An Infrared and Thermal Decomposition Study on Solid Deposits Originating from Heavy-Duty Diesel SCR Urea Injection Fluids
Abstract
1. Introduction
2. Materials and Methods
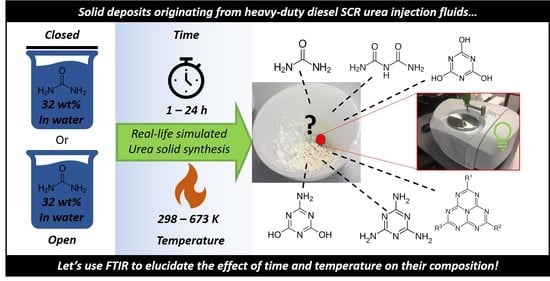
2.1. Deposit Synthesis
2.2. FTIR Analysis
2.3. TPD Experiments
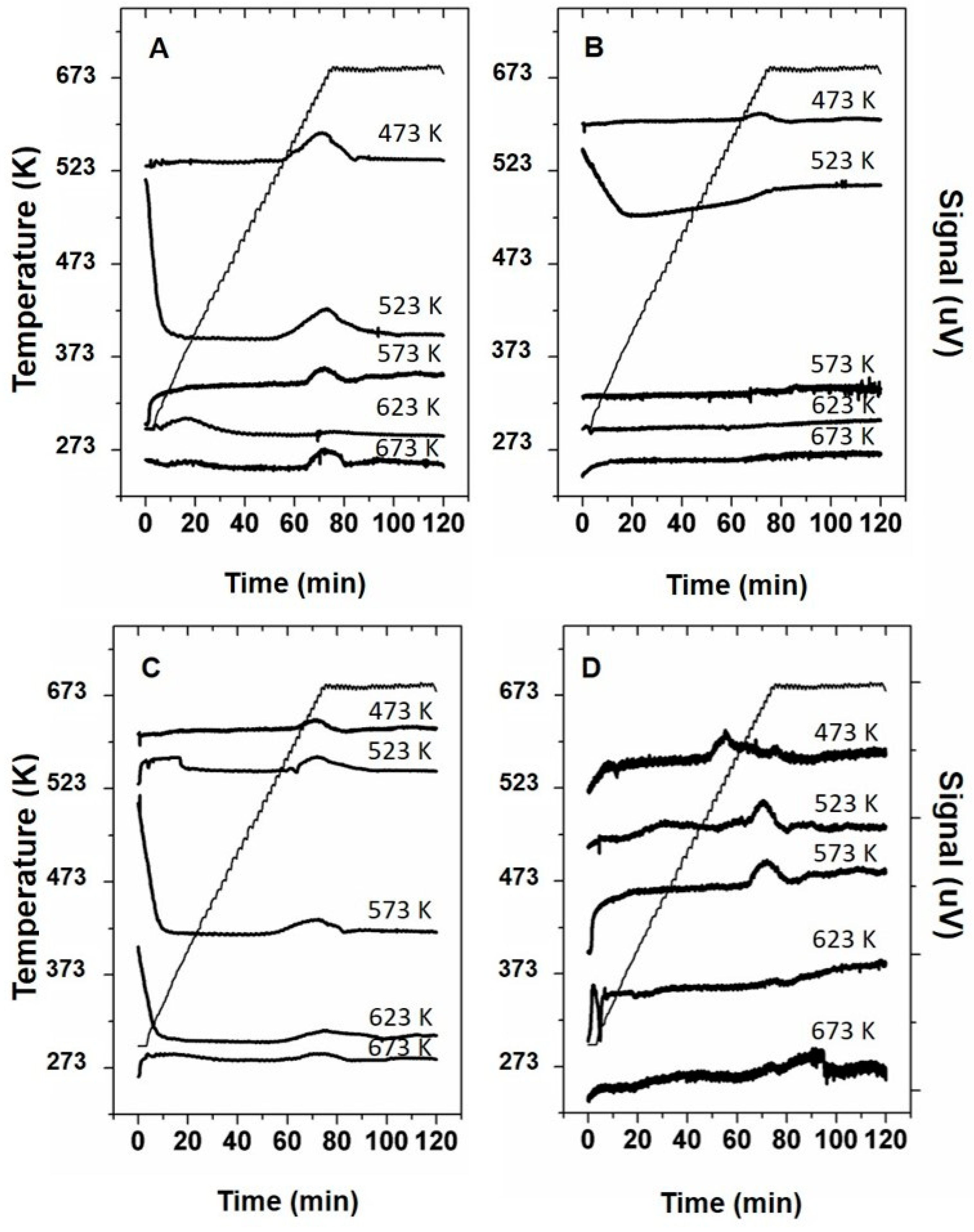
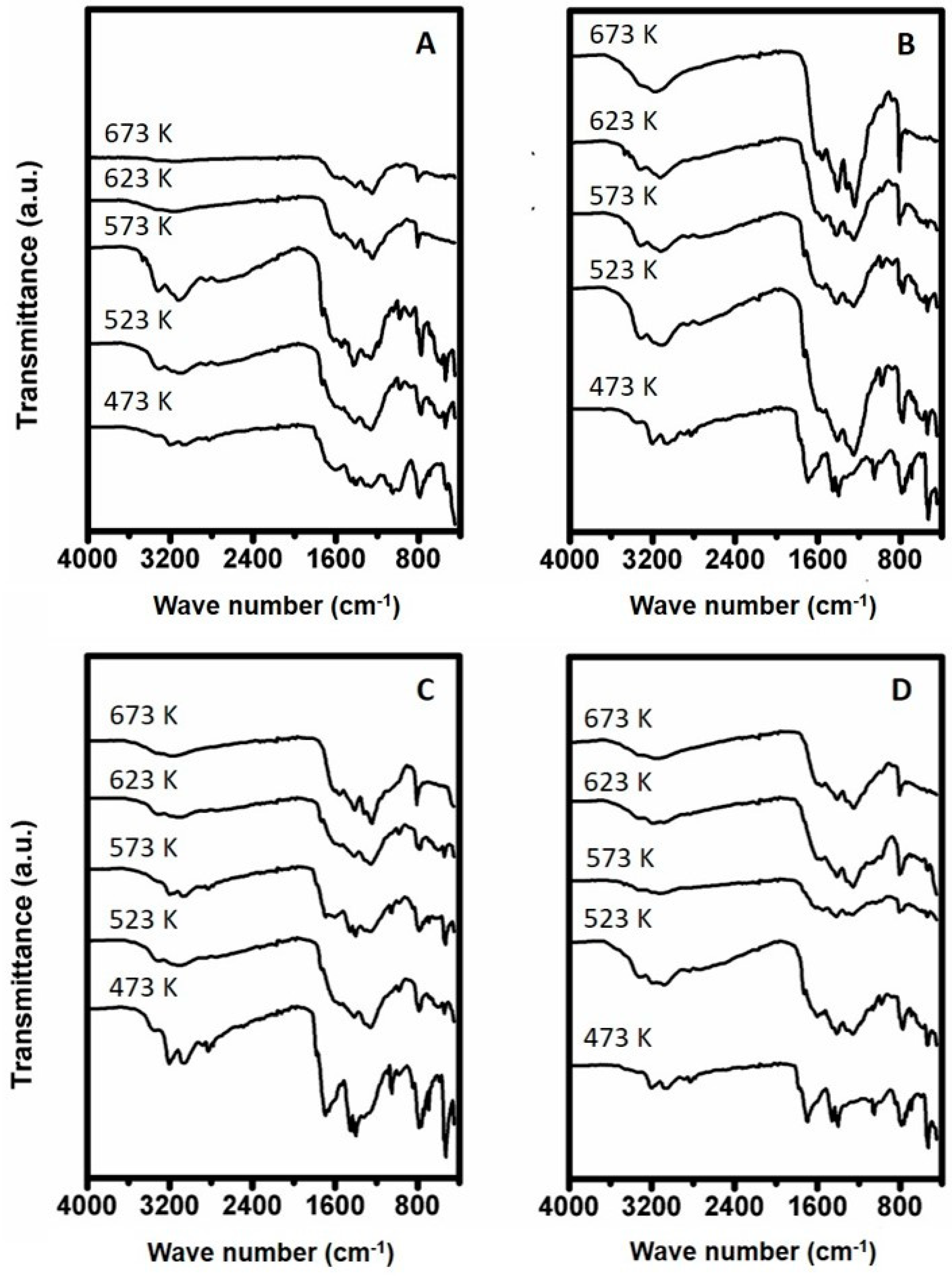
3. Results
3.1. FTIR Analysis of Synthesized Compounds
3.1.1. Urea Deposits Prepared Below and at 423 K
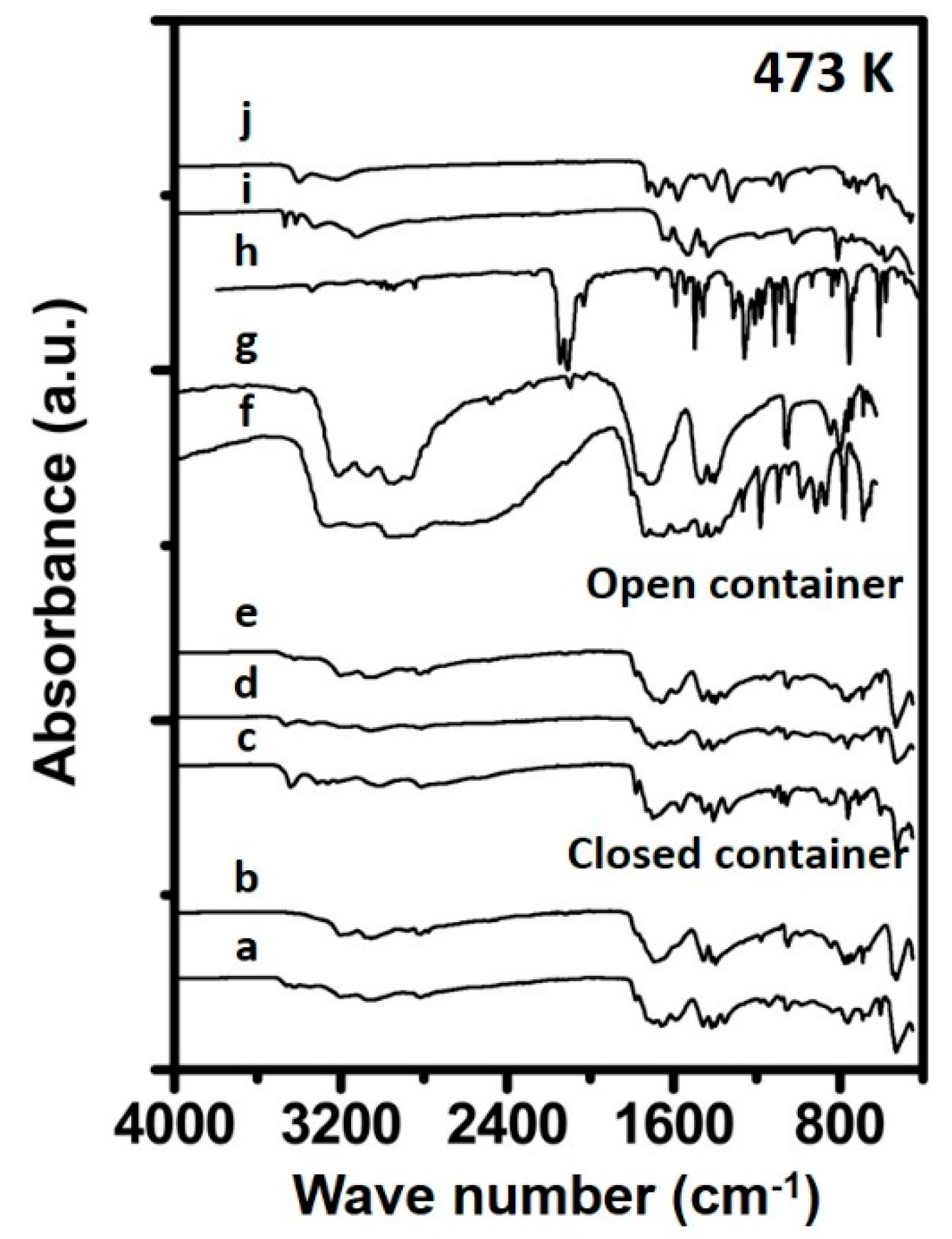
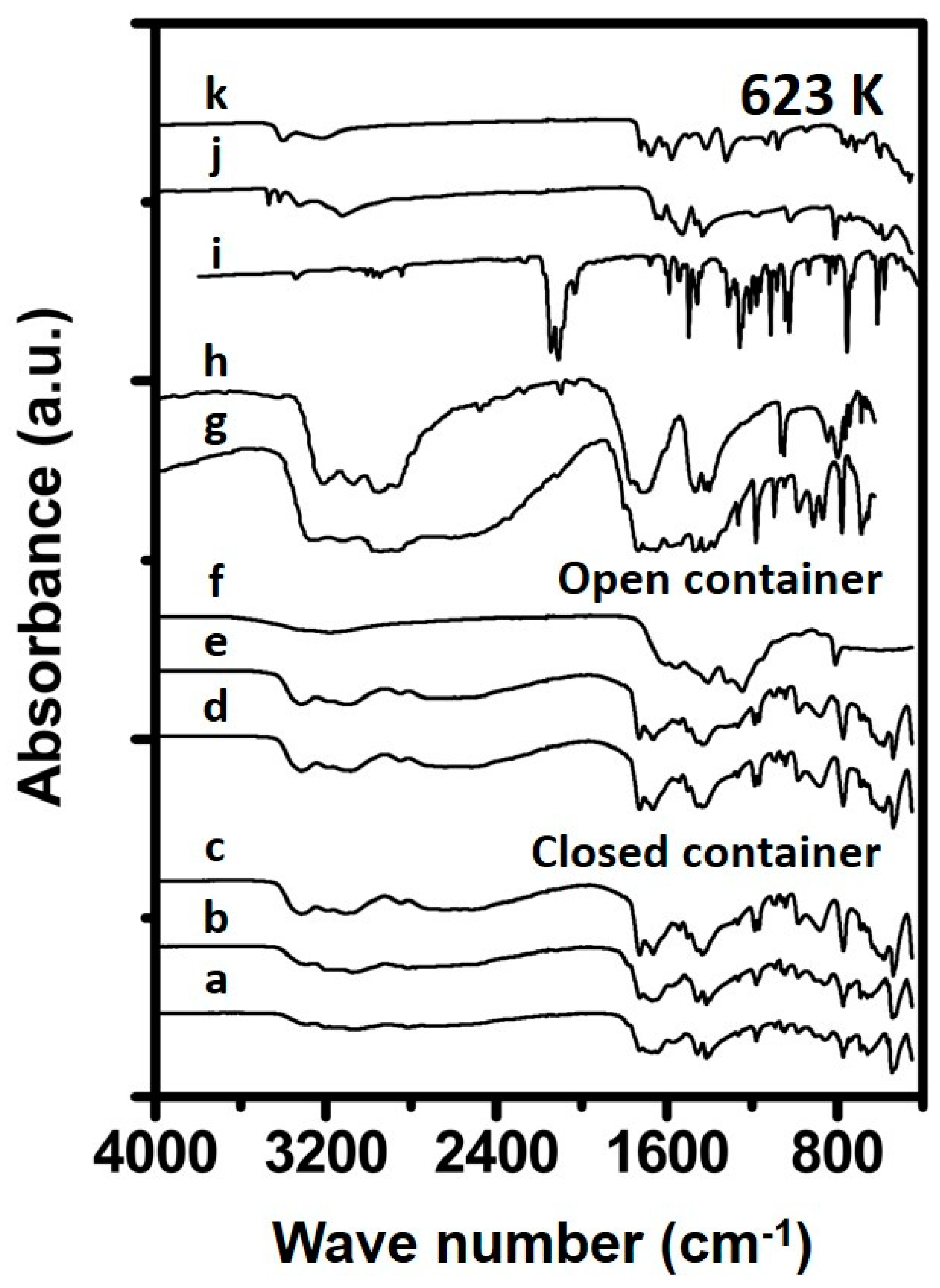
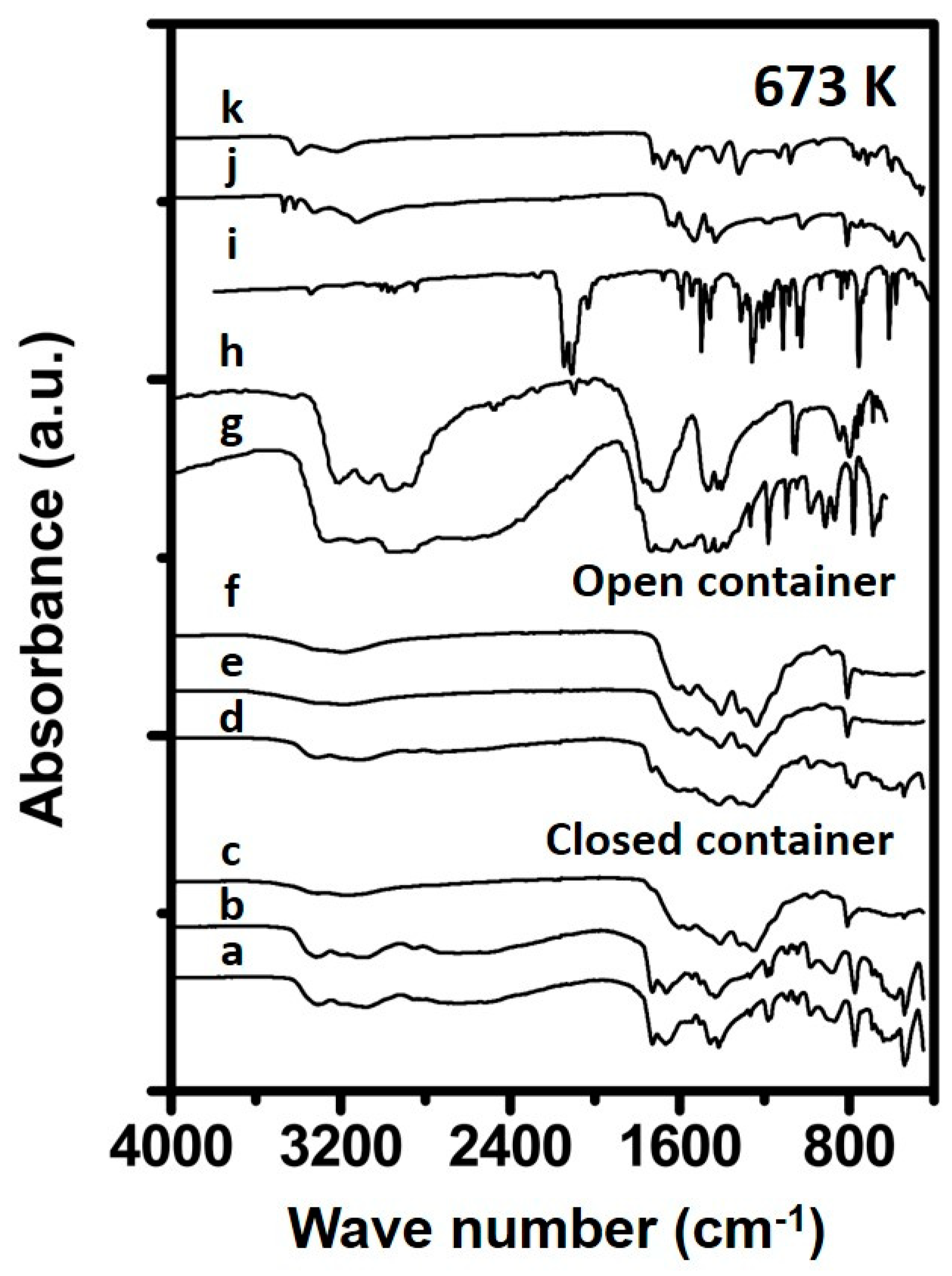
3.1.2. Urea Deposits Prepared Above 423 K
3.2. Thermal Decomposition Experiments
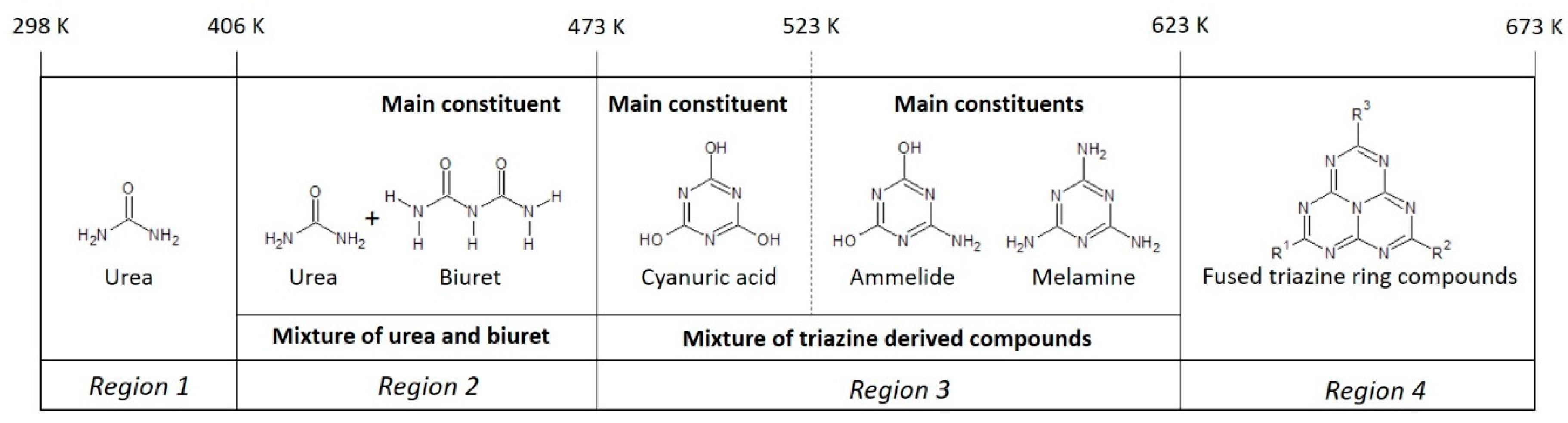
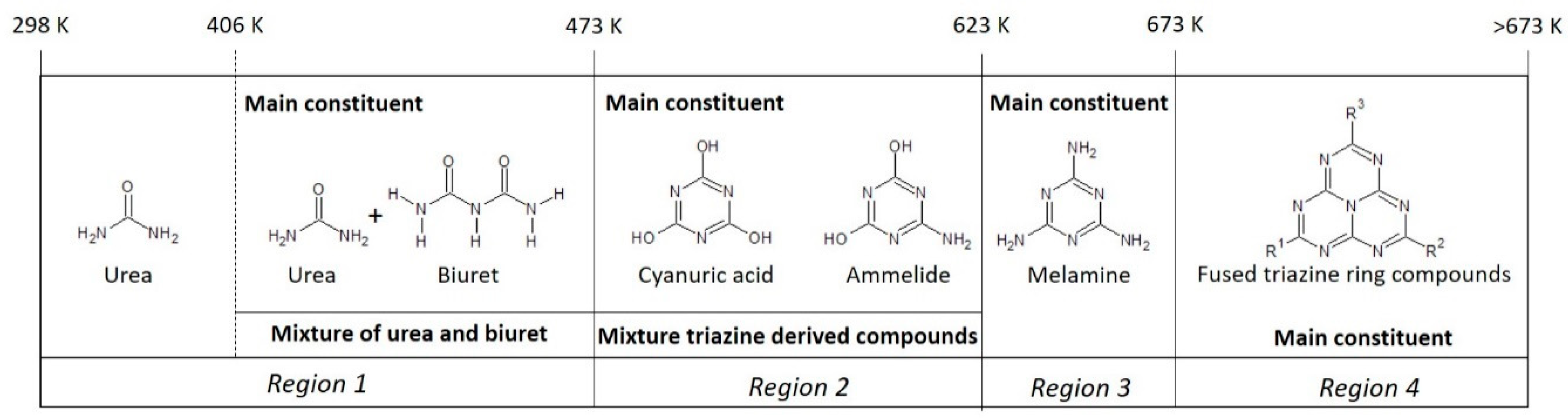
4. Discussion
4.1. Formation Temperature versus Urea Deposit Composition
4.2. Decomposition Behavior of Urea Deposits
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A. Local environment and nature of Cu active sites in zeolite-based catalysts for the selective catalytic reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Zeets, M.; Resasco, D.E.; Wang, B. Enhanced chemical activity and wettability at adjacent Brønsted acid sites in HZSM-5. Catal. Today 2018, 312, 44–50. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Jin, Y.; Zhang, R. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts. Catal. Today 2018, 327, 295–307. [Google Scholar] [CrossRef]
- Hug, H.T.; Mayer, A.; Hartenstein, A. Off-Highway Exhaust Gas After-Treatment: Combining Urea-SCR, Oxidation Catalysis and Traps; SAE Paper No. 930363; SAE International: Warrendale, PA, USA, 1993. [Google Scholar]
- Miller, W.R.; Klein, J.T.; Mueller, R.; Doelling, W.; Zuerbig, J. The Development of Urea SCR Technology for U.S. Heavy Duty Trucks; SAE Paper No. 2000-01-0190; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Yim, S.D.; Kim, S.J.; Baik, J.H.; Nam, I.S.; Mok, Y.S.; Lee, J.; Cho, B.K.; Oh, S.H. Decomposition of Urea into NH3 for the SCR Process. Ind. Eng. Chem. Res. 2004, 43, 4856–4863. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Dietz, E.; Thielen, D.; Anspach, B.; Brauer, J. Study of the urea thermal decomposition (pyrolysis) reaction and importance to cyanuric acid production. Am. Lab. 1999, 31, 3–21. [Google Scholar]
- Koebel, M.; Strutz, E.O. Thermal and hydrolytic decomposition of urea for automotive selective catalytic reduction systems: Thermochemical and practical aspects. Ind. Eng. Chem. Res. 2003, 42, 2093–2100. [Google Scholar] [CrossRef]
- Koryakin, A.; Gal’perin, V.; Sarbaev, A.N.; Finkel’shtein, A.I. Thermographic analysis of urea and products of its pyrolysis. Zhurnal. Org. Khimii 1971, 7, 972–977. [Google Scholar]
- Asaoka, T.; Shimasaki, C.; Toriyama, H.; Yamada, H.; Sakano, M. Influence of Some Pretreatments on Thermal Decomposition of Urea. J. Soc. Chem. Ind. Jpn. 1969, 72, 1056–1063. [Google Scholar]
- Bernhard, A.M.; Peitz, D.; Elsener, M.; Wokaun, A.; Kröcher, O. Hydrolysis and thermolysis of urea and its decomposition byproducts biuret, cyanuric acid and melamine over anatase TiO2. Appl. Catal. B Environ. 2012, 115, 129–137. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2, 5, 8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef]
- Zahn, Z.; Müllner, M.; Lercher, J.A. Catalytic hydrolysis of s-triazine compounds over Al2O3. Catal. Today 1996, 27, 167–173. [Google Scholar] [CrossRef]
- Devallencourt, B.; Saiter, J.M.; Fafet, A.; Ubrich, E. Thermogravimetry/Fourier transform infrared coupling investigations to study the thermal stability of melamine formaldehyde resin. Thermochim. Acta 1995, 259, 143–151. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F. Effects of zirconium silicate reinforcement on expandable graphite based intumescent fire retardant coating. Polym. Degrad. Stab. 2014, 103, 49–62. [Google Scholar]
- Piasek, Z.; Urbanski, T. The Infra-red Absorption Spectrum and Structure of Urea. Bull. Acad. Pol. Sci. 1962, 10, 113–120. [Google Scholar]
- Jung, Y.M.; Czarnik-Matusewicz, B.; Kim, S.B. Characterization of concentration-dependent infrared spectral variations of urea aqueous solutions by principal component analysis and two-dimensional correlation spectroscopy. J. Phys. Chem. B 2004, 108, 13008–13014. [Google Scholar] [CrossRef]
- Weeks, C.L.; Ibeling, D.R.; Han, S.; Ludwig, L.; Ayyappan, P. Analytical investigation of urea deposits in SCR system. SAE Int. J. Engines 2015, 8, 1219–1239. [Google Scholar] [CrossRef]
- Querioz, D.P.; de Pinho, M.N. Structural characteristics and gas permeation properties of polydimethylsiloxane/poly(propylene oxide) urethane/urea bi-soft segment membranes. Polymer 2005, 46, 2346–2353. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Hossenlopp, J.M.; Wilkie, C.A. Fire retardancy of melamine and zinc aluminum layered double hydroxide in poly (methyl methacrylate). Polym. Degrad. Stab. 2008, 93, 1855–1863. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Walters, R.; Lyon, R.E.; Savitski, E.P. Thermal decomposition of cyanate ester resins. Polym. Degrad. Stab. 2002, 78, 73–82. [Google Scholar] [CrossRef]
- El-Gamel, N.E.A.; Seyfarth, L.; Wagler, J.; Ehrenberg, H.; Schwarz, M.; Senker, J.; Kroke, E. The tautomeric forms of cyameluric acid derivatives. Chem. Eur. J. 2007, 13, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, A.; Saplinova, T.; Kroke, E. Tri-s-triazines (s-heptazines)—From a “mystery molecule” to industrially relevant carbon nitride materials. Coord. Chem. Rev. 2013, 257, 2032–2062. [Google Scholar] [CrossRef]




| Compound | Formation Temperature (K) | Decomposition Temperature (K) | Sublimation Temperature (K) |
|---|---|---|---|
| Urea | - | 406 [10,11,13,14] | - |
| Biuret | 423 [10] | 466 [10,11,13,14] | - |
| Cyanuric acid | 433 [10] | 593–603 [10,11,15] | 523 [11,15] |
| Ammelide | 523 [10] | 683 [10,11] | 583 [11] |
| Ammeline | 523 [10] | 703 [10,11] | 613 [11] |
| Melamine | 623 [10] | 873 [17] | 623 [18,19] |
| Melam | 633 [17] | 873 [17] | - |
| Melem | 673 [17] | 873 [17] | - |
| Melon | 773 [17] | 873 [17] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tempelman, C.; Warning, N.; van Geel, J.; van Bommel, F.; Lamers, K.; Hashish, M.; Schippers, J.; Gundlach, M.; Luijendijk, E. An Infrared and Thermal Decomposition Study on Solid Deposits Originating from Heavy-Duty Diesel SCR Urea Injection Fluids. Reactions 2020, 1, 72-88. https://doi.org/10.3390/reactions1020007
Tempelman C, Warning N, van Geel J, van Bommel F, Lamers K, Hashish M, Schippers J, Gundlach M, Luijendijk E. An Infrared and Thermal Decomposition Study on Solid Deposits Originating from Heavy-Duty Diesel SCR Urea Injection Fluids. Reactions. 2020; 1(2):72-88. https://doi.org/10.3390/reactions1020007
Chicago/Turabian StyleTempelman, Christiaan, Niels Warning, Jeffrey van Geel, Femke van Bommel, Kim Lamers, Mahmoud Hashish, Jaap Schippers, Max Gundlach, and Ezra Luijendijk. 2020. "An Infrared and Thermal Decomposition Study on Solid Deposits Originating from Heavy-Duty Diesel SCR Urea Injection Fluids" Reactions 1, no. 2: 72-88. https://doi.org/10.3390/reactions1020007
APA StyleTempelman, C., Warning, N., van Geel, J., van Bommel, F., Lamers, K., Hashish, M., Schippers, J., Gundlach, M., & Luijendijk, E. (2020). An Infrared and Thermal Decomposition Study on Solid Deposits Originating from Heavy-Duty Diesel SCR Urea Injection Fluids. Reactions, 1(2), 72-88. https://doi.org/10.3390/reactions1020007