Abstract
A range of hemiacetal esters were synthesized by the reaction between carboxylic acids and butyl vinyl ether using n-dodecyl dihydrogen phosphate as catalyst. Specifically, nonanoic, propionic, acrylic, sebacic, and fumaric acids were used as substrates to prepare the corresponding hemiacetal esters. These compounds were used as model molecules to demonstrate the ability of hemiacetal ester functional groups to undergo the exchange reaction in the presence of weak carboxylic acids without any catalyst. Kinetics studies examined the effect of the carboxylic acid concentration on the exchange rate, and revealed that the exchange reaction proceeds through an associative mechanism.
1. Introduction
Hemiacetal esters, also called acylated hemiacetals, (see Scheme 1), possess a very interesting functional group, which has yet to be fully studied. It can be synthesized by a number of methods, such as through the addition of a carboxylic acid onto a vinyl ether [1], through Baeyer–Villiger oxidation of a cyclic ketone bearing a vicinal methoxy group [2], or through reaction of a ketone or acetaldehyde with β-hydroxy acids [3]. This function has mostly been used for its ability to dissociate under specific conditions. In particular, hemiacetal esters spontaneously dissociate into carboxylic acids and vinyl ethers upon heating. This thermal dissociation has been demonstrated to be structure-dependent by Otsuka et al. [4,5,6]. In a first publication [4], they showed that hemiacetals formed from carboxylic acids bearing unsaturated groups have a lower dissociation temperature than their saturated aliphatic analogs due to inductive and mesomeric electronic effects. These authors also showed that cyclic aliphatic acids led to even higher dissociation temperature. In a second publication [5], they demonstrated that vinyl ethers with higher inductive donor effects (such as tert-butyl vinyl ether compared to cyclohexyl vinyl ether) led to lower dissociation temperature. Finally, in a third publication [6], hemiacetal esters, prepared by the reaction between methacrylic acid and butyl or phenoxyethyl vinyl ether, were integrated into a copolymer with vinyl comonomers of varying polarity. This last study underlined that the dissociation temperature decreased with the increase in comonomer polarity.

Scheme 1.
Acid-catalyzed reaction between carboxylic acids and vinyl ether leading to hemiacetal ester.
Thermal dissociation of the hemiacetal ester functional group that leads to the recovery of the carboxylic acid function [1] has been used for the preparation of recyclable [4] polymers. It has also been exploited to temporarily protect carboxylic acids [7,8,9,10,11]. This protecting strategy also proved useful to stabilize an epoxy-acid formulation by making the acid function available on demand [8]. Storage of epoxy-acid formulations at ambient temperature generally leads to a viscosity increase due to a slow reaction between the acids and the epoxides. However, Yamamoto and Ishidoya [8] demonstrated that when the acid group was protected with a hemiacetal ester function, the viscosity increase was significantly reduced during storage at 40 °C. In addition, the acid group could be regenerated and reacted with epoxides by heating the formulation at the dissociation temperature of the hemiacetal ester. Besides this stabilizing effect, the conversion of acid groups into hemiacetal ester has also been used to convert solid diacids into liquid dihemiacetal esters, which are easier to formulate [9] and better suited to trigger post-crosslinking reactions of thermoplastic polymers [10,11]. A similar protecting strategy has been notably used by Ruckenstein and Zang to produce amphiphilic copolymers [7]. They converted methacrylic acid into a hemiacetal ester, thus making copolymerization with highly hydrophobic styrene in toluene possible. The hydrophilic acidic group was then regenerated by hydrolysis in acidic medium (HCl 5 M) to yield an amphiphilic polymer otherwise difficult to prepare. Since then, this hydrolysis reaction was used in other studies to form water-soluble [12] and alternative [13] copolymers, but also de-crosslinkable [14,15] or recyclable/degradable [16,17] polymers.
Recently, hemiacetal esters have been used to induce cationic polymerization of vinyl ethers [18,19,20,21,22,23,24]. In the presence of Lewis acids, hemiacetal esters dissociate into a carboxylate and a vinyl ether carbocation able to initiate the living cationic polymerization of vinyl ethers. According to Ouchi et al. [18], this polymerization could be controlled by using a mild catalyst (SnBr4 or ZnCl2) and stopped with methanol. Furthermore, Neitzel et al. [25,26] reported the ability of cyclic hemiacetal esters to homopolymerize in the presence of a Lewis base. This led to the formation of either a polyester or a poly(esteracetal), depending on the conditions (Scheme S1). According to the authors, this polymerization required the addition of a primary alcohol as initiator. In the presence of a small quantity of diethyl zinc catalyst, the alcohol opens the ring by substitution of the acid group in the hemiacetal ester function. The polyesteracetal is then produced by ring-opening polymerization of the cyclic hemiacetal ester by the carboxylate propagating end-group. This poly(esteracetal) can be thermally degraded. In contrast, in the presence of a high amount of catalyst, the alcohol opens the ring by reaction with the carbonyl. This leads to the formation of an ester linkage and triggers the release of acetaldehyde by decomposition of the unstable ethylacetal end-group. The polymerization then proceeds by ring-opening and produces a polyester. These articles suggest that diethyl zinc catalyzes the substitution of acid groups in hemiacetal ester.
Finally, in 1982, Gallucci and Going [1] reported the ability of hemiacetal esters to spontaneously undergo a substitution reaction with stronger acid than the acid involved in the hemiacetal ester function. Specifically, they showed that when a hemiacetal ester derived from acetic acid was reacted, in the absence of any catalyst, with excess HCl or trifluoroacetic acid (TFA), the acetic acid was substituted by chlorine or TFA, respectively. This catalyst-free substitution reaction has not been further studied after the work of Galluci and Going, although it potentially opens the way to very interesting developments in the fields of re-processable, recyclable, or self-healing materials. These studies indeed suggest that hemiacetal esters may undergo an acid exchange reaction.
The aim of the present work is to further examine this substitution reaction in hemiacetal ester, find out if an equilibrium acid exchange reaction exists, and cast some light on the mechanism of this exchange. This article presents the preparation of mono and di-hemiacetal esters using a phosphoric acid derivative as catalyst and discusses the formation of some impurities during the synthesis. The subsequent section gives proof for the existence of a reaction of carboxylic acid substitution in hemiacetal esters. Finally, the last part of the article shows that this substitution reaction is in fact an exchange reaction. A kinetics study of this exchange reaction casts light on the mechanism of this reaction and on some kinetic parameters.
2. Materials and Methods
2.1. Materials
Nonanoic acid (>97%), acrylic acid (>99%), propionic acid (>99.5%), sebacic acid (>99%), fumaric acid (>99%), and butyl vinyl ether (>98%) were obtained from Sigma-Aldrich. Mono-n-dodecyl phosphate (n-dodecyl dihydrogen phosphate, 90%) was purchased from abcr GmbH, and deuterated chloroform (99.8% deuterated, <100 ppm water) and methanol (99.8% deuterated, <300 ppm water) from Eurisotop. Unless otherwise stated, all chemicals were used as received.
2.2. NMR Analyses
The structures of the synthesized molecules were determined by 1H NMR spectroscopy using a Bruker Avance 400 MHz spectrometer. The acquisition parameters were as follows: flip angle 30°, acquisition time 4 s, pulse delay 1 s, and number of scans 4. The analyses were recorded at room temperature in deuterated chloroform and chemical shifts were reported in part per millions (ppm) relative to tetramethylsilane. Spin multiplicity corresponded to: s—singlet; d—doublet; dd—doublet of doublet; t—triplet; tt—triplet of triplet; qa—quadruplet; qi—quintuplet; m—multiplet.
2.3. Flash Chromatography
A flash chromatography REVELERIS® apparatus from GRACE equipped with UV and evaporating light scattering (ELS) detectors was used to purify the samples. GRACE 40 µm silica columns and cyclohexane/ethyl acetate (97:3 v/v) binary mixture eluent were used at a flow rate recommended by the manufacturer (depending on the column size).
2.4. Hemiacetal Ester Syntheses
1-Butoxyethyl nonanoate (BEN) was synthesized by mixing nonanoic acid (1 eq, 10 g, 6.3 × 10−2 mol) with butyl vinyl ether (2 eq, 13 g, 1.3 × 10−1 mol) and n-dodecyl dihydrogen phosphate (0.004 eq, 6.7 × 10−2 g, 2.5 × 10−4 mol) as catalyst in a round bottom flask. After 6 h of stirring at 20 °C, the excess butyl vinyl ether was evaporated under vacuum and the conversion of nonanoic acid was determined using 1H NMR spectroscopy. Then, the crude product was purified by flash chromatography. After purification, the solvents were evaporated under vacuum at 60 °C.
1-Butoxyethyl nonanoate (BEN) 1H NMR (400 MHz, CDCl3, δ = ppm), Figure S1: δ = 0.86 (t, 3H, CH3-CH2, 3JHH = 7.1 Hz), δ = 0.90 (t, 3H, CH3-CH2, 3JHH = 7.4 Hz), δ = 1.25–1.45 (m, 2H, CH2-CH2-CH3; 10H, CH2-CH2-CH2), δ = 1.37 (d, 3H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 1.5–1.6 (m, 2H, CH2-CH2-CH2-O-CHCH3-O), δ = 1.61 (qi, 2H, CH2-CH2-CH2-COO), δ = 2.3 (dt, 2H, CH2-CH2-COO, 2JHH = 1.3 Hz, 3JHH = 7.5 Hz), δ = 3.45 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.7 Hz), δ = 3.62 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.6 Hz), δ = 5.91 (qa, 1H, OOC-CHCH3-O, 3JHH = 5.2 Hz).
1-Butoxyethyl propionate (BEP) and 1-butoxyethyl acrylate (BEA) were prepared using the same protocol from propionic acid and acrylic acid, respectively.
1-Butoxyethyl propionate (BEP) 1H NMR (400 MHz, CDCl3, δ = ppm), Figure S2: δ = 0.90 (t, 3H, CH3-CH2, 3JHH = 7.4 Hz), δ = 1.13 (t, 3H, CH3-CH2-COO), δ = 1.3–1.4 (m, 2H, CH2-CH2-CH3), δ = 1.37 (d, 3H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 1.5–1.6 (m, 2H, CH2-CH2-CH2-O-CHCH3-O), δ = 2.33 (dqa, 2H, CH3-CH2-COO, 2JHH = 2.7 Hz, 3JHH = 7.6 Hz), δ = 3.45 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.4 Hz, 3JHH = 6.7 Hz), δ = 3.63 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.6 Hz), δ = 5.91 (qa, 1H, OOC-CHCH3-O, 3JHH = 5.2 Hz).
1-Butoxyethyl acrylate (BEA) 1H NMR (400 MHz, CDCl3, δ = ppm), Figure S3: δ = 0.90 (t, 3H, CH3-CH2, 3JHH = 7.4 Hz), δ = 1.3–1.4 (m, 2H, CH2-CH2-CH3), δ = 1.41 (d, 3H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 1.5–1.6 (m, 2H, CH2-CH2-CH2-O-CHCH3-O), δ = 3.47 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.7 Hz), δ = 3.65 (dt, 1H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.6 Hz), δ = 5.84 (dd, 1H, CHAHB = CH-COO, 2JHH = 1.4 Hz, 3JHH = 10.4 Hz), δ = 5.99 (qa, 1H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 6.11 (dd, 1H, CHAHB = CH-COO, 3JHH = 17.3 Hz, 3JHH = 10.4 Hz), δ = 6.42 (dd, 1H, CHAHB = CH-COO, 2JHH = 1.4 Hz, 3JHH = 17.3 Hz).
2.5. Dihemiacetal Ester Syntheses
1,10-Dibutoxyethyl sebacate (DBES) was prepared by mixing sebacic acid (1 eq, 10 g, 4.9 × 10−2 mol) with butyl vinyl ether (4 eq, 19.8 g, 2 × 10−1 mol) in the presence of n-dodecyl dihydrogen phosphate (0.02 eq, 2.6 × 10−1 g, 9.8 × 10−4 mol) as catalyst in a round bottom flask, without any solvent. After 6 h of stirring at 20 °C, the butyl vinyl ether in excess was evaporated under vacuum and the conversion of sebacic acid was determined using 1H NMR spectroscopy. Then, the crude product was purified by flash chromatography. After purification, the solvents were evaporated under vacuum at 60 °C.
1,10-Dibutoxyethyl sebacate (DBES) 1HNMR (400 MHz, CDCl3, δ = ppm), Figure S4: δ = 0.90 (t, 6H, CH3-CH2, 3JHH = 7.4 Hz), δ = 1.25–1.45 (m, 4H, CH2-CH2-CH3; 8H, CH2-CH2-CH2), δ = 1.38 (d, 6H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 1.49–1.66 (m, “4H, CH2-CH2-CH2-COO”, “4H, CH2-CH2-CH2-O-CHCH3-O”), δ = 2.30 (dt, 4H, CH2-CH2-COO, 2JHH = 1.3 Hz, 3JHH = 7.5 Hz), δ = 3.45 (dt, 2H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH= 6.7 Hz), δ = 3.63 (dt, 2H, CH2-CHAHB-O-CHCH3-O, 2JHH = 9.5 Hz, 3JHH = 6.6 Hz), δ = 5.91 (qa, 2H, OOC-CHCH3-O, 3JHH = 5.2 Hz).
1,4-Dibutoxyethyl fumarate (DBEF) was synthesized from fumaric acid using the same protocol with a longer stirring time of 24 h. DBEF proved to degrade during the flash chromatography purification step; it was thus not purified further after removal of the excess butyl vinyl ether.
1,4-Dibutoxyethyl fumarate (DBEF) 1HNMR (400 MHz, CDCl3, δ = ppm), Figure S5: δ = 0.90 (t, 6H, CH3-CH2), δ = 1.30–1.40 (m, 4H, CH2-CH2-CH3), δ = 1.44 (d, 6H, OOC-CHCH3-O, 3JHH = 5.2 Hz), δ = 1.49–1.58 (m, 4H, CH2-CH2-CH2-COO), δ = 3.44–3.69 (m, 4H, CH2-CH2-O-CHCH3-O), δ = 6.00 (qa, 2H, OOC-CHCH3-O), δ = 6.85 (s, 2H, OOC-CH = CH-COO, 3JHH = 5.2 Hz).
2.6. Hemiacetal Ester Exchange Demonstration
Crude DBEF (1 eq, 1 g, 3.2 × 10−3 mol) was mixed with nonanoic acid (2 eq, 1 g, 6.4 × 10−3 mol) in a test tube for 30 min. The transparent liquid mixture quickly turned into a suspension of a white powder. After 30 min, the crystals and the liquid phase were separated by centrifugation and analyzed by 1H NMR. The crystals were washed 2 times with CDCl3 before NMR analysis. The same protocol was used to demonstrate hemiacetal ester exchange from DBES.
2.7. Kinetics of the Hemiacetal Ester Exchange Reaction
In an NMR tube containing 680 mg of CDCl3 (450 µL), 200 mg of BEN (1.6 mol.L−1) and 50 mg (1.5 mol.L−1) or 100 mg (3 mol.L−1) of acrylic acid were added. The exchange reaction was then followed by recording 1H NMR spectra every 15 min for 55 h at 25 °C. An example of the spectrum (acquired after 10 h of kinetic monitoring for the stoichiometric conditions) is given in Figure S9. Note that the formation of small amounts of 1-(1-butoxyethoxy)butane and acetaldehyde during this reaction was not significant and did not disturb the exchange process.
3. Results
3.1. Synthesis of Hemiacetal Esters
Mono- and difunctional hemiacetal esters were synthesized according to Scheme 1, following a protocol described by Kovash et al. [9] with several modifications: in particular, n-dodecyl dihydrogen phosphate was used as catalyst instead of phosphoric acid because the long aliphatic chain confers higher solubility in non-polar media. Nakane and Ishidoya [27] demonstrated that the cationic polymerization of vinyl ether, which was observed with acids of lower pKa, did not occur when phosphoric acid was used as catalyst of the reaction. Here, the pKa (~2) [28] of n-dodecyl dihydrogen phosphate is comparable to that of phosphoric acid (pKa = 2.16) [27] and does not trigger the cationic polymerization of the vinyl ether. The reactions were conducted at 20 °C using an excess of vinyl ether. The synthesis conditions as well as the conversions determined by 1H NMR are summarized in Table 1. These reactions were almost quantitative, reaching at least 94% conversion in 6 h (except in the case of fumaric acid, for which the conversion was measured after 24 h of reaction). The structures of the resulting hemiacetal esters are shown in Figure 1.

Table 1.
Synthesis conditions and conversions of mono and dihemiacetal esters.
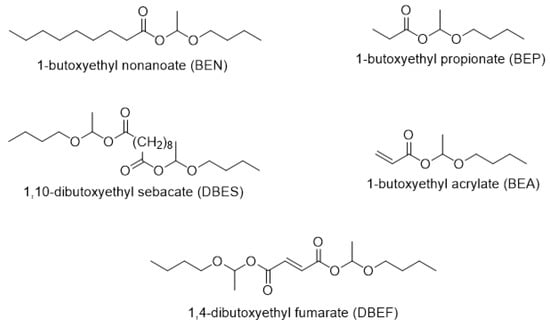
Figure 1.
Structures of the synthesized mono and dihemiacetal esters.
Although 0.4 mol% of catalyst was enough to promote quantitative conversion of the monoacids (Table 1, BEN, BEP, and BEA) in 6 h, a five-fold proportion of n-dodecyl dihydrogen phosphate was required to prepare the difunctional hemiacetal esters at a similar rate (Table 1, DBES and DBEF). This may be ascribed to the lower solubility of the diacids in butyl vinyl ether. In addition, the surfactant-like behavior of the catalyst may also help the solubilization of the diacids.
The crude products were examined by NMR spectroscopy (Figure S5) to determine the nature and source of the side products. Several side products were identified: acetaldehyde with characteristic signals at 2.19 and 9.78 ppm, 1-(1-butoxyethoxy)butane at 4.66 ppm, and butan-1-ol at 3.66 ppm (Figure S5 for the case of DBEF).
As proposed by Galucci and Going [1], these side products were likely formed by a reaction between the hemiacetal ester with either water or alcohol. The reaction of water (from moisture or present as a trace impurity in the reagents) with hemiacetal ester probably led to the formation of the constitutive acid and an unstable 1-butoxyethan-1-ol hemiacetal (Scheme 2a), which readily dissociates into acetaldehyde and butan-1-ol. Butan-1-ol can then react with another hemiacetal ester (Scheme 2b) to form 1-(1-butoxyethoxy)butane. However, the reaction of vinyl ether with water to form acetaldehyde and alcohol, followed by reaction of the alcohol with another vinyl ether, was also reported [29] and this could be another pathway to the side products.
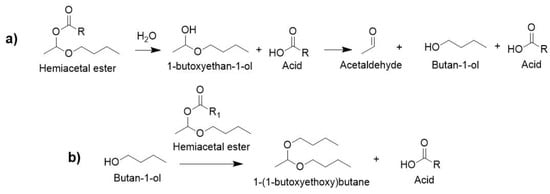
Scheme 2.
Formation of side products: (a) reaction of water with hemiacetal ester; (b) reaction of alcohol with hemiacetal ester.
Due to their high water sensitivity, the hemiacetal esters were purified by distillation of the excess vinyl ether followed by flash chromatography on silica columns, rather than by the washing protocol proposed by Kovash et al. [9]. Note that DBEF could not be purified by flash chromatography as it degraded on the silica column.
3.2. Exchange Reaction with Carboxylic Acids of Comparable Acid Strength
Gallucci and Going [1] demonstrated that, in the presence of strong acid in excess and without additional catalyst, hemiacetal esters undergo substitution with the strong acid. However, they did not investigate the effect of the relative strength of the acids on this substitution. In particular, no research works have reported acid substitution in hemiacetal ester with weak acids.
Here, the exchange with carboxylic acids was demonstrated by mixing pure DBEF (Figure 1), obtained from fumaric acid, with nonanoic acid. The clear liquid mixture quickly turned into a suspension of a white solid. After 30 min of stirring, the resulting solid and liquid phases were separated and each phase was analyzed by 1H NMR spectroscopy. The 1H NMR spectrum of the solid phase corresponded to pure fumaric acid (Figures S6 and S7), which suggested the degradation of DBEF. However, the 1H NMR spectrum of the liquid phase revealed a mixture of DBEF and BEN. These products were identified via the conjugated double bond in DBEF, which caused deshielding of the hemiacetal ester proton (COO-CH(CH3)-O) compared to those of DBES or BEN (Figure 2, signals b and c). The signal of the hemiacetal function at 5.91 ppm corresponding to BEN was more intense than that of DBEF at 6.00 ppm, which indicated that the liquid phase was richer in BEN than in DBEF. These observations indicate that an exchange reaction successfully and almost quantitatively occurred, leading to the formation of BEN and poorly soluble fumaric acid, where the latter precipitated out of the reaction medium. Precipitation was also observed when DBES (prepared from sebacic acid) was mixed in stoichiometric amounts with nonanoic acid. However, due to the identical chemical shifts of the BEN and DBES hemiacetal ester (COO-CH(CH3)-O) NMR signals, crystallization of sebacic acid was the only clear evidence of the exchange reaction. These experiments show that, in absence of catalyst, hemiacetal esters undergo acid exchange not only with strong acids but also with acids of comparable strength. In both cases, the formation of BEN (and disappearance of DBES or DBEF) was quantitative probably because the diacids formed were precipitating out of the reaction medium, thus driving the reaction towards complete exchange (Scheme 3).
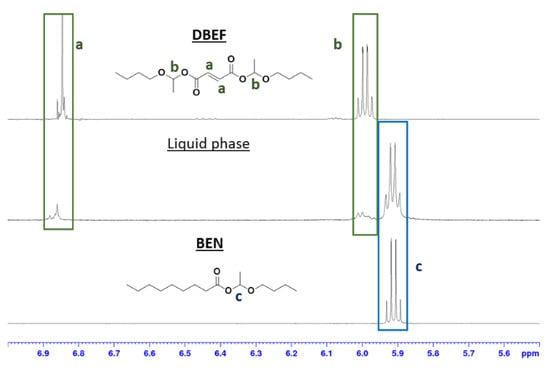
Figure 2.
1H NMR spectra of: BEN (bottom), the liquid phase resulting from the reaction of DBEF and nonanoic acid (middle), and DBEF (top).
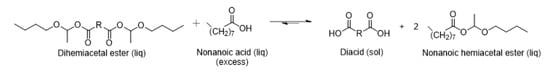
Scheme 3.
Reaction of nonanoic acid with dihemiacetal ester (neat reaction).
3.3. Exchange Mechanism
Exchange mechanisms of covalent bonds are classified as either dissociative or associative/concerted reactions [31]. The exchange in dissociative reactions involves the dissociation of a covalent bond, such as in the case of disulfide bonds [32] or bonds obtained from Diels–Alder cycloaddition [32], before the formation of new covalent bonds (Scheme 4a). In contrast, in associative exchange reactions, such as transesterification or imine metathesis for example [31], the exchange proceeds via the formation of an intermediate or a transition state in which a new covalent bond is formed before another is cleaved (Scheme 4b). Hemiacetal esters can be purified and stored at ambient temperature without spontaneous formation of vinyl ether, and have been used as a long-term storage protection of acids in the presence of otherwise reactive species [8]. However, as illustrated above, acid exchange does occur at ambient temperature. These observations suggest that the acid exchange likely proceeds via an associative mechanism.
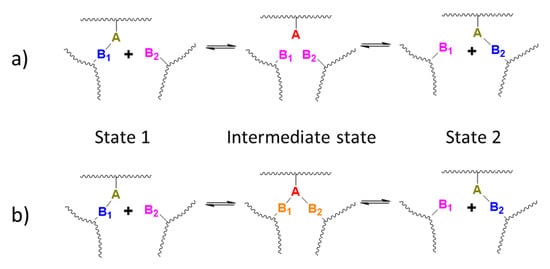
Scheme 4.
(a) Dissociative exchange reaction mechanism. (b) Associative exchange reaction mechanism.
To demonstrate the associative aspect of the acid exchange mechanism in hemiacetal ester, the reaction between BEN and acrylic acid (AA) (Figure 3a) was monitored by 1H NMR spectroscopy. This model reaction allows easy distinction between the hemiacetal ester proton of the two possible hemiacetal esters due to the deshielding effect of the double bond in AA. However, in contrast to the reaction between DBEF and nonanoic acid discussed above, the reaction medium remains homogenous and the reaction is not driven towards completion due to precipitation. Two experiments carried out using different initial molar ratios were monitored for 55 h to study the kinetics of the exchange reactions. The first experiment was carried out using near stoichiometric amounts of BEN and AA (1.07 mole of BEN for 1 mole of AA), whereas in the second experiment, the amount of AA was doubled. The evolutions of the concentrations of BEN and BEA as functions of time for each experiment are presented in Figure 3b. The NMR spectra obtained for 1, 10, and 55 h of monitoring in stoichiometric conditions are presented Figures S9–S11, respectively.
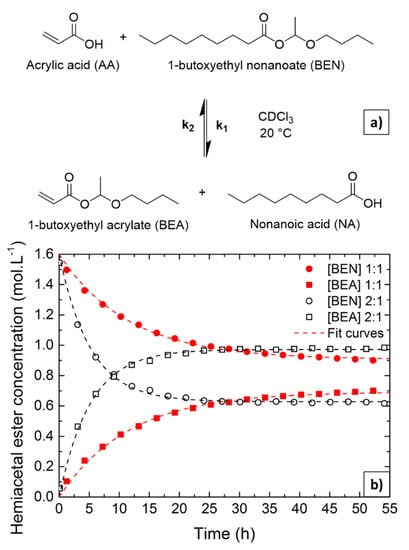
Figure 3.
(a) Hemiacetal ester acid exchange reaction, (b) Hemiacetal ester concentrations as function of time: [AA]0:[BEN]0 = 1:1 (red full squares and circles), [AA]0:[BEN]0 = 2:1 (black open squares and circles).
As expected, the acid exchange reaction revealed to be an equilibrium reaction. In stoichiometric conditions, the equilibrium did not reach an equimolar amount of each hemiacetal ester. This result is attributed to the lower stability of BEA compared to BEN, due to the presence of an unsaturated conjugate bond in the BEA structure [4]. To analyze the curves in Figure 3b, exponential laws (Equations (S4) and (S5)) [33,34,35] were used and the rate constants k and equilibrium concentrations for each system were determined. The equilibrium constant (K) was then calculated using Equation (S6) and assuming an elementary reaction. Initial rates of exchange reactions (v0) were also determined by fitting the derivatives of [BEA] as functions of time using Equation (S7). The values of the parameters are reported in Table 2.

Table 2.
Values of the parameters obtained from the monitoring of the hemiacetal acid exchange reactions.
The k and K values found were not identical for the two experiments. This indicates that the fitting equation does not reflect the exact mechanism of the reaction, and that the order of the exchange reaction is not 1 for all the species involved. The reaction kinetics were nonetheless also fitted with the kinetic law of bimolecular reversible elementary reactions (Equation (S8)). The obtained values of k1, k2, and K are also reported in Table 2. Here again, the k and K values were not identical for the two experiments, and k2 and K were even negative for the 1/2 reaction. These results confirm that these reactions are not elementary. The order of the reaction with respect to the acids and to the hemiacetal esters was then calculated using the initial rates methods (Equation (S18) and Equation (S21), respectively). These results were then used to determine the equilibrium constant using Equation (S6). The rate constant of BEA formation (k1) was obtained from Equation (S15) and the rate constant of BEN formation (k2) was determined from K and k1. All these calculated values are presented in Table 2.
The equilibrium constant K calculated using Equation (S6), and considering a non-elementary equation, was revealed to be smaller than unity in agreement with the lower stability of BEA compared to BEN. The presence of the conjugated double bond is likely responsible for the enhanced electrophilic character of the hemiacetal carbon in BEA. Indeed, as shown in previous reports, hemiacetal esters with conjugated double bonds are less stable than their saturated analogs due to mesomeric effects [4]. In addition, as shown in Table 1, the synthesis of BEA reached lower conversion than those of BEP or BEN, consistent with a slightly lower reactivity of acrylic acid compared to propionic or nonanoic acid. Perhaps more importantly, these experiments showed that increasing the acid concentration twofold resulted in a large increase in the initial exchange rate (Figure 3 and Table 2) as well as a significant increase in the BEA concentration at equilibrium. While in dissociative mechanisms, the kinetics are driven by the dissociation step, and thus, no influence of the reagent concentration can be observed, here, the acid concentration clearly affects the kinetics, indicating that the exchange reaction proceeds via an associative mechanism.
4. Conclusions
The ability of n-dodecyl dihydrogen phosphate to catalyze the reaction of carboxylic acids, including diacids, with butyl vinyl ether to form hemiacetal esters (acylated hemiacetals), and without undesirable cationic vinyl ether polymerization, was demonstrated. This synthesis proceeded to high conversion in 6–24 h at ambient temperature for a selection of mono and dicarboxylic acids. The sensitivities of these compounds to water and alcohol were highlighted by the formation of acetaldehyde and butanol, as well as 1(-1-butoxyethoxy)butane. Nevertheless, using dry conditions, the resulting hemiacetal esters could be isolated as pure compounds. These hemiacetal esters were used to study the ability of this functional group to undergo exchange reactions with carboxylic acids and to investigate the mechanism of this exchange. This study showed that hemiacetal esters can undergo exchange reactions with weak acids even in the absence of a Lewis base catalyst. This exchange reaction thus very likely occurs with any carboxylic acids. Interestingly, the relative strengths of the carboxylic acids did not seem to be the determining factor in this exchange. The relative stability of the hemiacetal ester governed by electronic effects is likely a more important parameter. The hemiacetal ester acid exchange rate was shown to be dependent on the acid concentration. This indicates that this exchange reaction proceeds via an associative mechanism. Hemiacetal esters that are otherwise known to dissociate at high temperature were thus shown to be able to undergo associative exchange of carboxylic acids at ambient temperature. This discovery opens the way to the design and development of new re-processable, recyclable, or self-healing materials. In the fast expanding field of covalent adaptable networks and vitrimers, this new associative exchange reaction combined with the thermal dissociation of hemiacetal esters, could lead to materials behaving as vitrimers at moderate temperature and as thermoplastics at temperatures above the dissociation temperature of hemiacetal ester.
Supplementary Materials
The following are available online at https://www.mdpi.com/2624-781X/1/2/8/s1. Figure S1: 1H NMR spectrum of 1-butoxyethyl nonanoate (BEN) in CDCl3; Figure S2: 1H NMR spectrum of 1-butoxyethyl propionate (BEP) in CDCl3; Figure S3: 1H NMR spectrum of 1-butoxyethyl acrylate (BEA) in CDCl3; Figure S4: 1H NMR spectrum of 1,10-dibutoxyethyl sebacate (DBES) in CDCl3; Figure S5: 1H NMR spectrum of non-purified 1,4-dibutoxyethyl fumarate (DBEF) in CDCl3; Figure S6: 1H NMR spectrum in CD3OD of the solid phase resulting from the reaction between DBEF and fumaric acid; Figure S7: 1H NMR spectrum of fumaric acid in CD3OD; Figure S8: 1H NMR spectrum in CDCl3 of the liquid phase resulting from the reaction between DBEF and fumaric acid; Figure S9: (a) 1H NMR spectrum (in CDCl3) obtained after 1 h of kinetic study in stoichiometric conditions and (b) Structures detected on the spectrum; Figure S10: (a) 1H NMR spectrum (in CDCl3) obtained after 10 h of kinetic study in stoichiometric conditions and (b) Structures detected on the spectrum; Figure S11: (a) 1H NMR spectrum (in CDCl3) obtained after 55 h of kinetic study in stoichiometric conditions and (b) Structures detected on the spectrum; Figure S12: Evolutions of BEN and BEA concentrations during the acid exchange reaction between BEN and acrylic acid; Figure S13: BEA formation rate as a function of time; Figure S14: BEN deviation from equilibrium as a function of time; Equations S1 to S21; Scheme S1: homopolymerization of 2-methyl-1,3-dioxane-4-one in presence of zinc catalyst, according to Neitzel et al. [26,27].
Author Contributions
D.B.: conceptualization, methodology, investigation, data curation, writing—original draft preparation, funding acquisition. J.M.: supervision, writing—review and editing. N.C.: supervision, writing—review and editing, funding acquisition. N.P.: supervision, writing—review and editing, funding acquisition. V.L.: conceptualization, methodology, supervision, writing—review and editing, funding acquisition. C.N.: supervision, project administration, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript
Funding
This project was supported by the Chimie Balard Cirimat Carnot institute through the ANR programme N°16 CARN 0008-01 and by the I-SITE MUSE (ANR managed “Investissements d’avenir” programme N° ANR-16-IDEX-0006) Explore#2 mobility grant.
Acknowledgments
The authors warmly thank Cédric Totée (ICGM) for the NMR spectroscopy technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallucci, R.R.; Going, R.C. Reaction of hemiacetal esters, acetals, and acylals with alcohols or acetic acid. J. Org. Chem. 1982, 47, 3517–3521. [Google Scholar] [CrossRef]
- Kammiyada, H.; Konishi, A.; Ouchi, M.; Sawamoto, M. Ring-Expansion Living Cationic Polymerization via Reversible Activation of a Hemiacetal Ester Bond. ACS Macro Lett. 2013, 2, 531–534. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Atsumi, M. Specific formation of a polymer containing five-membered oxalactone rings in the main chain in the cationic ring-opening polymerization of 6,8-dioxabicyclo[3.2.1]octan-7-one. Macromolecules 1984, 17, 1840–1843. [Google Scholar] [CrossRef]
- Otsuka, H.; Endo, T. Poly(hemiacetal ester)s: New Class of Polymers with Thermally Dissociative Units in the Main Chain. Macromolecules 1999, 32, 9059–9061. [Google Scholar] [CrossRef]
- Otsuka, H.; Fujiwara, H.; Endo, T. Thermal dissociation behavior of polymers with hemiacetal ester moieties in the side chain: The effect of structure on dissociation temperature. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4478–4482. [Google Scholar] [CrossRef]
- Otsuka, H.; Fujiwara, H.; Endo, T. Fine-tuning of thermal dissociation temperature using copolymers with hemiacetal ester moieties in the side chain: Effect of comonomer on dissociation temperature. React. Funct. Polym. 2001, 46, 293–298. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Zhang, H. Living Anionic Copolymerization of 1-(Alkoxy)ethyl Methacrylates with Polar and/or Nonpolar Monomers and the Preparation of Amphiphilic Block Copolymers Containing Poly(methacrylic acid) Hydrophilic Segments at Higher Temperatures Than Usually Employed. Macromolecules 1998, 31, 9127–9133. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ishidoya, M. New thermosetting coatings using blocked carboxyl groups. Prog. Org. Coat. 2000, 40, 267–273. [Google Scholar] [CrossRef]
- Kovash, C.S.; Pavlacky, E.; Selvakumar, S.; Sibi, M.P.; Webster, D.C. Thermoset Coatings from Epoxidized Sucrose Soyate and Blocked, Bio-Based Dicarboxylic Acids. ChemSusChem 2014, 7, 2289–2294. [Google Scholar] [CrossRef]
- Komatsu, H.; Hino, T.; Endo, T. Novel thermally latent self-crosslinkable copolymers bearing oxetane and hemiacetal ester moieties: The synthesis, self-crosslinking behavior, and thermal properties. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4260–4270. [Google Scholar] [CrossRef]
- Komatsu, H.; Ochiai, B.; Endo, T. Thermally latent synthesis of networked polymers from multifunctional hemiacetal ester and diepoxide catalyzed by Schiff-base-zinc chloride complex. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3682–3689. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Matsumoto, Y.; Zhang, T.; Matsuhashi, Y.; Kitayama, T. α-exomethylene lactone possessing acetal-ester linkage: Polymerization and postpolymerization modification for water-soluble polymer. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 955–961. [Google Scholar] [CrossRef]
- Ouchi, M.; Nakano, M.; Nakanishi, T.; Sawamoto, M. Alternating Sequence Control for Carboxylic Acid and Hydroxy Pendant Groups by Controlled Radical Cyclopolymerization of a Divinyl Monomer Carrying a Cleavable Spacer. Angew. Chem. Int. Ed. 2016, 55, 14584–14589. [Google Scholar] [CrossRef]
- Matsukawa, D.; Mukai, T.; Okamura, H.; Shirai, M. Photocurable oligo(hemiacetal ester)s having methacrylate side chains. Eur. Polym. J. 2009, 45, 2087–2095. [Google Scholar] [CrossRef]
- Matsukawa, D.; Okamura, H.; Shirai, M. Reworkable dimethacrylates with low shrinkage and their application to UV nanoimprint lithography. J. Mater. Chem. 2011, 21, 10407–10414. [Google Scholar] [CrossRef]
- Kazama, A.; Kohsaka, Y. Radical polymerization of ‘dehydroaspirin’ with the formation of a hemiacetal ester skeleton: A hint for recyclable vinyl polymers. Polym. Chem. 2019, 10, 2764–2768. [Google Scholar] [CrossRef]
- Neitzel, A.E.; Barreda, L.; Trotta, J.T.; Fahnhorst, G.W.; Haversang, T.J.; Hoye, T.R.; Fors, B.P.; Hillmyer, M.A. Hydrolytically-degradable homo- and copolymers of a strained exocyclic hemiacetal ester. Polym. Chem. 2019, 10, 4573–4583. [Google Scholar] [CrossRef]
- Ouchi, M.; Konishi, A.; Takenaka, M.; Sawamoto, M. Consecutive living polymerization from cationic to radical: A straightforward yet versatile methodology for the precision synthesis of “cleavable” block copolymers with a hemiacetal ester junction. Polym. Chem. 2012, 3, 2193–2199. [Google Scholar] [CrossRef]
- Kammiyada, H.; Ouchi, M.; Sawamoto, M. A Study on Physical Properties of Cyclic Poly(vinyl ether)s Synthesized via Ring-Expansion Cationic Polymerization. Macromolecules 2017, 50, 841–848. [Google Scholar] [CrossRef]
- Neitzel, A. Ring-Opening Polymerization of Cyclic Hemiacetal Esters for the Preparation of Hydrolytically and Thermally Degradable Polymers., University Of Minnesota, 2018. Available online: http://hdl.handle.net/11299/195385 (accessed on 29 October 2020).
- Kubota, H.; Yoshida, S.; Ouchi, M. Ring-expansion cationic cyclopolymerization for the construction of cyclic cyclopolymers. Polym. Chem. 2020, 11, 3964–3971. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Yamashita, M.; Matsuhashi, Y.; Yamashita, S. Synthesis of poly(conjugated ester)s by ring-opening polymerization of cyclic hemiacetal ester bearing acryl skeleton. Eur. Polym. J. 2019, 120, 109185. [Google Scholar] [CrossRef]
- Hyoi, K.; Kanazawa, A.; Aoshima, S. Cationic Ring-Opening Co- and Terpolymerizations of Lactic Acid-Derived 1,3-Dioxolan-4-ones with Oxiranes and Vinyl Ethers: Nonhomopolymerizable Monomer for Degradable Co- and Terpolymers. ACS Macro Lett. 2019, 8, 128–133. [Google Scholar] [CrossRef]
- Ouchi, M.; Kammiyada, H.; Sawamoto, M. Ring-expansion cationic polymerization of vinyl ethers. Polym. Chem. 2017, 8, 4970–4977. [Google Scholar] [CrossRef]
- Neitzel, A.E.; Petersen, M.A.; Kokkoli, E.; Hillmyer, M.A. Divergent Mechanistic Avenues to an Aliphatic Polyesteracetal or Polyester from a Single Cyclic Esteracetal. ACS Macro Lett. 2014, 3, 1156–1160. [Google Scholar] [CrossRef]
- Neitzel, A.E.; Haversang, T.J.; Hillmyer, M.A. Organocatalytic Cationic Ring-Opening Polymerization of a Cyclic Hemiacetal Ester. Ind. Eng. Chem. Res. 2016, 55, 11747–11755. [Google Scholar] [CrossRef]
- Nakane, Y.; Ishidoya, M. New crosslinking system using blocked carboxylic acid. Prog. Org. Coat. 1997, 31, 113–120. [Google Scholar] [CrossRef]
- Fiore, M. The synthesis of mono-alkyl phosphates and their derivatives: An overview of their nature, preparation and use, including synthesis under plausible prebiotic conditions. Org. Biomol. Chem. 2018, 16, 3068–3086. [Google Scholar] [CrossRef]
- Cho, C.G.; Feit, B.A.; Webster, O.W. Initiation of vinyl ether polymerization by trimethylsilyl triflate, dimethyl sulfide, and adventitious water. Macromolecules 1992, 25, 2081–2085. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Lorke, S.; Müller, U.; Meissl, R.; Brüggemann, O. Covalent cross-linking of polymers at room temperature. Int. J. Adhes. Adhes. 2019, 91, 150–159. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Nicolaÿ, R.; Winne, J.; Du Prez, F.E. Fluorinated Vitrimer Elastomers with a Dual Temperature Response. J. Am. Chem. Soc. 2018, 140, 13272–13284. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, N.; Maes, D.; Unal, K.; Guerre, M.; Winne, J.M.; Du Prez, F.E. Covalent Adaptable Networks with Tunable Exchange Rates Based on Reversible Thiol–yne Cross-Linking. Angew. Chem. Int. Ed. 2020, 59, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).