Long-Term Blueberry Storage by Ozonation or UV Irradiation Using Excimer Lamp
Abstract
1. Introduction
2. Materials and Methods
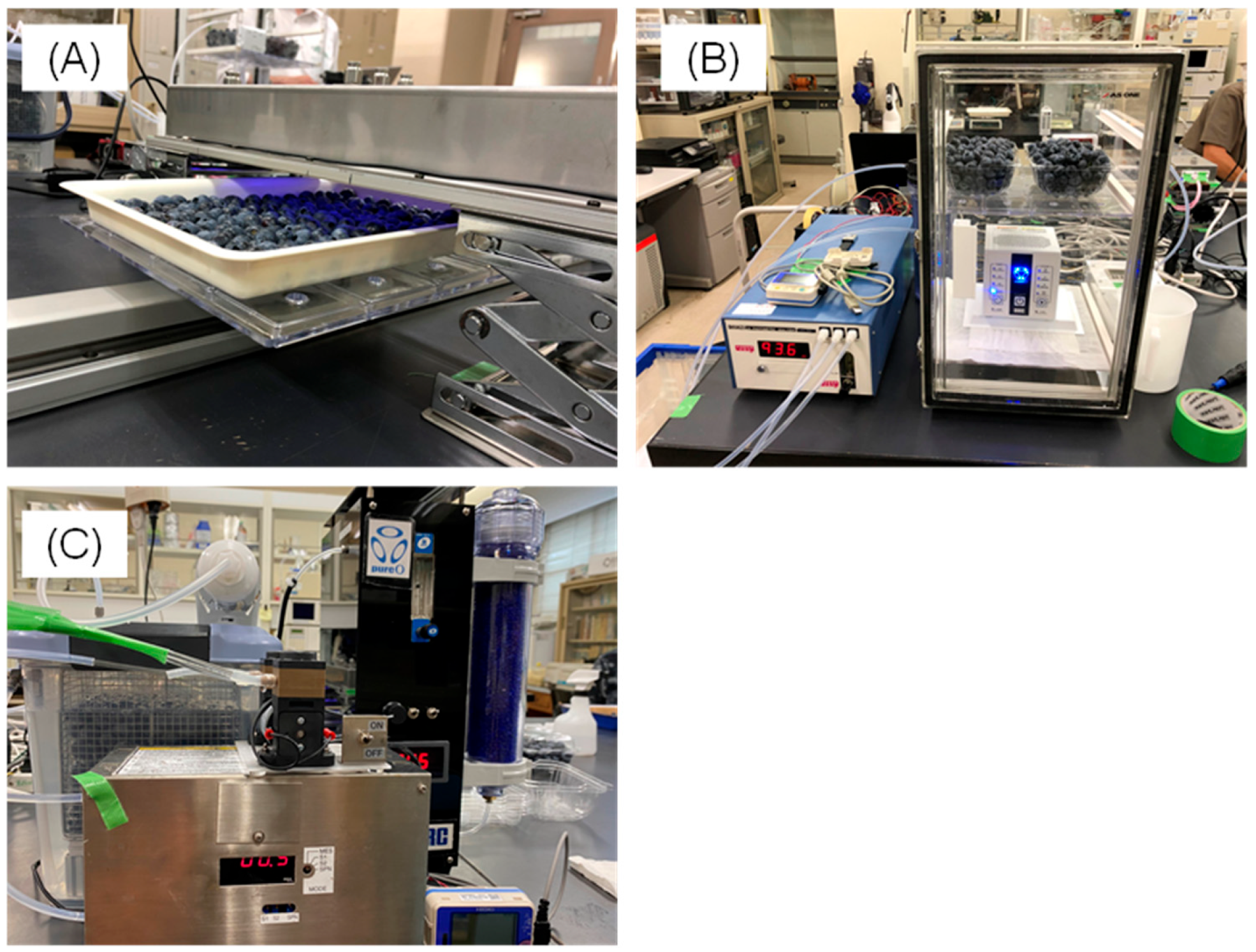
2.1. Sterilization Using Excimer Fluorescent Lamps
2.1.1. Plant Materials
2.1.2. Fruit Sterilization and Storage
2.1.3. Weight Loss
2.1.4. Soluble Solids Content (Brix) and Acidity
2.1.5. Anthocyanin Content
2.1.6. Mold Growth on Blueberry Surfaces
2.1.7. Evaluation of Appearance Quality
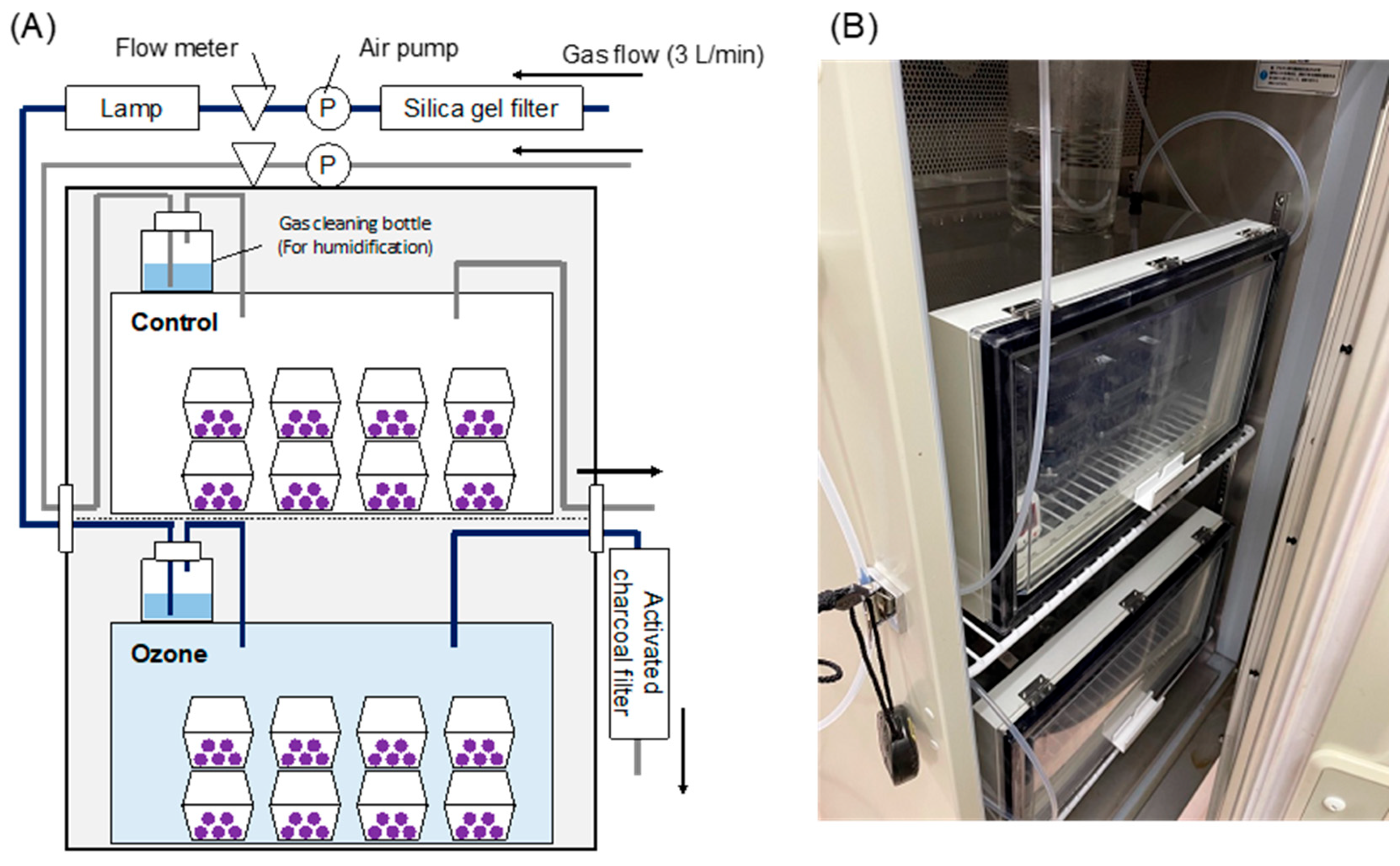
2.2. Continuous Ozone Treatment
2.2.1. Plant Materials
2.2.2. Fruit Sterilization and Storage
2.2.3. Weight Loss, Brix, Acidity, and Anthocyanin Content
2.2.4. Fruit Pericarp Hardness
2.2.5. Mold Growth on Fruit Surfaces
2.2.6. Evaluation of Appearance Quality
2.3. Statistical Analysis
3. Results
3.1. Effects of Sterilization Treatment Using Excimer Fluorescent Lamps on Blueberry Preservation and Storage
3.1.1. Weight Loss
3.1.2. Soluble Solids Content (Brix) and Acidity
3.1.3. Anthocyanin Content
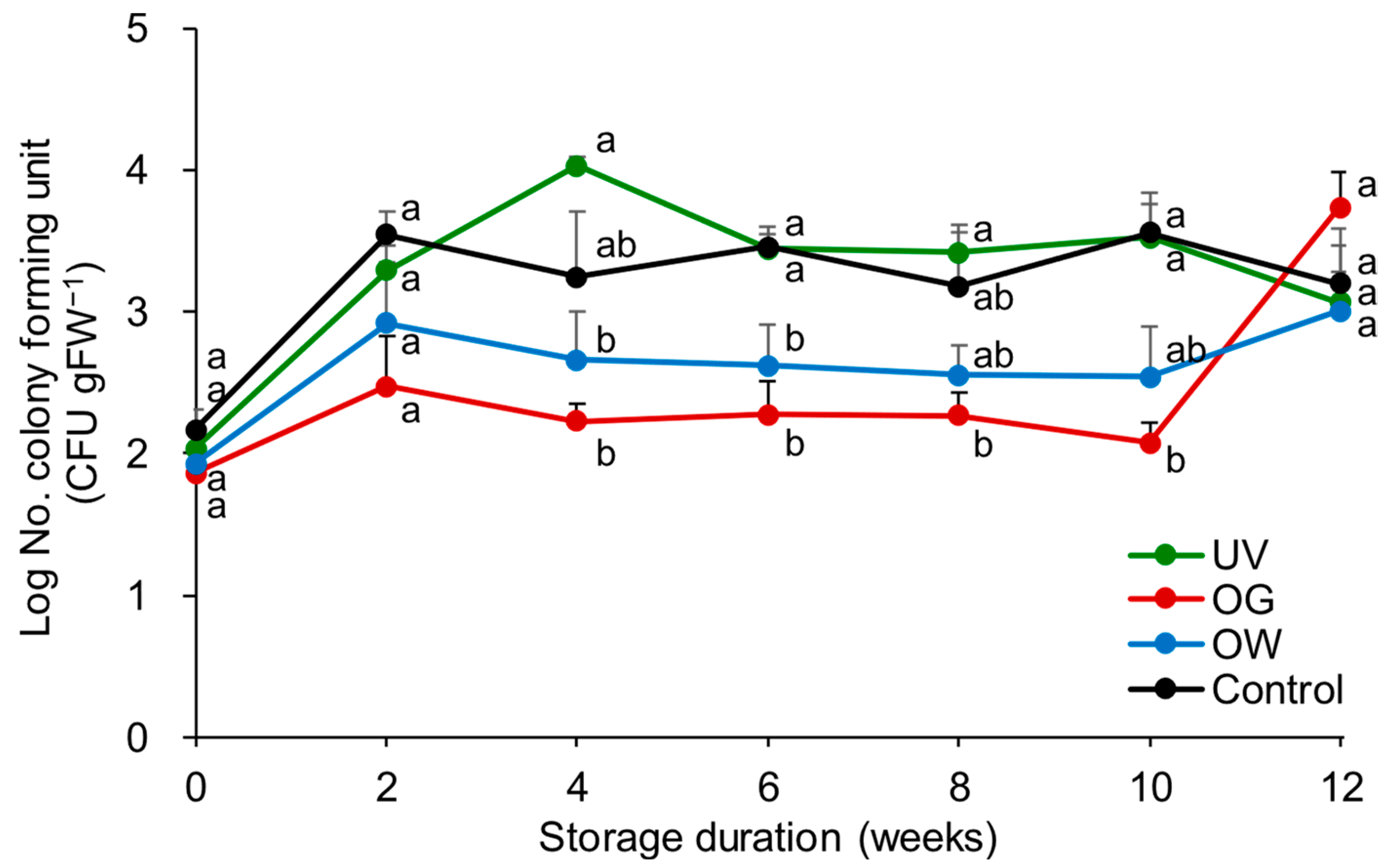
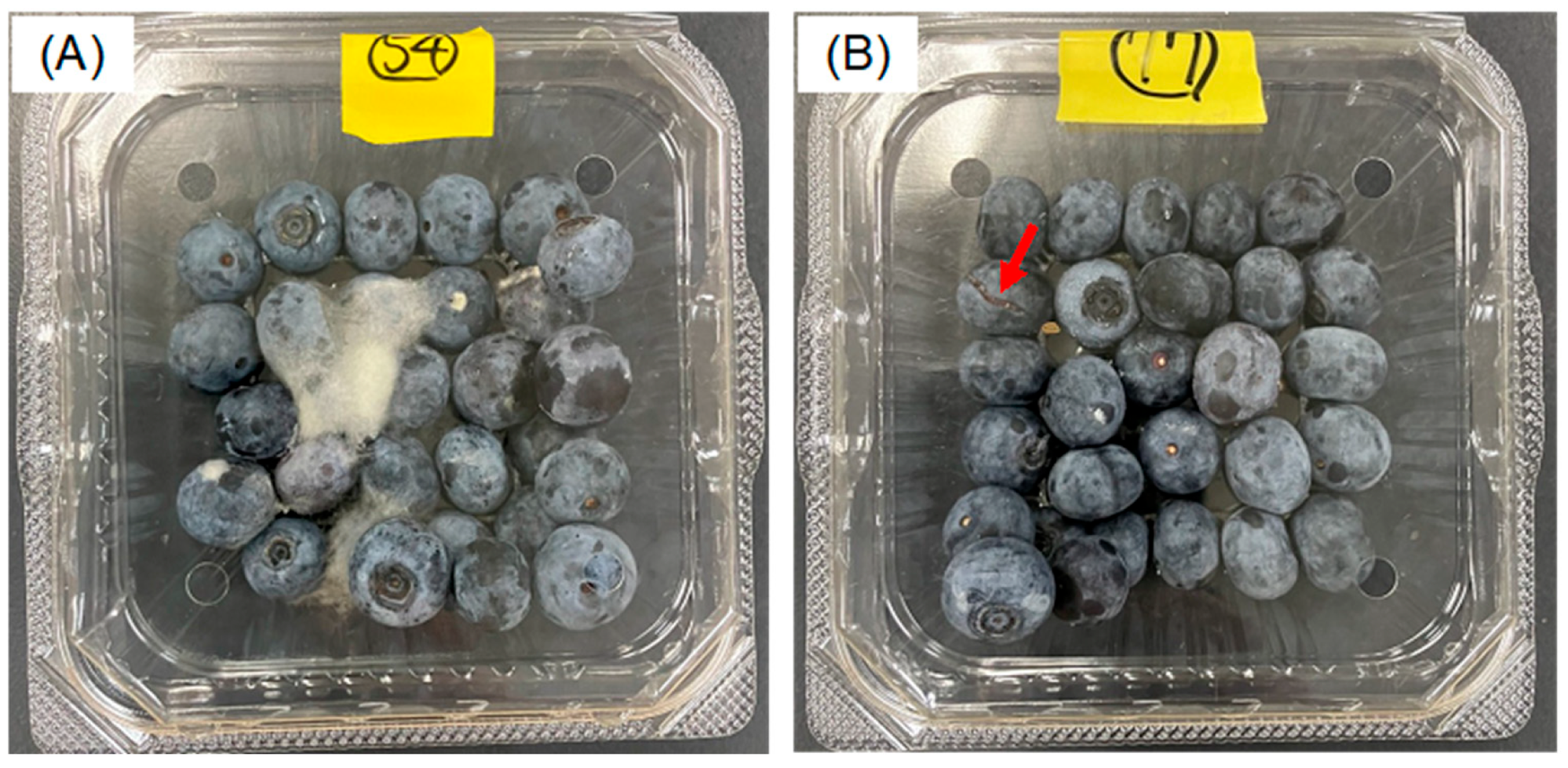
3.1.4. Mold Growth on Fruit Surfaces
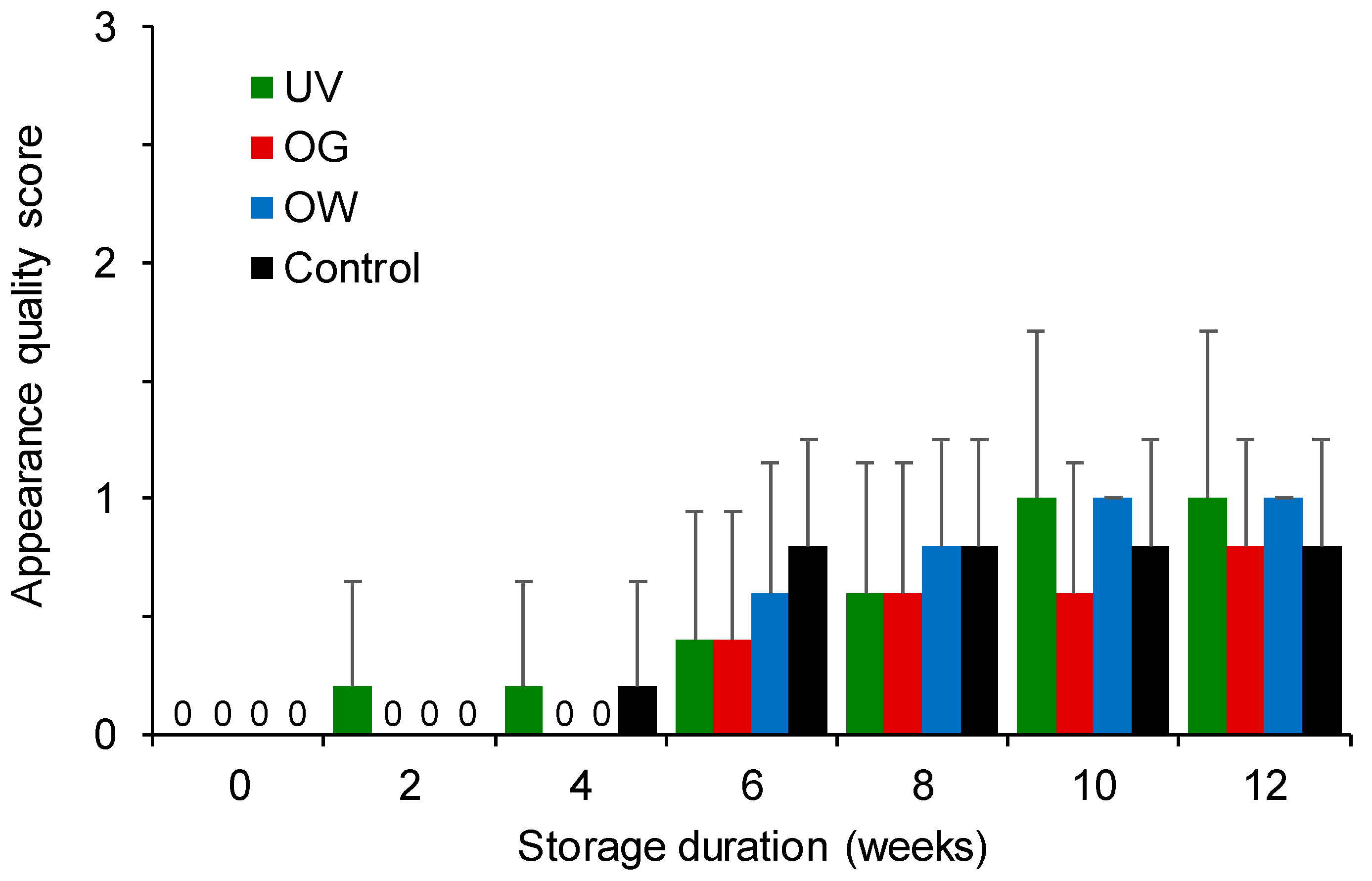
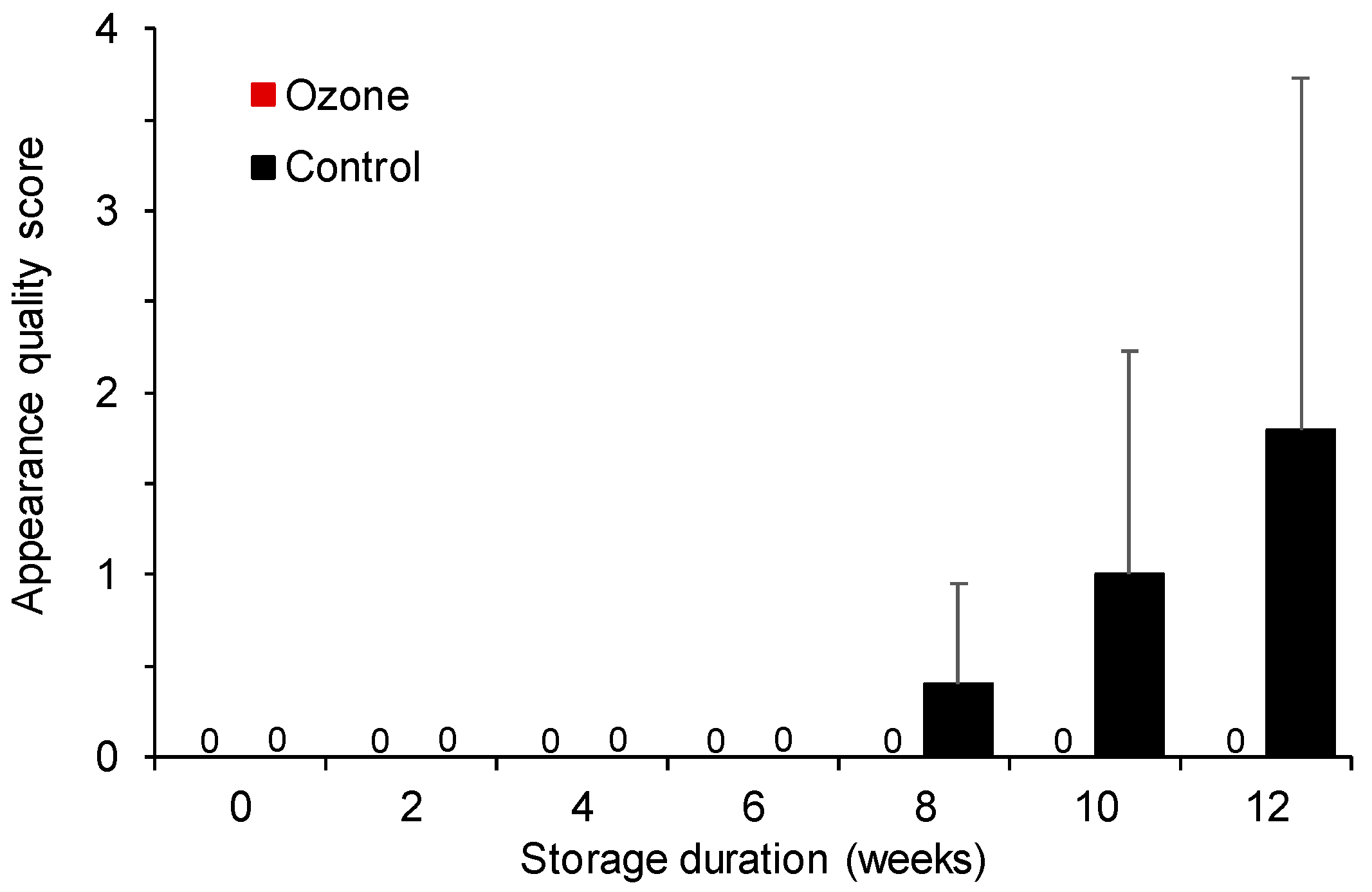
3.1.5. Evaluation of Appearance Quality
3.2. Effect of Continuous Ozone Treatment on Blueberry Fruit Storability
3.2.1. Weight Loss
3.2.2. Soluble Solids Content (Brix) and Acidity
3.2.3. Anthocyanin Content
3.2.4. Fruit Pericarp Hardness
3.2.5. Mold Growth on Fruit Surface
3.2.6. Evaluation of Fruit Appearance Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, L.; Williams, C.M. Blueberry benefits to cognitive function across the lifespan. Int. J. Food Sci. Nutr. 2020, 72, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Higashide, T.; Aoki, N.; Kinoshita, T.; Ibuki, T.; Kasahara, Y. Forcing culture of blueberry grown in a container using a hydroponics system suitable for use in hilly and mountainous areas. Hortic. Res. 2006, 5, 303–308. [Google Scholar] [CrossRef]
- Aung, T.; Muramatsu, Y.; Horiuchi, N.; Che, J.; Mochizuki, Y.; Ogiwara, I. Plant growth and fruit quality of blueberry in a controlled room under artificial light. J. Jpn. Soc. Hortic. Sci. 2014, 83, 273–281. [Google Scholar] [CrossRef]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Nadas, A.; Olmo, M.; García, J.M. Growth of Botrytis cinerea and strawberry quality in ozone-enriched atmospheres. J. Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Crisosto, H.C.; Smilanick, J.L.; Dokoozlian, N.K.; Luvisi, D.A. Maintaining table grape post-harvest quality for long distant markets. Int. Symp. Table Grape Prod. 1994, 195–199. [Google Scholar]
- Tournas, V.H.; Katsoudas, E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef]
- Hwang, C.; Huang, L.; Wu, V.C. In situ generation of chlorine dioxide for surface decontamination of produce. J. Food Prot. 2017, 80, 567–572. [Google Scholar] [CrossRef]
- Crowe, K.M.; Bushway, A.A.; Bushway, R.J.; Davis-Dentici, K.; Hazen, R.A. A comparison of single oxidants versus advanced oxidation processes as chlorine-alternatives for wild blueberry processing (Vaccinium angustifolium). Int. J. Food Microbiol. 2007, 116, 25–31. [Google Scholar] [CrossRef]
- Tadepalli, S.; Bridges, D.F.; Driver, R.; Wu, V.C.H. Effectiveness of different antimicrobial washes combined with freezing against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on blueberries. Food Microbiol. 2018, 74, 34–39. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Brandão, T.R.S.; Silva, C.L.M. Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. J. Food Eng. 2012, 108, 417–426. [Google Scholar] [CrossRef]
- Lim, W.; Harrison, M.A. Effectiveness of UV light as a means to reduce Salmonella contamination on tomatoes and food contact surfaces. Food Control 2016, 66, 166–173. [Google Scholar] [CrossRef]
- Takano, Y.; Ninohei, R.; Koike, A.; Serizawa, I.; Mochizuki, Y. Effects of excimer fluorescent UV lamps on mold and fruit quality in strawberries. AgriEngineering 2024, 6, 4889–4900. [Google Scholar] [CrossRef]
- Kikuchi, C. Bactericldal lamp and it’s application. J. IEI-J. 1974, 58, 143–146. [Google Scholar]
- Pangloli, P.; Hung, Y. Reducing microbiological safety risk on blueberries through innovative washing technologies. Food Control 2013, 32, 621–625. [Google Scholar] [CrossRef]
- Hayakawa, T.; Okazaki, A.; Takano, Y.; Serizawa, I. Measurement and effects of nitrogen oxide in ozone gas emitted from air-fed ozone generators. Bull. Med. Hyg. Ozone Res., Jpn. 2022, 29, 48–56. [Google Scholar]
- Alves, H.; Alencar, E.R.; Ferreira, W.F.S.; Silva, C.R.; Ribeiro, J.L. Microbiological and physical-chemical aspects of strawberry exposed to ozone gas at different concentrations during storage. Braz. J. Food Technol. 2019, 22, e2018002. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Kronberg, M.F.; Sanchez, G.J.; Gomez, P.L.; García-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone washing decreases strawberry susceptibility to Botrytis cinerea while maintaining antioxidant, optical and sensory quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G.; Smilanick, J.L. Application of low concentrations of ozone during the cold storage of table grapes. Postharvest Biol. Technol. 2014, 93, 38–48. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Santos-Pedro, D.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of aqueous ozone, blanching and combined treatments on microbial load of red bell peppers, strawberries and watercress. J. Food Eng. 2011, 105, 277–282. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Moczkowska, M.; Szpicer, A.; Wierzbicka, A. The impact of ozone on health-promoting, microbiological, and colour properties of Rubus ideaus raspberries. CyTA J. Food 2017, 15, 563–573. [Google Scholar] [CrossRef]
- Takano, Y.; Kobayashi, G.; Koike, A.; Serizawa, I. Comparison of ozone generation methods using UV lamp and discharging in water processing. In Proceedings of the 32nd Japan Ozone Association Annual Conference, Kyoto, Japan, 23 June 2023; Volume 32, pp. 135–138. [Google Scholar]
- Mochizuki, Y.; Meguro, I.; Fukuoka, A.; Miyashita, C.; Worarad, K.; Inoue, E. Storability of interspecific hybrid blueberry HoSp-S65G-13 suitable for cluster harvesting. Hortic. Res. 2021, 20, 463–468. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf life determination of fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. Int. J. Food Sci. 2015, 2015, 164143. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Silva, J.L.; Tokitkla, A.; Matta, F.B. Modified atmosphere storage influences quality parameters and shelf life of ‘Tifblue’ blueberries. J. Miss. Acad. Sci. 2010, 55, 143–148. [Google Scholar]
- Jaramillo-Sánchez, G.; Contigiani, E.V.; Castro, M.; Hodara, K.; Alzamora, S.M.; Loredo, A.G.; Nieto, A.B. Freshness maintenance of blueberries (Vaccinium corymbosum L.) during postharvest using ozone in aqueous phase: Microbiological, structure, and mechanical issues. Food Bioprocess Technol. 2019, 12, 2136–2147. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Sanford, K.A.; Lidster, P.D.; McRae, K.B.; Jackson, E.D.; Lawrence, R.A.; Stark, R.; Prange, R.K. Lowbush blueberry quality changes in response to mechanical damage and storage temperature. J. Am. Soc. Hortic. Sci. 1991, 116, 47–51. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. 2008. Postharvest shelf life extension of blueberries using a biodegradable package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Briano, R.; Girgenti, V.; Peano, C. Quality effect of ozone treatment for the red raspberries storage. Chem. Eng. Trans. 2015, 44, 25–30. [Google Scholar]
- Tabakoglu, N.; Karaca, H. Effects of ozone-enriched storage atmosphere on postharvest quality of black mulberry fruits (Morus nigra L.). LWT-Food Sci. Technol. 2018, 92, 276–281. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Piechowiak, T. Effect of ozone treatment on glutathione(GSH)status in selected berry fruit. Phytochemistry 2021, 187, 112767. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, H.; Dong, C.; Ji, H.; Zhang, X.; Ban, Z.; Zhang, N.; Xue, W. Effect of ozone treatment on the phenylpropanoid biosynthesis of postharvest strawberry. RSC Adv. 2019, 9, 25429–25438. [Google Scholar] [CrossRef]
- Allende, A.; Marín, A.; Buendía, B.; Tomás-Barberán, F.; Gil, M.I. Impact of combined postharvest treatments (UV-C light, gaseous O3, superatmospheric O2 and high CO2) on health promoting compounds and shelf-life of strawberries. Postharvest Biol. Technol. 2007, 46, 201–211. [Google Scholar] [CrossRef]
- Bank, H.L.; John, J.; Schmehl, M.K.; Dratch, R.J. Bactericidal effectiveness of modulated UV light. Appl. Environ. Microbiol. 1990, 56, 3888–3889. [Google Scholar] [CrossRef]
- Bridges, D.F.; Rane, B.; Wu, V.C.H. The effectiveness of closed-circulation gaseous chlorine dioxide or ozone treatment against bacterial pathogens on produce. Food Control 2018, 91, 261–267. [Google Scholar] [CrossRef]
- Cappellini, R.A.; Ceponis, M.J. Vulnerability of stem-end scars of blueberry fruits to postharvest decays. Phytopathology 1977, 67, 118–119. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef]
- Sarron, E.; Widehem, P.G.; Aussenac, T. Ozone treatments for preserving fresh vegetables quality: A critical review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]

| SS | DF | MS | F | p | ||
|---|---|---|---|---|---|---|
| Weight loss (g) | Treat. | 0.412 | 3 | 0.138 | 8.53 | 0.001 |
| Storage | 10.127 | 5 | 2.025 | 761.31 | <0.001 | |
| Treat. × Storage | 0.008 | 15 | 0.001 | 0.21 | 0.999 | |
| error | 0.213 | 80 | 0.003 | |||
| Soluble solids content (Brix) | Treat. | 0.001 | 3 | 0.000 | 1.49 | 0.255 |
| Storage | 0.005 | 6 | 0.001 | 2.90 | 0.012 | |
| Treat. × Storage | 0.008 | 18 | 0.000 | 1.63 | 0.067 | |
| error | 0.025 | 96 | 0.000 | |||
| Acidity (%) | Treat. | 0.010 | 3 | 0.003 | 0.81 | 0.509 |
| Storage | 0.040 | 6 | 0.007 | 0.99 | 0.438 | |
| Treat. × Storage | 0.110 | 18 | 0.006 | 0.92 | 0.557 | |
| error | 0.559 | 84 | 0.007 | |||
| Anthocyanin content (mg gFW−1) | Treat. | 0.002 | 3 | 0.001 | 0.12 | 0.949 |
| Storage | 0.057 | 6 | 0.010 | 2.70 | 0.024 | |
| Treat. × Storage | 0.039 | 18 | 0.002 | 0.62 | 0.866 | |
| error | 0.168 | 48 | 0.004 | |||
| No. colony-forming unit (CFU gFW−1) | Treat. | 18.620 | 3 | 6.207 | 17.24 | <0.001 |
| Storage | 19.576 | 6 | 3.263 | 9.05 | <0.001 | |
| Treat. × Storage | 13.494 | 18 | 0.750 | 2.08 | 0.012 | |
| error | 34.605 | 96 | 0.361 |
| Treatment | Storage Duration (Weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| Weight loss (g) (n = 5) | UV A | (50.0 ± 0.78) | 0.07 ± 0.03 | 0.13 ± 0.03 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.39 ± 0.04 | 0.47 ± 0.03 |
| OG AB | (50.4 ± 0.36) | 0.07 ± 0.01 | 0.15 ± 0.04 | 0.23 ± 0.02 | 0.33 ± 0.02 | 0.41 ± 0.03 | 0.49 ± 0.03 | |
| OW C | (49.5 ± 0.84) | 0.09 ± 0.02 | 0.19 ± 0.01 | 0.30 ± 0.02 | 0.41 ± 0.03 | 0.53 ± 0.03 | 0.65 ± 0.05 | |
| Control BC | (49.7 ± 0.44) | 0.09 ± 0.02 | 0.17 ± 0.01 | 0.28 ± 0.02 | 0.39 ± 0.02 | 0.50 ± 0.03 | 0.61 ± 0.04 | |
| Soluble solids content (Brix) (n = 5) | UV | 14.5 ± 1.96 | 14.0 ± 1.04 | 12.6 ± 1.62 | 13.9 ± 0.43 | 12.8 ± 0.85 | 11.8 ± 0.93 | 12.9 ± 2.12 |
| OG | 13.8 ± 1.68 | 14.5 ± 1.34 | 13.9 ± 1.23 | 13.6 ± 1.91 | 13.3 ± 1.15 | 11.5 ± 1.89 | 13.1 ± 1.50 | |
| OW | 13.4 ± 1.36 | 13.4 ± 2.53 | 11.5 ± 0.71 | 12.3 ± 1.66 | 12.0 ± 2.85 | 13.1 ± 1.43 | 12.7 ± 0.68 | |
| Control | 14.3 ± 0.94 | 12.7 ± 2.32 | 13.8 ± 1.40 | 12.9 ± 1.45 | 13.5 ± 1.15 | 13.7 ± 2.12 | 10.4 ± 0.99 | |
| Acidity (%) (n = 5) | UV | 0.38 ± 0.10 | 0.36 ± 0.03 | 0.34 ± 0.10 | 0.39 ± 0.06 | 0.39 ± 0.05 | 0.38 ± 0.05 | 0.45 ± 0.07 |
| OG | 0.39 ± 0.09 | 0.37 ± 0.04 | 0.35 ± 0.01 | 0.40 ± 0.14 | 0.33 ± 0.03 | 0.43 ± 0.13 | 0.35 ± 0.04 | |
| OW | 0.39 ± 0.04 | 0.42 ± 0.09 | 0.41 ± 0.09 | 0.39 ± 0.13 | 0.38 ± 0.08 | 0.43 ± 0.08 | 0.40 ± 0.03 | |
| Control | 0.36 ± 0.02 | 0.40 ± 0.04 | 0.38 ± 0.06 | 0.43 ± 0.08 | 0.35 ± 0.04 | 0.39 ± 0.05 | 0.45 ± 0.07 | |
| Anthocyanin content (mg gFW−1) (n = 3) | UV | 0.84 ± 0.06 | 0.82 ± 0.04 | 0.93 ± 0.10 | 0.84 ± 0.03 | 0.76 ± 0.08 | 0.74 ± 0.05 | 0.74 ± 0.16 |
| OG | 0.84 ± 0.06 | 0.97 ± 0.08 | 0.86 ± 0.10 | 0.79 ± 0.05 | 0.78 ± 0.10 | 0.77 ± 0.10 | 0.76 ± 0.15 | |
| OW | 0.84 ± 0.04 | 0.90 ± 0.10 | 0.86 ± 0.10 | 0.79 ± 0.05 | 0.75 ± 0.20 | 0.83 ± 0.23 | 0.83 ± 0.06 | |
| Control | 0.79 ± 0.07 | 0.94 ± 0.02 | 0.82 ± 0.07 | 0.89 ± 0.14 | 0.82 ± 0.20 | 0.83 ± 0.05 | 0.70 ± 0.10 | |
| SS | Df | MS | F | p | ||
|---|---|---|---|---|---|---|
| Weight loss (g) | Ozone | 0.291 | 1 | 0.291 | 4.882 | 0.078 |
| Storage | 1.284 | 5 | 0.257 | 11.616 | <0.001 | |
| Ozone × Storage | 0.074 | 5 | 0.015 | 0.670 | 0.650 | |
| error | 0.553 | 25 | 0.022 | |||
| Soluble solids content (Brix) | Ozone | 0.000 | 1 | <0.001 | 3.401 | 0.102 |
| Storage | 0.011 | 6 | 0.002 | 13.171 | <0.001 | |
| Ozone × Storage | 0.001 | 6 | <0.001 | 1.475 | 0.207 | |
| error | 0.007 | 48 | <0.001 | |||
| Acidity (%) | Ozone | 0.000 | 1 | 0.000 | 0.090 | 0.772 |
| Storage | 0.847 | 6 | 0.141 | 62.568 | <0.001 | |
| Ozone × Storage | 0.011 | 6 | 0.002 | 0.803 | 0.573 | |
| error | 0.108 | 48 | 0.002 | |||
| Anthocyanin content (mg gFW−1) | Ozone | 0.104 | 1 | 0.104 | 12.904 | 0.007 |
| Storage | 0.528 | 6 | 0.088 | 16.850 | <0.001 | |
| Ozone × Storage | 0.023 | 6 | 0.004 | 0.725 | 0.631 | |
| error | 0.251 | 48 | 0.005 | |||
| Pericarp firmness (gf) | Ozone | 0.113 | 1 | 0.113 | 9.727 | 0.014 |
| Storage | 0.128 | 6 | 0.021 | 0.893 | 0.508 | |
| Ozone × Storage | 0.129 | 6 | 0.022 | 0.906 | 0.499 | |
| error | 1.143 | 48 | 0.024 | |||
| No. colony forming unit (CFU gFW−1) | Ozone | 14.167 | 1 | 14.167 | 59.524 | <0.001 |
| Storage | 10.828 | 6 | 1.805 | 5.106 | <0.001 | |
| Ozone × Storage | 9.919 | 6 | 1.653 | 4.678 | <0.001 | |
| error | 29.687 | 84 | 0.353 |
| Treat. | Storage Duration (Weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| Weight loss (g) (n = 4) | Ozone | (51.9 ± 0.26) | 0.29 ± 0.08 | 0.36 ± 0.18 | 0.39 ± 0.57 | 0.67 ± 0.33 | 0.82 ± 0.31 | 0.96 ± 0.26 |
| Control | (52.6 ± 0.27) | 0.33 ± 0.05 | 0.33 ± 0.16 | 0.41 ± 0.12 | 0.60 ± 0.11 | 0.65 ± 0.06 | 0.75 ± 0.07 | |
| Soluble solids content (Brix) (n = 5) | Ozone | 14.5 ± 1.24 | 16.2 ± 1.05 | 13.0 ± 1.57 | 13.4 ± 1.51 | 13.6 ± 1.02 | 12.3 ± 0.70 | 11.3 ± 0.78 |
| Control | 14.8 ± 1.47 | 11.8 ± 1.16 | 13.4 ± 0.93 | 11.8 ± 1.35 | 12.4 ± 0.52 | 11.9 ± 1.36 | ||
| Acidity (%) (n = 5) | Ozone | 0.54 ± 0.05 | 0.55 ± 0.05 | 0.35 ± 0.04 | 0.36 ± 0.06 | 0.30 ± 0.05 | 0.30 ± 0.02 | 0.28 ± 0.04 |
| Control | 0.50 ± 0.04 | 0.33 ± 0.04 | 0.37 ± 0.06 | 0.29 ± 0.04 | 0.31 ± 0.05 | 0.31 ± 0.04 | ||
| Anthocyanin content (mg gFW−1) (n = 5) | Ozone A | 1.07 ± 0.23 | 1.12 ± 0.13 | 0.86 ± 0.06 | 0.93 ± 0.13 | 0.76 ± 0.14 | 0.69 ± 0.06 | 0.68 ± 0.17 |
| Control B | 0.85 ± 0.11 | 0.71 ± 0.11 | 0.79 ± 0.18 | 0.65 ± 0.05 | 0.59 ± 0.17 | 0.52 ± 0.06 | ||
| Pericarp firmness (gf) (n = 5) | Ozone A | 184.2 ± 52.0 | 172.7 ± 54.9 | 181.1 ± 64.2 | 189.5 ± 44.7 | 172.5 ± 30.4 | 235.4 ± 73.8 | 171.5 ± 30.1 |
| Control B | 168.9 ± 17.0 | 185.0 ± 41.1 | 159.7 ± 56.7 | 142.4 ± 62.1 | 157.6 ± 75.7 | 125.4 ± 68.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, Y.; Hojo, D.; Sato, K.; Inubushi, N.; Miyashita, C.; Inoue, E.; Mochizuki, Y. Long-Term Blueberry Storage by Ozonation or UV Irradiation Using Excimer Lamp. AgriEngineering 2025, 7, 269. https://doi.org/10.3390/agriengineering7080269
Takano Y, Hojo D, Sato K, Inubushi N, Miyashita C, Inoue E, Mochizuki Y. Long-Term Blueberry Storage by Ozonation or UV Irradiation Using Excimer Lamp. AgriEngineering. 2025; 7(8):269. https://doi.org/10.3390/agriengineering7080269
Chicago/Turabian StyleTakano, Yujiro, Daichi Hojo, Kosuke Sato, Noe Inubushi, Chieto Miyashita, Eiichi Inoue, and Yuya Mochizuki. 2025. "Long-Term Blueberry Storage by Ozonation or UV Irradiation Using Excimer Lamp" AgriEngineering 7, no. 8: 269. https://doi.org/10.3390/agriengineering7080269
APA StyleTakano, Y., Hojo, D., Sato, K., Inubushi, N., Miyashita, C., Inoue, E., & Mochizuki, Y. (2025). Long-Term Blueberry Storage by Ozonation or UV Irradiation Using Excimer Lamp. AgriEngineering, 7(8), 269. https://doi.org/10.3390/agriengineering7080269






