Abstract
Diverse studies have showed that the pesticides can cause important damages in ecosystem. Therefore, the development of bio pesticides through nanotechnology can increase efficacy and limit the negative impacts in the environmental that traditionally seen through the use of chemical pesticides. Nanoparticles obtained from plants’ extracts can be used for effective pest management as a combined formulation of metal and some other organic material present in the plants. In the present study, our evaluated biosynthesis of nanoparticles of copper used two plant extracts (Acacia cornigera and Annona purpurea), and the Taguchi method was adopted for the synthesis optimization of the following variables of biosynthesis: temperature, pH, extract concentration, and reaction times to maximize the insecticidal activity on Tribolium castaneum. Our results showed that the nanoparticles were successfully synthesized using Acacia cornigera and Anona purpurea extract under optimum conditions under Taguchi L 9 orthogonal design, where copper nanoparticles were obtained with a size of 63–153 nm for using A. cornigera extract, 87–193 nm for A. purpurea extract, and a zeta potential of 9.6 mV and −32.7 mV, respectively. The nanoparticles of copper from A. cornigera showed effective insecticidal activity against Tribolium castaneum, and 90% mortality compared to the 76.6% obtained from nanoparticles of copper from A. purpurea. The results suggest that Cu-nanoparticles derived from both plants could be used as a biocontrol agent of Tribolium castaneum, a pest of stored grain with great economic importance.
1. Introduction
The red flour beetle Tribolium castaneum is considered a major agriculture pest, damaging stored grains and cereal products. This species can infest a large diversity of cereal flours, principally wheat flour, on which this insect can complete egg-to-adult development within ~4–6 weeks [1,2,3]. Recent reports also recorded their presence on diverse raw commodities (e.g., spices, oilseeds, cottonseeds, dried fruits, and processed foods) [4]. This insect can cause serious problems that affect the quality of stored products as a result of alteration of chemical composition. For example, the production of quinone substances by T. castaneum adults may cause allergic reactions or a carcinogenic effect in the products infested by this insect [5].
Generally, chemical fumigation with gaseous pesticides is used for the control of this insect; however, this method is very dangerous for ecosystems, and expensive [4,5]. Several reports showed that continuous use of diverse types of insecticides has accelerated the presence of resistance in this insect. The presence of resistance in different classes of insecticides may be the result of the capacity to produce detoxification enzymes such as cytochrome P450 proteins [6]. Zhu et al. [7] mention the expression of P450 genes in the brain of T. castaneum, which is involved in deltamethrin resistance. On the other hand, multiple resistance towards two different classes of insecticides (pyrethroid and organophosphate) has been reported in the Brazilian population of the red flour beetle [8]. Similar results have been reported in the Nigerian population, which was resistant to dichlorvos, cypermethrin, DDT, and malathion [9]. This resistance involves the presence of different mechanisms of insecticide resistance, for example, T. castaneum treated with malathion showed that the induction of the antioxidant enzyme catalase could have helped to withstand oxidative stresses, contributing to increased immunity of the insecticide [10]. Therefore, the development of new strategies to control Tribolium castaneum that are both eco-friendly and cost-effective are necessary.
In this sense, the development of new agriculture products incorporating metallic nanoparticles (NPs) has recently become of scientific and technological interest worldwide [11]. Metallic NPs presented important remarkable applications as antimicrobial and pesticide control agents, thus far proposing a potential function for managing plant diseases and reducing losses in agricultural lands [12]. Specifically, the use of phytochemicals from plants as reducing agents of various types of metal-based nanoparticles (green synthesis) represent a viable option because of its eco-friendly nature, cost-effectiveness, and non-toxic manner. In this context, the use of chemical pesticides can caused several problems such as environmental contamination, pest resistance, and bioaccumulation represent great risks to ecosystem and human health hazards [13]. The nanotechnology represents an appealing tool for pest control with the use of a minimal amount of pesticide, as a result of new methods for the formulation and delivery of existing active ingredients, as well as novel active ingredients derived from the interaction between metals and phytochemicals from plants [14]. Recent studies demonstrated that CuNPs obtained from Pseudomonas fluorescens showed toxicity against Tribolium castaneum, and that optimized growth conditions are necessary [15]. However, the consumed of time to the growth culture of microorganisms and the process of synthesis of nanoparticles, could are limited with respect to synthesis of nanoparticles obtained of phytochemicals from plants [16]. In this context, Acacia cornigera and Annona purpurea are plants that showed high content of nutrients, minerals, and phytochemicals, and they can constitute a source of functional compounds like tannins, alkaloids, and terpenoids, among others, which could act as stabilizing and reducing agents during the biosynthesis of metallic nanoparticles [17,18]. Therefore, the aim of the present study was to evaluate the insecticide activity of green synthesis of nanoparticles of copper obtained from Acacia cornigera and Annona purpurea extracts against Tribolium castaneum, a relevant pest of stored grain. For this study, a statistical design with a Taguchi L9 (34) orthogonal array was used to optimize the synthetic conditions of CuNPs (temperature, pH, extract concentration, and reaction time) according to the mortality of T. castaneum.
2. Materials and Methods
For the synthesis of phytonanoparticles, leaves of Acacia cornigera and Annona purpurea plants were collected from La Concordia, Chiapas, Mexico (16°07′05.97″ N and 92°41′30.08″ W). The leaves were dried at 40 °C, and when finished the drying process, they were ground to obtain a fine powder. An aqueous solution from each plant was prepared using 10 g of powder leaves mixed with 100 mL of distilled water and heated at 60 °C for 30 min. Finalized the incubation, the mixture was centrifuged at 4000 rpm for 20 min, and the supernatant was recovered and stored in dark conditions at −20 °C.
2.1. Biosynthesis of Copper Nanoparticles (CuNPs)
For the biosynthesis of metallic nanoparticles, aqueous solution from each plant was mixed with 10 mM copper sulfate (CuSO4) and water relation 1:4 v/v [16]. To evaluate the effect of temperature (30, 40, 50 °C), pH (5, 7, 9), concentration of extract of each plant (50, 75, 100%), and reaction time (20, 30, and 40 min) in the bio reduction process of CuNPs, an orthogonal experimental was designed (Table 1). The variable considered for the optimal conditions was the T. castaneum mortality response.

Table 1.
Orthogonal array L9 for CuNPs from A. cornigera and CuNPs from A. purpurea. Evaluation and results obtained for temperature, pH, extract concentration, and reaction time on the mortality of T. castaneum.
2.2. CuNPs Physicochemical Characterization
The bio-synthesis of Cu and the production of CuNPs, using the two extracts of each plant, were measured with a spectrophotometer (DR6000™ Hach, UV VIS Spectrophotometer, Loveland, CO, USA) at 350–500 nm, with a resolution of 5 nm. For the characterization of size and zeta potential of the CuNPs from Acacia cornigera and Annona purpurea plants, the Dynamic Light Scattering (DLS) and laser Doppler velocimetry (LDV) were calculated using a Nanotrac Wave instrument (Microtrac, North Wales, PA, USA) in base, and the method proposed by Abdelmoteleb et al. [18]. The measurements were made using DLS within the range of 0.1–1000 μm at 25 °C, employing a laser wavelength of 780 nm with a scattering angle of 90°. The data obtained were analyzed using Microtrac FLEX operating software (DIMENSIONS LS). For the determination of size and morphology of the synthesized CuNPs, the SEM (scanning electron microscope) JEOL 6010 at 10 kV, coupled with the elemental analysis and energy-dispersive X-ray spectroscopy (EDX; XFlash 6I30 Brucker, Billerica, MA, USA), was used [19].
2.3. Determination of Total Phenols and Flavonoids
The Folin–Ciocalteu method was used for the determination of phenolic compounds in the extract plants (A. purpurea and A. cornigera) and Cu-phyto nanoparticle [19]. A 500 µL from each sample was added to 2.5 mL Folin–Ciocalteu’s reagent solution (0.25 M) and incubated for 5 min; after this, aqueous solution of 20% sodium carbonate (2 mL) was added. Finally, each sample was incubated under room temperature for 40 min, and changes in the absorbance were registered at 725 nm. A calibration curve was prepared with gallic acid (0–200 μg/mL) to quantify the phenol content. The analysis was performed in triplicate, and the results are expressed in mg equivalents of gallic acid (mg/g dry matter).
The flavonoid content from each sample was evaluated by mixing 0.25 mL of separate extract samples, diluted in 0.75 mL of distilled water, 0.05 mL of 10% aluminum chloride in ethanol, 0.05 mL of 1 M potassium acetate, and 1.4 mL of distilled water [20]. Then, the reactions were incubated at room temperature for 30 min, and absorbance was registered at 415 nm. A calibration curve was prepared using a quercetin standard solution (0–40 μg/mL), and flavonoid content was expressed as mg equivalents of quercetin (mg/g of dry matter).
2.4. Insecticidal Activity of CuNPs
T. castaneum insects were reared at 27 ± 2 °C and 68 ± 2% relative humidity in 12 h:12 h (light:dark) conditions. Nine groups of ten adult beetles were separated and sprayed with 2 mL of the aqueous solution of CuNPs obtained in the experimental units of the orthogonal Taguchi L9 (34) matrix. On the other hand, a group was treated with A. cornigera, A. purpurea extracts, and another group was treated only with the CuSO4 solution. Then, the beetles were placed separately in Petri dishes, and the mortality rate was observed from 1 day to 6 days after the treatment [21,22]. A group of insects was used as a control, which was sprayed with 2 mL of distilled water.
2.5. Determination of Cell Viability
The effect of treatment of CuNPs from both plants in the viability cell (membrane damage) of T. castaneum was analyzed using the Evans blue staining protocol described by Mendez-Trujillo et al. [23], and the absorbance was quantified at 600 nm. The experiments were performed in triplicate and water was used as a control.
2.6. Statistical Analysis
Taguchi analysis was applied to establish the optimal conditions for synthesizing CuNPs, resulting in the highest T. castaneum mortality. For the design of the Taguchi array and the optimization process, the MINITAB® version 19 software was used.
3. Results
3.1. Synthesis of Copper Nanoparticles from Plants’ Extracts
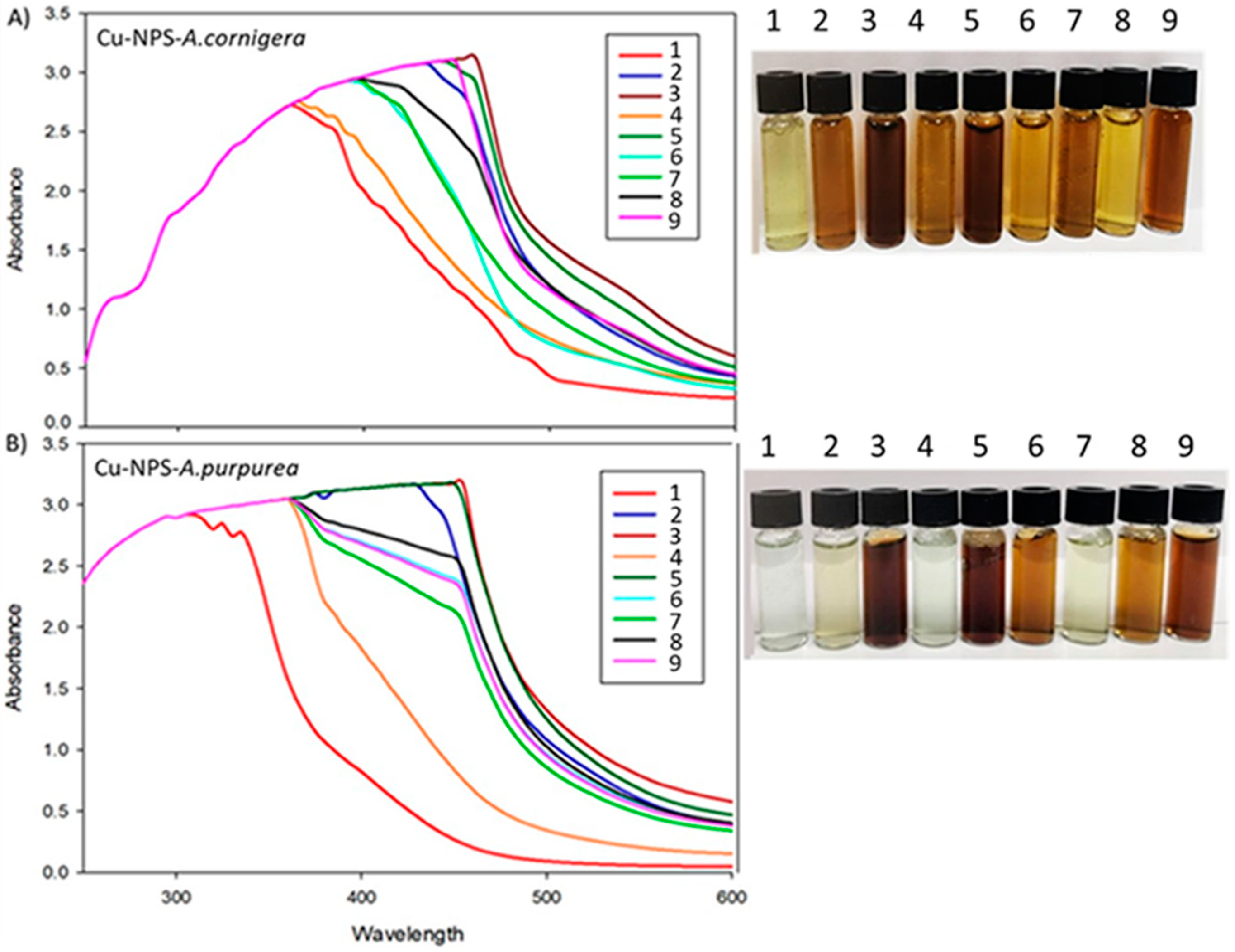
CuNPs synthesis from A. cornigera and A. purpurea was confirmed in all experimental units mainly by a color change of the solution from green to dark brown at the end of the reaction (Figure 1). The CuNPs formed from A. cornigera and A. purpurea extracts were measured through a UV-visible spectrum analysis for each experimental unit at a wavelength of 200–600 nm. The UV-vis spectra showed centered peaks ranging from 340 nm to 460 nm, which are characteristic ranges of CuNPs due to their surface plasmon resonance absorption band (Figure 1). Our results showed the importance of optimization of variables such as temperature, pH, extract concentration, and reaction time in the optimal conditions for copper nanoparticle formation, concerning biological effects on T. castaneum (Table 1).
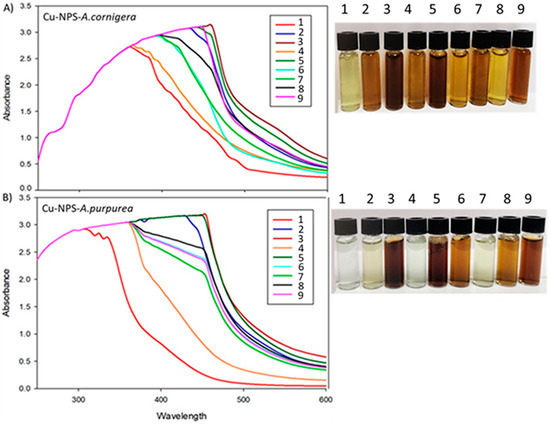
Figure 1.
Color change and UV-vis spectra in experimental units of the orthogonal Taguchi L9 (34), observed during CuNPs biosynthesis in each experimental unit: (A) CuNPs from A. cornigera and (B) CuNPs from A. purpurea of the orthogonal Taguchi L9.
3.2. Insecticidal Activity of Copper Nanoparticles from Plants’ Extracts
The results for the individual effects of each parameter involved in the biosynthesis of CuNPs and their effect in T. castaneum mortality are shown in Table 1. Our results showed that experiments 2, 6, and 7 present the major mortality in the insect, while experiments 1, 4, and 9 occasionally decreased in this parameter. The results showed that the two factors of temperature (40 °C to 50 °C) and pH (5–7) increased the mortality rate. In contrast, increased temperature (50 °C to 60 °C) and variation of pH of 7–9 caused low mortality of insects. Finally, the variation in the reaction time from 20 min to 30 min increased the mortality; however, variation in time from 30 min to 40 min caused a decrease in mortality. Therefore, of the experiments, six have a major insecticidal effect, with a mortality rate of 80% and 70% for A. cornigera and A. purpurea, respectively. Therefore, the optimal conditions for the biosynthesis of CuNPs from A. cornigera and A. purpurea extracts are show in Table 2.

Table 2.
Optimal procedure conditions for the CuNPs synthesis based on statistical estimations after conducting the tests.
The results of the assay that employed the optimal conditions are shown in the Table 2, which shows that mortality caused by CuNPs from A. cornigera and A. purpurea was 90% and 76.6%, respectively, and this was similar to the experimental value of 91.6% and 77.3% for each extract plant (Table 2). Therefore, the predicted and actual results were minimal (minor to 2% in both cases), considering acceptable.
3.3. Determination of Cell Viability
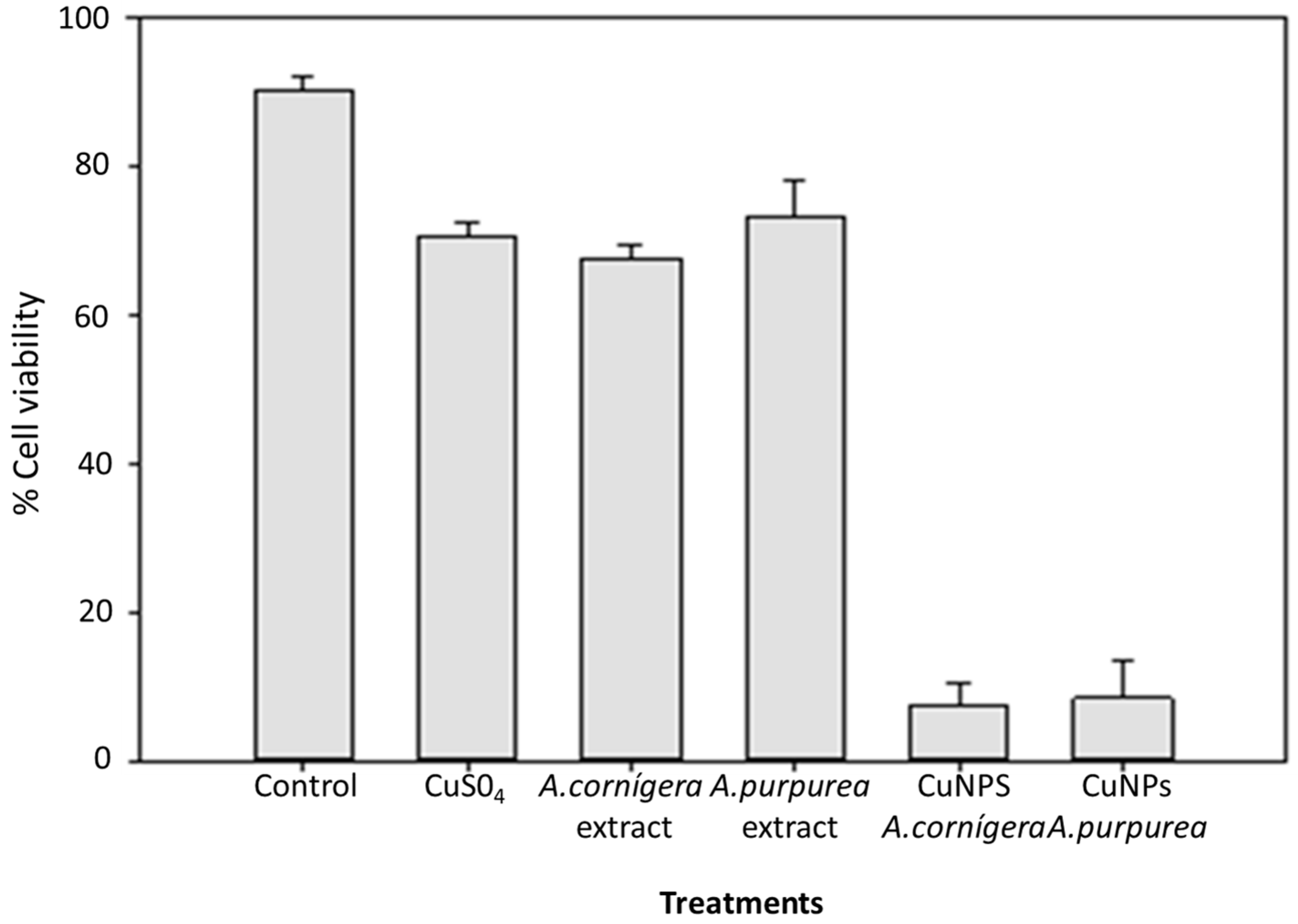
A diverse report showed that Evan’s blue staining technique can be used to assess cell death or membrane damage. The relevance of this technique lies in the fact that the membrane stability is the result of altered environmental conditions, and hence, can be used as a biomarker to assess stress-induced cell damage [23]. The results of cell viability with A. cornigera and A. purpurea CuNPs are shown in Figure 2. The results show that the use of CuNPs biosynthesized under optimal conditions caused a significant reduction in cell viability in insects treated with CuNPs from both extract plants (95% for A. cornigera and 90% for A. purpurea). In contrast, an insignificant reduction in T. castaneum mortality was registered when using the Cu solution and both A. cornigera and A. purpurea extracts after 6 days of exposure. On the other hand, copper and extracts of both plants were not shown to significantly effect the mortality of the insects.
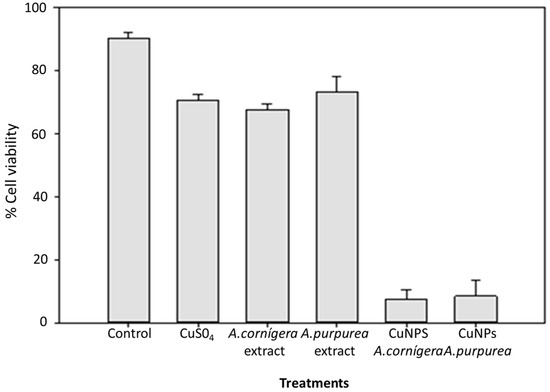
Figure 2.
Effect of CuNPs from A. cornigera and A. purpurea in T. castaneum cell viability after six days of exposure.
3.4. Characterization of Cu-Nanoparticles in Optimal Conditions
3.4.1. Scanning Electron Microscopy
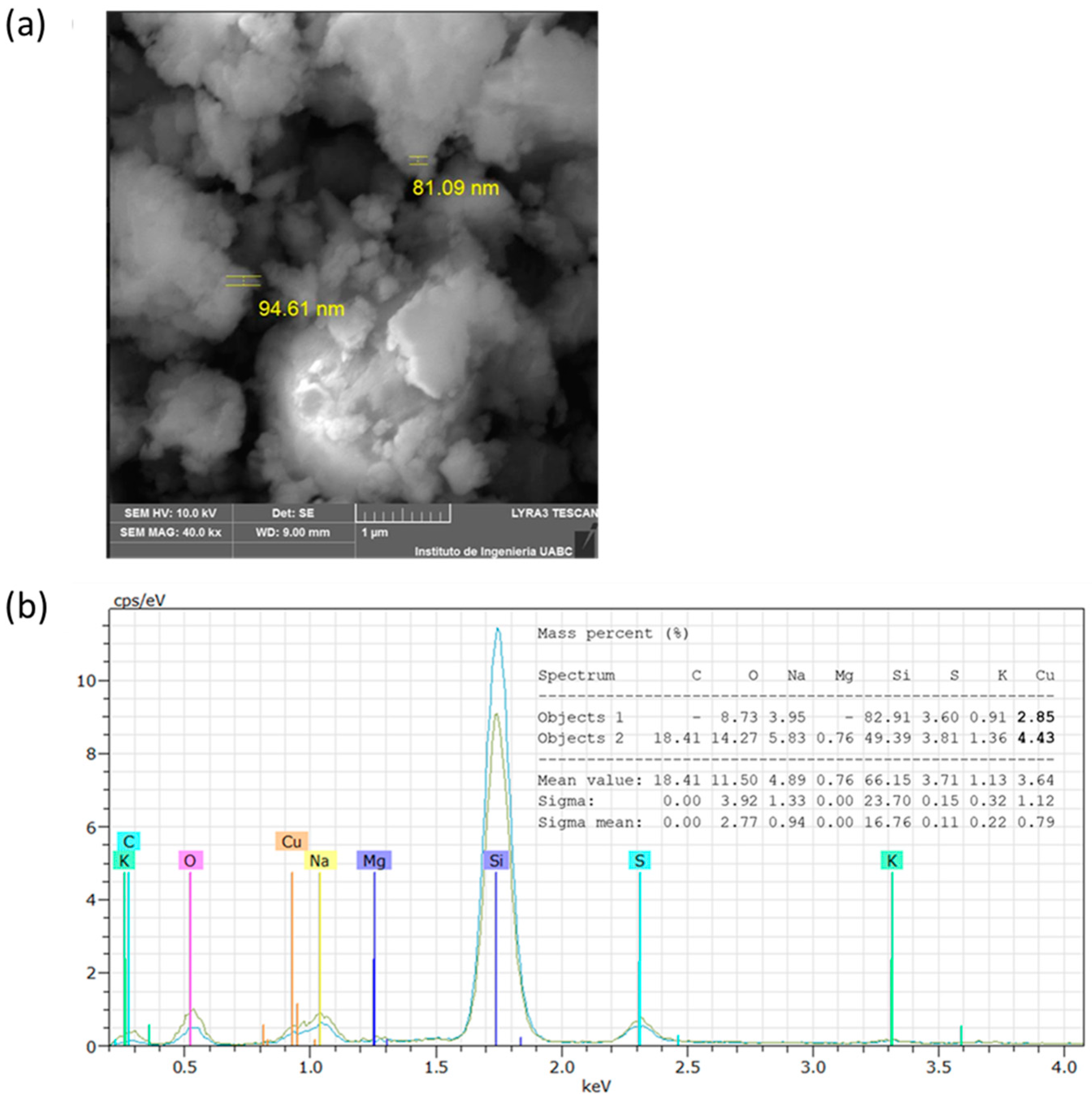
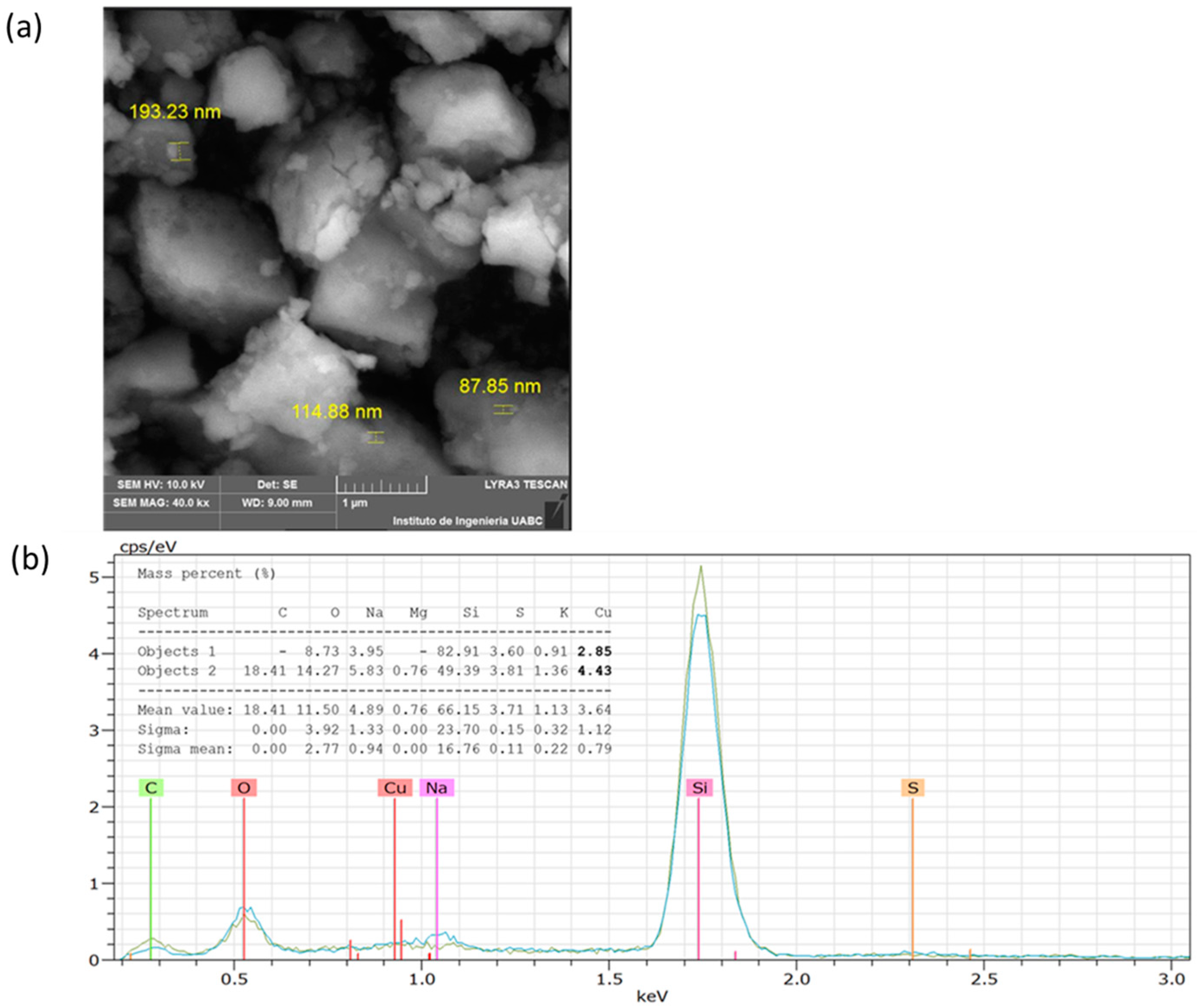
The analysis by SEM showed that the CuNPs from A. cornigera and A. purpurea were non-spherical in shape, with variable sizes for both phytonanoparticles. Our results showed that CuNPs from A. cornigera have sizes ranging from 81–94 nm (Figure 3a). On the other hand, CuNPs from A. purpurea have sizes ranging from 87–193 nm (Figure 4b). The results of SEM in combination with EDX revealed the existence of the Cu metal in both CuNPs. The presence of other elements, such as C, O, Na, Mg, Si, S, and K, were observed, and this may be due to the sample holder and the mineral composition of each plant extract (Figure 3b and Figure 4b).
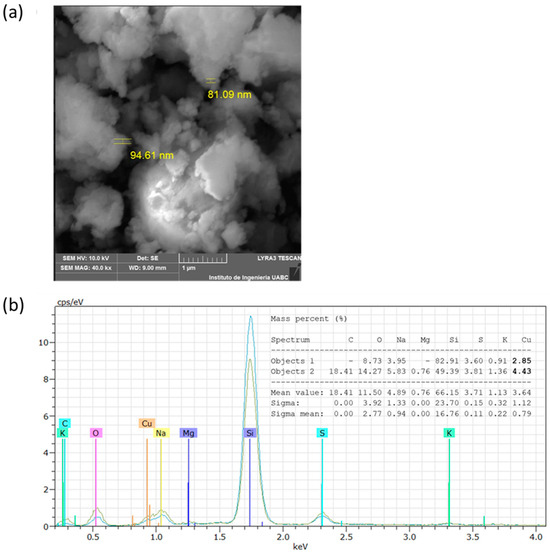
Figure 3.
SEM (a) and EDX (b) analysis of CuNPs from A. cornigera.
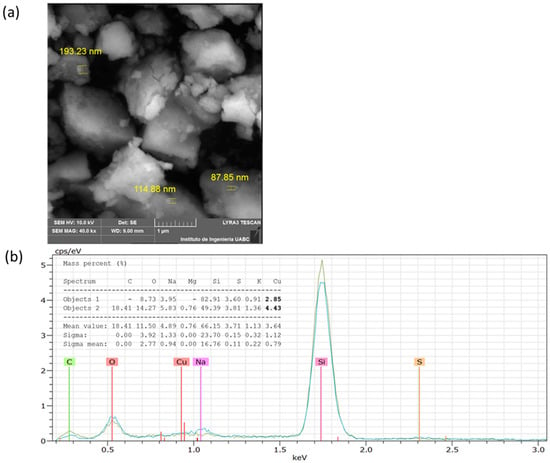
Figure 4.
SEM (a) and EDX (b) analysis of CuNPs from A. purpurea.
3.4.2. Dynamic Light Scattering (DLS)
The particle size in the solution for CuNPs from A. cornigera and CuNPs from A. purpurea is shown in Table 3. Our results showed that CuNPs from both plants tended to form agglomerates of different sizes. According to an analysis of DLS CuNPs from both plants, they exhibited a different pattern by agglomerating, 26.28 nm, when dispersed in water. CuNPs from A. cornigeras showed high instability and a tendency to agglomerate as results of their zeta potential (+9.6 mV) and electrophoretic mobility (0.75 μmcm/(Vs)). In contrast, for synthesized CuNPs from A. purpurea, present a zeta potential negative (−32.7 mv), and 2.55 μmcm/(Vs) demonstrates that the dispersed phase is more stable than CuNPs from A. cornigera (Table 3).

Table 3.
DLS and LDV data from CuNPs from A. cornigera and CuNPs from A. purpurea.
3.5. Determination of Total Phenols and Flavonoids
Total phenolic and flavonoid contents are shown in Table 4. A significant difference in total phenolic content and flavonoids content (p < 0.05) in CuNPs obtained from both plants compared with extract plants were observed. The extracts of A. cornigera show a minor value of content of phenols compared to A. purpurea, but the flavonoids content was higher than A. purpurea. In the case of phenolic total and flavonoids content of CuNPs from both plants, the results not show a significant difference for both CuNPs. Finally, CuNPs from both plants showed a lower content of phenols and flavonoids (p < 0.05) with respect to only aqueous extracts of plants.

Table 4.
Total content of phenols and flavonoids in extracts and CuNPs from A. cornigera and A. purpurea.
4. Discussion
In the present study, the color change that occurred during the synthesis of copper nanoparticles with extracts in each experimental unit confirms the CuNPs formation (Figure 1). In this aspect, the phytochemicals present in A. cornigera and A. purpurea have the capacity of reducing the Cu2+ to Cu0, and encourage the formation of CuNPs with their respective variation in color intensity due to the excitation of surface plasmon vibrations [19,20]. The UV-visible spectra of the CuNPs synthesized from both extracts recorded absorption peaks in the range of 340–460 nm, which coincides with previous studies [24,25]. On the other hand, copper is one essential micronutrient that plays an important role in the growth and development of plants. Recent reports showed that copper can affect the development of insects (e.g., dragonfly larvae) as a result of inhibition of the activity of acetylcholinesterase [26]. On the other hand, recent studies reported that CuNPs have attracted a great deal of attention from all over the world due to their insecticide activity [27]. Rahman et al. [28] mention that copper oxide nanoparticles demonstrated high larvicidal in Spodoptera frugiperda (J.E. Smith). Therefore, studies about optimization of the biosynthesis process are necessary. Recently, copper nanoparticles have been prepared using different extract of plants [29].
To the best of our knowledge, no study has been reported on the green synthesis of copper nanoparticles using A. cornigera and A. purpurea leaves extract. In addition, the green synthesis of copper nanoparticles has not been optimized earlier, as conducted in the present study through the Taguchi optimization technique. The Taguchi design method has been used in different fields of scientific research, including nanotechnology [30]. Authors mention the importance of used of Taguchi optimization method to the synthesis of silver nanoparticles [13]. In our study, the use of the Taguchi L9 (34) orthogonal matrix shows that experiment 6 was the most effective against T. castaneum due to exposure to CuNPs from A. cornigera and A. purpurea, where higher mortality was of 80% and 70%, respectively. In this sense, the variation of temperature, pH, and reaction time has a positive effect on the mortality of T. castaneum. The Taguchi technique has been reported in the optimization of the synthesis of AuNPs using factors such as reducing agent, additive, sonication time, and temperature [31]. Our results demonstrate that it is possible optimize the green synthesis of CuNPs process with greater biological activity; in this case, in T. castaneum mortality, which can be observed in Table 1. The use of Taguchi orthogonal design was similar in mortality of 91% for A. cornigera and 77% for A. purpurea, compared with the experimental mortality of 90% and 76.6%. It was also possible to obtain a difference of less than 2% between the predicted results and those obtained. Zeta potential plays a key role in determining the stability of nanoparticles and colloidal systems [32]. In this sense, the positive values of zeta potential and electrophretic mobility of CuNPs from A. cornigera could explain the decreased effectiveness observed in the mortality of the insect studied, compared to CuNPs from A. purpurea that show negative values of zeta potential. In this sense, nanoparticles that possess negative zeta potentials of −30 mV are considered as a stable colloidal suspension system that prevents nanoparticles aggregation [33]. Therefore, the CuNPs from A. purpurea can be more stable, and have major insecticidal activity, although the high content of compounds phenolic may be more important than flavonoids in the stability of nanoparticles compared to the CuNPs from A. cornigera. Rodriguez et al. [34] mention that the total phenolic compound can be employed as bioinsecticides against Sitophilus zeamais, and their toxic effect is a result of inhibition of acetylcholinesterase (AChE) activity. In this sense, it is possible that the insecticidal effect of CuNPs from both extract plants can be attributed to two possible modes of action: (a) insecticidal action as a result of Cu-nanoparticles that can easily cross epithelial and endothelial cells through transcytosis [34,35] and (b) inhibition of enzymes such as AChE by interaction with phenolic compounds present in CuNPs. In addition, green CuNPs can cause oxidative stress since they can easily advance along the dendrites, axons, and lymphatic vessels of insects [36,37,38]. The difference in insecticidal activity between the Cu-nanoparticles of A. cornigera and A. purpurea could be attributed to variations in the composition of each of the extracts with which they were synthesized, since the secondary metabolites of each of the extracts, in addition to being responsible for the formation of the nanoparticles, surround them, giving them stability and bioactivity.
5. Conclusions
Our results showed the relevance of using the Taguchi design in the formulation of phytonanoparticles of copper from A. cornigera and A. purpurea, two plants of importance in Latin America. Although both CuNPs from these plants showed an important effect on the mortality of Tribolium castaneum, only, CuNPs from A. purpurea had the higher levels of phenolic compounds and major insecticidal effect compared to nanoparticles obtained from A. cornigera extracts. The exact mechanism of action of CuNPs obtained in this study is not clear; hence, future studies are necessary to evaluate their effect in the inhibition of enzymes in relation to the resistance to insecticides in this insect, as previously shown through their evaluation in field studies.
Author Contributions
Conceptualization, R.S.T. and D.G.-M.; methodology, R.S.T., F.G.-M. and B.V.-S.; software, E.B.-P., O.G.-J., A.B.-C. and O.T.-C.; formal analysis, E.B.-P., B.V.-S. and D.G.-M.; investigation, D.G.-M., B.V.-S. and E.B.-P.; writing—original draft preparation, B.V.-S. and D.G.-M.; writing—review and editing, E.B.-P., A.B.-C., O.T.-C. and D.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to Conahcyt for the fellowship of Rogelio Solorzano Toala.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pimentel, D. Pesticides and Pest control. In Integrated Pest Management: Innovation-Development Process; Springer Science: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Dhaliwall, G.S.; Jindal, V.; Dhawan, A.K. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2010, 37, 1–7. [Google Scholar]
- Stathas, I.G.; Sakellaridis, A.C.; Papadelli, M.; Kapolos, J.; Papadimitriou, K.; Stathas, G.J. The Effects of Insect Infestation on Stored Agricultural Products and the Quality of Food. Foods 2023, 12, 2046. [Google Scholar] [CrossRef] [PubMed]
- Hiruy, B.; Getu, E. Host type and textures on the survival of Tribolium castaneum (Coleoptera: Tenebrionidae) parental and filial generations. J. Entomol. Zool. 2018, 6, 622–626. [Google Scholar]
- Duarte, S.; Magro, A.; Tomás, J.; Hilário, C.; Ferreira, R.B.; Carvalho, M.O. Antifungal Activity of Benzoquinones Produced by Tribolium castaneum in Maize-Associated Fungi. Insects 2022, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, M.; Wang, Y.; Song, W.; Tang, P. Identification and functional analysis of cytochrome P450 CYP346 family genes associated with phosphine resistance in Tribolium castaneum. Pestic. Biochem. Phys. 2020, 168, 104622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [PubMed]
- Alif, A.A.S.; Thangapandiyan, S. Comparative bioassay of silver nanoparticles and malathion on infestation of red flour beetle, Tribolium castaneum. J. Basic Appl. Zool. 2019, 80, 55. [Google Scholar]
- Mukhtar, M.M.; Mustapha, M.A.; Aliyu, M.; Ibrahim, S.S. Multiple Pesticide Resistance in Rust-Red Flour Beetle (Tribolium castaneum, Herbst 1797) from Northern Nigeria Is Probably Driven by Metabolic Mechanisms. Agrochemicals 2023, 2, 170–180. [Google Scholar] [CrossRef]
- Rauf, A.; Wilkins, R.M. Malathion-resistant Tribolium castaneum has enhanced response to oxidative stress, immunity, and fitness. Pestic. Biochem. Physiol. 2022, 184, 105128. [Google Scholar] [CrossRef]
- Julio, A.H.; Gigliolli, A.A.; Cardoso, K.A.; Drosdoski, S.D.; Kulza, R.A.; Seixas, F.A.; Ruvolo-Takasusuki, M.C.; de Souza, C.G.; Lapenta, A.S. Multiple resistance to pirimiphos-methyl and bifenthrin in Tribolium castaneum involves the activity of lipases, esterases, and laccase2. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 195, 27–43. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, D.; Valdez-Salas, B.; Bernardo-Mazariegos, E.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Ruíz-Valdiviezo, V.; Rodríguez-Hernández, L.; Sanchez-Viveros, G. Influence of Monometallic and Bimetallic Phytonanoparticles on Physiological Status of Mezquite. Open Life Sci. 2019, 14, 62–68. [Google Scholar] [CrossRef]
- Subekti, N.; Saputri, R. The application of Cinnamomum aromaticum nanoparticle and chlorpyrifos for controlling Tribolium castaneum. AIP Conf. Proc. 2019, 2155, 020018. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; N. Maodaa, S.; Allam, A.A.; Elshaer, N. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Murthy, H.A.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic synthesis of metal/metal oxide nanoparticles for degradation of dyes. J. Renew. Mater. 2021, 10, 1911–1930. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Valdez-Salas, B.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Grimaldo-Juarez, O.; González-Mendoza, D. Silver nanoparticles from Prosopis glandulosa and their potential application as biocontrol of Acinetobacter calcoaceticus and Bacillus cereus. Chem. Speciat. Bioavailab. 2016, 29, 1–5. [Google Scholar] [CrossRef]
- Rajendran, M.; Muthukumarasamy, E.; Paulraj, B. Antioxidant Assay of Gold and silver nanoparticles from edible basidiomycetes mushroom fungi. Free Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- León-Jiménez, E.; Valdés-Salas, B.; González-Mendoza, D.; Tzintzun-Camacho, O. Synthesis and insecticide activity of Cunanoparticles from Prosopis juliflora (Sw) DC and Pluchea sericea (Nutt) on Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Rev. Soc. Entomol. Arg. 2019, 78, 1851–7471. [Google Scholar] [CrossRef]
- Mendez-Trujillo, V.; Valdez-Salas, B.; Carrillo-Beltran, M.; Curiel-Alvarez, M.A.; Tzintzun-Camacho, O.; Ceceña-Duran, C.; Gonzalez-Mendoza, D. Green Synthesis of Bimetallic nanoparticles from Prosopis juliflora (sw) dc., and its effect against cotton mealybug, Phenacoccus solenopsis (hemiptera: Pseudococcidae). Phyton Int. J. Exp. Bot. 2019, 88, 269–275. [Google Scholar] [CrossRef]
- Halimi, M.; Nasrabadi, M.; Soleamani, N.; Rouhani, N. Green synthesis of nanosilver particles from extract of Dracocephalum lindbergii. Asian J. Nanosci. Mater. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Ullah, S.; Shujaat, N.; Khan, R.U.; Khan, R.A.; Bilal, H.; Khan, M.; Bilal, H.; Khan, I.U.; Ahmad, M.; Khan, M.; et al. Ruellia nudiflora-mediated biological synthesis of silver nanoparticles and their potential antioxidant, antifungal and antibacterial applications against selected multidrug resistant bacteria. Pak-Euro J. Med. Life Sci. 2021, 4, 229–239. [Google Scholar]
- Amer, N.R.; Lawler, S.P.; Zohdy, N.M.; Younes, A.; ElSayed, W.M.; Wos, G.; Abdelrazek, S.; Omer, H.; Connon, R.E. Copper Exposure Affects Anti-Predatory Behaviour and Acetylcholinesterase Levels in Culex pipiens (Diptera, Culicidae). Insects 2022, 13, 1151. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Pittarate, S.; Perumal, V.; Rajula, J.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response. Insects 2022, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-T.; Wang, J.-C. Properties Related to the HLB Value of Hybrid Thermoelectric Nanofluids at Different Temperatures. Polymers 2024, 16, 509. [Google Scholar] [CrossRef]
- Korukonda, J.R.; Korumilli, T.O. Au nanoparticles by instant green chemicals and optimising key synthesis parameters using Taguchi method. Micro Nano Lett. 2019, 14, 1198–1203. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Mahmoud, S.Y.; Farrag, E.S. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Krameria sp. Root Extract and Its Applications. Molecules 2023, 28, 4629. [Google Scholar] [CrossRef]
- Prathap, N.; Dravid, N.; Kaarmukhilnilavan, S.R.; Shivakumar, M.S.; Venkatesan, S.; Shaik, M.R.; Shaik, B. Copper Oxide Nanoparticles Synthesized from Indigofera linnaei Ali and This Plant’s Biological Applications. Inorganics 2023, 11, 462. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.L.; Zygadlo, J.A.; Dambolena, J.; Zunino, M. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Photocatalytic and antibacterial activityof CuO nanoparticlesbiosynthesized using Verbascum thapsus leaves extract. Optik 2020, 204, 164230. [Google Scholar] [CrossRef]
- Avis Tresa, B.; Antony, R. Green synthesis of silver doped nano metal oxides of zinc & copper for antibacterial properties, adsorption, catalytic hydrogenation & photodegradation of aromatics. J. Environ. Chem. Eng. 2019, 7, 102840. [Google Scholar]
- Chan-Chan, L.H.; Martínez-Barbosa, M.E.; Vásquez-Alfaro, M.M.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Lagarda-Diaz, I.; Maldonado, A. Segmented Poly(urea) urethane Nanoparticles: Size Optimization Using Taguchi Experimental Design and Nanoprecipitation Method. Curr. Nanosci. 2021, 17, 70–80. [Google Scholar] [CrossRef]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2019, 44, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).