Conductive Textiles for Signal Sensing and Technical Applications
Abstract
:1. Introduction
2. Materials Used to Impart Conductivity on Textiles
2.1. Conductive Polymers
2.1.1. Polypyrrole (PPy)
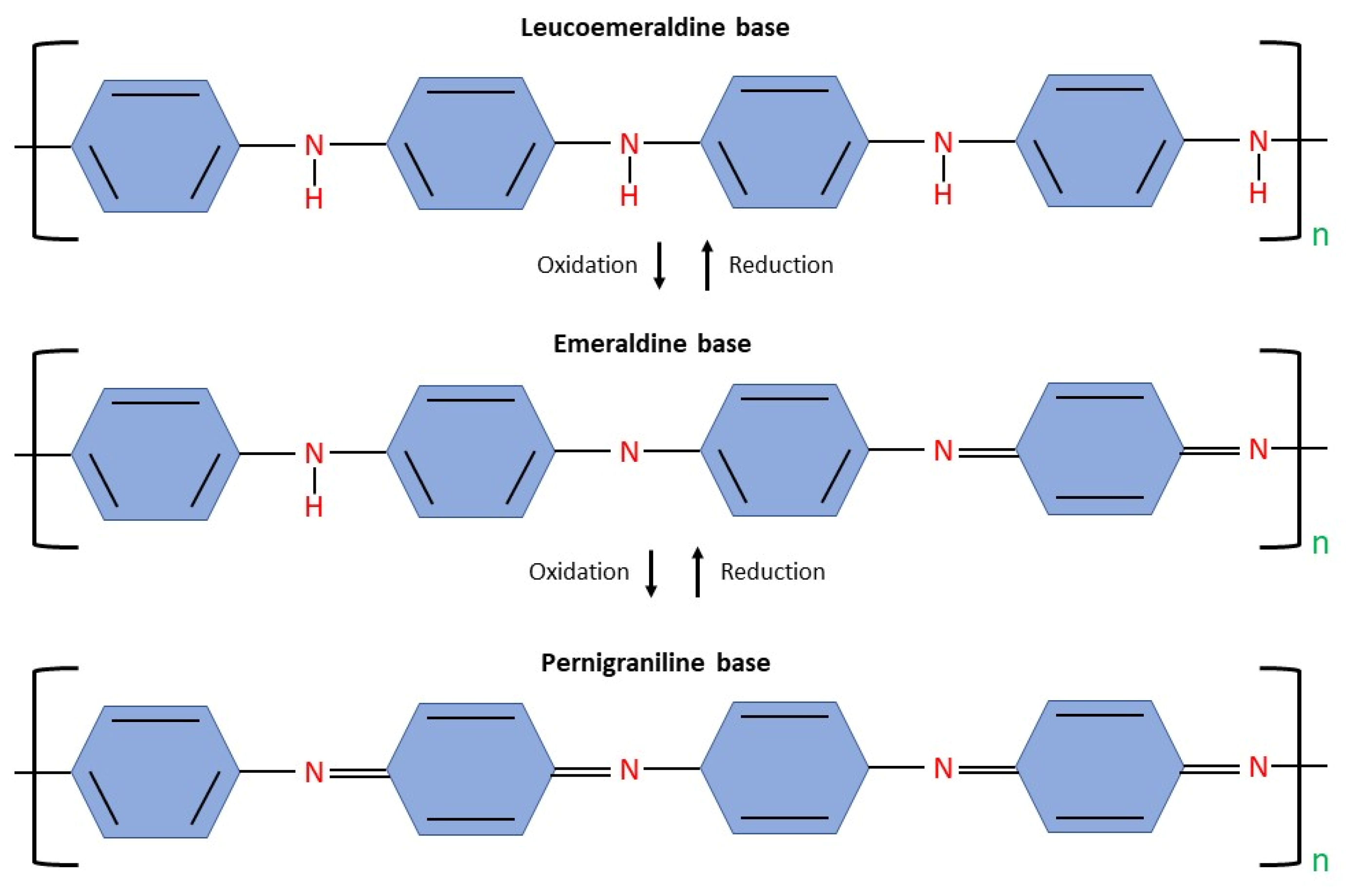
2.1.2. Polyaniline (PANI)
- (a).
- Leucoemeraldine (C6H4NH)n—100% reduction level
- (b).
- Emeraldine ([C6H4NH]2[C6H4N]2)n—50% oxidation, 50% reduction
- (c).
- Pernigraniline (C6H4N)n—100% oxidation level
2.1.3. Poly(3,4-ethylenedioxythiophene) (PEDOT)
2.2. Conductive Metals
2.2.1. Silver Nanoparticles (AgNPs)
2.2.2. Gold Nanoparticles (AuNPs)
2.2.3. Copper Nanoparticles (CuNPs)
2.2.4. Metal Oxide Nanoparticles (MONPs)
2.3. Conductive Non-Metals
2.3.1. Carbon Nanotube (CNTs)
2.3.2. Graphene
3. Fabrication Processes of Conductive Textiles
3.1. Deposition Method
3.1.1. Vapor Deposition
- Physical Vapor Deposition (PVD)
- 2.
- Chemical Vapor Deposition (CVD)
3.1.2. Layer-by-Layer Deposition (LbLD)
3.1.3. Electrochemical Deposition
3.1.4. Electroless Deposition
3.2. In Situ Polymerization
3.3. Coating
3.3.1. Dip Coating
3.3.2. Rod Coating
3.3.3. Roller Coating
3.4. Printing
3.4.1. Screen Printing
3.4.2. Inkjet Printing
3.4.3. 3D Printing
4. Testing for Conductive Textiles
4.1. Conductivity Testing
4.2. Electromagnetic Shielding (EMS) Testing
- -
- Reflection coefficient, which depicts the amount of reflected EM wave under an impedance discontinuity in the transmission medium;
- -
- Absorption coefficient, which signifies a parameter on how much energy from an EMR (electromagnetic radiation) wave a material can absorb;
- -
- Transmission coefficient, which indicates the quantity of EMR penetration through a shield.
4.2.1. Open-Field Test Method
- Calibration measurement of the transmitted signal without material (P0);
- Measurements of the transmitted signal as a function of incident angle with material (P1);
- The ratio of P1/P0 is the power transmittance required to determine complex permittivity.
4.2.2. Shielded Box Method
- Low range—9 kHz to 20 MHz—applicable for magnetic components (H);
- Resonant range—20 MHz to 300 MHz—applicable for electrical components (E);
- High range—300 MHz-18 GHz (100 GHz)—in case of plane wave power (P).
4.2.3. Shielded Room Method
4.2.4. Coaxial Transmission Line Method (Transverse Electromagnetic Cell Method)
5. Application of Conductive Textiles
5.1. As Electronic Textiles (E-Textiles) for Bio-Sensing and Health Care
5.2. Soft Robotics
5.3. Other E-textiles Applications
5.4. EM Shielding Textiles
5.5. Supercapacitor
6. Challenges and Future Outlook
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mattila, H. Chapter 15—Yarn to Fabric: Intelligent Textiles. In Textiles and Fashion; Sinclair, R., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 355–376. [Google Scholar] [CrossRef]
- Guler, S.D.; Gannon, M.; Sicchio, K. (Eds.) A Brief History of Wearables. In Crafting Wearables: Blending Technology with Fashion; Apress: Berkeley, CA, USA, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT:PSS-Based Conductive Textiles and Their Applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, A.R. Challenges for eco-design of emerging technologies: The case of electronic textiles. Mater. Des. 2013, 51, 51–60. [Google Scholar] [CrossRef]
- Cherenack, K.; van Pieterson, L. Smart textiles: Challenges and opportunities. J. Appl. Phys. 2012, 112, 091301. [Google Scholar] [CrossRef] [Green Version]
- Hughes-Riley, T.; Dias, T.; Cork, C. A Historical Review of the Development of Electronic Textiles. Fibers 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Varnaitė-Žuravliova, S. The Types, Properties, and Applications of Conductive Textiles; Cambridge Scholars Publishing: Newcastle Upon Tyne, UK, 2019. [Google Scholar]
- Grancarić, A.M.; Jerkovic, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive polymers for smart textile applications. J. Ind. Text. 2017, 48, 612–642. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.-J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar] [CrossRef]
- Tatari, A.; Shekarian, E.; Ghaffari, M.; Akbarpour, I. Production and applications of paper-conductive polymer composites. J. Prot. Exploit. Nat. Resour. 2017, 6, 27–37. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Machida, S.; Miyata, S.; Techagumpuch, A. Chemical synthesis of highly electrically conductive polypyrrole. Synth. Met. 1989, 31, 311–318. [Google Scholar] [CrossRef]
- Noh, K.A.; Kim, D.-W.; Jin, C.-S.; Shin, K.-H.; Kim, J.H.; Ko, J.M. Synthesis and pseudo-capacitance of chemically-prepared polypyrrole powder. J. Power Sources 2003, 124, 593–595. [Google Scholar] [CrossRef]
- Stankovic, R.; Laninović, V.; Vojnović, M.; Pavlović, O.; Krstajić, N.; Jovanovic, S. Synthesis and Electrochemical Properties of Polypyrrole. Mater. Sci. Forum 1996, 214, 147–154. [Google Scholar] [CrossRef]
- Park, J.H.; Ko, J.M.; Park, O.; Kim, D.-W. Capacitance properties of graphite/polypyrrole composite electrode prepared by chemical polymerization of pyrrole on graphite fiber. J. Power Sources 2002, 105, 20–25. [Google Scholar] [CrossRef]
- An, K.H.; Jeon, K.K.; Heo, J.K.; Lim, S.C.; Bae, D.J.; Lee, Y.H. High-Capacitance Supercapacitor Using a Nanocomposite Electrode of Single-Walled Carbon Nanotube and Polypyrrole. J. Electrochem. Soc. 2002, 149, A1058–A1062. [Google Scholar] [CrossRef]
- An, K.H.; Jeong, S.Y.; Hwang, H.R.; Lee, Y.H. Enhanced Sensitivity of a Gas Sensor Incorporating Single-Walled Carbon Nanotube–Polypyrrole Nanocomposites. Adv. Mater. 2004, 16, 1005–1009. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Tang, Q.; Lan, Z.; Li, P.; Lin, J.; Fan, L. Application of microporous polyaniline counter electrode for dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1299–1302. [Google Scholar] [CrossRef]
- Kulkarni, V.G.; Campbell, L.D.; Mathew, W.R. Thermal stability of polyaniline. Synth. Met. 1989, 30, 321–325. [Google Scholar] [CrossRef]
- Mengoli, G.; Musiani, M.M.; Zotti, G.; Valcher, S. Potentiometric investigation of the kinetics of the polyaniline—Oxygen reaction. J. Electroanal. Chem. Interfacial Electrochem. 1986, 202, 217–230. [Google Scholar] [CrossRef]
- Stejskal, J.; Kratochvíl, P.; Jenkins, A.D. Polyaniline: Forms and Formation. Collect. Czechoslov. Chem. Commun. 1995, 60, 1747–1755. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Chapter 14—Application of polyaniline and its derivatives. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–272. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.; Lim, H. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tian, M.; Qu, L.; Zhu, S.; Guo, X.; Han, G.; Sun, K.; Hu, X.; Wang, Y.; Xu, X. Functionalization of cotton fabric with graphene oxide nanosheet and polyaniline for conductive and UV blocking properties. Synth. Met. 2015, 202, 82–88. [Google Scholar] [CrossRef]
- Wu, W.; Wang, B.; Segev-Bar, M.; Dou, W.; Niu, F.; Horev, Y.D.; Deng, Y.; Plotkin, M.; Huynh, T.-P.; Jeries, R.; et al. Free-Standing and Eco-Friendly Polyaniline Thin Films for Multifunctional Sensing of Physical and Chemical Stimuli. Adv. Funct. Mater. 2017, 27, 1703147. [Google Scholar] [CrossRef]
- McCullough, R.D. The Chemistry of Conducting Polythiophenes: From Synthesis to Self-Assembly to Intelligent Materials. In Handbook of Oligo- and Polythiophenes; John Wiley & Sons: New York, NY, USA, 1998; pp. 1–44. [Google Scholar] [CrossRef]
- Sotzing, G.A.; Reynolds, J.R.; Steel, P.J. Poly(3,4-ethylenedioxythiophene) (PEDOT) prepared via electrochemical polymerization of EDOT, 2,2′-Bis(3,4-ethylenedioxythiophene) (BiEDOT), and their TMS derivatives. Adv. Mater. 1997, 9, 795–798. [Google Scholar] [CrossRef]
- Fabretto, M.; Jariego-Moncunill, C.; Autere, J.-P.; Michelmore, A.; Short, R.; Murphy, P. High conductivity PEDOT resulting from glycol/oxidant complex and glycol/polymer intercalation during vacuum vapour phase polymerisation. Polymer 2011, 52, 1725–1730. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- O’Connor, T.F.; Zaretski, A.V.; Savagatrup, S.; Printz, A.D.; Wilkes, C.D.; Diaz, M.I.; Sawyer, E.J.; Lipomi, D.J. Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol. Energy Mater. Sol. Cells 2016, 144, 438–444. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Ge, R.; Qin, F.; Dong, X.; Meng, W.; Liu, T.; Tong, J.; Jiang, F.; Zhou, Y.; et al. Free-Standing Conducting Polymer Films for High-Performance Energy Devices. Angew. Chem. Int. Ed. 2015, 55, 979–982. [Google Scholar] [CrossRef]
- Ding, Y.; Invernale, M.A.; Sotzing, G.A. Conductivity Trends of PEDOT-PSS Impregnated Fabric and the Effect of Conductivity on Electrochromic Textile. ACS Appl. Mater. Interfaces 2010, 2, 1588–1593. [Google Scholar] [CrossRef]
- Hamedi, M.; Herland, A.; Karlsson, R.H.; Inganäs, O. Electrochemical Devices Made from Conducting Nanowire Networks Self-Assembled from Amyloid Fibrils and Alkoxysulfonate PEDOT. Nano Lett. 2008, 8, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Tao, X.-M.; Wang, R.-X. Fabrication and characterization of OLEDs using PEDOT:PSS and MWCNT nanocomposites. Compos. Sci. Technol. 2008, 68, 2837–2841. [Google Scholar] [CrossRef]
- Li, Z.; Sinha, S.K.; Treich, G.M.; Wang, Y.; Yang, Q.; Deshmukh, A.A.; Sotzing, G.A.; Cao, Y. All-organic flexible fabric antenna for wearable electronics. J. Mater. Chem. C 2020, 8, 5662–5667. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene–metallic textile composite electrodes. Nat. Commun. 2015, 6, 7260. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Fukuyama, Y.; Sunda, T.; Lin, B.; Zhou, J.; Takizawa, J.; Ohmori, A.; Kimura, M. Foldable Textile Electronic Devices Using All-Organic Conductive Fibers. Adv. Eng. Mater. 2014, 16, 550–555. [Google Scholar] [CrossRef]
- Liu, N.; Fang, G.; Wan, J.; Zhou, H.; Long, H.; Zhao, X. Electrospun PEDOT:PSS–PVA nanofiber based ultrahigh-strain sensors with controllable electrical conductivity. J. Mater. Chem. 2011, 21, 18962–18966. [Google Scholar] [CrossRef]
- Lock, J.P.; Im, S.G.; Gleason, K.K. Oxidative Chemical Vapor Deposition of Electrically Conducting Poly(3,4-ethylenedioxythiophene) Films. Macromolecules 2006, 39, 5326–5329. [Google Scholar] [CrossRef]
- Brooke, R.; Mitraka, E.; Sardar, S.; Sandberg, M.; Sawatdee, A.; Berggren, M.; Crispin, X.; Jonsson, M.P. Infrared electrochromic conducting polymer devices. J. Mater. Chem. C 2017, 5, 5824–5830. [Google Scholar] [CrossRef] [Green Version]
- Fabretto, M.; Autere, J.-P.; Hoglinger, D.; Field, S.; Murphy, P. Vacuum vapour phase polymerised poly(3,4-ethyelendioxythiophene) thin films for use in large-scale electrochromic devices. Thin Solid Films 2011, 519, 2544–2549. [Google Scholar] [CrossRef]
- Kaushik, V.; Lee, J.; Hong, J.; Lee, S.; Lee, S.; Seo, J.; Mahata, C.; Lee, T. Textile-Based Electronic Components for Energy Applications: Principles, Problems, and Perspective. Nanomaterials 2015, 5, 1493–1531. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Cai, K.; Du, Y.; Chen, L. Preparation and thermoelectric properties of SWCNT/PEDOT:PSS coated tellurium nanorod composite films. J. Alloy. Compd. 2018, 778, 163–169. [Google Scholar] [CrossRef]
- Chen, M.; Duan, S.; Zhang, L.; Wang, Z.; Li, C. Three-dimensional porous stretchable and conductive polymer composites based on graphene networks grown by chemical vapour deposition and PEDOT:PSS coating. Chem. Commun. 2014, 51, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, Y.; Neudeck, A.; Möhring, U. Metal-coated fibers. In Inorganic and Composite Fibers: Production, Properties, and Applications; Elsevier: Cambridge, UK, 2018; pp. 243–276. [Google Scholar] [CrossRef]
- Montazer, M.; Harifi, T. Nanofinishing of Textile Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Atwa, Y.; Maheshwari, N.; Goldthorpe, I.A. Silver nanowire coated threads for electrically conductive textiles. J. Mater. Chem. C 2015, 3, 3908–3912. [Google Scholar] [CrossRef]
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.S.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials 2019, 9, 1064. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Mechael, S.S.; Chen, Y.; Carmichael, T.B. Solution Deposition of Conformal Gold Coatings on Knitted Fabric for E-Textiles and Electroluminescent Clothing. Adv. Mater. Technol. 2018, 3, 1700292. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yan, C.; Hu, H.; Zhou, X.; Guo, R.; Liu, X.; Xie, Z.; Huang, Z.; Zheng, Z. Aqueous and Air-Compatible Fabrication of High-Performance Conductive Textiles. Chem.-Asian J. 2014, 9, 2170–2177. [Google Scholar] [CrossRef]
- Irene, G.; Georgios, P.; Ioannis, C.; Anastasios, T.; Diamantis, P.; Marianthi, C.; Philippe, W.; Maria, S. Copper-coated textiles: Armor against MDR nosocomial pathogens. Diagn. Microbiol. Infect. Dis. 2016, 85, 205–209. [Google Scholar] [CrossRef]
- Ali, A.; Baheti, V.; Militky, J.; Khan, Z.; Tunakova, V.; Naeem, S. Copper coated multifunctional cotton fabrics. J. Ind. Text. 2017, 48, 448–464. [Google Scholar] [CrossRef]
- Gouda, M.; Aljaafari, A.; Al-Fayz, Y.; Boraie, W.E. Preparation and Characterization of Some Nanometal Oxides Using Microwave Technique and Their Application to Cotton Fabrics. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Li, L.; Fan, T.; Hu, R.; Liu, Y.; Lu, M. Surface micro-dissolution process for embedding carbon nanotubes on cotton fabric as a conductive textile. Cellulose 2016, 24, 1121–1128. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Pasha, S.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Choudhary, N.; Hwang, S.; Choi, W. Carbon Nanomaterials: A Review in Handbook of Nanomaterials Properties; Springer: Berlin, Germany, 2014; pp. 709–769. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Dai, H.; Rinzler, A.G.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Single-wall nanotubes produced by metal-catalyzed disproportionation of carbon monoxide. Chem. Phys. Lett. 1996, 260, 471–475. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Yu, Q.; Connell, D.W.; Yu, G. Titania/carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technol. Environ. Policy 2013, 15, 871–880. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Ajayan, P.M. Reliability and current carrying capacity of carbon nanotubes. Appl. Phys. Lett. 2001, 79, 1172–1174. [Google Scholar] [CrossRef]
- Dürkop, T.; Kim, B.M.; Fuhrer, M.S. Properties and applications of high-mobility semiconducting nanotubes. J. Phys. Condens. Matter 2004, 16, R553–R580. [Google Scholar] [CrossRef]
- Terrones, M. Science and technology of the twenty first century: Synthesis, properties, and applications of carbon nanotubes. Ann. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Gao, B.; Bower, C.; Lorentzen, J.; Fleming, L.; Kleinhammes, A.; Tang, X.; McNeil, L.; Wu, Y.; Zhou, O. Enhanced saturation lithium composition in ball-milled single-walled carbon nanotubes. Chem. Phys. Lett. 2000, 327, 69–75. [Google Scholar] [CrossRef]
- Raffaelle, R.; Landi, B.; Harris, J.; Bailey, S.; Hepp, A. Carbon nanotubes for power applications. Mater. Sci. Eng. B 2005, 116, 233–243. [Google Scholar] [CrossRef]
- Vairavapandian, D.; Vichchulada, P.; Lay, M.D. Preparation and modification of carbon nanotubes: Review of recent advances and applications in catalysis and sensing. Anal. Chim. Acta 2008, 626, 119–129. [Google Scholar] [CrossRef]
- Artukovic, E.; Kaempgen, M.; Hecht, D.S.; Roth, A.S.; Grüner, G. Transparent and Flexible Carbon Nanotube Transistors. Nano Lett. 2005, 5, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.U.; Gipp, P.P.; Heller, C.M. Carbon nanotube p-n junction diodes. Appl. Phys. Lett. 2004, 85, 145–147. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Wang, L.; Zhou, Y.; Grüner, G.; Marks, T.J. Organic Light-Emitting Diodes Having Carbon Nanotube Anodes. Nano Lett. 2006, 6, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dharap, P.; Nagarajaiah, S.; Barrera, E.V.; Kim, J.D. Carbon Nanotube Film Sensors. Adv. Mater. 2004, 16, 640–643. [Google Scholar] [CrossRef]
- Mahar, B.; Laslau, C.; Yip, R.; Sun, Y. Development of Carbon Nanotube-Based Sensors—A Review. IEEE Sens. J. 2007, 7, 266–284. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallbank, J.R.; Patel, A.; Mucha-Kruczynski, M.; Geim, A.K.; Fal’Ko, V. Generic miniband structure of graphene on a hexagonal substrate. Phys. Rev. B 2013, 87, 245408. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, A.; Sasaki, T.; Pumera, M. Platelet Graphite Nanofibers for Electrochemical Sensing and Biosensing: The Influence of Graphene Sheet Orientation. Chem.-Asian J. 2010, 5, 266–271. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef]
- Meng, F.; Lu, W.; Li, Q.; Byun, J.-H.; Oh, Y.; Chou, T.-W. Graphene-Based Fibers: A Review. Adv. Mater. 2015, 27, 5113–5131. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Karim, N.; Vallés, C.; Afroj, S.; Novoselov, K.S.; Yeates, S.G. Ultraflexible and robust graphene supercapacitors printed on textiles for wearable electronics applications. 2D Mater. 2017, 4, 035016. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-Processed Graphene/MnO2 Nanostructured Textiles for High-Performance Electrochemical Capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Molina, J.; Fernández, J.; Inés, J.C.; del Río, A.I.; Bonastre, J.; Cases, F. Electrochemical characterization of reduced graphene oxide-coated polyester fabrics. Electrochim. Acta 2013, 93, 44–52. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; He, P.; Fernando, A.; Carr, C.; Novoselov, K.S. Scalable Production of Graphene-Based Wearable E-Textiles. ACS Nano 2017, 11, 12266–12275. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Preparation of superhydrophobic electroconductive graphene-coated cotton cellulose. Cellulose 2013, 20, 963–972. [Google Scholar] [CrossRef]
- Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Thermally reduced graphene oxide-coated fabrics for flexible supercapacitors and self-powered systems. Nano Energy 2015, 15, 587–597. [Google Scholar] [CrossRef]
- Yakubu, A.; Abbas, Z.; Danjumma, S.G. Graphene Synthesis by Chemical Vapour Deposition (CVD): A Review on Growth Mechanism and Techniques. Int. J. Eng. Res. 2019, 8, 15–26. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Shukla, A.; Vankar, V.; Kumar, V. Growth, structure and field emission characteristics of petal like carbon nano-structured thin films. Thin Solid Films 2005, 492, 124–130. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Outlaw, R.A.; Hou, K.; Manos, D.M.; Holloway, B.C. Synthesis of carbon nanosheets and carbon nanotubes by radio frequency plasma enhanced chemical vapor deposition. Diam. Relat. Mater. 2007, 16, 196–201. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Asen, P. Preparation of graphene/nickel-iron hexacyanoferrate coordination polymer nanocomposite for electrochemical energy storage. Electrochim. Acta 2015, 160, 337–346. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2013, 2, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, N.; Sun, X.; Lin, X.; Shen, X.; Jia, J.; Zhang, B.; Tang, B.; Chan, M.; Kim, J.-K. Highly Aligned Graphene/Polymer Nanocomposites with Excellent Dielectric Properties for High-Performance Electromagnetic Interference Shielding. Adv. Mater. 2014, 26, 5480–5487. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Duan, F.; Liao, Y.; Zeng, Z.; Jin, H.; Zhou, L.; Zhang, Z.; Su, Z. Graphene-based nanocomposite strain sensor response to ultrasonic guided waves. Compos. Sci. Technol. 2019, 174, 42–49. [Google Scholar] [CrossRef]
- Stoller, M.D.; Murali, S.; Quarles, N.; Zhu, Y.; Potts, J.R.; Zhu, X.; Ha, H.-W.; Ruoff, R.S. Activated graphene as a cathode material for Li-ion hybrid supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Song, J.; Yang, W.; Wang, M.; Zhu, C.; Zhao, W.; Zheng, J.; Lin, Y. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2011, 1, 107–131. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Lai, H.; Li, Z.; Dong, X.; Cai, S.; Chu, X.; Gao, C. Capacitive charge storage enables an ultrahigh cathode capacity in aluminum-graphene battery. J. Energy Chem. 2019, 45, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Hayner, C.M.; Kung, M.C.; Kung, H.H. In-Plane Vacancy-Enabled High-Power Si-Graphene Composite Electrode for Lithium-Ion Batteries. Adv. Energy Mater. 2011, 1, 1079–1084. [Google Scholar] [CrossRef]
- Shi, Q.; Li, J.; Hou, C.; Shao, Y.; Zhang, Q.; Li, Y.; Wang, H. A remote controllable fiber-type near-infrared light-responsive actuator. Chem. Commun. 2017, 53, 11118–11121. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhao, Y.; Hu, C.; Zhang, Z.; Chen, N.; Jiang, L.; Qu, L. Graphene Fibers with Predetermined Deformation as Moisture-Triggered Actuators and Robots. Angew. Chem. Int. Ed. 2013, 52, 10482–10486. [Google Scholar] [CrossRef]
- Qiao, J.; Di, J.; Zhou, S.; Jin, K.; Zeng, S.; Li, N.; Fang, S.; Song, Y.; Li, M.; Baughman, R.H.; et al. Large-Stroke Electrochemical Carbon Nanotube/Graphene Hybrid Yarn Muscles. Small 2018, 14, e1801883. [Google Scholar] [CrossRef]
- Sasikala, S.P.; Lee, K.E.; Lim, J.; Lee, H.J.; Koo, S.H.; Kim, I.H.; Jung, H.J.; Kim, S.O. Interface-Confined High Crystalline Growth of Semiconducting Polymers at Graphene Fibers for High-Performance Wearable Supercapacitors. ACS Nano 2017, 11, 9424–9434. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-Graphene Core-Sheath Microfibers for All-Solid-State, Stretchable Fibriform Supercapacitors and Wearable Electronic Textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D Honeycomb-Like Structured Graphene and Its High Efficiency as a Counter-Electrode Catalyst for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Sun, H.; Chen, T.; Qiu, L.; Luo, Y.; Peng, H. Photovoltaic Wire Derived from a Graphene Composite Fiber Achieving an 8.45 % Energy Conversion Efficiency. Angew. Chem. 2013, 125, 7693–7696. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Hyun, W.J.; Park, O.O.; Chin, B.D. Foldable Graphene Electronic Circuits Based on Paper Substrates. Adv. Mater. 2013, 25, 4729–4734. [Google Scholar] [CrossRef]
- Bonanni, A.; Pumera, M. Graphene Platform for Hairpin-DNA-Based Impedimetric Genosensing. ACS Nano 2011, 5, 2356–2361. [Google Scholar] [CrossRef]
- Kasry, A.; Kuroda, M.A.; Martyna, G.J.; Tulevski, G.S.; Bol, A.A. Chemical Doping of Large-Area Stacked Graphene Films for Use as Transparent, Conducting Electrodes. ACS Nano 2010, 4, 3839–3844. [Google Scholar] [CrossRef]
- De Arco, L.G.; Zhang, Y.; Schlenker, C.W.; Ryu, K.; Thompson, M.; Zhou, C. Continuous, Highly Flexible, and Transparent Graphene Films by Chemical Vapor Deposition for Organic Photovoltaics. ACS Nano 2010, 4, 2865–2873. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.-F.; Yang, Y.; Sanchez Casalongue, H.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4−Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef] [Green Version]
- Ang, P.K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-Gated Epitaxial Graphene as pH Sensor. J. Am. Chem. Soc. 2008, 130, 14392–14393. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.-J. Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Patel, P.C.; Vasavada, D.A.; Mankodi, H.R. Applications of electrically conductive yarns in technical textiles. In Proceedings of the 2012 IEEE International Conference on Power System Technology (POWERCON), Auckland, New Zealand, 30 October–2 November 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Lin, T. 6—Conductive coatings for textiles. In Smart Textile Coatings and Laminates; Smith, W.C., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 155–188. [Google Scholar] [CrossRef]
- Irwin, M.D.; Roberson, D.A.; Olivas, R.I.; Wicker, R.B.; Macdonald, E. Conductive polymer-coated threads as electrical interconnects in e-textiles. Fibers Polym. 2011, 12, 904–910. [Google Scholar] [CrossRef]
- Pawlak, R.; Lebioda, M.; Tomczyk, M.; Rymaszewski, J.; Korzeniewska, E.; Walczak, M. Modelling and applications of conductive elements on textile materials. COMPEL-Int. J. Comput. Math. Electr. Electron. Eng. 2018, 37, 1645–1656. [Google Scholar] [CrossRef]
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302. [Google Scholar] [CrossRef]
- Liu, J.L.; Bashir, S. Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Filho, J.M.C.D.S.; Ermakov, V.A.; Marques, F.C. Perovskite Thin Film Synthesised from Sputtered Lead Sulphide. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ostroverkhova, O. Handbook of Organic Materials for Electronic and Photonic Devices, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Pawlak, R.; Korzeniewska, E.; Koneczny, C.; Hałgas, B. Properties Of Thin Metal Layers Deposited On Textile Composites By Using The Pvd Method For Textronic Applications. Autex Res. J. 2017, 17, 229–237. [Google Scholar] [CrossRef]
- Miśkiewicz, P.; Frydrych, I.; Cichocka, A. Application of Physical Vapor Deposition in Textile Industry. Autex Res. J. 2022, 22, 42–54. [Google Scholar] [CrossRef]
- Shishkovsky, I.; Lebedev, P. 3—Chemical and physical vapor deposition methods for nanocoatings. In Nanocoatings and Ultra-Thin Films; Makhlouf, A.S.H., Tiginyanu, I., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 57–77. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Silva, N.L.; Gonçalves, L.M.; Carvalho, H. Deposition of conductive materials on textile and polymeric flexible substrates. J. Mater. Sci. Mater. Electron. 2012, 24, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Jilani, A.; Abdel-Wahab, M.S.; Hammad, A.H. Advance Deposition Techniques for Thin Film and Coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N.N., Ed.; IntechOpen: London, UK, 2017; pp. 137–149. [Google Scholar] [CrossRef] [Green Version]
- Aghamiri, S.; Rabiee, N.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Karimi, M. Microfluidic devices: Synthetic approaches. In Biomedical Applications of Microfluidic Devices; Academic Press: London, UK, 2021; pp. 23–36. [Google Scholar] [CrossRef]
- Krishna, J.; Perumal, A.S.; Khan, I.; Chelliah, R.; Wei, S.; Swamidoss, C.M.A.; Oh, D.-H.; Bharathiraja, B. Synthesis of nanomaterials for biofuel and bioenergy applications. In Nanomaterials; Academic Press: London, UK, 2021; pp. 97–165. [Google Scholar] [CrossRef]
- Renken, A.; Kiwi-Minsker, L. Microstructured catalytic reactors. In Advances in Catalysis; Academic Press: Amsterdam, The Netherlands, 2010; Volume 53, pp. 47–122. [Google Scholar] [CrossRef]
- Vančo, M.; Krmela, J.; Pešlová, F. The Use of PVD Coating on Natural Textile Fibers. Procedia Eng. 2016, 136, 341–345. [Google Scholar] [CrossRef]
- Esen, M.; Ilhan, I.; Karaaslan, M.; Esen, R. Investigation of electromagnetic and ultraviolet properties of nano-metal-coated textile surfaces. Appl. Nanosci. 2019, 10, 551–561. [Google Scholar] [CrossRef]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.W.; Choi, H.J.; Jang, W.G.; Kim, T.S.; Suh, D.S.; Jeong, H.Y.; Chang, S.Y.; Roh, J.C.; Yoo, C.S.; et al. Electromagnetic interference shielding effectiveness of sputtered NiFe/Cu multi-layer thin film at high frequencies. Thin Solid Films 2019, 677, 130–136. [Google Scholar] [CrossRef]
- Jang, S.; Cho, J.; Jeong, K.; Cho, G. Exploring possibilities of ECG electrodes for bio-monitoring smartwear with Cu sputtered fabrics. In Proceedings of the International Conference on Human-Computer Interaction, Beijing, China, 22–27 July 2007; pp. 1130–1137. [Google Scholar]
- Wang, K.; Huang, Y.; Wang, M.; Yu, M.; Zhu, Y.; Wu, J. PVD amorphous carbon coated 3D NiCo2O4 on carbon cloth as flexible electrode for both sodium and lithium storage. Carbon 2017, 125, 375–383. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Wang, M.; Wang, K.; Yu, M.; Chen, X.; Zhang, Z. Novel carbon coated core-shell heterostructure NiCo2O4@NiO grown on carbon cloth as flexible lithium-ion battery anodes. Ceram. Int. 2018, 44, 21690–21698. [Google Scholar] [CrossRef]
- Choy, K. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- de Lodyguine, A. Illuminesenz for Incandescent Lamps. U.S. Patent US575002A, 12 January 1897. Available online: https://patents.google.com/patent/US575002A/en (accessed on 30 August 2022).
- Malinauskas, A. Chemical deposition of conducting polymers. Polymer 2001, 42, 3957–3972. [Google Scholar] [CrossRef]
- Tan, S.N.; Ge, H. Investigation into vapour-phase formation of polypyrrole. Polymer 1996, 37, 965–968. [Google Scholar] [CrossRef]
- Dall’Acqua, L.; Tonin, C.; Peila, R.; Ferrero, F.; Catellani, M. Performances and properties of intrinsic conductive cellulose–polypyrrole textiles. Synth. Met. 2004, 146, 213–221. [Google Scholar] [CrossRef]
- Xia, Y.; Lu, Y. Fabrication and properties of conductive conjugated polymers/silk fibroin composite fibers. Compos. Sci. Technol. 2008, 68, 1471–1479. [Google Scholar] [CrossRef]
- Gregory, R.; Kimbrell, W.; Kuhn, H. Electrically Conductive Non-Metallic Textile Coatings. J. Coat. Fabr. 1991, 20, 167–175. [Google Scholar] [CrossRef]
- Jang, J.; Chang, M.; Yoon, H. Chemical Sensors Based on Highly Conductive Poly(3,4-ethylenedioxythiophene) Nanorods. Adv. Mater. 2005, 17, 1616–1620. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and Oxidative Chemical Vapor Deposition of Polymeric Thin Films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- Yang, X.; Shang, S.; Li, L.; Tao, X.-M.; Yan, F. Vapor phase polymerization of 3,4-ethylenedioxythiophene on flexible substrate and its application on heat generation. Polym. Adv. Technol. 2009, 22, 1049–1055. [Google Scholar] [CrossRef]
- Bashir, T.; Skrifvars, M.; Persson, N.-K. Synthesis of high performance, conductive PEDOT-coated polyester yarns by OCVD technique. Polym. Adv. Technol. 2011, 23, 611–617. [Google Scholar] [CrossRef]
- Hyde, K.; Dong, H.; Hinestroza, J. Effect of surface cationization on the conformal deposition of polyelectrolytes over cotton fibers. Cellulose 2007, 14, 615–623. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Qi, Q.; Xu, N.; Yu, D. Layer-by-layer assembly of PDMS-coated nickel ferrite/multiwalled carbon nanotubes/cotton fabrics for robust and durable electromagnetic interference shielding. Cellulose 2020, 27, 2829–2845. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Alimohammadi, F.; Song, G.; Kiumarsi, A. Characterization of nanocomposite coatings on textiles: A brief review on Microscopic technology. Curr. Microsc. Contrib. Adv. Sci. Technol. 2012, 2, 1424–1437. [Google Scholar]
- Gulrajani, M.; Gupta, D. Emerging techniques for functional finishing of textiles. Indian J. Fibre Text Res. 2011, 36, 388–397. [Google Scholar]
- Parsons, G.N.; Atanasov, S.E.; Dandley, E.C.; Devine, C.K.; Gong, B.; Jur, J.S.; Lee, K.; Oldham, C.J.; Peng, Q.; Spagnola, J.C.; et al. Mechanisms and reactions during atomic layer deposition on polymers. Coord. Chem. Rev. 2013, 257, 3323–3331. [Google Scholar] [CrossRef]
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. A Vac. Surfaces Films 2016, 34, 010801. [Google Scholar] [CrossRef]
- Saetia, K.; Schnorr, J.M.; Mannarino, M.M.; Kim, S.Y.; Rutledge, G.C.; Swager, T.M.; Hammond, P.T. Spray-Layer-by-Layer Carbon Nanotube/Electrospun Fiber Electrodes for Flexible Chemiresistive Sensor Applications. Adv. Funct. Mater. 2013, 24, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yang, R. Efficient preparation and characterization of paraffin-based microcapsules by emulsion polymerization. J. Appl. Polym. Sci. 2019, 136, 47552. [Google Scholar] [CrossRef]
- Tian, M.; Du, M.; Qu, L.; Chen, S.; Zhu, S.; Han, G. Electromagnetic interference shielding cotton fabrics with high electrical conductivity and electrical heating behavior via layer-by-layer self-assembly route. RSC Adv. 2017, 7, 42641–42652. [Google Scholar] [CrossRef] [Green Version]
- Shakir, I.; Ali, Z.; Bae, J.; Park, J.; Kang, D.J. Layer by layer assembly of ultrathin V2O5 anchored MWCNTs and graphene on textile fabrics for fabrication of high energy density flexible supercapacitor electrodes. Nanoscale 2014, 6, 4125–4130. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Emam, H.E. Layer by layer assembly of nanosilver for high performance cotton fabrics. Fibers Polym. 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Prakash, S.; Yeom, J. Chapter 4—Advanced Fabrication Methods and Techniques. In Nanofluidics and Microfluidics; William Andrew Publishing: Waltham, MA, USA, 2014; pp. 87–170. [Google Scholar] [CrossRef]
- Shang, S.; Zeng, W. 4—Conductive nanofibres and nanocoatings for smart textiles. In Multidisciplinary Know-How for Smart-Textiles Developers; Kirstein, T., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 92–128. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings—A review. J. Alloy. Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Lincot, D. Electrodeposition of semiconductors. Thin Solid Films 2005, 487, 40–48. [Google Scholar] [CrossRef]
- Bicelli, L.; Bozzini, B.; Mele, C.; D’Urzo, L. A Review of Nanostructural Aspects of Metal Electrodeposition. Int. J. Electrochem. Sci. 2008, 3, 356–408. [Google Scholar]
- Biswal, H.J.; Vundavilli, P.R.; Gupta, A. Perspective—Electrodeposition of Graphene Reinforced Metal Matrix Composites for Enhanced Mechanical and Physical Properties: A Review. J. Electrochem. Soc. 2020, 167, 146501. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2019, 69, 7–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Zhang, Q.-Z.; Zhang, D.; Miao, Z.-C.; Zhang, X.-L.; Chou, S.-L. Research Progress in MnO2-Carbon Based Supercapacitor Electrode Materials. Small 2018, 14, 1702883. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Zheng, X.; Yang, S. A scalable electrodeposition route to the low-cost, versatile and controllable fabrication of perovskite solar cells. Nano Energy 2015, 15, 216–226. [Google Scholar] [CrossRef]
- Sreedeviamma, D.K.; Remadevi, A.; Sruthi, C.V.; Pillai, S.; Peethambharan, S.K. Nickel electrodeposited textiles as wearable radar invisible fabrics. J. Ind. Eng. Chem. 2020, 88, 196–206. [Google Scholar] [CrossRef]
- Darvishzadeh, A.; Nasouri, K. Manufacturing, modeling, and optimization of nickel-coated carbon fabric for highly efficient EMI shielding. Surf. Coat. Technol. 2021, 409, 126957. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tian, Y.; Liu, Y.; Wen, J.; Huang, Y.; Hang, C.; Zheng, Z.; Wang, C. Electrodeposition fabrication of Cu@Ni core shell nanowire network for highly stable transparent conductive films. Chem. Eng. J. 2020, 390, 124495. [Google Scholar] [CrossRef]
- Habib, M.A.; Rahman, M. Analysis of Electrolyte Flow in Localized Electrochemical Deposition. Procedia Eng. 2013, 56, 766–771. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Shang, W.; Chen, G.; Wang, H.; Tan, P.; Deng, X.; Song, H.; Xu, Z.; Huang, J.; Zhou, X. Environmentally Stable, Highly Conductive, and Mechanically Robust Metallized Textiles. ACS Appl. Electron. Mater. 2021, 3, 1477–1488. [Google Scholar] [CrossRef]
- Qi, K.; Hou, R.; Zaman, S.; Qiu, Y.; Xia, B.Y.; Duan, H. Construction of Metal–Organic Framework/Conductive Polymer Hybrid for All-Solid-State Fabric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 18021–18028. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, W.; Xie, X.; Liu, N.; Yang, Y.; Wu, H.; Yao, Y.; Pasta, M.; Alshareef, H.N.; Cui, Y. Symmetrical MnO2–Carbon Nanotube–Textile Nanostructures for Wearable Pseudocapacitors with High Mass Loading. ACS Nano 2011, 5, 8904–8913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Zang, J.; Xin, G.; Yuan, Y.; Qu, X. Electrophoretic deposition of MnO2-coated carbon nanotubes on a graphite sheet as a flexible electrode for supercapacitors. Carbon 2012, 50, 5196–5202. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, P.; Guo, B.; Cheng, Z.; Fan, H.; Yang, M.; Yang, X.; Li, J. Electrodeposition of manganese dioxide film on activated carbon paper and its application in supercapacitors with high rate capability. RSC Adv. 2014, 4, 64187–64192. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.-H.; Kim, S.; Park, H.; Lim, S.K. ZnO nanostructure electrodeposited on flexible conductive fabric: A flexible photo-sensor. Sens. Actuators B Chem. 2017, 240, 1106–1113. [Google Scholar] [CrossRef]
- Rosa-Ortiz, S.M.; Takshi, A. Copper Electrodeposition by Hydrogen Evolution Assisted Electroplating (HEA) for Wearable Electronics. In Proceedings of the 2020 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, HI, USA, 10–13 February 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Chen, H.; Liao, F.; Yuan, Z.; Han, X.; Xu, C. Simple and fast fabrication of conductive silver coatings on carbon fabrics via an electroless plating technique. Mater. Lett. 2017, 196, 205–208. [Google Scholar] [CrossRef]
- Cao, S.; Pedraza, A.; Allard, L. Laser-induced microstructural changes and decomposition of aluminum nitride. J. Mater. Res. 1995, 10, 54–62. [Google Scholar] [CrossRef]
- Staňo, M.; Fruchart, O. Magnetic nanowires and nanotubes. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 155–267. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, A.; Pulletikurthi, G.; Endres, F. A Review on the Electroless Deposition of Functional Materials in Ionic Liquids for Batteries and Catalysis. Front. Chem. 2019, 7, 85. [Google Scholar] [CrossRef]
- Root, W.; Aguiló-Aguayo, N.; Pham, T.; Bechtold, T. Conductive layers through electroless deposition of copper on woven cellulose lyocell fabrics. Surf. Coat. Technol. 2018, 348, 13–21. [Google Scholar] [CrossRef]
- Gan, X.; Wu, Y.; Liu, L.; Shen, B.; Hu, W. Electroless plating of Cu–Ni–P alloy on PET fabrics and effect of plating parameters on the properties of conductive fabrics. J. Alloy. Compd. 2008, 455, 308–313. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, S.; Huang, Y. Ultrasonic-assisted electroless deposition of Ag on PET fabric with low silver content for EMI shielding. Surf. Coat. Technol. 2010, 204, 2829–2833. [Google Scholar] [CrossRef]
- Montazer, M.; Allahyarzadeh, V. Electroless Plating of Silver Nanoparticles/Nanolayer on Polyester Fabric Using AgNO3/NaOH and Ammonia. Ind. Eng. Chem. Res. 2013, 52, 8436–8444. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.-L.; He, Y.; Cheng, B.-W. A novel method for fabricating elastic conductive polyurethane filaments by in-situ reduction of polydopamine and electroless silver plating. Mater. Des. 2017, 113, 254–263. [Google Scholar] [CrossRef]
- Lin, X.; Wu, M.; Zhang, L.; Wang, D. Superior Stretchable Conductors by Electroless Plating of Copper on Knitted Fabrics. ACS Appl. Electron. Mater. 2019, 1, 397–406. [Google Scholar] [CrossRef]
- Guo, R.H.; Jiang, S.Q.; Yuen, C.W.M.; Ng, M.C.F. An alternative process for electroless copper plating on polyester fabric. J. Mater. Sci. Mater. Electron. 2008, 20, 33–38. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Kong, J.-S.; An, S.-K.; Kim, H.-D. Surface modification of polymers and improvement of the adhesion between evaporated copper metal film and a polymer. I. Chemical modification of PET. J. Adhes. Sci. Technol. 2000, 14, 1119–1130. [Google Scholar] [CrossRef]
- Lu, Y. Electroless copper plating on 3-mercaptopropyltriethoxysilane modified PET fabric challenged by ultrasonic washing. Appl. Surf. Sci. 2009, 255, 8430–8434. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva-Acuña, C. Multi-phase microstructures drive exciton dissociation in neat semicrystalline polymeric semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Baheti, V.; Vik, M.; Militky, J. Copper electroless plating of cotton fabrics after surface activation with deposition of silver and copper nanoparticles. J. Phys. Chem. Solids 2019, 137, 109181. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Guan, T.; Xu, F.; Sun, J. Durable, Highly Electrically Conductive Cotton Fabrics with Healable Superamphiphobicity. ACS Appl. Mater. Interfaces 2018, 10, 12042–12050. [Google Scholar] [CrossRef]
- Hassan, Z.; Atalay, O.; Kalaoglu, F. Conductive cotton fabric using laser pre-treatment and electroless plating. J. Text. Inst. 2021, 113, 737–747. [Google Scholar] [CrossRef]
- Grothe, J.; Kaskel, S.; Leuteritz, A. Nanocomposites and hybrid materials. In Polymer Science: A Comprehensive Reference; 10 Volume Set; Elsevier: Amsterdam, The Netherlands, 2012; pp. 177–209. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A. Conductive polymer-based electro-conductive textile composites for electromagnetic interference shielding: A review. J. Ind. Text. 2016, 47, 2228–2252. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Han, W.; Zhang, J.; Ding, G.; Dong, S.; Cui, G. Review—In Situ Polymerization for Integration and Interfacial Protection Towards Solid State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070527. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Raju, K.; Lee, J.; Cho, M.H. Enhanced Thermal Stability under DC Electrical Conductivity Retention and Visible Light Activity of Ag/TiO2@Polyaniline Nanocomposite Film. ACS Appl. Mater. Interfaces 2014, 6, 8124–8133. [Google Scholar] [CrossRef]
- Hong, K.H.; Oh, K.W.; Kang, T.J. Preparation and properties of electrically conducting textiles byin situ polymerization of poly(3,4-ethylenedioxythiophene). J. Appl. Polym. Sci. 2005, 97, 1326–1332. [Google Scholar] [CrossRef]
- Grishina, T.R. The effect of the table salt substitute sanasol on arterial pressure, water-salt metabolism and kidney functions in experimental pathology of circulatory homeostasis. Eksp. Klin. Farmakol. 1992, 55, 21–24. [Google Scholar]
- Dey, M.; Doumenc, F.; Guerrier, B. Numerical simulation of dip-coating in the evaporative regime. Eur. Phys. J. E 2016, 39, 19. [Google Scholar] [CrossRef] [Green Version]
- Gaulding, E.A.; Diroll, B.T.; Goodwin, E.D.; Vrtis, Z.J.; Kagan, C.R.; Murray, C.B. Deposition of Wafer-Scale Single-Component and Binary Nanocrystal Superlattice Thin Films Via Dip-Coating. Adv. Mater. 2015, 27, 2846–2851. [Google Scholar] [CrossRef]
- Ceratti, D.R.; Louis, B.; Paquez, X.; Faustini, M.; Grosso, D. A New Dip Coating Method to Obtain Large-Surface Coatings with a Minimum of Solution. Adv. Mater. 2015, 27, 4958–4962. [Google Scholar] [CrossRef]
- Roland, S.; Pellerin, C.; Bazuin, C.G.; Prud’Homme, R.E. Evolution of Small Molecule Content and Morphology with Dip-Coating Rate in Supramolecular PS–P4VP Thin Films. Macromolecules 2012, 45, 7964–7972. [Google Scholar] [CrossRef]
- Jittavanich, K.; Clemons, C.; Kreider, K.; Aljarrah, M.; Evans, E.; Young, G. Modeling, simulation and fabrication of coated structures using the dip coating technique. Chem. Eng. Sci. 2010, 65, 6169–6180. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2016, 81, 378–404. [Google Scholar] [CrossRef]
- He, S.; Xin, B.; Chen, Z.; Liu, Y. Flexible and highly conductive Ag/G-coated cotton fabric based on graphene dipping and silver magnetron sputtering. Cellulose 2018, 25, 3691–3701. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Tata, J.; Carosio, F.; Malucelli, G. Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol-gel treatments. J. Appl. Polym. Sci. 2010, 119, 1961–1969. [Google Scholar] [CrossRef]
- Gorenšek, M.; Recelj, P. Nanosilver Functionalized Cotton Fabric. Text. Res. J. 2007, 77, 138–141. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol–gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Combination of silica sol and dyes on textiles. J. Sol-Gel Sci. Technol. 2006, 39, 111–118. [Google Scholar] [CrossRef]
- Schramm, C.; Binder, W.; Tessadri, R. Durable Press Finishing of Cotton Fabric with 1,2,3,4-Butanetetracarboxylic Acid and TEOS/GPTMS. J. Sol-Gel Sci. Technol. 2004, 29, 155–165. [Google Scholar] [CrossRef]
- Kampeerapappun, P.; Visatchok, K.; Wangarsa, D. Preparation and properties of superhydrophobic cotton fabrics. J. Met. Mater. Miner. 2010, 20, 79–83. [Google Scholar]
- Nasirizadeh, N.; Dehghani, M.; Yazdanshenas, M.E. Preparation of hydrophobic and conductive cotton fabrics using multi-wall carbon nanotubes by the sol–gel method. J. Sol-Gel Sci. Technol. 2014, 73, 14–21. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, W.; Kim, H.; Choi, S.; Park, B.-C.; Kang, S.-H.; Choi, K.C. High Luminance Fiber-Based Polymer Light-Emitting Devices by a Dip-Coating Method. Adv. Electron. Mater. 2015, 1, 1500103. [Google Scholar] [CrossRef]
- Pu, D.; Zhou, W.; Li, Y.; Chen, J.; Chen, J.; Zhang, H.; Mi, B.; Wang, L.; Ma, Y. Order-enhanced silver nanowire networks fabricated by two-step dip-coating as polymer solar cell electrodes. RSC Adv. 2015, 5, 100725–100729. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Zhang, H.; Wang, Q.; Guan, F.; Yu, Z. Flexible and Multifunctional Silk Textiles with Biomimetic Leaf-Like MXene/Silver Nanowire Nanostructures for Electromagnetic Interference Shielding, Humidity Monitoring, and Self-Derived Hydrophobicity. Adv. Funct. Mater. 2019, 29, 1905197. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Pan, N. Graphene based supercapacitor fabricated by vacuum filtration deposition. J. Power Sources 2012, 206, 476–482. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.; Meng, C.; He, J.; Dong, X. Fabrication of highly conductive and multifunctional polyester fabrics by spray-coating with PEDOT:PSS solutions. Prog. Org. Coat. 2018, 121, 89–96. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, Q.; Jian, M.; Zhang, Y. Sheath–Core Graphite/Silk Fiber Made by Dry-Meyer-Rod-Coating for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2016, 8, 20894–20899. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Carbon nanotube polymer coatings for textile yarns with good strain sensing capability. Sens. Actuators A Phys. 2012, 179, 83–91. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Brzezinski, S.; Kaminska, I.; Wrobel, S.; Mizerska, U.; Fortuniak, W.; Piorkowska, E.; Svyntkivska, M.; Makowski, T. Electrically conductive composite textiles modified with graphene using sol-gel method. J. Alloy. Compd. 2019, 784, 22–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, W.; Liu, L.; Cheng, W.; Wang, W.; Liew, K.M.; Wang, B.; Hu, Y. Eco-friendly flame retardant and electromagnetic interference shielding cotton fabrics with multi-layered coatings. Chem. Eng. J. 2019, 372, 1077–1090. [Google Scholar] [CrossRef]
- Shim, E. Coating and laminating processes and techniques for textiles. In Smart Textile Coatings and Laminates; Elsevier: Cambridge, UK, 2019; pp. 11–45. [Google Scholar] [CrossRef]
- Yang, K.; Torah, R.; Wei, Y.; Beeby, S.; Tudor, J. Waterproof and durable screen printed silver conductive tracks on textiles. Text. Res. J. 2013, 83, 2023–2031. [Google Scholar] [CrossRef]
- Paul, G.; Torah, R.; Yang, K.; Beeby, S.; Tudor, J. An investigation into the durability of screen-printed conductive tracks on textiles. Meas. Sci. Technol. 2014, 25, 025006. [Google Scholar] [CrossRef]
- Sadi, S.; Yang, M.; Luo, L.; Cheng, D.; Cai, G.; Wang, X. Direct screen printing of single-faced conductive cotton fabrics for strain sensing, electrical heating and color changing. Cellulose 2019, 26, 6179–6188. [Google Scholar] [CrossRef]
- Stempien, Z.; Rybicki, E.; Lesnikowski, J. Inkjet-printing deposition of silver electro-conductive layers on textile substrates at low sintering temperature by using an aqueous silver ions-containing ink for textronic applications. Sens. Actuators B Chem. 2016, 224, 714–725. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Dumitrescu, D.; Loghin, C.; Chen, Y.; Wang, L.; Nierstrasz, V. 3D printing of NinjaFlex filament onto PEDOT: PSS-coated textile fabrics for electroluminescence applications. J. Electron. Mater. 2018, 47, 2082–2092. [Google Scholar] [CrossRef] [Green Version]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Virkki, J.; Björninen, T.; Merilampi, S.; Sydänheimo, L.; Ukkonen, L. The effects of recurrent stretching on the performance of electro-textile and screen-printed ultra-high-frequency radio-frequency identification tags. Text. Res. J. 2014, 85, 294–301. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chuang, M.-C.; Lou, S.-L.; Wang, J. Thick-film textile-based amperometric sensors and biosensors. Analyst 2010, 135, 1230–1234. [Google Scholar] [CrossRef]
- Torah, R.; Wei, Y.; Li, Y.; Yang, K.; Beeby, S.; Tudor, J. Printed textile-based electronic devices. In Handbook of Smart Textiles; Springer: Singapore, 2015; pp. 653–687. [Google Scholar] [CrossRef]
- Fukuda, K.; Someya, T. Recent Progress in the Development of Printed Thin-Film Transistors and Circuits with High-Resolution Printing Technology. Adv. Mater. 2016, 29, 1602736. [Google Scholar] [CrossRef]
- Matavž, A.; Bobnar, V.; Malič, B. Tailoring Ink–Substrate Interactions via Thin Polymeric Layers for High-Resolution Printing. Langmuir 2017, 33, 11893–11900. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Lee, W.H.; Lee, H.S.; Lee, J.H.; Park, Y.D.; Cho, K. Self-Organization of Ink-jet-Printed Triisopropylsilylethynyl Pentacene via Evaporation-Induced Flows in a Drying Droplet. Adv. Funct. Mater. 2008, 18, 229–234. [Google Scholar] [CrossRef]
- Kim, I.; Shahariar, H.; Ingram, W.F.; Zhou, Y.; Jur, J.S. Inkjet Process for Conductive Patterning on Textiles: Maintaining Inherent Stretchability and Breathability in Knit Structures. Adv. Funct. Mater. 2018, 29, 1807573. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2012, 51, 16–34. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Malandraki, A.; Butterworth, S.; Beach, C.; Rigout, M.; Novoselov, K.S.; Casson, A.J.; Yeates, S.G. All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. C 2017, 5, 11640–11648. [Google Scholar] [CrossRef] [Green Version]
- Beecroft, M. 3D printing of weft knitted textile based structures by selective laser sintering of nylon powder. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 012017. [Google Scholar] [CrossRef]
- Hamzah, H.H.; Shafiee, S.A.; Abdalla, A.; Patel, B.A. 3D printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem. Commun. 2018, 96, 27–31. [Google Scholar] [CrossRef]
- Dip, T.M.; Emu, A.S.; Nafiz, M.N.H.; Kundu, P.; Rakhi, H.R.; Sayam, A.; Akhtarujjman, M.; Shoaib, M.; Ahmed, M.S.; Ushno, S.T.; et al. 3D printing technology for textiles and fashion. Text. Prog. 2021, 52, 167–260. [Google Scholar] [CrossRef]
- Chizari, K.; Daoud, M.A.; Ravindran, A.R.; Therriault, D. 3D Printing of Highly Conductive Nanocomposites for the Functional Optimization of Liquid Sensors. Small 2016, 12, 6076–6082. [Google Scholar] [CrossRef]
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2015, 26, 698–703. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; van Bennekom, S.; Woldhuis, H.; Wijnia, S.; de With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Gao, T.; Yang, Z.; Chen, C.; Li, Y.; Fu, K.; Dai, J.; Hitz, E.M.; Xie, H.; Liu, B.; Song, J.; et al. Three-Dimensional Printed Thermal Regulation Textiles. ACS Nano 2017, 11, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Akbari, M.; Sydänheimo, L.; Ukkonen, L.; Virkki, J. 3D-Printed Graphene Antennas and Interconnections for Textile RFID Tags: Fabrication and Reliability towards Humidity. Int. J. Antennas Propag. 2017, 2017, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Yamaura, M.; Hagiwara, T.; Murata, K.; Takumoto, M. Study on the electrical conduction mechanism of polypyrrole films. Synth. Met. 1991, 40, 35–48. [Google Scholar] [CrossRef]
- Abthagir, P.; Saraswathi, R. Charge transport and thermal properties of polyindole, polycarbazole and their derivatives. Thermochim. Acta 2004, 424, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Gasana, E.; Westbroek, P.; Hakuzimana, J.; De Clerck, K.; Priniotakis, G.; Kiekens, P.; Tseles, D. Electroconductive textile structures through electroless deposition of polypyrrole and copper at polyaramide surfaces. Surf. Coat. Technol. 2006, 201, 3547–3551. [Google Scholar] [CrossRef]
- Long, Y.; Duvail, J.; Li, M.; Gu, C.; Liu, Z.; Ringer, S.P. Electrical Conductivity Studies on Individual Conjugated Polymer Nanowires: Two-Probe and Four-Probe Results. Nanoscale Res. Lett. 2009, 5, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Rubežienė, V.; Varnaitė-Žuravliova, S. EMI shielding textile materials. In Materials for Potential EMI Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–378. [Google Scholar] [CrossRef]
- Celozzi, S.; Araneo, R.; Lovat, G. Electromagnetic Shielding; John Wiley & Sons: New York, NJ, USA, 2008; Volume 192. [Google Scholar]
- Cheng, K.B. Production and electromagnetic shielding effectiveness of the knitted stainless steel/polyester fabrics. J. Text. Engr. 2000, 46, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Geetha, S.; Kumar, K.K.S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C.K. EMI shielding: Methods and materials-A review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- Bagavathi, M.; Priyadarshini, R. A review on the production methods and testing of textiles for electro magnetic interference (EMI) shielding. Int. J. Eng. Res. Appl. 2015, 5, 2248–9622. [Google Scholar]
- Tong, X.C. Advanced Materials and Design for Electromagnetic Interference Shielding, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tao, Y.; Li, P.; Shi, S.Q. Effects of Carbonization Temperature and Component Ratio on Electromagnetic Interference Shielding Effectiveness of Woodceramics. Materials 2016, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.K.; Padbury, R.; Toprakci, O.; Dirican, M.; Zhang, X. Conductive textiles. In Engineering of High-Performance Textiles; Woodhead Publishing: Cambridge, UK, 2018; pp. 305–334. [Google Scholar] [CrossRef]
- Tezel, S.; Kavuşturan, Y.; Vandenbosch, G.A.; Volski, V. Comparison of electromagnetic shielding effectiveness of conductive single jersey fabrics with coaxial transmission line and free space measurement techniques. Text. Res. J. 2013, 84, 461–476. [Google Scholar] [CrossRef]
- Hassan, Z.; Kalaoglu, F.; Atalay, O. Development and characterization of conductive textile (cotton) for wearable electronics and soft robotic applications. Text. Res. J. 2020, 90, 1792–1804. [Google Scholar] [CrossRef]
- Gonçalves, C.; da Silva, A.F.; Gomes, J.; Simoes, R. Wearable E-Textile Technologies: A Review on Sensors, Actuators and Control Elements. Inventions 2018, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Fishlock, D. A panacea for all bodily ills. IEE Rev. 2001, 23–28. [Google Scholar] [CrossRef]
- Thorp, E. The invention of the first wearable computer. In Proceedings of the Digest of Papers. Second International Symposium on Wearable Computers (Cat. No.98EX215), Pittsburgh, PA, USA, 19–20 October 1998; pp. 4–8. [Google Scholar] [CrossRef]
- Gopalsamy, C.; Park, S.; Rajamanickam, R.; Jayaraman, S. The Wearable Motherboard?: The first generation of adaptive and responsive textile structures (ARTS) for medical applications. Virtual Real. 1999, 4, 152–168. [Google Scholar] [CrossRef]
- Paradiso, R.; Loriga, G.; Taccini, N.; Gemignani, A.; Ghelarducci, B. WEALTHY-a wearable healthcare system: New frontier on e-textile. J. Telecommun. Inf. Technol. 2005, 4, 105–113. [Google Scholar]
- Pirotte, F.; Depré, A.; Shishoo, R.; De Jonckheere, J.; Grillet, A. Smart textiles embedded with optical fibre sensors for health monitoring of patients. In Medical and Healthcare Textiles; Woodhead Publishing: Cambridge, UK, 2010; pp. 463–471. [Google Scholar] [CrossRef]
- Asada, H.H.; Shaltis, P.; Mccombie, D.B.; Reisner, A.T. Wearable Blood Pressure Sensor and Method of Calibration. U.S. Patent 7,641,614 B2, 5 January 2010. [Google Scholar]
- Teng, X.-F.; Zhang, Y.-T.; Poon, C.C.Y.; Bonato, P. Wearable Medical Systems for p-Health. IEEE Rev. Biomed. Eng. 2008, 1, 62–74. [Google Scholar] [CrossRef]
- Edmison, J.; Jones, M.; Lockhart, T.; Martin, T. An e-textile system for motion analysis. Stud. Health Technol. Inform. 2004, 108, 292–301. [Google Scholar]
- Enokibori, Y.; Ito, Y.; Suzuki, A.; Mizuno, H.; Shimakami, Y.; Kawabe, T.; Mase, K. SpiroVest: An e-textile-based wearable spirometer with posture change adaptability. In Proceedings of the 2013 ACM Conference on Pervasive and Ubiquitous Computing Adjunct Publication, Zurich, Switzerland, 8–12 September 2013; pp. 203–206. [Google Scholar] [CrossRef]
- Enokibori, Y.; Mase, K. Human joint angle estimation with an e-textile sensor. In Proceedings of the 2014 ACM International Symposium on Wearable Computers, Seattle, WA, USA, 13–17 September 2014; pp. 129–130. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Yang, J.; Li, X.; Wu, Y.; Wei, W.; Liu, J.; Chen, J.; Yang, J. Large-Scale and Washable Smart Textiles Based on Triboelectric Nanogenerator Arrays for Self-Powered Sleeping Monitoring. Adv. Funct. Mater. 2017, 28, 1704112. [Google Scholar] [CrossRef]
- Li, X.; Koh, K.H.; Farhan, M.; Lai, K.W.C. An ultraflexible polyurethane yarn-based wearable strain sensor with a polydimethylsiloxane infiltrated multilayer sheath for smart textiles. Nanoscale 2020, 12, 4110–4118. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, X.; Andalib, S.; Xu, J.; Zhou, Y.; Tat, T.; Lin, K.; Chen, J. Discovering giant magnetoelasticity in soft matter for electronic textiles. Matter 2021, 4, 3725–3740. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Fleury, A.; Sugar, M.; Chau, T. E-textiles in Clinical Rehabilitation: A Scoping Review. Electronics 2015, 4, 173–203. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, Y.T.; Tokita, M.; Murata, H.; Hirasawa, Y.; Yodogawa, K.; Iwasaki, Y.-K.; Asai, K.; Shimizu, W.; Nakashima, H.; Tsukada, S. Validation of wearable textile electrodes for ECG monitoring. Heart Vessel. 2019, 34, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Esfahani, M.I.M. Smart textiles in healthcare: A summary of history, types, applications, challenges, and future trends. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–107. [Google Scholar] [CrossRef]
- Axisa, F.; Schmitt, P.; Gehin, C.; Delhomme, G.; McAdams, E.; Dittmar, A. Flexible Technologies and Smart Clothing for Citizen Medicine, Home Healthcare, and Disease Prevention. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 325–336. [Google Scholar] [CrossRef]
- Shishoo, R. Textiles in Sport, 1st ed.; Elsevier: Cambridge, UK, 2005. [Google Scholar]
- Munro, B.J.; Campbell, T.E.; Wallace, G.G.; Steele, J.R. The intelligent knee sleeve: A wearable biofeedback device. Sens. Actuators B Chem. 2008, 131, 541–547. [Google Scholar] [CrossRef]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.E.; Roberts, W.O.; Stone, J.A. National athletic trainers’ association position statement: Fluid replacement for athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar] [PubMed]
- Morris, D.; Schazmann, B.; Wu, Y.; Coyle, S.; Brady, S.; Hayes, J.; Slater, C.; Fay, C.; Lau, K.T.; Wallace, G.; et al. Wearable sensors for monitoring sports performance and training. In Proceedings of the 2008 5th International Summer School and Symposium on Medical Devices and Biosensors, Hong Kong, China, 1–3 June 2008; pp. 121–124. [Google Scholar] [CrossRef]
- Seyedin, S.; Razal, J.M.; Innis, P.C.; Jeiranikhameneh, A.; Beirne, S.; Wallace, G.G. Knitted Strain Sensor Textiles of Highly Conductive All-Polymeric Fibers. ACS Appl. Mater. Interfaces 2015, 7, 21150–21158. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, M.; Xu, T.; Sun, X.; Sun, B.; Sun, C.; Liu, X.; Zhang, X.; Qu, L. Multiscale Disordered Porous Fibers for Self-Sensing and Self-Cooling Integrated Smart Sportswear. ACS Nano 2019, 14, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Totaro, M.; Beccai, L. Toward Perceptive Soft Robots: Progress and Challenges. Adv. Sci. 2018, 5, 1800541. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Lee, P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2020, 33, e2002640. [Google Scholar] [CrossRef]
- Furuse, A.; Hashimoto, M. Development of novel textile and yarn actuators using plasticized PVC gel. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2017, Portland, OR, USA, 25–29 March 2017; Volume 10163, p. 1016327. [Google Scholar] [CrossRef]
- Guo, J.; Xiang, C.; Helps, T.; Taghavi, M.; Rossiter, J. Electroactive textile actuators for wearable and soft robots. In Proceedings of the 2018 IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018; pp. 339–343. [Google Scholar] [CrossRef]
- Cianchetti, M.; Renda, F.; Licofonte, A.; Laschi, C. Sensorization of continuum soft robots for reconstructing their spatial configuration. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 634–639. [Google Scholar] [CrossRef]
- Atalay, A.; Sanchez, V.; Atalay, O.; Vogt, D.M.; Haufe, F.; Wood, R.J.; Walsh, C.J. Batch Fabrication of Customizable Silicone-Textile Composite Capacitive Strain Sensors for Human Motion Tracking. Adv. Mater. Technol. 2017, 2, 1700136. [Google Scholar] [CrossRef] [Green Version]
- Kanik, M.; Orguc, S.; Varnavides, G.; Kim, J.; Benavides, T.; Gonzalez, D.; Akintilo, T.; Tasan, C.C.; Chandrakasan, A.P.; Fink, Y.; et al. Strain-programmable fiber-based artificial muscle. Science 2019, 365, 145–150. [Google Scholar] [CrossRef]
- Pei, Z.; Xiong, X.; He, J.; Zhang, Y. Highly Stretchable and Durable Conductive Knitted Fabrics for the Skins of Soft Robots. Soft Robot. 2019, 6, 687–700. [Google Scholar] [CrossRef]
- Nashed, M.-N.; Hardy, D.A.; Hughes-Riley, T.; Dias, T. A Novel Method for Embedding Semiconductor Dies within Textile Yarn to Create Electronic Textiles. Fibers 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Lian, Y.; Yu, H.; Wang, M.; Yang, X.; Li, Z.; Yang, F.; Wang, Y.; Tai, H.; Liao, Y.; Wu, J.; et al. A multifunctional wearable E-textile via integrated nanowire-coated fabrics. J. Mater. Chem. C 2020, 8, 8399–8409. [Google Scholar] [CrossRef]
- Poupyrev, I.; Gong, N.-W.; Fukuhara, S.; Karagozler, M.E.; Schwesig, C.; Robinson, K.E. Project Jacquard: Interactive digital textiles at scale. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; pp. 4216–4227. [Google Scholar] [CrossRef] [Green Version]
- Australian Radiation Protection and Nuclear Safety Agency. Mobile Phones and Health. Available online: https://www.arpansa.gov.au/understanding-radiation/radiation-sources/more-radiation-sources/mobile-phones?fbclid=IwAR3M-JEZTmbLZExqYD0C8YcFq23l-EsQH3pfYys-hyn4fUUkdrFhorJdaHo (accessed on 14 January 2022).
- International Commission on Non-Ionizing Radiation. Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–521. [Google Scholar]
- Hirata, A.; Matsuyama, S.-I.; Shiozawa, T. Temperature rises in the human eye exposed to EM waves in the frequency range 0.6–6 GHz. IEEE Trans. Electromagn. Compat. 2000, 42, 386–393. [Google Scholar] [CrossRef]
- Mille, M.W. Electrical Wiring Configurations and Childhood Cancer. Volume 112. 1980. Available online: https://www.osti.gov/biblio/7055661 (accessed on 14 January 2022).
- Wdowiak, A.; Mazurek, P.; Wdowiak, A.; Bojar, I. Effect of electromagnetic waves on human reproduction. Ann. Agric. Environ. Med. 2017, 24, 13–18. [Google Scholar] [CrossRef]
- Zou, L.; Lan, C.; Yang, L.; Xu, Z.; Chu, C.; Liu, Y.; Qiu, Y. The optimization of nanocomposite coating with polyaniline coated carbon nanotubes on fabrics for exceptional electromagnetic interference shielding. Diam. Relat. Mater. 2020, 104, 107757. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, M.; Qiang, Z.; Song, J.; Wang, Y.; Fan, Y.; You, Z.; Liao, Y.; Zhu, M.; Ye, C. Multi-functional and highly conductive textiles with ultra-high durability through ‘green’ fabrication process. Chem. Eng. J. 2020, 406, 127140. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Y.; Wang, W.; Yu, D. Multilayer structured PANI/MXene/CF fabric for electromagnetic interference shielding constructed by layer-by-layer strategy. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 125047. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Y.; Wang, W.; Qu, Z.; Yu, D. A Flexible Electromagnetic Interference Shielding Fabric Prepared by Construction of PANI/MXene Conductive Network via Layer-by-Layer Assembly. Adv. Mater. Interfaces 2021, 8, 2001893. [Google Scholar] [CrossRef]
- Jia, L.-C.; Ding, K.-Q.; Ma, R.-J.; Wang, H.-L.; Sun, W.-J.; Yan, D.-X.; Li, B.; Li, Z.-M. Highly Conductive and Machine-Washable Textiles for Efficient Electromagnetic Interference Shielding. Adv. Mater. Technol. 2018, 4, 1800503. [Google Scholar] [CrossRef]
- Zong, J.-Y.; Zhou, X.-J.; Hu, Y.-F.; Yang, T.-B.; Yan, D.-X.; Lin, H.; Lei, J.; Li, Z.-M. A wearable multifunctional fabric with excellent electromagnetic interference shielding and passive radiation heating performance. Compos. Part B Eng. 2021, 225, 109299. [Google Scholar] [CrossRef]
- Jia, L.-C.; Xu, L.; Ren, F.; Ren, P.-G.; Yan, D.-X.; Li, Z.-M. Stretchable and durable conductive fabric for ultrahigh performance electromagnetic interference shielding. Carbon 2018, 144, 101–108. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganguly, S.; Das, P.; Das, T.K.; Bose, M.; Singha, N.K.; Das, A.K.; Das, N.C. Fabrication of Reduced Graphene Oxide/Silver Nanoparticles Decorated Conductive Cotton Fabric for High Performing Electromagnetic Interference Shielding and Antibacterial Application. Fibers Polym. 2019, 20, 1161–1171. [Google Scholar] [CrossRef]
- Ghouri, A.S.; Aslam, R.; Siddiqui, M.S.; Sami, S.K. Recent Progress in Textile-Based Flexible Supercapacitor. Front. Mater. 2020, 7, 58. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Y.; Li, N.; Zhang, H.; Zhi, C. Recent Progress on Flexible and Wearable Supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zeng, L.; Chen, Y.; Zhang, R.; Yang, R.; Pang, J.; Ding, L.; Liu, H.; Zhou, W. Ni-Co-N hybrid porous nanosheets on graphene paper for flexible and editable asymmetric all-solid-state supercapacitors. Nano Energy 2019, 61, 18–26. [Google Scholar] [CrossRef]
- Dai, S.; Xu, W.; Xi, Y.; Wang, M.; Gu, X.; Guo, D.; Hu, C. Charge storage in KCu7S4 as redox active material for a flexible all-solid-state supercapacitor. Nano Energy 2016, 19, 363–372. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Huang, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2019, 139, 100520. [Google Scholar] [CrossRef]
- Ren, M.; Di, J.; Chen, W. Recent Progress and Application Challenges of Wearable Supercapacitors. Batter. Supercaps. 2021, 4, 1279–1290. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Xie, M.; Tao, J.; Shen, X.; Ji, Z.; Yan, Z.; Zhai, L.; Yuan, A. Polyaniline wrapped graphene functionalized textile with ultrahigh areal capacitance and energy density for high-performance all-solid-state supercapacitors for wearable electronics. Compos. Sci. Technol. 2020, 198, 108305. [Google Scholar] [CrossRef]
- Kim, T.; Samuel, E.P.; Park, C.; Kim, Y.-I.; Aldalbahi, A.; Alotaibi, F.; Yoon, S.S. Wearable fabric supercapacitors using supersonically sprayed reduced graphene and tin oxide. J. Alloy. Compd. 2020, 856, 157902. [Google Scholar] [CrossRef]
- Lu, C.; Wang, D.; Zhao, J.; Han, S.; Chen, W. A Continuous Carbon Nitride Polyhedron Assembly for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606219. [Google Scholar] [CrossRef]
- Lv, Z.; Luo, Y.; Tang, Y.; Wei, J.; Zhu, Z.; Zhou, X.; Li, W.; Zeng, Y.; Zhang, W.; Zhang, Y.; et al. Editable Supercapacitors with Customizable Stretchability Based on Mechanically Strengthened Ultralong MnO2 Nanowire Composite. Adv. Mater. 2017, 30, 1704531. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Bonderover, E.; Jordan, W.B.; Sturm, J.C. Electrotextiles: Concepts and challenges. Int. J. High Speed Electron. Syst. 2002, 12, 391–399. [Google Scholar] [CrossRef]
- Islam, G.M.N.; Ali, M.A.; Collie, S. Textile sensors for wearable applications: A comprehensive review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Badghaish, H.S.; Hussain, M.M. Textile Electronics–Prospects, Advances, Challenges and Opportunities. MRS Adv. 2020, 5, 2359–2379. [Google Scholar] [CrossRef]
- Molla, T.I.; Compton, C.; Dunne, L.E. Identifying Challenges of Fabricating E-textile Garments Via a Case Study. In Proceedings of the International Textile and Apparel Association Annual Conference Proceedings, Las Vegas, NV, USA, 25–29 October 2019; Volume 76. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, C.; Zheng, Z. Textile Composite Electrodes for Flexible Batteries and Supercapacitors: Opportunities and Challenges. Adv. Energy Mater. 2020, 11, 2002838. [Google Scholar] [CrossRef]
- Levitt, A.; Hegh, D.; Phillips, P.; Uzun, S.; Anayee, M.; Razal, J.M.; Gogotsi, Y.; Dion, G. 3D knitted energy storage textiles using MXene-coated yarns. Mater. Today 2020, 34, 17–29. [Google Scholar] [CrossRef]
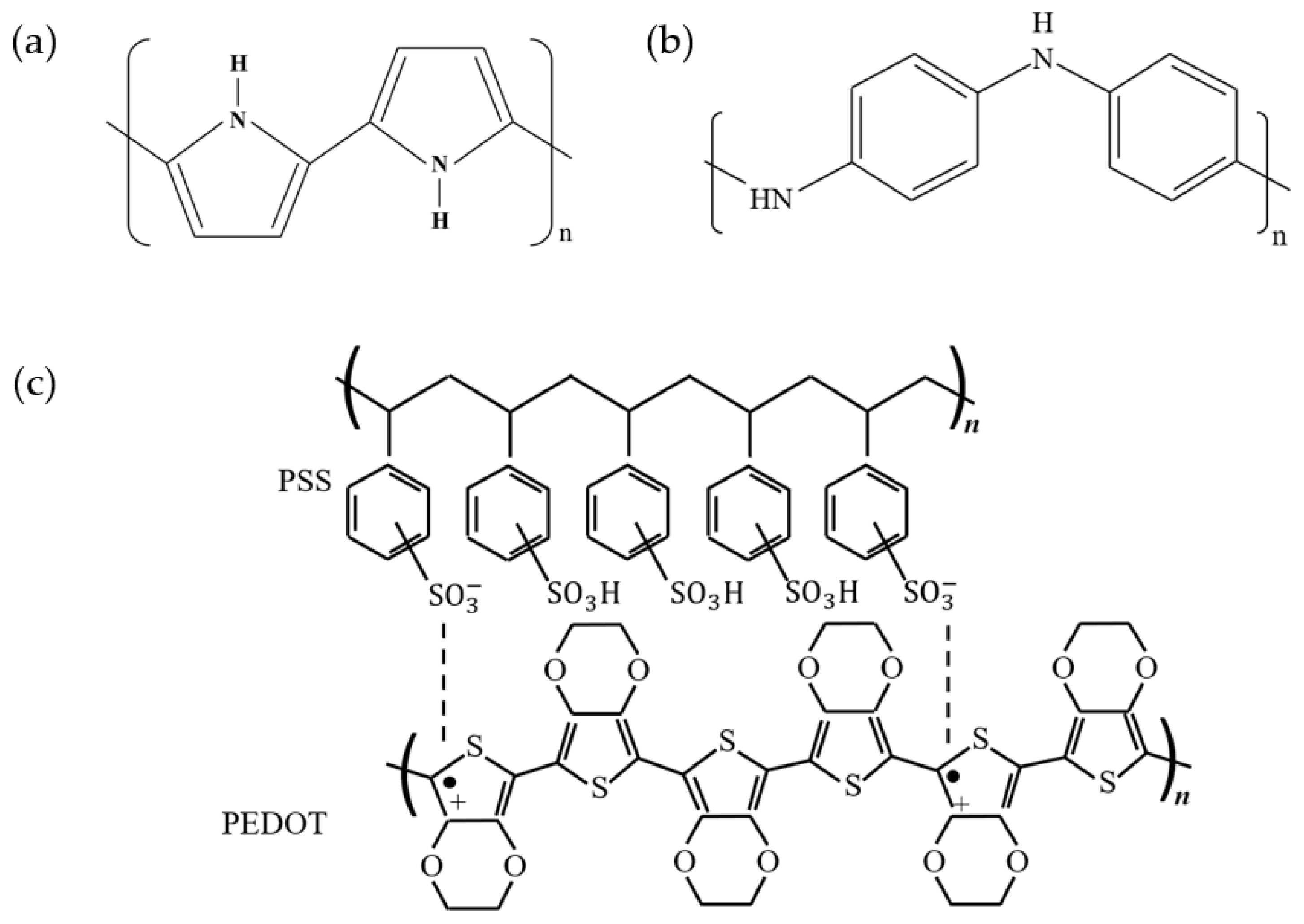
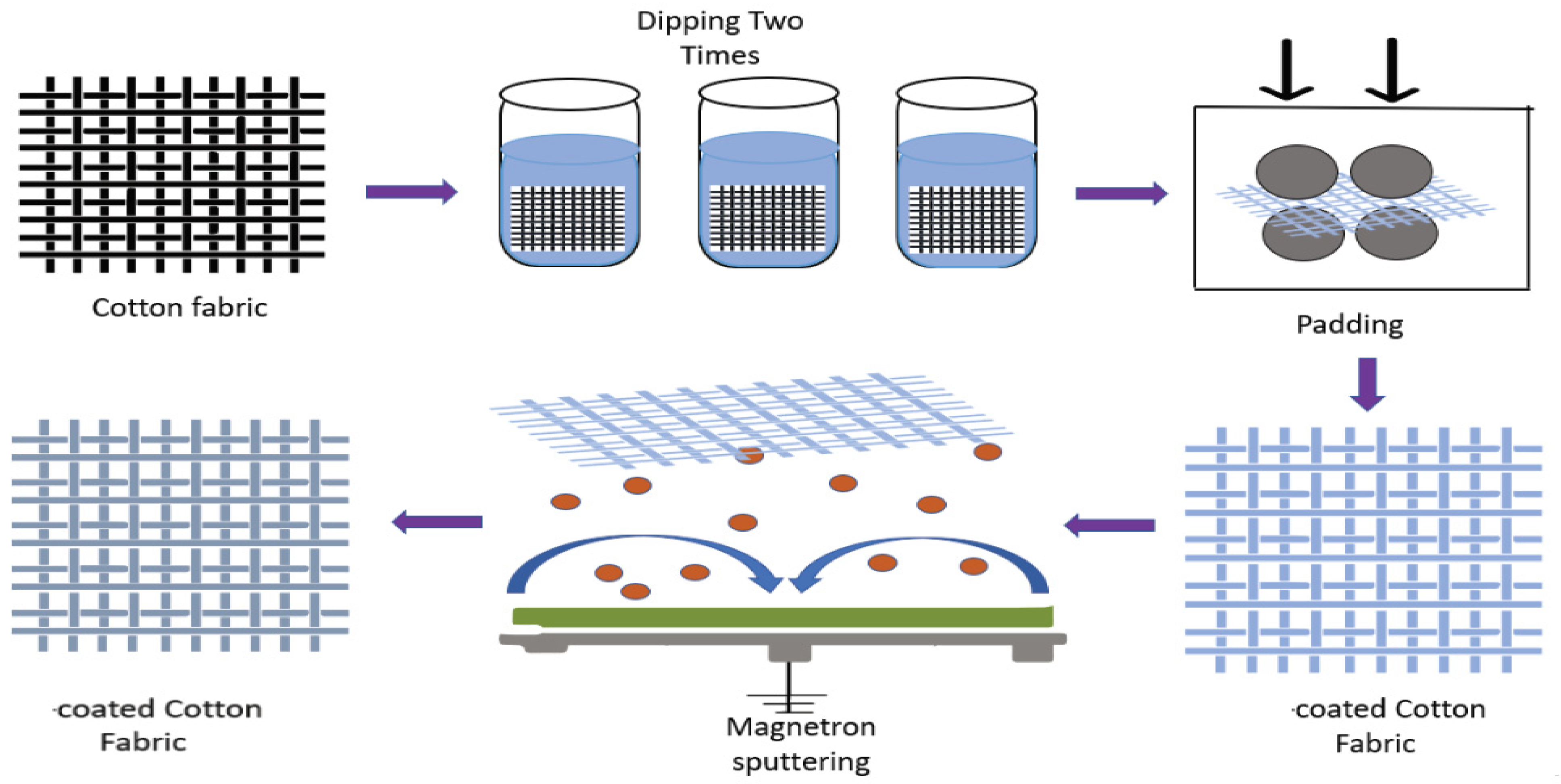
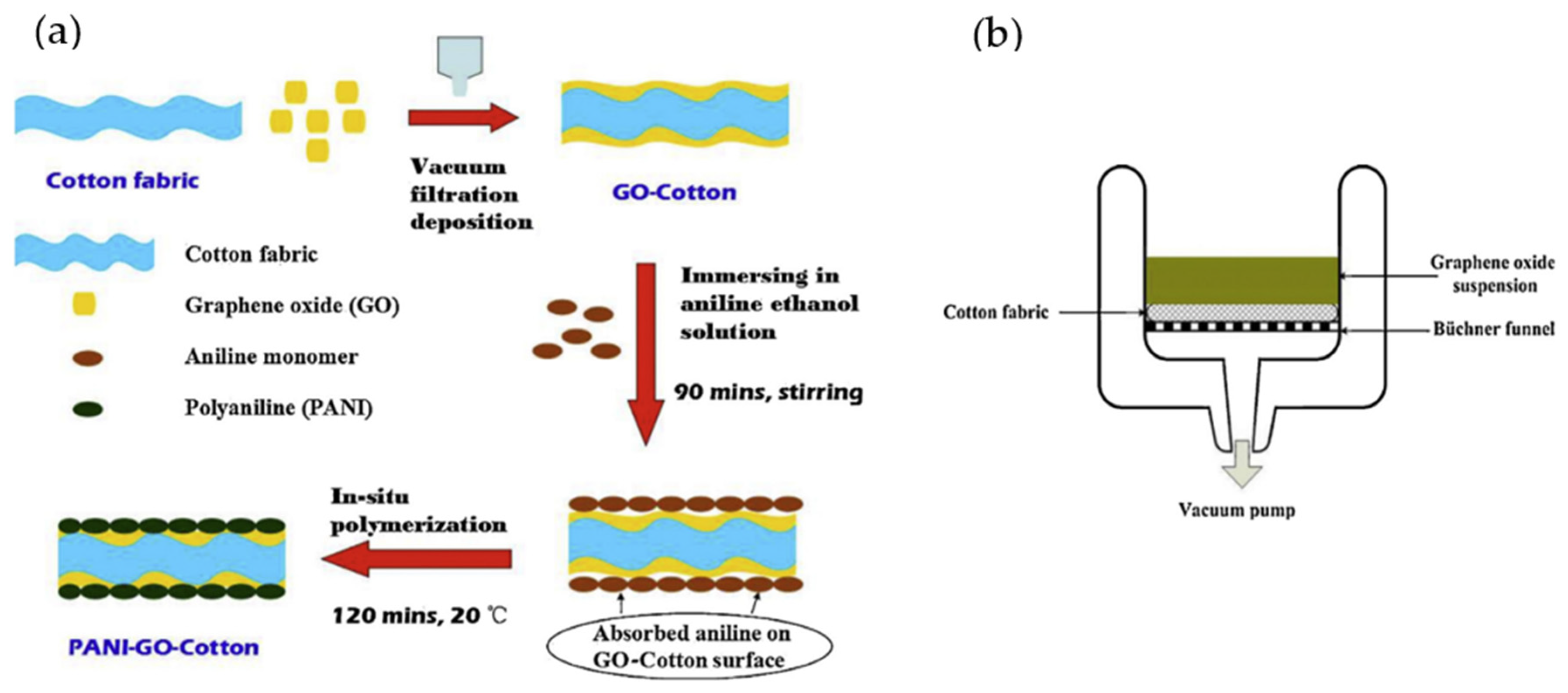
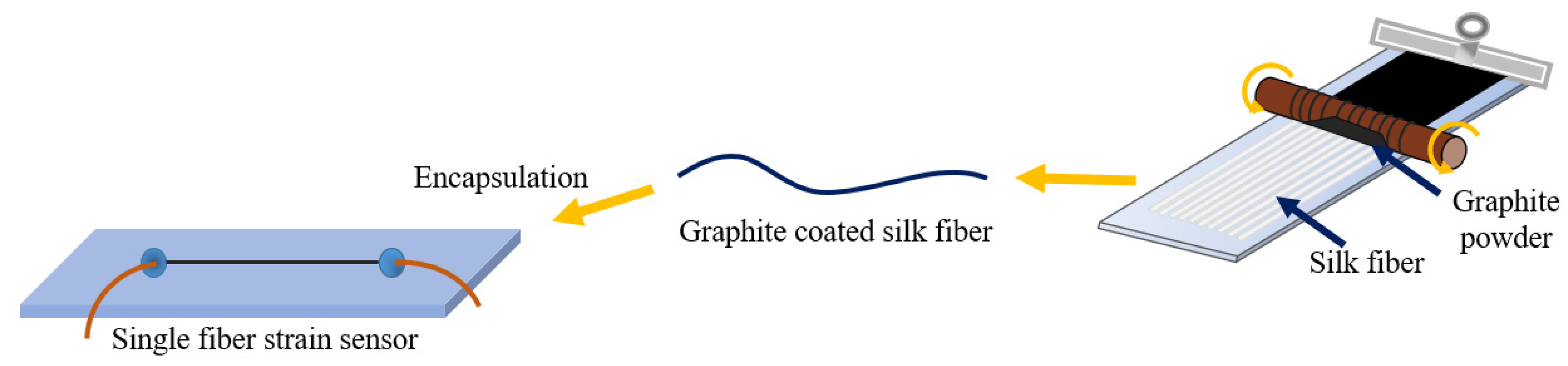
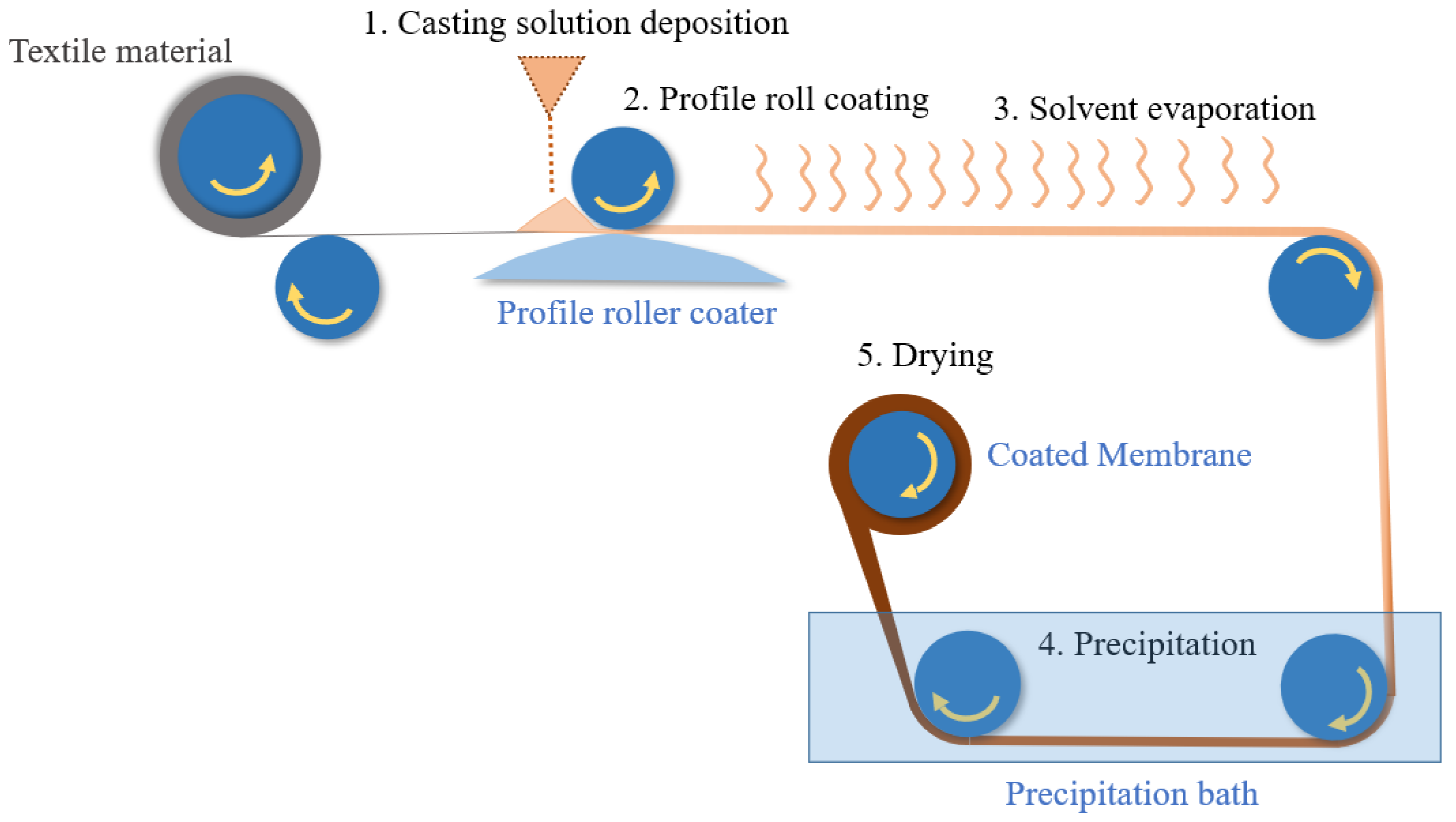
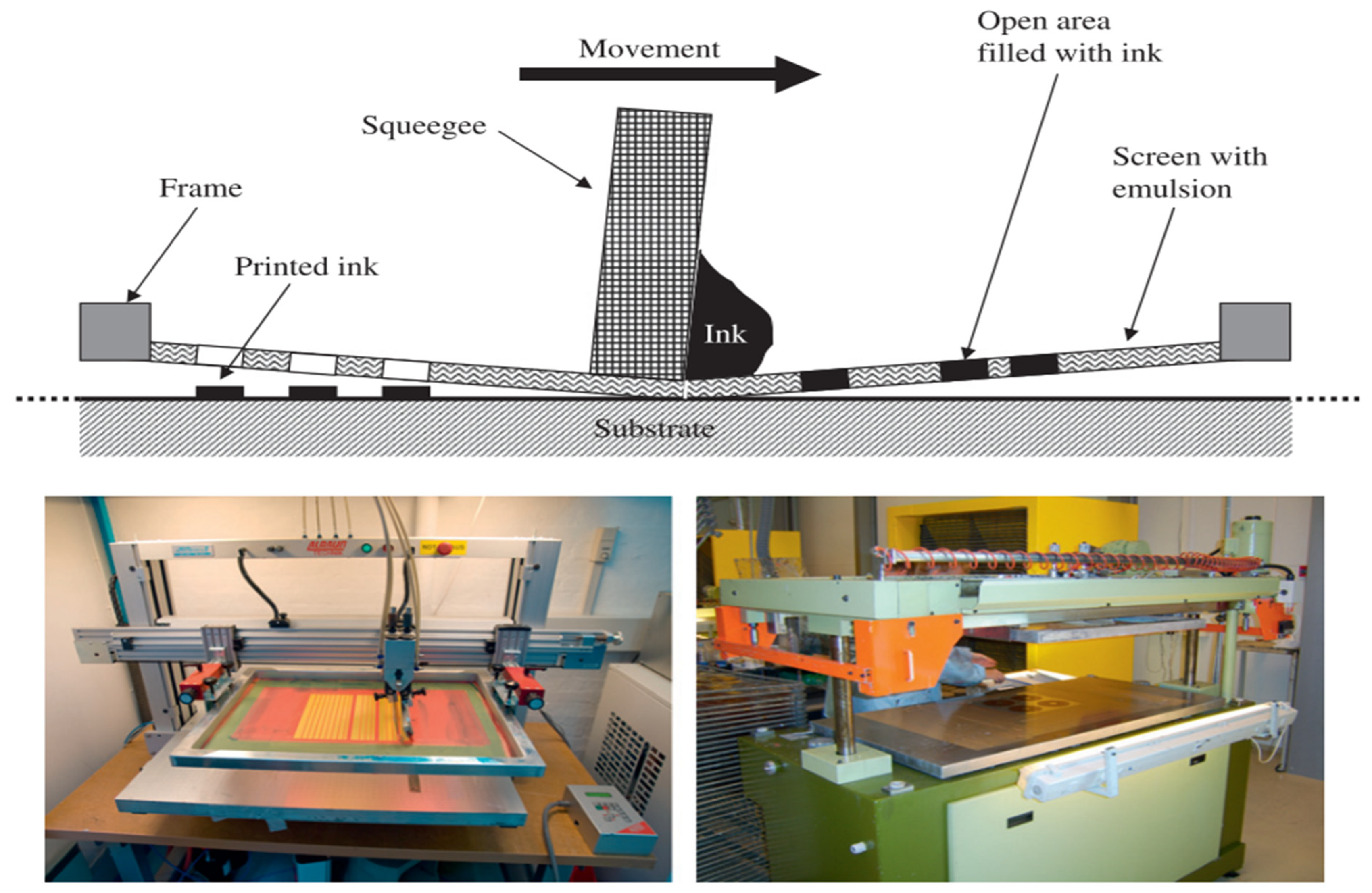



| Processes | Material | Coating Parameters | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Directly solution dip coating | Silver/graphene oxide/cotton fabric | Dipping cycle: 2 | user-friendly process | weak uniformity | [230] |
| Material to liquor ratio: 1:40 | high efficiency rate | poor bonding state | |||
| impressive conductive nature with the least surface resistance of 2.71 ohm/sq. | |||||
| Gas Pressure: 0.8 Pa | |||||
| Sol-gel dip coating | Organo-silicon/graphene/PET fabrics/polypropylene fabrics | Padding rate: 1 m/min | coated at nearly room temperature | more complex dynamic method | [246] |
| Squeezing roll pressure: 15 kg/cm | harsh chemical reaction on fibrous material | ||||
| Drying temperature: 105 °C | |||||
| Drying time: 20 min | |||||
| Multi-layered dip coating | AgNWs/cotton fabrics | Dipping Cycles:1–4 | good uniformity | relative low efficiency | [247] |
| multi-layer structure coating | |||||
| Dipping time: 1 min | high electrical conductivity (2416.46 Sm−1) | ||||
| Temperature: 50 °C | eco friendly | ||||
| Vacuum-assited dip coating | AgNW/MXene/silk fabric | Dipping Cycles: 10–50 | wide range of substrates | complicated dip coating devices | [240] |
| facilitate coating solution deposition | |||||
| Spray coating | PEDOT:PSS/PET fabrics | Drying temperature: 130 | excellent control over the conductive thickness | isolated droplets | [243] |
| suitable for the deposition of all types of conductive materials | Non-uniform surface | ||||
| Liquid flow rate: 2.5 mL/min | large scale production | ||||
| Air pressure: 3 bars | |||||
| Rod coating | Graphite/silk fiber | Coating cycles: 10 times | relatively low-cost and highly efficient fabrication of GSF fibers | ineffective with high viscosity liquid. | [244] |
| Roller coating | Thermoplastic Polyurethane (TPU)/CNT/Spandex Multifilament | Inlet temperature: 200 ± 5 °C | simple and cost-effective process | ineffective with low viscose liquids | [245] |
| Dropping temperature: 150 ± 5 °C | continuous process | [248] | |||
| Roller speed: 0.45 m/min | higher speed | ||||
| Process | Materials | Printing Parameters | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Screen printing | CNT/cotton fabrics |
|
|
| [251] |
| Inkjet printing | Reactive silver ink/cotton fabric/PET fabric/wool fabric |
|
|
| [252] |
| 3D printing | Ninja flex filament/PEDOT:PSS/Polyester fabric |
|
|
| [253,254] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayhan, M.G.S.; Khan, M.K.H.; Shoily, M.T.; Rahman, H.; Rahman, M.R.; Akon, M.T.; Hoque, M.; Khan, M.R.; Rifat, T.R.; Tisha, F.A.; et al. Conductive Textiles for Signal Sensing and Technical Applications. Signals 2023, 4, 1-39. https://doi.org/10.3390/signals4010001
Rayhan MGS, Khan MKH, Shoily MT, Rahman H, Rahman MR, Akon MT, Hoque M, Khan MR, Rifat TR, Tisha FA, et al. Conductive Textiles for Signal Sensing and Technical Applications. Signals. 2023; 4(1):1-39. https://doi.org/10.3390/signals4010001
Chicago/Turabian StyleRayhan, Md. Golam Sarower, M. Khalid Hasan Khan, Mahfuza Tahsin Shoily, Habibur Rahman, Md. Rakibur Rahman, Md. Tusar Akon, Mahfuzul Hoque, Md. Rayhan Khan, Tanvir Rayhan Rifat, Fahmida Akter Tisha, and et al. 2023. "Conductive Textiles for Signal Sensing and Technical Applications" Signals 4, no. 1: 1-39. https://doi.org/10.3390/signals4010001
APA StyleRayhan, M. G. S., Khan, M. K. H., Shoily, M. T., Rahman, H., Rahman, M. R., Akon, M. T., Hoque, M., Khan, M. R., Rifat, T. R., Tisha, F. A., Sumon, I. H., Fahim, A. W., Uddin, M. A., & Sayem, A. S. M. (2023). Conductive Textiles for Signal Sensing and Technical Applications. Signals, 4(1), 1-39. https://doi.org/10.3390/signals4010001











