Phenolic Compounds from Pineapple Crown: Comparative Assessment of Fermentation and Conventional Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Drying the Pineapple Crown
2.2. Extraction Methods of Phenolic Compounds
2.2.1. Dynamic Maceration Extraction
2.2.2. Soxhlet Extraction
2.2.3. Extraction by Submerged Fermentation
2.2.4. Enzyme Assay
2.3. Analysis of Total Phenolic Compounds
2.3.1. Analysis of Phenols
2.3.2. Phenol Analysis by the FastBlue BB Method
2.4. Evaluation of the Chemical Profile of Methanolic Extracts by High-Performance Liquid Chromatography (HPLC)
2.5. Statistical Analysis
3. Results
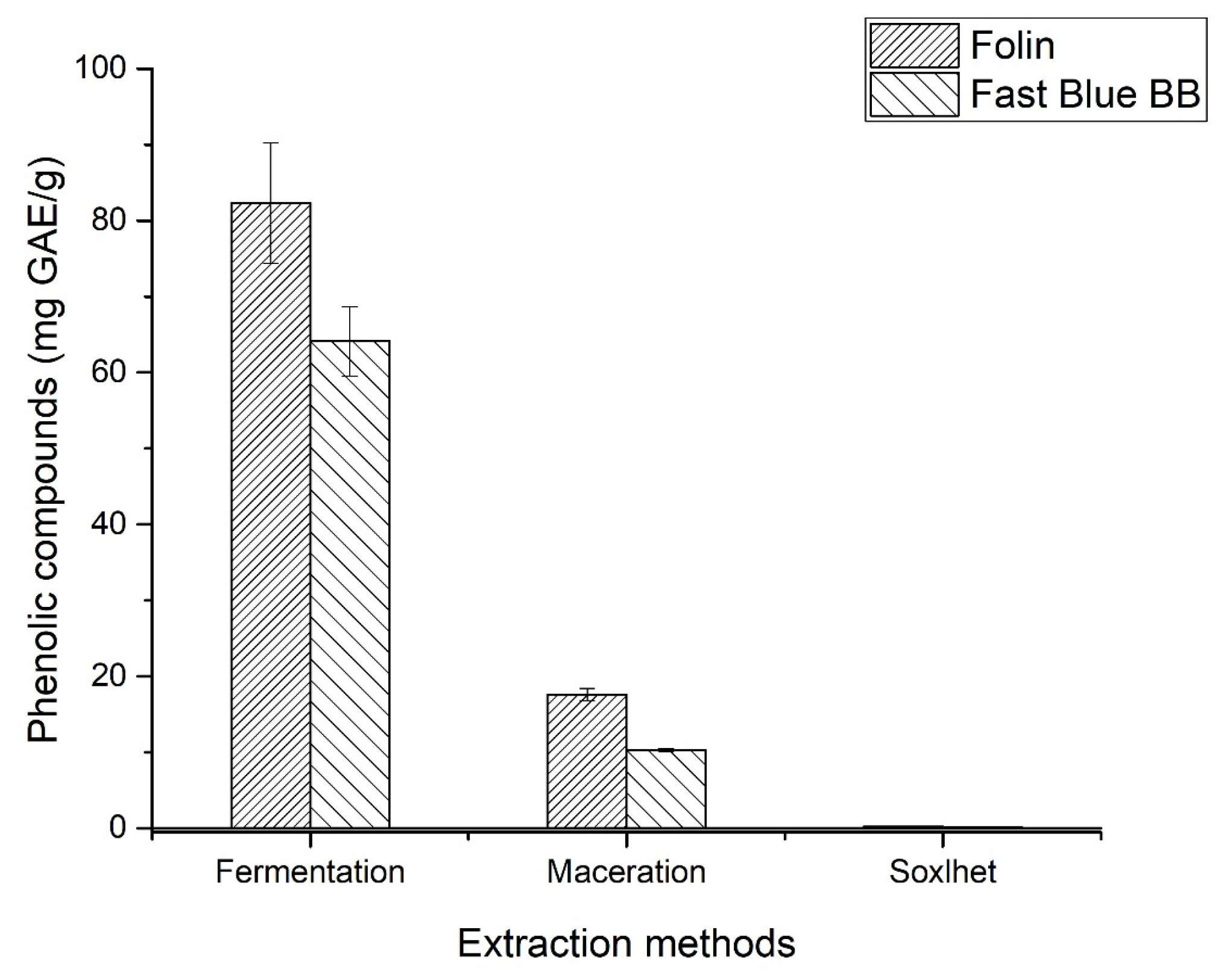
3.1. Quantification of Total Phenolic Compounds
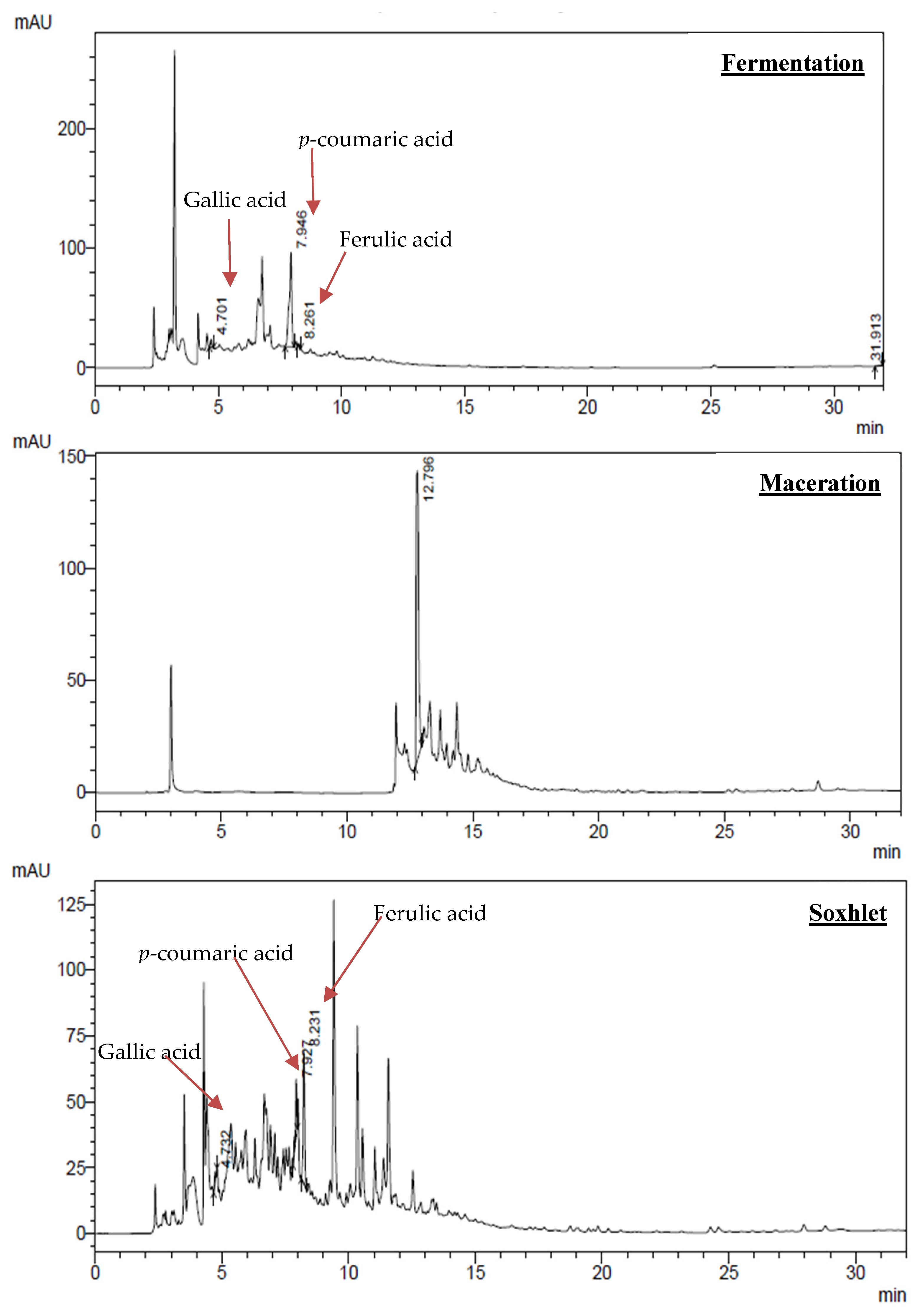
3.2. Chemical Profiling by High-Performance Liquid Chromatography (HPLC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UENF | Northern Fluminense State University Darcy Ribeiro |
| FTL | Food Technology Laboratory |
| SDG | Sustainable Development Goal |
| HPLC | High-Performance Liquid Chromatography |
| PFR | Pineapple crown residue flour |
References
- Embrapa-Brazilian Agricultural Research Corporation (2021) Technical Bulletin; Embrapa-Brazilian Agricultural Research Corporation: Brasília, Brazil, 2021; 12p.
- Matos, A.P. Abacaxi: O Produtor Pergunta, a Embrapa Responde, 2nd ed.; Associação Brasileira de Milho e Sorgo: Sete Lagoas, Brazil; Inovações, Milho e Sorgo: Bento Gonçalves, Brazil, 2013. [Google Scholar]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Mardawati, E.; Putri, S.; Fitriana, H.; Nurliasari, D.; Rahmah, D.R.; Maulana, I.; Dewantoro, A.; Hermiati, E.; Balia, R. Aplicação do conceito de biorrefinaria à produção de bromelaína, etanol e xilitol a partir de resíduos de abacaxi. Fermentation 2023, 9, 816. [Google Scholar] [CrossRef]
- Hamzah, A.; Hamzah, M.; Man, C.; Jamali, N.; Siajam, S.; Ismail, M. Atualizações recentes sobre a conversão de resíduos de abacaxi (Ananas comosus) em produtos de valor agregado, perspectivas futuras e desafios. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- Castillo-González, E.; Giraldi-Díaz, M.; De Medina-Salas, L.; Sánchez-Castillo, M. Pré-Compostagem e Vermicompostagem de Abacaxi (Ananas comosus) e Resíduos Vegetais. Appl. Sci. 2019, 9, 3564. [Google Scholar] [CrossRef]
- Bernardo, S.P.C.; Rosana, A.R.R.; de Souza, A.N.; Chiorean, S.; Martins, M.L.L.; Vederas, J.C. Draft genome sequence of the thermophilic bacterium Bacillus licheniformis SMIA-2, an antimicrobial-and thermostable enzyme-producing isolate from Brazilian soil. Microbiol. Resour. Announc. 2020, 9, e00106-20. [Google Scholar] [CrossRef]
- Souza, A.N.D.; Martins, M.L.L. Isolation, properties and kinetics of growth of a thermophilic Bacillus. Braz. J. Microbiol. 2001, 32, 271–275. [Google Scholar] [CrossRef]
- Barbosa, J.B.; Gentil, N.O.; Ladeira, S.A.; Martins, M.L.L. Addendum to Issue 1-ENZITEC 2012 Cheese whey and passion fruit rind flour as substrates for protease production by Bacillus sp. SMIA-2 strain isolated from Brazilian soil. Biocatal. Biotransform. 2014, 32, 244–250. [Google Scholar] [CrossRef]
- Lim, Z.E.; Thai, Q.B.; Le, D.K.; Luu, T.P.; Nguyen, P.T.; Do, N.H.; Duong, H.M. Functionalized pineapple aerogels for ethylene gas adsorption and nickel (II) ion removal applications. J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Jalil, A.A.; Lee, T. A critical review on pineapple (Ananas comosus) wastes for water treatment, challenges and future prospects towards circular economy. Sci. Total Environ. 2023, 856, 158817. [Google Scholar] [CrossRef]
- Barretto, L.C.D.O.; Moreira, J.D.J.D.S.; Santos, J.A.B.D.; Narendra, N.; Santos, R.A.R.D. Characterization and extraction of volatile compounds from pineapple (Ananas comosus L. Merril) processing residues. Food Sci. Technol. 2013, 33, 638–645. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kumar, V.; Singh, K.; Kumar, R.; Kumar, V. Pineapple (Ananas cosmosus) product processing: A review. J. Pharmacogn. Phytochem. 2019, 8, 4642–4652. [Google Scholar]
- Roda, A.; Lambri, M. Food uses of pineapple waste and byproducts: A review. Int. J. Food Sci. Technol. 2019, 54, 1009–1017. [Google Scholar] [CrossRef]
- Huang, Y.; Chow, C.; Fang, Y. Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J. Food Drug Anal. 2011, 19, 4. [Google Scholar] [CrossRef]
- Seenak, P.; Kumphune, S.; Malakul, W.; Chotima, R.; Nernpermpisooth, N. Pineapple consumption reduced cardiac oxidative stress and inflammation in high cholesterol diet-fed rats. Nutr. Metab. 2021, 18, 36. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (byproducts). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Cong-Cong, X.U.; Bing, W.A.N.G.; Yi- Qiong, P.U.; Jian-Sheng, T.A.O. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef]
- Susanti, I.; Pratiwi, R.; Rosandi, Y.; Hasanah, A.N. Separation methods of phenolic compounds from plant extract as antioxidant agents candidate. Plants 2024, 13, 965. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.D.M. Extraction systems and analytical Techniques for food phenolics compounds: A review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Şen, U.; Viegas, C.; Duarte, M.P.; Maurício, E.M.; Nobre, C.; Correia, R.; Gonçalves, M. Maceration of Waste Cork in Binary Hydrophilic Solvents for the Production of Functional Extracts. Environments 2023, 10, 142. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food waste. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from the food industry byproducts. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- de Los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial byproducts. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial Wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Li, L.; Gu, T.; Chen, S.; Wang, J.; Gao, M. The Release of Bound Phenolics to Enhance the Antioxidant Activity of Cornmeal by Liquid Fermentation with Bacillus subtilis. Foods 2025, 14, 499. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Gulsunoglu-Konuskan, Z.; e Kilic-Akyilmaz, M. Microbial bioconversion of phenolic compounds in agro-industrial wastes: A review of mechanisms and effective factors. J. Agric. Food Chem. 2022, 70, 6901–6910. [Google Scholar] [CrossRef]
- Madeira Junior, J.V.; Teixeira, C.B.; e Macedo, G.A. Biotransformation and bioconversion of phenolic compounds obtainment: An overview. Crit. Rev. Biotechnol. 2015, 35, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, P.K.; Singh, T.A.; Singh, N.J.; Shadangi, K.P.; Srivastava, R.K.; Singh, A.K.; Vivekanand, V. Sustainable utilization of pineapple waste for production of bioenergy, biochemicals and value-added products: A review. Bioresour. Technol. 2022, 351, 127085. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent progress and future perspectives for zero agriculture waste Technologies: Pineapple waste as a case study. Sustainability 2023, 15, 3575. [Google Scholar] [CrossRef]
- Sukruansuwan, V.; Napathorn, S.C. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain A-04. Biotechnol. Biofuels 2018, 11, 202. [Google Scholar] [CrossRef]
- Chaudhary, S.; Singh, B. Pineapple byproducts utilization: Progress towards the circular economy. Food Humanit. 2024, 2, 100243. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; de Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009, 115, 469–475. [Google Scholar] [CrossRef]
- Jenssen, P.H.; Peek, K.; Morgan, H.W. Effect of culture conditions on the production of an extracellular proteinase by Thermus sp. Rt41A. Appl. Microbiol. Biotechnol. 1994, 41, 400–406. [Google Scholar] [CrossRef]
- Cruz, E. Cellulases from Bacillus sp. SMIA-2: Drying and Use for Formulation Development Ecological Cleaning. Ph.D. Thesis, University State of Northern Fluminense Darcy Ribeiro, Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Zhou, Y.; Cao, Y.; Li, J.; Agar, O.T.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Screening and characterization of phenolic compounds by LC-ESI-QTOF-MS/MS and their antioxidant potentials in papaya fruit and their byproduct activities. Food Biosci. 2023, 52, 102480. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L. Plants 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Pismag, R.; Laudouze, M.; Martinez, M. Systematic evaluation of the Folin-Ciocalteu and Fast Blue BB reactions during the analysis of total phenolics in vegetables, nuts and plant seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.; Lewers, K.; Medina, M.; Saftner, R. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How does it Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Rudiana, T.; Nurbayti, S.; Ashari, T.H.; Zhorif, S.A.; Suryani, N. Comparison of maceration and Soxhletation methods on the antioxidant activity of the Bouea macrophylla Griff plant. J. Kim. Val. 2023, 9, 244–252. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperature on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Maillard, M.N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Strieder, M.; De Oliveira, I.; Bragagnolo, F.; Sanches, V.; Pizani, R.; De Souza Mesquita, L.; Rostagno, M. Consistency of Phenolic Compounds in Plant Residues Parts: A Review of Primary Sources, Key Compounds, and Extraction Trends. J. Agric. Food Chem. 2025, 73, 11515–11534. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef]
- Leite, P.; Belo, I.; Salgado, J.M. Enhancing Antioxidants Extraction from Agro-Industrial Byproducts by Enzymatic Treatment. Foods 2022, 11, 3715. [Google Scholar] [CrossRef]
- Brito, T.B.N.; Moreira, R.C.; Carvalho, M.G.O.; Arruda, H.S.; Neri-Numa, I.A.; Pastore, G.M.; Ferreira, M.S.L.; Fai, A.E.C.; Bicas, J.L. from pineapple crown to multi-targeting bioactive compounds by solid-state fermentation using Aspergillus tubingensis. Food Res. Int. 2025, 220, 117060. [Google Scholar] [CrossRef]
- Li, N.; Du, B.; Ren, X.; Yang, L.; Du, P.; Li, P.; Wang, J.; Li, J.; Xiao, J.; Wang, J.; et al. Intracellular self-assembly and metabolite analysis of key enzymes for L-lysine synthesis based on key components of cellulosomes. Front. Microbiol 2025, 16, 1596240. [Google Scholar] [CrossRef]
- Fowler, Z.L.; Koffas, M.A. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [Google Scholar] [CrossRef]
- Steingass, C.; Glock, M.; Schweiggert, R.; Carle, R. Studies on the phenolic patterns of different tissues of the pineapple (Ananas comosus [L.] Merr.) by HPLC-DAD-ESI-MSn and GC-MS analyses. Anal. Bioanal. Chem. 2015, 407, 6463–6479. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2018, 18, 336–341. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Mulțescu, M.; Marinaș, I.; Susman, I.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Campos, M.G.; Markham, K.R. Structure Information from HPLC and On-Line Measured Absorption Spectra; Coimbra University Press: Coimbra, Portugal, 2007; pp. 1–122. [Google Scholar]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Molaei, S.; Tehrani, A.D.; Shamlouei, H. Antioxidant activates of new carbohydrate based gallate derivatives: A DFT study. J. Mol. Liq. 2023, 377, 121506. [Google Scholar] [CrossRef]
- de Souza, G.L.; Peterson, K.A. Benchmarking antioxidant-related properties for gallic acid through the use of DFT, MP2, CCSD, and CCSD (T) approaches. J. Phys. Chem. A 2021, 125, 198–208. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The antioxidant properties, metabolism, application and mechanism of ferulic acid in medicine, food, cosmetics, livestock and poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Mancuso, A.; Cristiano, M.C.; Pandolfo, R.; Greco, M.; Fresta, M.; Paolino, D. Improvement of ferulic acid antioxidant activity by multiple emulsions. Nanomaterials 2021, 11, 425. [Google Scholar] [CrossRef]
- Lambruschini, C.; Demori, I.; El Rashed, Z.; Rovegno, L.; Canessa, E.; Cortese, K.; Moni, L. Synthesis, photoisomerization, antioxidant activity, and lipid-lowering effect of ferulic acid and feruloyl amides. Molecules 2020, 26, 89. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 115, 719–724. [Google Scholar] [CrossRef]
- Plazonić, A.; Bucar, F.; Maleš, Ž.; Mornar, A.; Nigović, B.; Kujundẑić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef]
- Shui, G.; Leong, L. Separation and determination of organic acids and phenolic compounds in fruit juices and beverages by high-performance liquid chromatography. J. Chromatography. A 2002, 977, 89–96. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.B.; Guo, B. Quantitative and qualitative analysis of flavonoids and phenolic acids in snow chrysanthemum (Coreopsis dyeing Nutt) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [Google Scholar] [CrossRef]
| Avicelase activity | 0.384 U mL−1 |
| CMCase activity | 0.03 U mL−1 |
| Polygalacturonase activity | 2.839 U mL−1 |
| Phenolic compounds | 82.3 mg GAE g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.T.M.d.; Carvalho, A.L.P.B.; Pereira, S.M.d.F.; Martins, M.L.L.; Oliveira, E.R.M.d.; Silva, T.C.d.; Vieira, H.D.; Oliveira, D.B.d. Phenolic Compounds from Pineapple Crown: Comparative Assessment of Fermentation and Conventional Extraction Methods. AgriEngineering 2025, 7, 409. https://doi.org/10.3390/agriengineering7120409
Souza TTMd, Carvalho ALPB, Pereira SMdF, Martins MLL, Oliveira ERMd, Silva TCd, Vieira HD, Oliveira DBd. Phenolic Compounds from Pineapple Crown: Comparative Assessment of Fermentation and Conventional Extraction Methods. AgriEngineering. 2025; 7(12):409. https://doi.org/10.3390/agriengineering7120409
Chicago/Turabian StyleSouza, Taynara Thais Manhães de, Ana Lúcia Paes Barbosa Carvalho, Silvia Menezes de Faria Pereira, Meire Lelis Leal Martins, Emilly Rita Maria de Oliveira, Tuane Cristina da Silva, Henrique Duarte Vieira, and Daniela Barros de Oliveira. 2025. "Phenolic Compounds from Pineapple Crown: Comparative Assessment of Fermentation and Conventional Extraction Methods" AgriEngineering 7, no. 12: 409. https://doi.org/10.3390/agriengineering7120409
APA StyleSouza, T. T. M. d., Carvalho, A. L. P. B., Pereira, S. M. d. F., Martins, M. L. L., Oliveira, E. R. M. d., Silva, T. C. d., Vieira, H. D., & Oliveira, D. B. d. (2025). Phenolic Compounds from Pineapple Crown: Comparative Assessment of Fermentation and Conventional Extraction Methods. AgriEngineering, 7(12), 409. https://doi.org/10.3390/agriengineering7120409







