Valorization of (Bio)Ethanol over MoO3/(WO3-ZrO2) Sol-Gel-like Catalysts
Abstract
1. Introduction
2. Experimental
2.1. Preparation and Characterization of the Catalysts
2.2. Catalytic Tests of Dehydration Dehydrogenation of Ethanol
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Posada, J.A.; Patel, A.D.; Roes, A.; Blok, K.; Faaij, A.P.; Patel, M.K. Potential of bioethanol as a chemical building block for biorefineries: Preliminary sustainability assessment of 12 bioethanol-based products. Bioresour. Technol. 2013, 135, 490–499. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Dagle, V.L.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- Frosi, M.; Tripodi, A.; Conte, F.; Ramis, G.; Mahinpey, N.; Rossetti, I. Ethylene from renewable ethanol: Process optimization and economic feasibility assessment. J. Ind. Eng. Chem. 2021, 104, 272–285. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.-C.; Sun, D.-H.; Li, W.-C.; Lv, W.-L.; Wang, J.; Liang, Y.-Q.; Lu, A.-H. Catalytic Conversion of Ethanol to Oxygen-Containing Value-Added Chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Shinoharaa, Y.; Nakajimaa, T.; Suzukia, S.; Mishimab, S.; Ishikawac, H. A Computational Chemical Investigation of the Dehydration and Dehydrogenation of Ethanol on Oxide Catalysts. J. Chem. Softw. 1998, 4, 89–100. Available online: https://web.archive.org/web/20181102054541id_/https://www.jstage.jst.go.jp/article/jchemsoft1992/4/3/4_3_89/_pdf (accessed on 10 December 2023). [CrossRef][Green Version]
- Mabate, T.P.; Maqunga, N.P.; Ntshibongo, S.; Maumela, M.; Bingwa, N. Metal oxides and their roles in heterogeneous catalysis: Special emphasis on synthesis protocols, intrinsic properties, and their influence in transfer hydrogenation reactions. SN Appl. Sci. 2023, 5, 196. [Google Scholar] [CrossRef]
- Phung, T.K.; Hernández, L.P.; Busca, G. Conversion of ethanol over transition metal oxide catalysts: Effect of tungsta addition on catalytic behaviour of titania and zirconia. Appl. Catal. A Gen. 2015, 489, 180–187. [Google Scholar] [CrossRef]
- Zhou, W.; Soultanidis, N.; Xu, H.; Wong, M.S.; Neurock, M.; Kiely, C.J.; Wachs, I.E. Nature of Catalytically Active Sites in the Supported WO3/ZrO2 Solid Acid System: A Current Perspective. ACS Catal. 2017, 7, 2181–2198. [Google Scholar] [CrossRef]
- Rousseau, R.; Dixon, D.A.; Kay, B.D.; Dohnálek, Z. Dehydration, dehydrogenation, and condensation of alcohols on supported oxide catalysts based on cyclic (WO3)3 and (MoO3)3 clusters. Chem. Soc. Rev. 2014, 43, 7664–7680. [Google Scholar] [CrossRef] [PubMed]
- Chuklina, S.; Chuklina, S.; Zhukova, A.; Zhukova, A.; Fionov, Y.; Fionov, Y.; Kadyko, M.; Kadyko, M.; Fionov, A.; Fionov, A.; et al. Selectivity of Ethanol Conversion on Al/Zr/Ce Mixed Oxides: Dehydration and Dehydrogenation Pathways Based on Surface Acidity Properties. ChemistrySelect 2022, 7, e202203031. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Selective Catalytic Oxidation of Ethanol to Acetic Acid on Dispersed Mo-V-Nb Mixed Oxides. Chem.–A Eur. J. 2007, 13, 9324–9330. [Google Scholar] [CrossRef]
- Xiang, N.; Xu, P.; Ran, N.; Ye, T. Production of acetic acid from ethanol over CuCr catalysts via dehydrogenation-(aldehyde–water shift) reaction. RSC Adv. 2017, 7, 38586–38593. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in catalytic dehydrogenation of ethanol to acetaldehyde. Green Chem. 2021, 23, 7902–7916. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Dimitrov, L.D.; Oliveira, M.C.-R.; Zǎvoianu, R.; Fernandes, A.; Portela, M.F. Oxidative dehydrogenation of butane over substoichiometric magnesium vanadate catalysts prepared by citrate route. J. Non-Crystalline Solids 2010, 356, 1488–1497. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kourieh, R.; Bennici, S.; Marzo, M.; Gervasini, A.; Auroux, A. Investigation of the WO3/ZrO2 surface acidic properties for the aqueous hydrolysis of cellobiose. Catal. Commun. 2012, 19, 119–126. [Google Scholar] [CrossRef]
- Signoretto, M.; Scarpa, M.; Pinna, F.; Strukul, G.; Canton, P.; Benedetti, A. WO3/ZrO2 catalysts by sol–gel processing. J. Non-Cryst. Solids 1998, 225, 178–183. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, S.; Achariya, A.; Pramanik, P. A novel sol–gel synthesis of mesoporous ZrO2–MoO3/WO3 mixed oxides. Microporous Mesoporous Mater. 2008, 115, 426–431. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Zhang, Y.; Wei, Y.; Fang, W.; Yang, Y. Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts. J. Mol. Catal. A Chem. 2012, 365, 60–65. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Lv, L.; Shao, J.; Du, Y.; Li, Y.; Chang, W. Enhanced triethylamine sensing characteristics of In-doped WO3 cubic nanoblocks at low operating temperature. Vacuum 2023, 218, 112640. [Google Scholar] [CrossRef]
- Zhou, H.; Zou, X.; Zhang, K.; Sun, P.; Islam, S.; Gong, J.; Zhang, Y.; Yang, J. Molybdenum–Tungsten Mixed Oxide Deposited into Titanium Dioxide Nanotube Arrays for Ultrahigh Rate Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 18699–18709. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Brown, H.M.; Marin-Flores, O.G.; Bays, J.T.; Karim, A.M.; Wang, Y. Aqueous phase hydrodeoxygenation of polyols over Pd/WO3-ZrO2: Role of Pd-WO3 interaction and hydrodeoxygenation pathway. Catal. Today 2016, 269, 103–109. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Gao, J.; Fu, Q.; Tang, Y. Structure Characterization of W03/Zr02 Catalysts by Raman Spectroscopy. J. Raman Spectrosc. 1996, 27, 549–554. [Google Scholar] [CrossRef]
- Kondrachova, L.; Hahn, B.P.; Vijayaraghavan, G.; Williams, R.D.; Stevenson, K.J. Cathodic Electrodeposition of Mixed Molybdenum Tungsten Oxides from Peroxo-polymolybdotungstate Solutions. Langmuir 2006, 22, 10490–10498. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shin, C.-H.; Suh, Y.-W. Higher Brønsted acidity of WOx/ZrO2 catalysts prepared using a high-surface-area zirconium oxyhydroxide. Mol. Catal. 2017, 438, 272–279. [Google Scholar] [CrossRef]
- Chan, X.; Akter, N.; Yang, P.; Ooi, C.; James, A.; Boscoboinik, J.A.; Parise, J.B.; Kim, T. Fundamental study of furfuryl alcohol dehydration reaction over molybdenum oxide catalyst. Mol. Catal. 2019, 466, 19–25. [Google Scholar] [CrossRef]
- Hahn, T.; Bentrup, U.; Armbrüster, M.; Kondratenko, E.V.; Linke, D. The Enhancing Effect of Brønsted Acidity of Supported MoOxSpecies on their Activity and Selectivity in Ethylene/trans-2-Butene Metathesis. ChemCatChem 2014, 6, 1664–1672. [Google Scholar] [CrossRef]
- Barton, D.G.; Soled, S.L.; Iglesia, E. Solid acid catalysts based on supported tungsten oxides. Top. Catal. 1998, 6, 87–99. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Rijo, B.; Kiennemann, A.; Portela, M.F. Methanol oxidation over iron molybdate catalysts. Main and side reactions kinetics. Appl. Catal. A Gen. 2023, 658, 119118. [Google Scholar] [CrossRef]



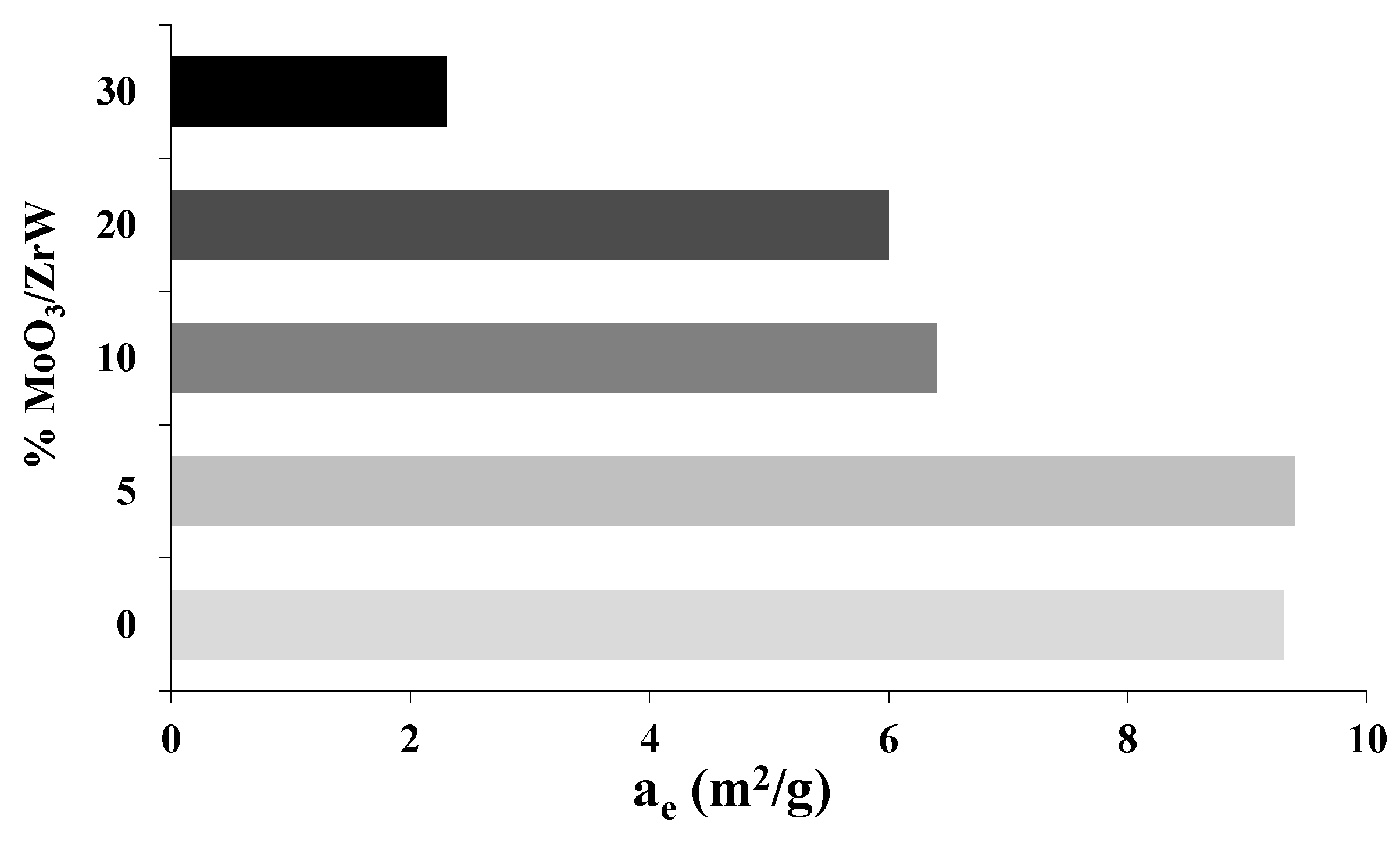
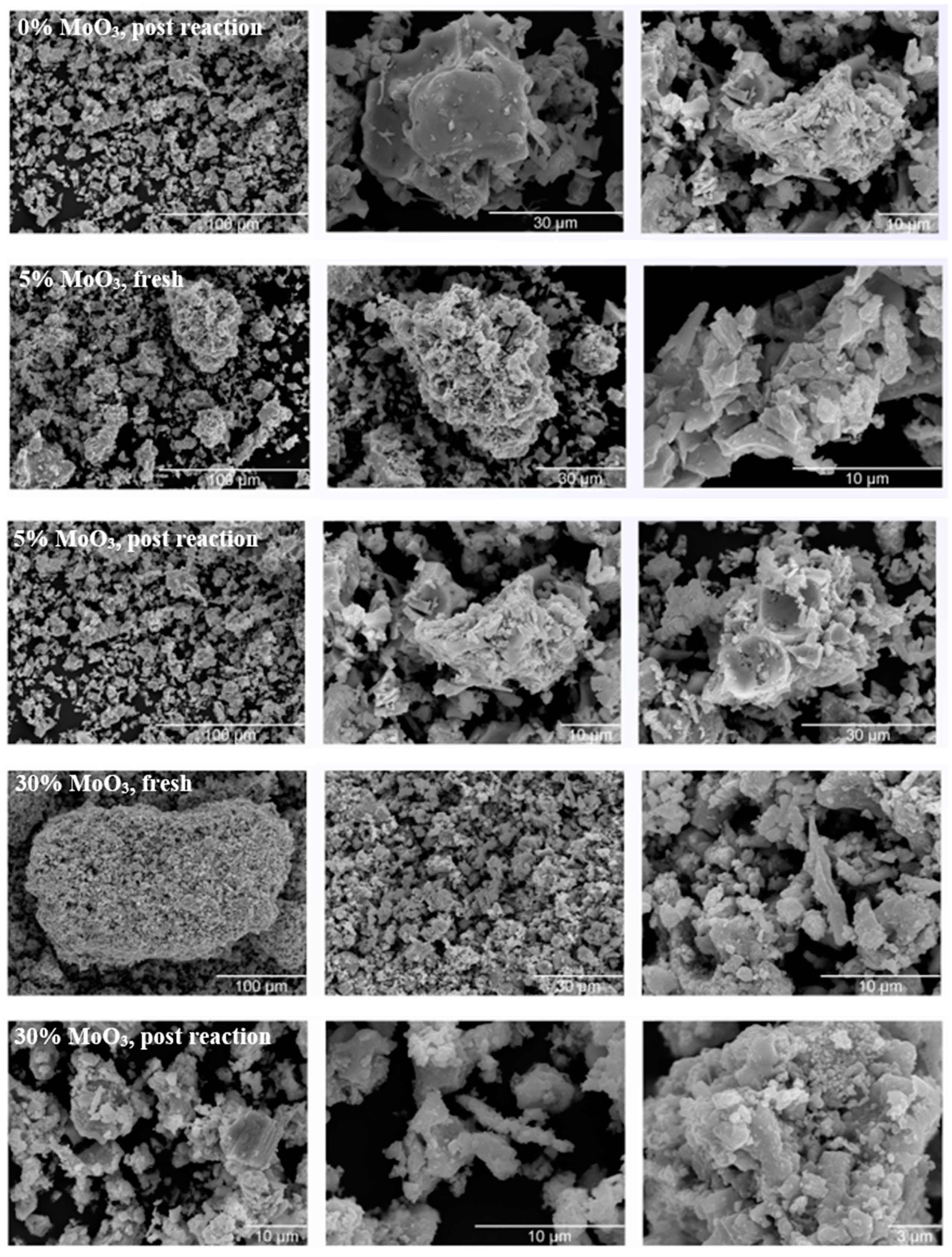
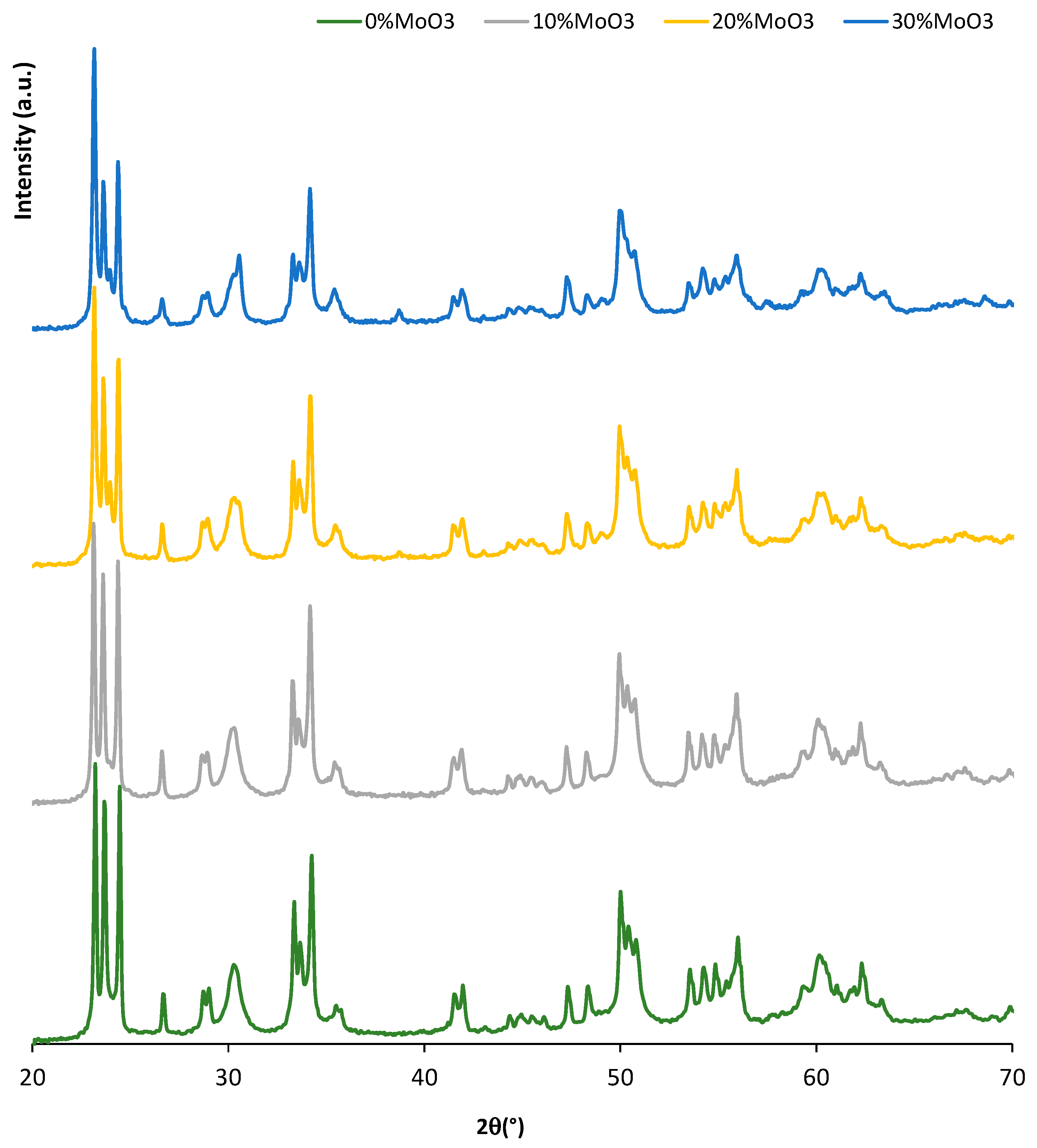
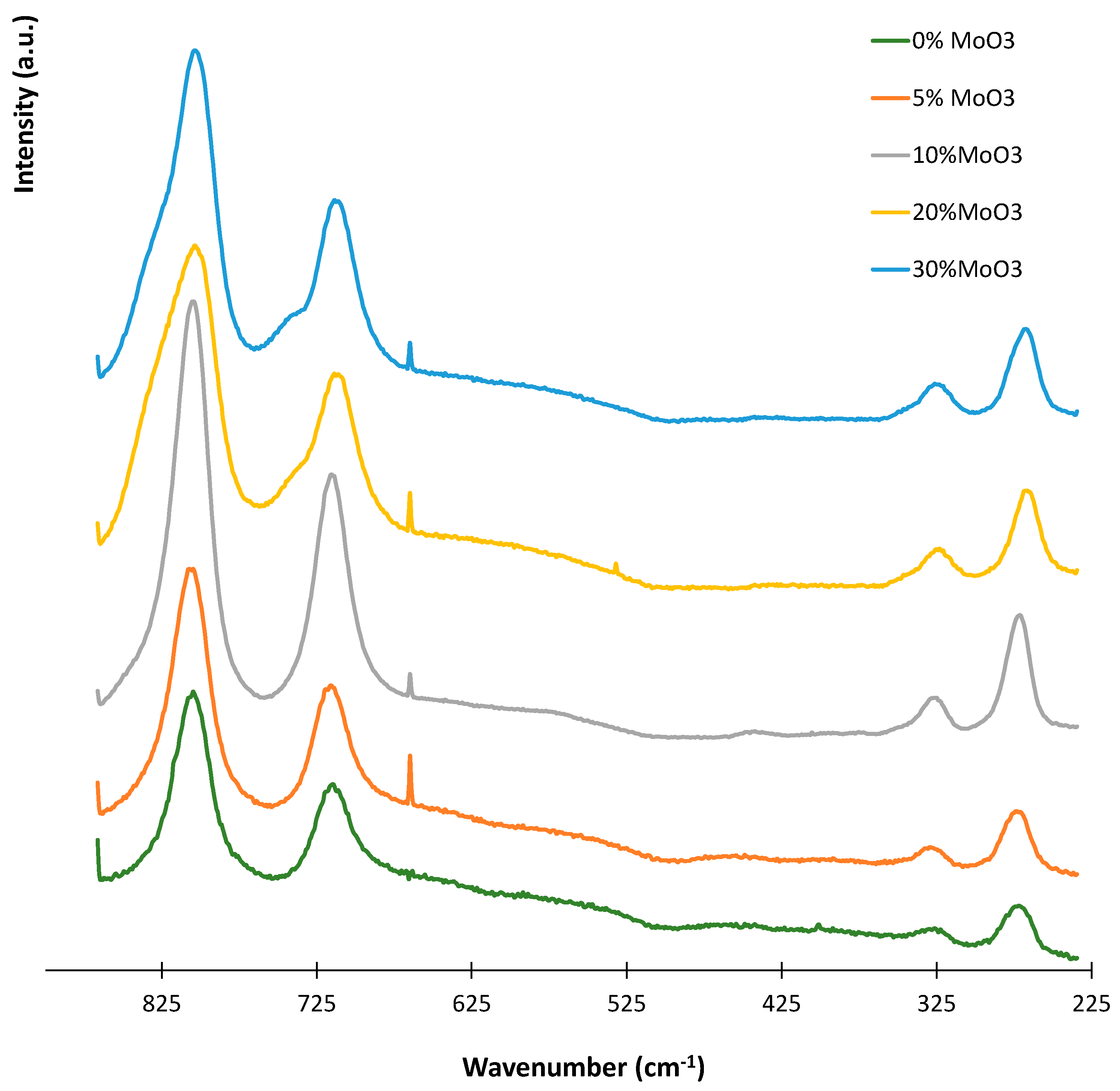
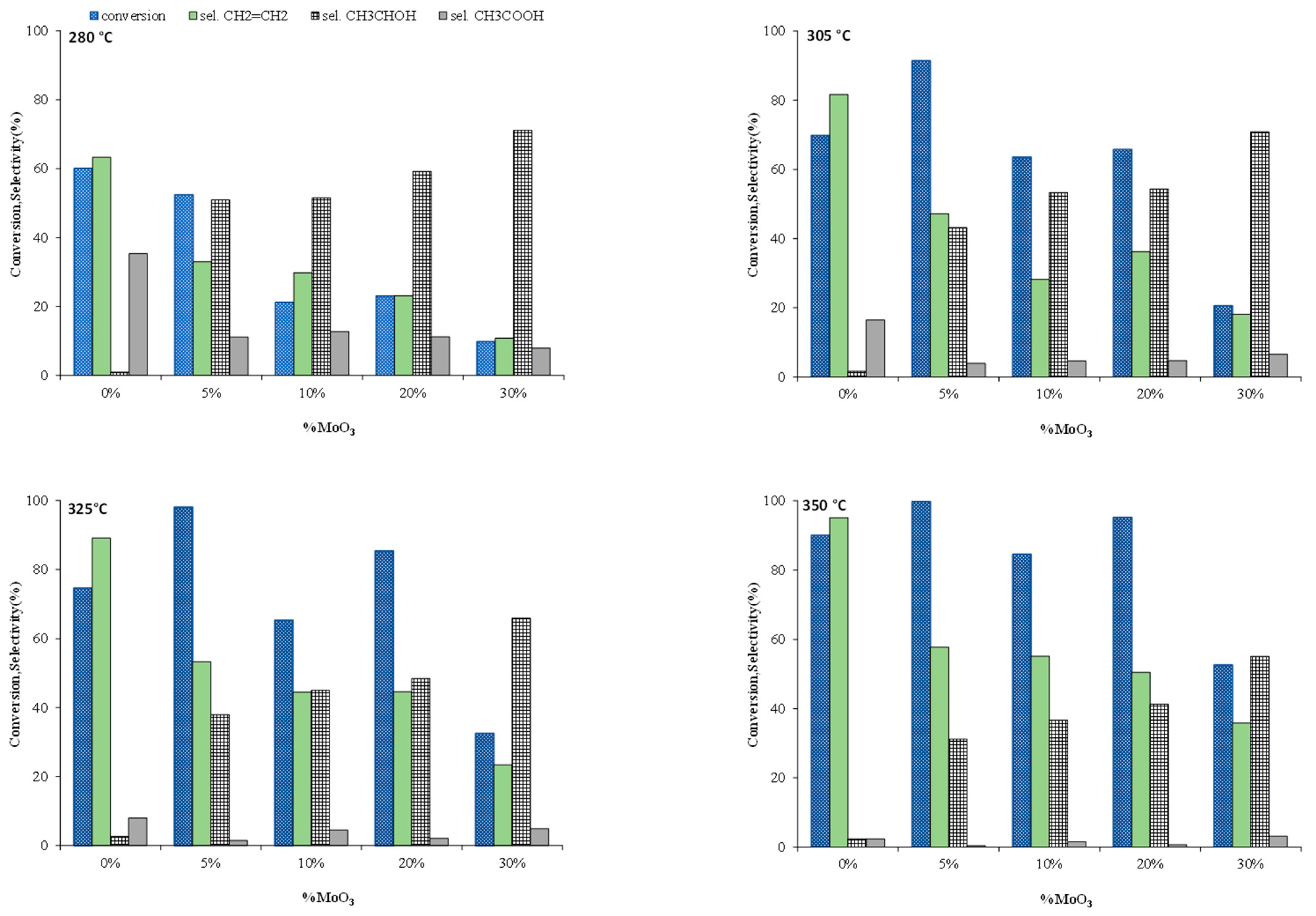
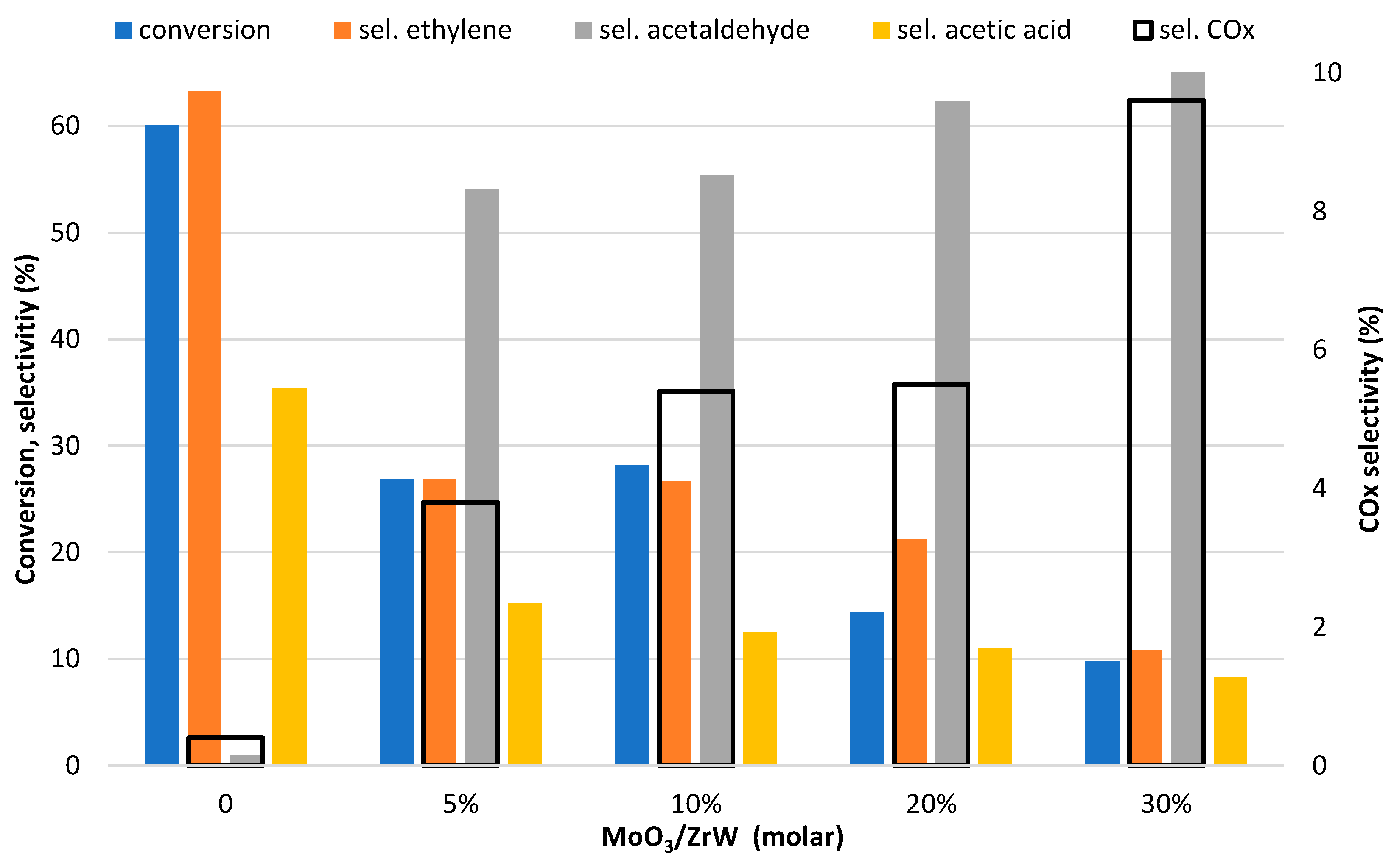

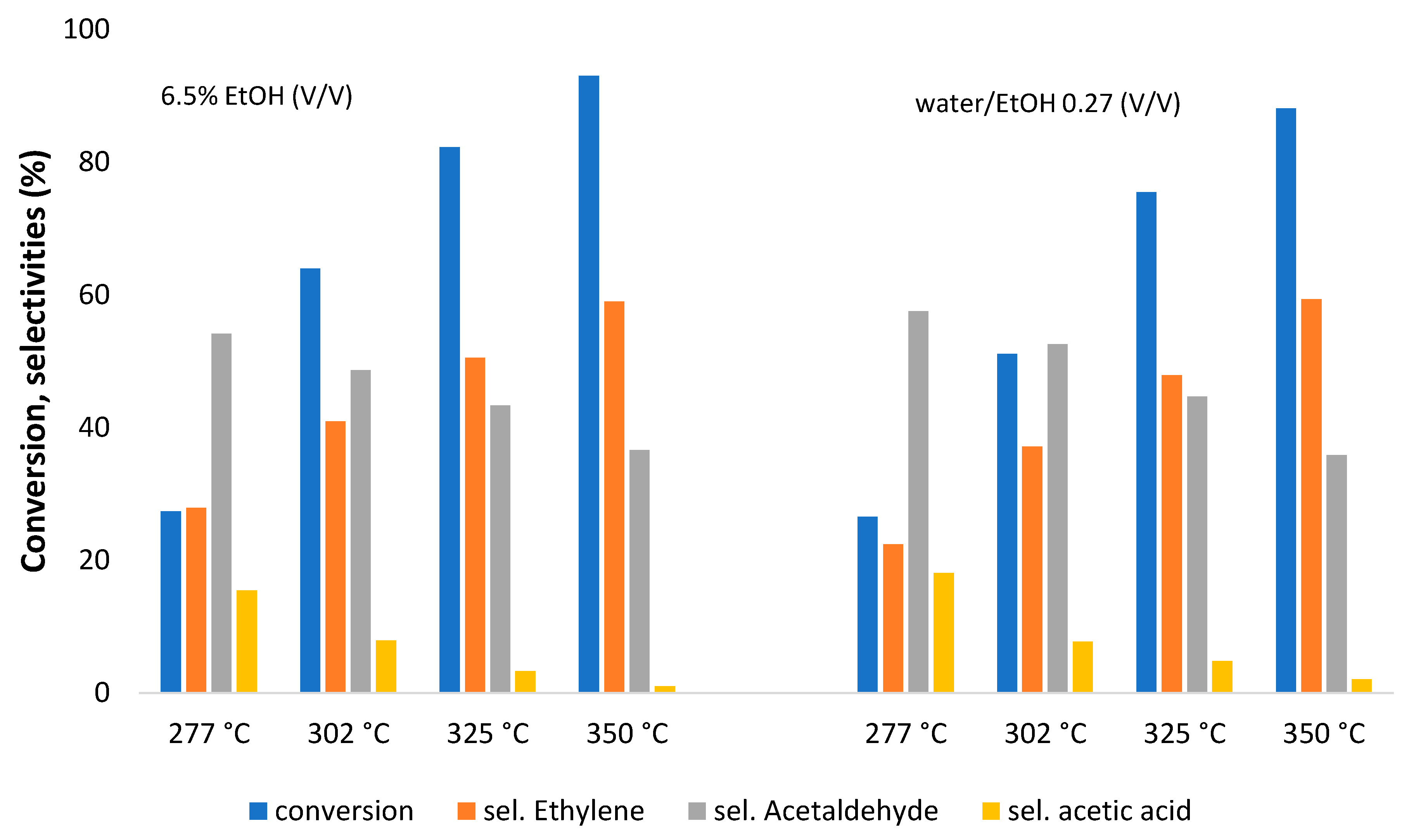
| Metal Oxide | Dehydration (%) | Dehydrogenation (%) |
|---|---|---|
| ThO2 | 100 | trace |
| Al2O3 | 98.5 | 1.5 |
| W2O4 | 98.5 | 1.5 |
| Cr2O3 | 91 | 9 |
| SiO2 | 84 | 16 |
| TiO2 | 63 | 37 |
| BeO | 45 | 55 |
| ZrO2 | 45 | 55 |
| UO2 | 24 | 76 |
| Mo2O5 | 23 | 77 |
| Fe2O3 | 14 | 86 |
| V2O5 | 9 | 91 |
| ZnO | 5 | 95 |
| MnO | 0 | 100 |
| SnO | 0 | 100 |
| CdO | 0 | 100 |
| Mn3O5 | 0 | 100 |
| MgO | 0 | 100 |
| Catalysts | |||||||
|---|---|---|---|---|---|---|---|
| Atomic (%) | 0% MoO3 | 5% MoO3 | 30% MoO3 | ||||
| Point | #1 | #2 | #3 | #1 | #2 | #3 | #1 |
| Zr | 46.3 | 42.6 | 28.2 * | 25.0 | 12.0 | 12.2 | 28.4 |
| W | 53.7 | 57.4 | 71.8 * | 67.5 | 78.8 | 77.3 | 27.3 |
| Mo | 0.0 | 0.0 | 0.0 | 7.5 | 9.2 | 10.5 | 44.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares Dias, A.P.; Rijo, B.; Costa Pereira, M.F.; Zăvoianu, R.; Pavel, O.D. Valorization of (Bio)Ethanol over MoO3/(WO3-ZrO2) Sol-Gel-like Catalysts. Reactions 2024, 5, 260-273. https://doi.org/10.3390/reactions5010012
Soares Dias AP, Rijo B, Costa Pereira MF, Zăvoianu R, Pavel OD. Valorization of (Bio)Ethanol over MoO3/(WO3-ZrO2) Sol-Gel-like Catalysts. Reactions. 2024; 5(1):260-273. https://doi.org/10.3390/reactions5010012
Chicago/Turabian StyleSoares Dias, Ana Paula, Bruna Rijo, Manuel Francisco Costa Pereira, Rodica Zăvoianu, and Octavian Dumitru Pavel. 2024. "Valorization of (Bio)Ethanol over MoO3/(WO3-ZrO2) Sol-Gel-like Catalysts" Reactions 5, no. 1: 260-273. https://doi.org/10.3390/reactions5010012
APA StyleSoares Dias, A. P., Rijo, B., Costa Pereira, M. F., Zăvoianu, R., & Pavel, O. D. (2024). Valorization of (Bio)Ethanol over MoO3/(WO3-ZrO2) Sol-Gel-like Catalysts. Reactions, 5(1), 260-273. https://doi.org/10.3390/reactions5010012











