J. Catal. 1999, 185, 120–130. [Google Scholar] [CrossRef]
- Zhang, H.; Lancelot, C.; Chu, W.; Hong, J.; Khodakov, A.Y.; Chernavskii, P.A.; Zheng, J.; Tong, D. The nature of cobalt species in carbon nanotubes and their catalytic performance in Fischer–Tropsch reaction. J. Mater. Chem. 2009, 19, 9241–9249. [Google Scholar]
- Davis, B.H. Fischer—Tropsch Synthesis: Comparison of Performances of Iron and Cobalt Catalysts. Ind. Eng. Chem. Res. 2007, 46, 8938–8945. [Google Scholar] [CrossRef]
- Yao, Y.; Hildebrandt, D.; Glasser, D.; Liu, X. Fischer—Tropsch Synthesis Using H2/CO/CO2 Syngas Mixtures over a Cobalt Catalyst. Ind. Eng. Chem. Res. 2010, 49, 11061–11066. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X.; Hildebrandt, D.; Glasser, D. The effect of CO2 on a cobalt-based catalyst for low temperature Fischer—Tropsch synthesis. Chem. Eng. J. 2012, 193–194, 318–327. [Google Scholar] [CrossRef]
- Warayanon, W.; Tungkamani, S.; Sukkathanyawat, H.; Phongaksorn, M.; Ratana, T.; Sornchamni, T. Effect of Manganese Promoter on Cobalt Supported Magnesia Catalyst for Fischer-Tropsch Synthesis. Energy Procedia 2015, 79, 163–168. [Google Scholar] [CrossRef]
- Vosoughi, V.; Badoga, S.; Dalai, A.K.; Abatzoglou, N. Effect of Pretreatment on Physicochemical Properties and Performance of Multiwalled Carbon Nanotube Supported Cobalt Catalyst for Fischer − Tropsch Synthesis. Ind. Eng. Chem. Res. 2016, 55, 6049–6059. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar]
- Šťastný, M.; Issa, G.; Popelková, D.; Ederer, J.; Kormunda, M.; Kříženecká, S.; Henych, J. Nanostructured manganese oxides as highly active catalysts for enhanced hydrolysis of bis(4-nitrophenyl)phosphate and catalytic decomposition of methanol. Catal. Sci. Technol. 2021, 11, 1766–1779. [Google Scholar] [CrossRef]
- Saib, A.; Claeys, M.; van Steen, E. Silica supported cobalt Fischer-Tropsch catalysts: Effect of pore diameter of support. Catal. Today 2001, 71, 395–402. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Bechara, R.; Griboval-Constant, A. Fischer—Tropsch synthesis over silica supported cobalt catalysts: Mesoporous structure versus cobalt surface density. Appl. Catal. A Gen. 2003, 254, 273–288. [Google Scholar] [CrossRef]
- Intarasiri, S.; Ratana, T.; Sornchamni, T.; Tungkamani, S. Pore size effect of mesoporous support on metal particle size of Co / SiO2 catalyst in Fischer-Tropsch synthesis. Int. J. Adv. Appl. Sci. 2018, 5, 80–85. [Google Scholar]
- Song, D.; Li, J. Effect of catalyst pore size on the catalytic performance of silica supported cobalt Fischer—Tropsch catalysts. J. Mol. Catal. A Chem. 2006, 247, 206–212. [Google Scholar] [CrossRef]
- Borg, Ø.; Eri, S.; Blekkan, E.A.; Storsæter, S.; Wigum, H.; Rytter, E.; Holmen, A. Fischer-Tropsch synthesis over γ-alumina-supported cobalt catalysts: Effect of support variables. J. Mol. Catal. A Chem. 2006, 248, 206–212. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Wang, S.; Li, J. Fischer—Tropsch synthesis: The effect of Al2O3 porosity on the performance of Co/Al2O3 catalyst. Catal. Commun. 2005, 6, 512–516. [Google Scholar] [CrossRef]
- Puskas, I. Metal-Support Interactions in Precipitated, Magnesium-Promoted Cobalt-Silica Catalysts. J. Catal. 1992, 135, 615–628. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nagata, K.; Adachi, T.; Imanaka, T.; Inamura, K. Preparation and Characterization of Highly Dispersed Cobalt Oxide and Sulfide Catalysts Supported on SiO2. J. Phys. Chem. 1991, 95, 310–319. [Google Scholar] [CrossRef]
- Ming, H.; Baker, B.G. A: Characterization of cobalt Fischer-Tropsch catalysts I. Unpromoted cobalt-silica gel catalysts. Appl. Catal. A Gen. 1995, 123, 23–36. [Google Scholar] [CrossRef]
- Khodakov, A.; Ducreux, O.; Lynch, J.; Rebours, B.; Chaumette, P. Structural Modification of Cobalt Catalysts: Effect of Wetting Studied by X-Ray and Infrared Techniques. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 1999, 54, 525–536. [Google Scholar] [CrossRef]
- Stranick, M.A.; Stranick, M.A. Mn2O3 by XPS. J. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Jahan, M.; Tominaka, S.; Henzie, J. Phase pure α- Mn2O3 prisms and their bifunctional electrocatalytic activity in oxygen evolution and reduction reactions. J. Roy Soc. Chem. 2016, 45, 18494–18501. [Google Scholar] [CrossRef]
- Das, T.K.; Jacobs, G.; Davis, B.H. Fischer—Tropsch synthesis: Deactivation of promoted and unpromoted cobalt—Alumina catalysts. Catal. Lett. 2005, 101, 187–190. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Karaca, H.; Safonova, O.; Fongarland, P.; Chambrey, S.; Roussel, P.; Griboval-Constant, A.; Lacroix, M.; Curulla-Ferré, D.; Luck, F.; et al. Identification of the active species in the working alumina-supported cobalt catalyst under various conditions of Fischer—Tropsch synthesis. Catal. Today 2011, 164, 62–67. [Google Scholar] [CrossRef]
- Enache, D.I.; Rebours, B.; Roy-Auberger, M.; Revel, R. In Situ XRD Study of the Influence of Thermal Treatment on the Characteristics and the Catalytic Properties of Cobalt-Based Fischer—Tropsch Catalysts. J. Catal. 2002, 205, 346–353. [Google Scholar] [CrossRef]
- Ducreux, O.; Rebours, B.; Lynch, J.; Roy-Auberger, M.; Bazin, D. Microstructure of Supported Cobalt Fischer-Tropsch Catalysts. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 2008, 64, 49–62. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Fischer—Tropsch synthesis: Activity of metallic phases of cobalt supported on silica. Catal. Today 2013, 215, 13–17. [Google Scholar] [CrossRef]
- Pedersen, E.; Svenum, I.-H.; Blekkan, A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins—An experimental and theoretical study. J. Catal. 2018, 361, 23–32. [Google Scholar] [CrossRef]
- Tyson, W.R. Surface free energies of solid metals” estimation from liquid. J. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Akbarzadeh, O.; Mohd Zabidi, N.A.; Aljunid Merican, Z.M.; Sagadevan, S.; Kordijazi, A.; Das, S.; Amani Babadi, A.; Ab Rahman, M.; Hamizi, N.A.; Abdul Wahab, Y.; et al. Effect of Manganese on Co—Mn/CNT Bimetallic Catalyst Performance in Fischer—Tropsch Reaction. J. Symmetry 2019, 11, 1328. [Google Scholar] [CrossRef]
- Breejen, J.P.D.; Frey, A.M.; Yang, J.; Holmen, A.; van Schooneveld, M.M.; de Groot, F.M.F.; Stephan, O.; Bitter, J.H.; de Jong, K.P. A Highly Active and Selective Manganese Oxide Promoted Cobalt-on-Silica Fischer—Tropsch Catalyst. Top. Catal. 2011, 54, 768–777. [Google Scholar] [CrossRef]
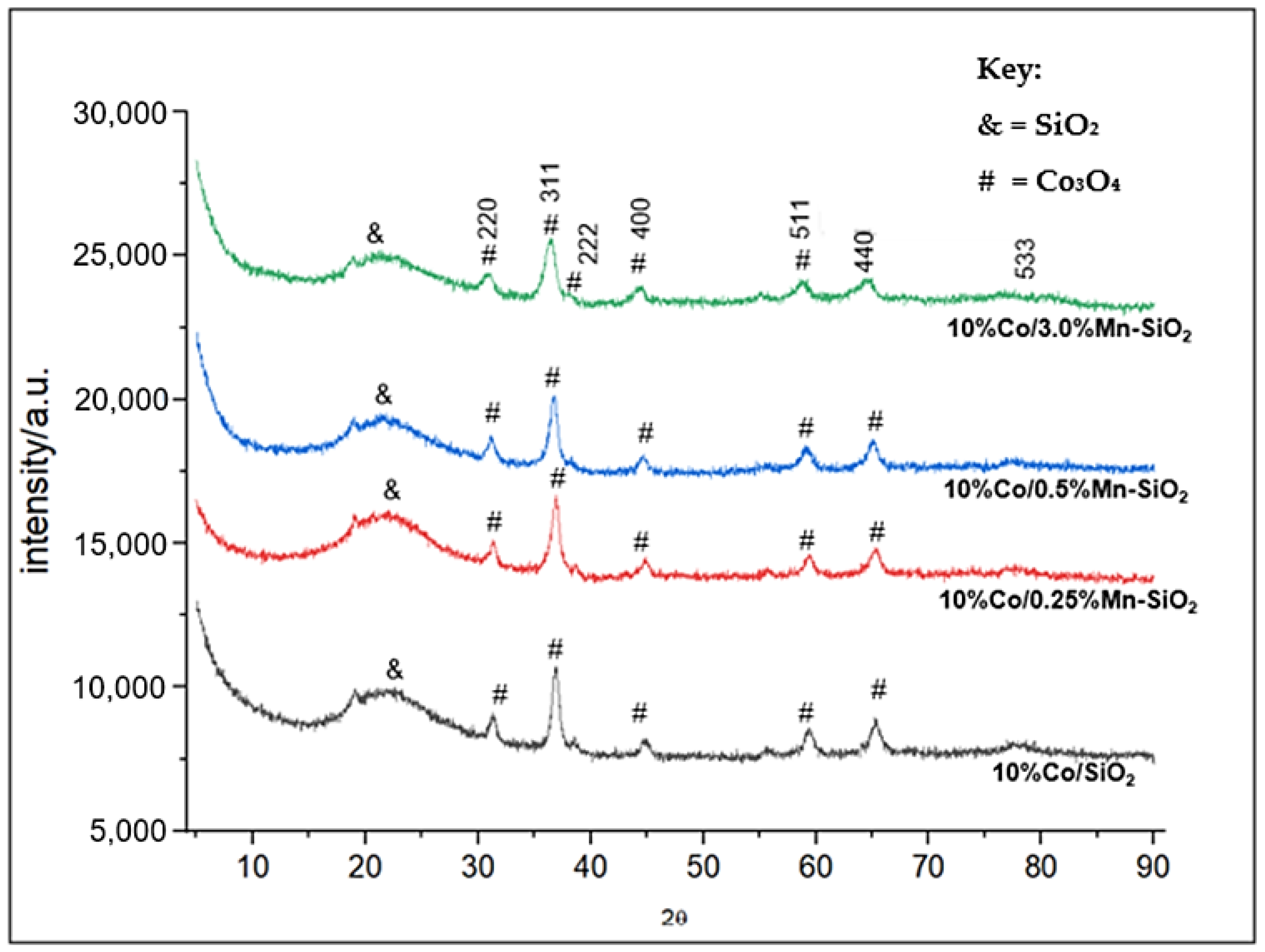
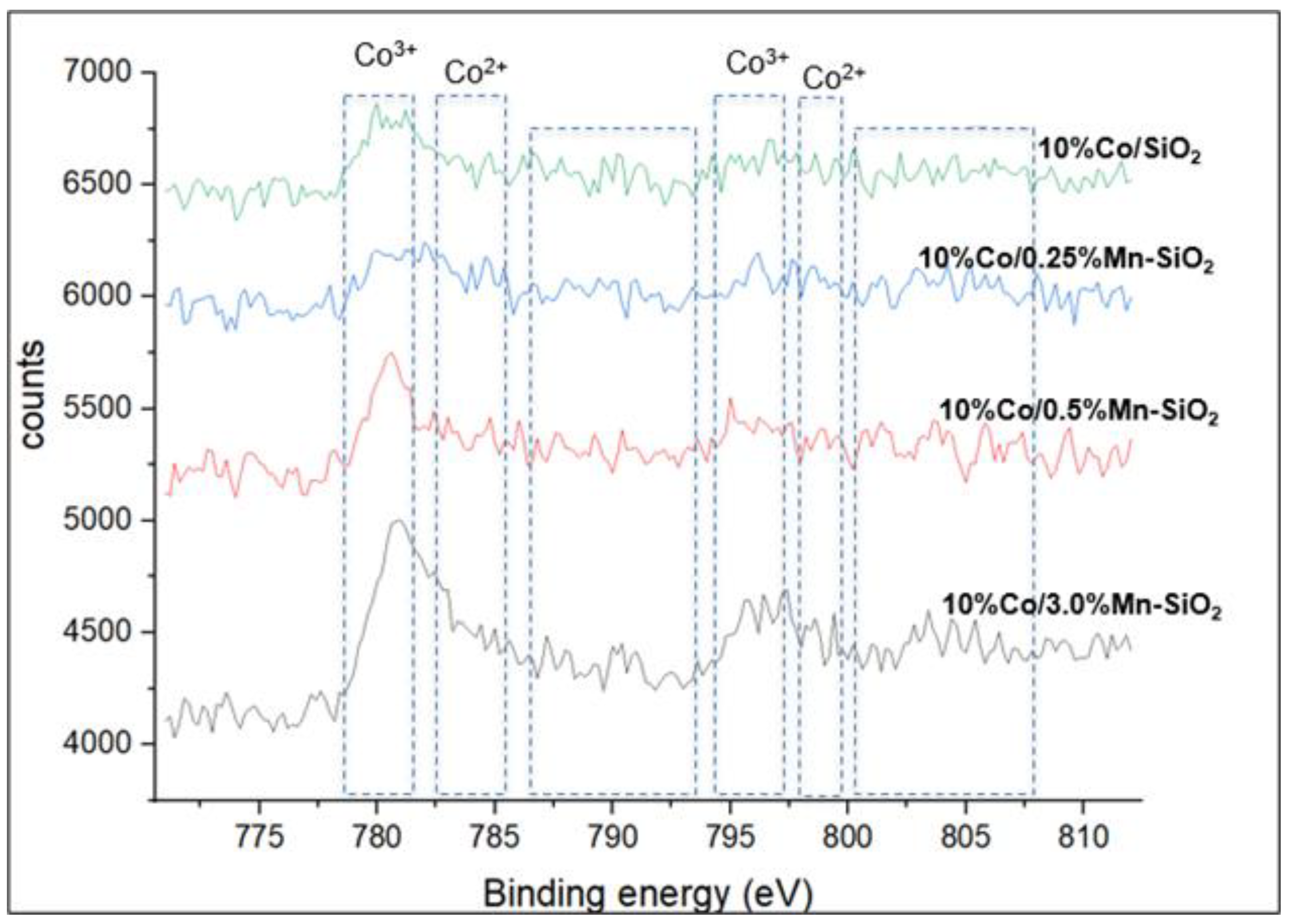
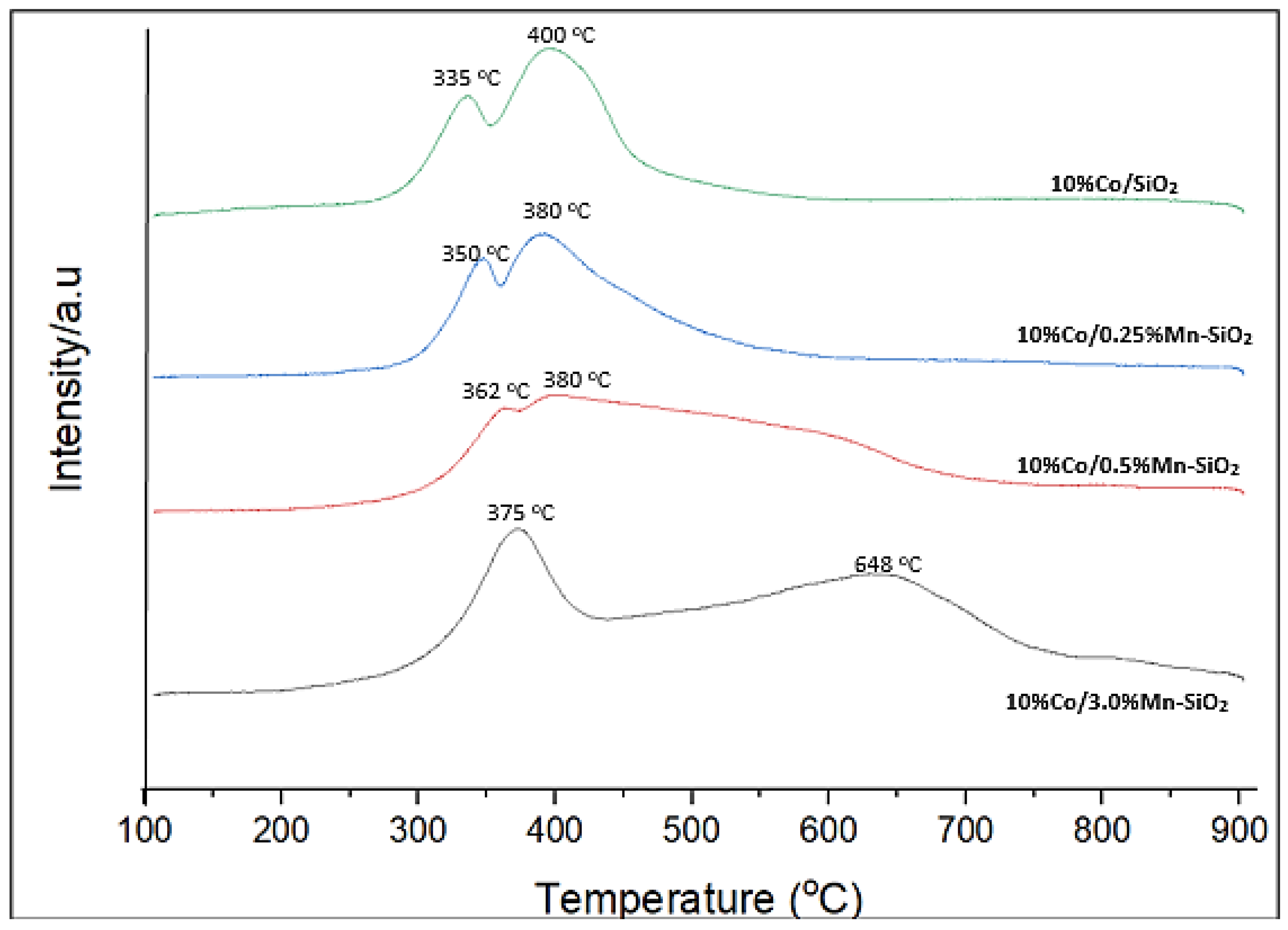
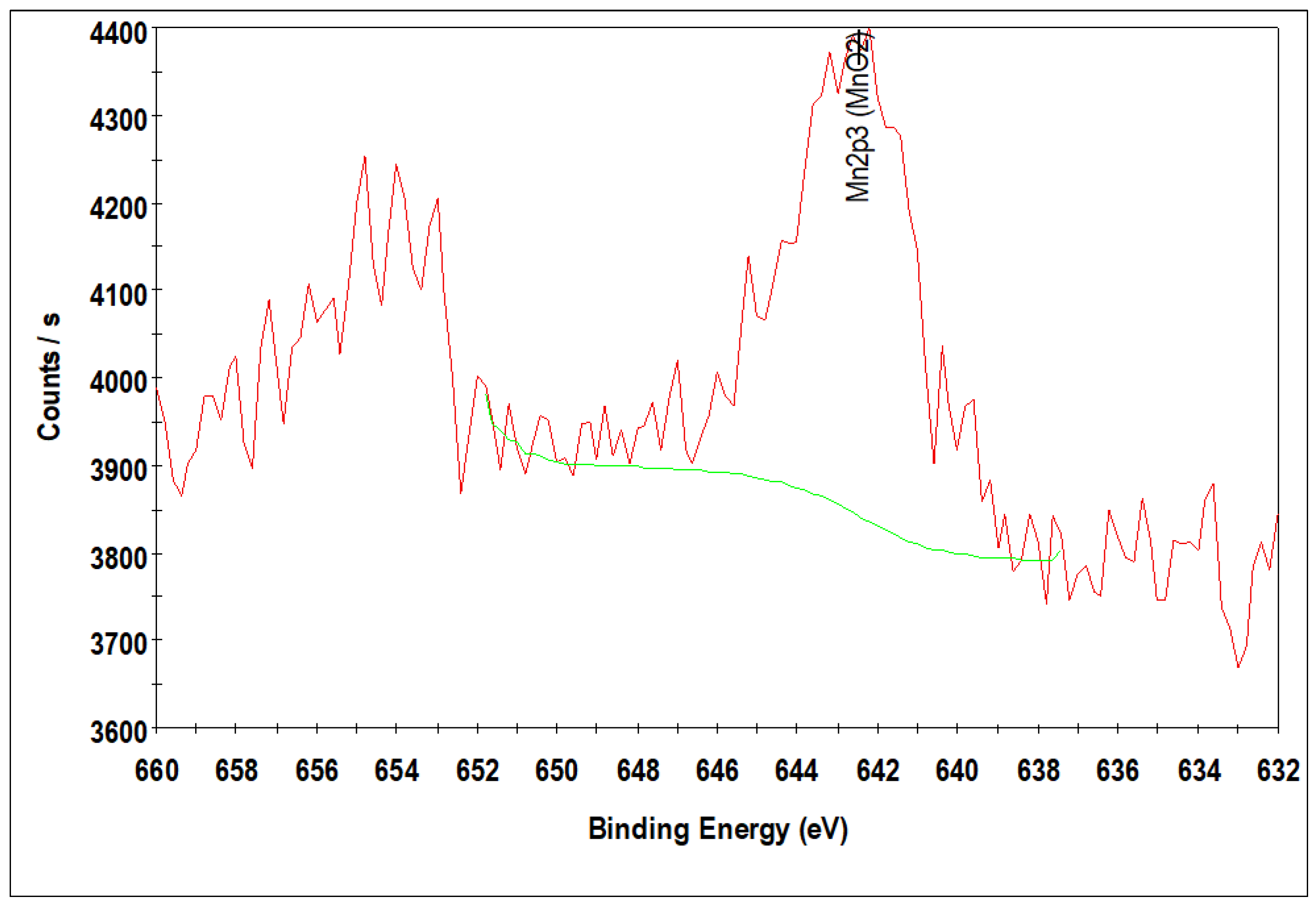
| Sample | ICP-AES Loadings (wt.%) | BET Surface Area (m2/g) | Pore Volume (m3/g) | Pore Sizes (nm) | |
|---|---|---|---|---|---|
| Co | Mn | ||||
| SiO2 | - | - | 317.0 | 1.15 | 15.0 |
| 10%Co/SiO2 | 9.55 | - | 312.2 | 0.75 | 14.1 |
| 10%Co/0.25%Mn-SiO2 | 9.86 | 0.25 | 308.4 | 0.51 | 6.4 |
| 10%Co/0.5%Mn-SiO2 | 9.09 | 0.57 | 309.6 | 0.51 | 6.1 |
| 10%Co/3.0%Mn-SiO2 | 9.68 | 3.09 | 320.4 | 0.46 | 5.4 |
| 10%Co/SiO2 | 10%Co/0.25%Mn-SiO2 | 10%Co/0.5%Mn-SiO2 | 10%Co/3.0%Mn-SiO2 | |
|---|---|---|---|---|
| Co2p3/2 (BE) | 781.1 | 781.1 | 780.9 | 781.3 |
| Co2p (atomic %) | 0.5 | 0.4 | 0.4 | 0.8 |
| Mn2p (atomic %) | - | - | - | 0.7 |
| Si2p (atomic %) | 27.9 | 26.4 | 19.6 | 26 |
| C1s (atomic %) | 13.3 | 15.9 | 31.8 | 14.9 |
| Catalyst | Metal Sizes | |||||||
|---|---|---|---|---|---|---|---|---|
| XRD | H2 Chemisorption | CO Chemisorption | ||||||
| d(Co3O4) (nm) a | d(Co0) (nm) | Dispersion (%) | d(Co0) (nm)b | Metal Surface Area (m2/g) b | DOR (%) c | H2 Uptake (×10−4 moles/g) d | CO Uptake (×10−4 moles/g) e | |
| 10%Co/SiO2 | 11.03 | 8.27 | 7.87 | 12.19 | 8.025 | 96.0 | 1.77 | 0.64 |
| 10%Co/0.25%Mn-SiO2 | 11.20 | 8.40 | 10.43 | 9.21 | 10.63 | 94.3 | 1.34 | 0.70 |
| 10%Co/0.5%Mn-SiO2 | 10.79 | 8.09 | 11.80 | 8.03 | 12.02 | 94.0 | 2.01 | 0.68 |
| 10%Co/3.0%Mn-SiO2 | 8.45 | 6.34 | 15.39 | 6.23 | 13.39 | 92.3 | 2.61 | 0.65 |
| 10%Co/SiO2 | 10%Co/0.25%Mn-SiO2 | 10%Co/0.5%Mn-SiO2 | 10%Co/3.0%Mn-SiO2 | |
|---|---|---|---|---|
| CO conversion (%) | 31.8 | 20.1 | 18.9 | 24.7 |
| Selectivity/C1 (%) | 4.77 | 1.66 | 4.72 | 2.97 |
| C2–C4 | 6.10 | 3.90 | 5.48 | 4.23 |
| CO2 | - | - | - | - |
| C5+ | 89.2 | 94.4 | 89.8 | 92.8 |
| rCO 10−5) | 24.1 | 15.4 | 7.4 | 19.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibanyane, N.; Gorimbo, J.; Yao, Y. Impact of Inverse Manganese Promotion on Silica-Supported Cobalt Catalysts for Long-Chain Hydrocarbons via Fischer–Tropsch Synthesis. Reactions 2024, 5, 607-622. https://doi.org/10.3390/reactions5030030
Thibanyane N, Gorimbo J, Yao Y. Impact of Inverse Manganese Promotion on Silica-Supported Cobalt Catalysts for Long-Chain Hydrocarbons via Fischer–Tropsch Synthesis. Reactions. 2024; 5(3):607-622. https://doi.org/10.3390/reactions5030030
Chicago/Turabian StyleThibanyane, Ntebogang, Joshua Gorimbo, and Yali Yao. 2024. "Impact of Inverse Manganese Promotion on Silica-Supported Cobalt Catalysts for Long-Chain Hydrocarbons via Fischer–Tropsch Synthesis" Reactions 5, no. 3: 607-622. https://doi.org/10.3390/reactions5030030
APA StyleThibanyane, N., Gorimbo, J., & Yao, Y. (2024). Impact of Inverse Manganese Promotion on Silica-Supported Cobalt Catalysts for Long-Chain Hydrocarbons via Fischer–Tropsch Synthesis. Reactions, 5(3), 607-622. https://doi.org/10.3390/reactions5030030







