Abstract
Chlorides have long been held responsible for the initiation and progression of the corrosion of reinforcing steels in concrete structures, with higher concentrations assumed to cause earlier and more severe subsequent reinforcement corrosion. However, extensive field observations and detailed experimental results show that, in well-compacted, low-permeability concretes, reinforcement corrosion often does not occur even in the presence of high concentrations of chlorides. If corrosion does occur, it has been observed as pitting (and crevice) corrosion primarily at air voids in the concrete at the steel–concrete interface. Herein, it is shown that this is consistent with thermodynamic principles (Pourbaix) for the pitting of steel in practical concretes with high pH and air voids, irrespective of chloride concentration. Any subsequent corrosion becomes inhibited, in part through the formation of corrosion products. The experimental observations also show that there is a separate, concurrent process of the dissolution of calcium hydroxide and its leaching from the concrete. The rate of dissolution is accelerated proportionally to the concentration of chlorides. This is the primary mechanism for longer-term reinforcement corrosion, eventually producing circum-neutral pH at the steel and thereby setting up the thermodynamics permitting general corrosion. The findings question the relevance of a critical chloride concentration as an indicator of the commencement of reinforcement corrosion. Concrete permeability, remaining alkali reserves (or pH), and physical observation of evidence of rust damage are better indicators.
1. Introduction
The corrosion of steel reinforcements in concrete structures exposed to seawater has long been associated with elevated concentrations of chlorides, and it is assumed that chlorides are directly responsible for reinforcement corrosion once the chloride concentration at the steel surface is sufficiently high to break down the high-pH passive film that the concrete usually provides around the steel reinforcement bars [1]. That concentration has been termed the ‘critical chloride concentration’. Once the break-down occurs, corrosion is assumed to proceed, at a non-zero rate, eventually damaging the surrounding concrete. The literature invariably invokes the (empirical) Tuutti model [2] (although earlier proposed by Clear [3]) to describe this behaviour. Efforts over many years to obtain numerical estimates for the ‘critical chloride concentration’ have been (and remain) extensive. A high degree of scatter has been observed for the values estimated from experimental tests, leading to recent efforts to invoke probability theory as a way forward [4]. In this context, it might be noted that, from a probability point of view, the large scatter immediately indicates that not all relevant factors at play have been taken into account [5]. This is consistent with the ‘critical chloride concentration’ more recently not being considered the sole durability assessment tool for structures exposed to chloride [4].
One of the factors not considered in the notion of the critical chloride concentration is the potential role of the concrete–steel interface [4,6]. The fact that this is an important factor was already foreshadowed in 1950 by Friedland [7], who pointed to the influence of the quality of mortar and concrete regarding the corrosion of steel reinforcements, and in 1975 by Verbeck [8], who observed that reinforcement corrosion was closely associated with air voids in the concrete at the steel surface. It also likely explains some observations from experiments meant to examine the role of chlorides for actual concretes [9], using model concretes made with added chlorides and containing a single steel bar to simulate reinforcement. These tests showed significant corrosion of the steel bar within a few (1–3) years [9]. However, subsequent efforts to replicate these results, including for a much wider range of concrete properties, showed little to no corrosion for many years for well-compacted, low-aggregate–cement-ratio concretes [10]. The reason for the poor performance of the original model concretes [9] was eventually attributed [10] to little or no concrete compaction and with the reinforcement bar being ‘placed’ into the concretes [9]. Although it cannot now be confirmed, it is likely that the resulting concrete cover provided little or no protection and a high presence of air voids within the concrete and particularly at the concrete–steel interface. Thus, in terms of conventional wisdom, the steel bars would have been exposed to a high concentration of chlorides and, by implication, this resulted in the observed severe corrosion within a few years of first exposure. However, these observations are not necessarily typical of actual field experience.
Extensive, long-term experience for many practical reinforced concrete structures exposed to various chloride-rich environments has shown little or no evidence of reinforcement corrosion even after exposures for 80 years or more [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. This experience includes reinforced concretes in marine immersion, tidal, splash, and atmospheric zones and reinforced concrete exposed to road bridge de-icing salts. In the latter case, eventual reinforcement corrosion was eventually attributed to damage to concrete cover as a result of vehicular impact rather than chloride-induced damage [24,25]. Other cases include physical damage to the concrete being the result of severe storm events [22,26] or alkali–aggregate activity [27,28].
The above-noted observations are mainly for long-term exposures. The question arises how the various observations are related to shorter-term corrosion. Very few controlled experiments are available to throw light on this relationship. One of these is a set of experiments on 3 m long reinforced concrete beams, 280 mm × 150 mm in cross-section with 10 mm cover each, with two 12 mm diam. steel reinforcement bars. The beams were simply supported at their ends, had an imposed vertical load along them, and were exposed in a chloride-rich laboratory environment for more than 28 years [29]. After an initial high rate of the corrosion of the steel bars, the rate decreased significantly [30]. This was attributed to a build-up of rust products and calcium carbonate on the bars. Corrosion occurred mainly along the bottom of the bars. After 10–15 years, the various beams showed a marked increase in corrosion of the steel reinforcement, mainly as pitting corrosion, sufficient to cause concrete cracking and spalling [31].
The other longer-term study covers 56 sets of concrete mix combinations (aggregate–cement, water–cement ratios), each consisting of 20 essentially identical specimens cast at the same time using the same concrete mix. All the specimens were 40 mm × 40 mm in cross-section, 160 mm long, with a longitudinal 6 mm diam steel bar [10]. Mostly, these were made with seawater as mixing water, some with added chlorides and some with freshwater. The concretes with low aggregate–cement ratios showed little or no corrosion of the steel even after 15 or more years of continuous exposure. Where corrosion of the steel reinforcement bar was observed, it was almost always as pitting or crevice corrosion within (wet) air voids in the concrete at the steel–concrete interface [10]. These experiments showed that (a) the voids varied significantly in size from barely observable under the (optical) microscope [10] and (b) that, without the presence of visible air voids, there was no visible evidence of corrosion initiation, despite the elevated chloride concentration. These laboratory observations on model concretes concur with multiple observations of no corrosion initiation for concrete with a high degree of steel–concrete interface contact, even after decades of marine exposure [16,17,18,20,21,23]. However, there are also some reports of the initiation of corrosion for steel reinforcements in experiments lasting 180 days and with increasing chloride concentrations, which reported that only 12–16% of cases of corrosion initiation that appeared to correspond with air voids, classified by the authors as ‘coarse’ voids, were visually observable in the concrete at the concrete–steel interface ([6] Section 3.4.1, [32]). The possibility of imperfect steel–concrete interface contact was noted as a potential factor for the presence of oxygen and water but not elaborated. Importantly, the specimens were not monitored to ascertain whether corrosion continued after the observed initiation, that is, whether the initial corrosion under chloride conditions then became longer-term active corrosion. The possible mechanisms and thermodynamics involved in the cases where there appeared to be no voids of any size are subject to ongoing investigation [33,34].
In addition to the observations for the steel–concrete interface, the laboratory experiments [10] showed the exterior surfaces of the concrete being subject to the loss of calcium hydroxide, with the slow-dissolution-rate concrete alkali holding the concrete pH at around 12. The experiments also showed that the loss of calcium hydroxide penetrates further into the concrete with continued exposure. The rate of this loss is accelerated by the increased solubility of calcium hydroxide in the presence of higher concentrations of chlorides [35]. Once the loss of calcium hydroxide and the associated circum-neutral pH of the porewaters had reached the steel, general corrosion was observed [36]. Figure 1 shows an example of the interior of the concrete specimens after 7 years of exposure in continued high-chloride conditions and the condition of the reinforcement of the concrete. Other examples are provided in [23,36], including details of how and where the pH measurements were made and the measurement of calcium hydroxide concentration. These references as well as [10,37] might be consulted as a prelude to considering the following exposition.
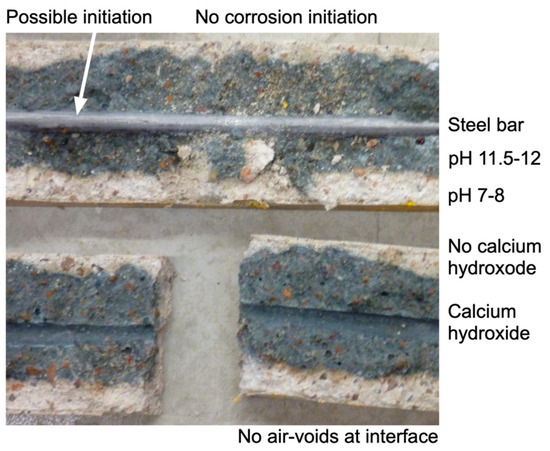
Figure 1.
Example of the interior of concrete and reinforcement bar for concrete specimen 40 mm × 40 mm in cross-section split lengthwise along the 6 mm diam. steel bar after 7 years of exposure in the laboratory fog-room (25 °C, very high relative humidity). Also shown are typical readings of concrete pH, and presence or otherwise of calcium hydroxide and the associated ‘front’ between high and low values, and the lack of steel corrosion.
Observations similar to those of Figure 1 confirm that there are two quite different processes acting on concretes exposed to chloride-rich environments—(a) the initiation and early development of corrosion and (b) loss of calcium hydroxide to the external environment with an associated drop in concrete pH. The extent to which these two processes are influenced by chloride concentration is examined below.
The next section examines whether the notion of a ‘critical chloride concentration’ is consistent with the basic thermodynamics applicable to corrosion initiation. It also considers why air voids in the concrete and other imperfect concrete–steel interface zones appear to permit corrosion initiation and why such corrosion appears as pitting or crevice corrosion as observed experimentally [10]. The section that follows considers the mechanisms involved in the second mode of behaviour—the progressive loss of calcium hydroxide as apparent from experimental observations. Neither of these aspects is considered explicitly in the classical models such as Tuutti [2]. The Discussion section reviews the development of reinforcement corrosion and its modelling, based on the principal mechanisms outlined herein. Other factors potentially involved are considered briefly. Comments are made on the practical implications.
Overall, this paper presents a completely new interpretation of the available data using the science of corrosion applied to the initiation and early corrosion of steel reinforcements in practical concretes. It shows that the conventional concept of a ‘critical chloride concentration’ leading to the commencement of serious damaging corrosion lacks both experimental and theoretical validity. Also, a further exposition is provided for a new model previously outlined and proposed for the development of the corrosion of steel reinforcements in concrete, including how that model relates to the classical Tuutti model.
2. Corrosion Feasibility
Water-soluble chlorides in concrete are held primarily in the porewater within the pore structure of the concrete [1]. Particularly for permeable concretes and for extended periods of exposure and for seawater exposures, the concentration of chloride within those pores may reach or even exceed the concentration of chlorides in seawater itself. It could be lower for exposures to the atmosphere owing to rainwater dilution or longer-term leaching out in drier conditions. Irrespective, there is a range of chloride concentrations that is possible. In the following, the chloride concentration in aerated seawater is used as a starting point for analysis. The aerated water condition is relevant since, as noted above, the corrosion of steel in concrete has mostly been identified as occurring at and within wet air voids in the concrete at the concrete–steel interface [4,8,10]. The possibility [32,33] that corrosion might initiate at locations on the steel without the presence of air voids is not considered herein, although some comments are made in the Discussion.
The feasibility of corrosion as an (electro-)chemical reaction is best considered in terms of the Gibbs free energy criterion [38] but, for most corrosion analyses, can be considered using a suitable Pourbaix diagram. For steels, the Pourbaix diagram for iron is commonly adopted, noting that, for general corrosion, there is little difference. Figure 2 shows the Pourbaix diagram that also includes pitting corrosion [39].
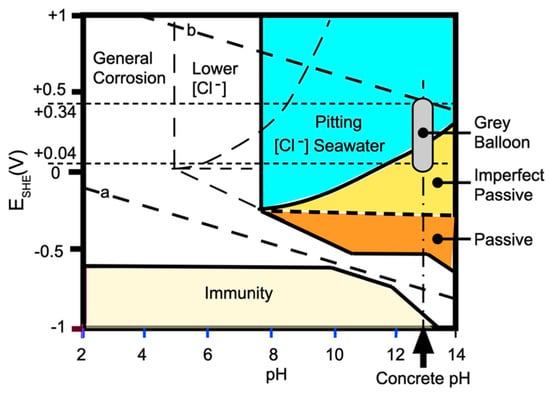
Figure 2.
Potential (ESHE)–pH diagram (Pourbaix diagram) for iron in seawater showing region in which pitting is feasible, and the balloon region (grey ellipse) representing the conditions at the steel–concrete interface at a wet air void or similar local imperfection.
For the corrosion of iron inside fresh or new concrete in wet aerated conditions with a pH around 13, the corrosion potential is a function of the degree of saturation or, equivalently, the availability of oxygen in the water. Electrochemical observations have shown that the range is around ESHE +0.04 to +0.34 V [40]. This range is shown in Figure 2 with the grey ellipse or ‘balloon’. It is seen that the lower part of this balloon lies inside the region marked ‘imperfect passive’. This denotes that pitting corrosion develops further only in the presence of any pre-existing pits or localized corrosion. (The ‘imperfect passivation’ region was not included in the original version of the Pourbaix diagram [41].) The (near-)horizontal orientation of the boundary between the passive and ‘imperfect passive’ regions is not greatly affected by chloride concentration [39]. The upper part of the grey balloon lies in the region denoted ‘pitting’, and essentially, this is independent of the chloride concentration, as seen by reference to the high- and low-chloride-concentration regions marked schematically in Figure 2.
The pitting of steels is affected by inclusions and associated ‘differential aeration’, which is essentially local galvanic corrosion initiated by the local potential difference between the Fe in the steel and the composition of the inclusion. For steels, the precise steel composition has minimal effect on general corrosion [42], but for pitting corrosion, by far the most common inclusion to cause pitting is MnS, including for modern steels [43]. These are important for facilitating initial pitting in the imperfect passive zone in Figure 2, particularly for lower chloride concentrations, when the pitting region moves to the left and the imperfect passive zone becomes dominant.
The critical aspect in the above is that the pitting referred to can occur in locations where both water and oxygen are available at the steel surface. As noted, for most practical reinforced concretes, those locations are at imperfections in the steel–concrete interface and, as experimental evidence shows, these are mainly seen as air voids in the concrete [4,8,10]. With the presence of water, Figure 2 shows that, even with a pH at around 13, the pitting of steel is feasible irrespective of chloride concentration. Owing to the inhomogeneity of the concrete at the steel–concrete interface, this scenario is likely to be met at somewhat different points in time along a steel bar.
Within the voids and at the steel–concrete interface, the solution almost certainly will be stagnant or very close to it. This means that the above observations are comparable with classical experimental results [44]. These showed no obvious evidence of corrosion, and thus no initiation, for iron immersed in stagnant (quiescent) solutions of different salts, including sodium chloride. That the stagnant condition was important was illustrated in laboratory experiments that showed that increased rates of stirring increased the amount of corrosion, from zero at initiation onwards [45].
Pitting corrosion will be able to continue immediately after initiation provided that the conditions for feasibility remain. In the presence of air voids, this means the continued (or semi-continuous) availability of water for the (initial) cathodic oxygen reduction reaction (ORR) [46] and the pits not being blocked up by the deposition of corrosion products. The first depends very much on the permeability of the concrete (and the thickness of cover to the reinforcement). The second depends on the rate of the build-up of rusts, noting that pitting creates the corrosion product Fe(OH)2 and others. These will deposit at cathodic regions, initially by the ORR in the interior of the void cavity and, in addition, also within the pits, near their mouths when local conditions there have become cathodic [43,46]. Further into pits, the conditions become increasingly acidic with the result that, eventually, the hydrogen evolution reaction becomes thermodynamically feasible within the pits [43,46]. This only requires water. The internal deposition and build-up of corrosion product can contribute to the inhibition of pit growth and passivation [47].
Throughout this early process, the pH external to the pits and outside the voids is still too high to permit general corrosion. The slowing and eventual cessation of pit growth has been observed [30] and is more readily achieved for less-permeable concretes with substantial concrete cover on the reinforcement [10]. As corrosion continues, the cathodic reaction on the inside of the mouths of pits and external to them can be expected to change gradually from oxygen reduction to water reduction (hydrogen evolution reaction), consistent with the corrosion behaviour of bare steel in (sea)water [48], thereby releasing hydrogen, as long observed for pitting corrosion [49].
From this description, it should be clear that air void size can play a role in the severity of the very early pitting corrosion of the steel at the concrete–steel interface and, also, in contributing to the amount of corrosion products generated. This, together with the corrosion product generated by the later corrosion under hydrogen evolution, could, if sufficient, eventually cause fracture of the surrounding concrete. The latter is consistent with the conventional view of damage caused by the build-up of rust products. But as shown here, the situation is more complex than simply the external corrosion of reinforcement bars.
For older concretes that have undergone some loss of pH, the balloon in Figure 2 will lie to the left of where it is shown. When the pH drops to about 9 anywhere along the concrete–steel interface, general corrosion becomes feasible at that location. As noted, for real concretes, this can come about through the loss of concrete alkalis, principally the loss of the longer-retained alkali Ca(OH)2. This is also the dominant mechanism for longer-term reinforcement corrosion, as discussed further in the next section.
3. Effect of Loss of Alkalis
Irrespective of the processes just considered, concretes exposed in some way to the atmosphere or aerated waters (including seawater) suffer from the slow loss, by leaching, of the alkalis in the concrete. The process commences at the exterior surfaces and moves inwards. Figure 1 shows an example for specimens exposed for 7 years in the laboratory fog-room. For these specimens, the possibility of carbonation was essentially excluded through the high relative humidity of the atmosphere [36].
The specimen shown in Figure 1 is one of a batch of 20 identical specimens made with low-heat cement that has the interesting property that concretes made using it change colour from dark grey to light grey within hours of being exposed to the atmosphere [50]. For the specimens such as that in Figure 1, while still in the fog-room and before being broken open to inspect the interior concrete and the state of the steel bar, the interior concrete could not have been exposed to the atmosphere, with the important exception of concrete exposed where the concrete alkali Ca(OH)2 had leached out to the external environment. Such leaching leaves a more permeable exterior zone [35] that would permit the entry of oxygen and thus allow oxidation of the lighter-colour concrete. In other words, the depth of the lighter-coloured concrete is a direct indicator of the depth of alkali leaching. Notionally, this may be interpreted as a ‘front’ of loss of Ca(OH)2 advancing into the concrete matrix from the specimen exterior, even though the ‘front’ is actually a gradual transition from high Ca(OH)2 (and thus high concrete pH) to low Ca(OH)2 (and thus low concrete pH). A similar gradual transition in loss of Ca(OH)2 and pH can be seen for the carbonation of concrete [51]. Subsequent to these observations, it was noted that, for specimens made with standard (local) blended cement, a change in colour could be observed but much less clearly.
In Figure 1, the pH at the lighter-coloured exterior zones is around circum-neutral, consistent with the observed very low concentration of calcium hydroxide. Further in, and around the reinforcing bar, the pH is still at around pH 12, consistent in this case with the presence of calcium hydroxide in that concrete. Other experimental observations at longer periods of exposure for concretes made in the same batch as that in Figure 1 show a narrower dark (high-pH) zone, with, after even longer exposures, a lack of dark concrete, a circum-neutral pH, and clear evidence of reinforcement corrosion. As might be expected, parallel findings have been reported for concretes of greater permeability [36] since, for these, the leaching-out, by diffusion, of calcium hydroxide occurs at a faster rate. Also, the rate of outward diffusion increases proportionally with a greater chloride concentration in the concrete. As noted, this is the direct result of the increase in the solubility of calcium hydroxide with higher chloride concentrations [35].
When the circum-neutral pH front (i.e., the ‘front’ of the light-coloured concrete in Figure 1) reaches the steel, general corrosion becomes feasible. Thus, the time it takes for the loss of Ca(OH)2 to move progressively from the outer parts of the specimen to the steel determines the (increasing) thermodynamic feasibility for corrosion to occur.
4. Model for Corrosion Progression
The above exposition shows that two distinct processes are involved in the development of the corrosion of steel reinforcements in concrete and that the role of chlorides differs in each. In the early stages, in the high-pH concrete environment, the corrosion of steel is feasible only as pitting (or crevice) corrosion facilitated by the presence of inclusions such as MnS. As noted above in relation to Figure 2, this feasibility is unaffected by the presence of chlorides. General corrosion is not feasible until the pH of the porewaters reduces to around 9 or less.
The progression of pitting corrosion will result in corrosion product build-up, initially mainly within the air voids [10], with the result that the pitting corrosion process tends to become inhibited, at least for low-permeability concretes, owing to the (near-)exhaustion of oxygen. For more permeable concretes with thin or modest concrete cover on the reinforcement, inward diffusion of oxygen (and water) may permit the corrosion processes within the voids to continue with the gradual expulsion of corrosion products that then may cause concrete damage. As noted, this scenario is supported by experimental evidence [10,30,31] and is consistent with theory.
Drawing on the observations given above, the development of corrosion loss as a function of the period of exposure can be represented as in Figure 3. In this model, there is an early period 0–A that may be considered as the period for water diffusion through the concrete from the external environment to the steel reinforcement, with oxygen already available at voids or other imperfections (of whatever size); a short period A–B during which pitting corrosion increases in air voids and other regions of non-perfect concrete–steel contact, with the magnitude of the corrosion dependent on the availability of water and the volume of oxygen in the air voids; and the period B–C in which the development of corrosion and corrosion products is facilitated by the inward diffusion of oxygen (and water). This part of the model is rate-governed by the diffusivity of the concrete, with near-passivation resulting for low-permeability concretes. The period C–D represents the likely extensive general corrosion that becomes possible when concrete protection is lost due to the destructive action of corrosion products and the reduction in concrete pH. This latter part can be considered to correspond to active corrosion in the Tuutti model [1,2]. However, in the Tuutti model, phases 0–A, A–B, and B–C in Figure 3 are not distinguished.
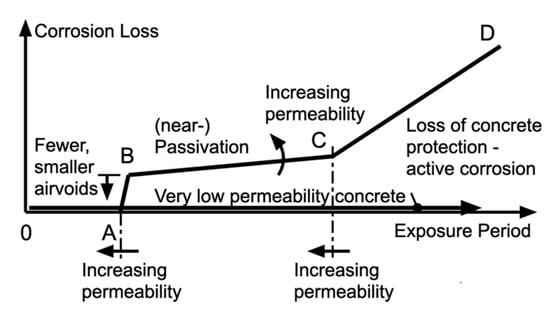
Figure 3.
Schematic evolution of corrosion loss of reinforcement in concrete as a function of marine environment exposure [23,28] but now with additional notation to indicate the adverse effect of greater concrete permeability.
As noted above, the corrosion process B–C in Figure 3 is governed by the rate of the loss of concrete alkalis, and this process is influenced by the diffusion and dissolution of calcium hydroxide and influenced by the effect of chloride concentration on the rate of the dissolution of calcium hydroxide. The above exposition, as reflected in Figure 3, shows that there is no causal or mechanistic link between the feasibility of corrosion as reflected in the Pourbaix diagram (Figure 2) (phases 0–A–B–C) and the loss of alkalis by outward leaching (phase C–D). The latter is influenced by the presence of chlorides. It follows immediately that the chloride concentration in the concrete is not a sufficient criterion (absolute or as a surrogate) for indicating when serious corrosion damage is likely to commence (i.e., as in the classical Tuutti model).
5. Discussion
The exposition above for the initiation of corrosion is based primarily on oxygen being already available in air voids and similar imperfections (of whatever size) at the steel–concrete interface, with pitting corrosion becoming feasible when water becomes available. This includes pitting in the imperfect passive zone of the Pourbaix diagram (Figure 2). In the context of the latter, it is noted that the precise mechanisms involved in the contribution of MnS and other inclusions to the initiation and the subsequent development of pitting remains for clarification, including for steels in concrete [52,53]. However, these mechanisms are not of direct concern herein.
As noted also above, the presence of air voids [54] or similar imperfections at the concrete–steel interface appears, from experimental observations, to be the most likely and widely occurring practical scenario [55]. This includes the possible effect of steel surface imperfections having a similar role at the concrete–steel interface [56,57]. Whether other mechanisms and characteristics of the concrete–steel interface could satisfy thermodynamic requirements for the commencement of corrosion remains an area for further investigation [33,34]. In this context, Wranglen [43] has noted that the sulphide in MnS inclusions can be oxidized, eventually leading to localized acidification and thus to pit initiation under low-oxygen conditions. The extent to which this is important for practical concretes remains to be investigated. This also applies to other potential mechanisms causing or leading to reinforcement corrosion in chloride environments [28], including physical damage, damage to aggregates from alkali–aggregate reactivity, a lack of adequate concrete compaction, the depth of concrete cracking, and the role of carbonation [50]. Further, the possibility that rolling scale as occurs on practical hot-rolled steel bars plays a role in inhibiting initiation compared with cold-drawn bars widely used in experimental studies also warrants investigation. However, it is noted that practical observations show that hot-rolled bars corrode relatively quickly (days) after being exposed to (moist) atmospheric exposure conditions.
Of course, for the progression of corrosion after initiation, as in phases B–C and C–D in Figure 3, the amount of water and the rate at which it is available will influence the rate of those two processes. Estimating the rates for these is a calibration exercise against various exposure environments. Some preliminary estimates are available for concretes likely to be continuously wet [28], but this will need to be extended to more general exposure conditions. Thus, further work is required for a comprehensive calibration exercise, noting again that this will require long-term experiments or observations to capture the longer-term rates. As should now be clear, they cannot be validly extrapolated from shorter-term observations.
It should be clear from the above exposition that concrete permeability and the presence of air voids and other oxygen-containing imperfections at the concrete–steel interface can play a significant, if not major, part in reinforcement corrosion, both in the short term and, for water, also in the long term. It is now clear that chlorides play a role only in longer-term corrosion through increasing the rate of the dissolution and loss of calcium hydroxide. Herein, only the principles involved have been discussed. This should provide guidance for the directions for further research, including the quantification of the various effects and influences. Although much remains to be done to calibrate the model of Figure 3 to experimental and field observations, initial attempts have been made to estimate the long-term rates [28].
6. Conclusions
The following conclusions are drawn from the material presented herein:
- Field experience for practical concretes and for laboratory tests on model concretes confirms that the initiation of the corrosion of steel reinforcements is directly associated with the presence of air pockets, air voids, or similar imperfections in the concrete at the steel–concrete interface, irrespective of the size of those voids or the local concentration of chlorides in the concrete.
- These practical observations are consistent with thermodynamic principles for the feasibility of the corrosion of iron, including with the presence of MnS inclusions typical of steels, again irrespective of the local concentration of chlorides.
- Field evidence and experimental observations over many years show that, for low-permeability, well-compacted concretes with adequate cover, any corrosion that may initiate likely will become inhibited as a result of the decreasing availability of water and/or oxygen and the build-up of corrosion products over and within the mouths of pits.
- Experimental observations over many years show that the primary mechanism for the long-term corrosion of reinforcing steel in concretes is the loss of alkalinity imparted principally by calcium hydroxide in the concrete around the steel, noting that the rate of loss is proportional to the concentration of chlorides and that it increases with concrete permeability and reduces with increasing concrete cover thickness.
- It follows that, in design practice, attention should be given to achieving well-compacted, low-permeability concretes, while for the analysis of existing reinforced concrete structures, the focus should be on assessing remaining alkali reserves (or pH) and evidence of rust damage.
Author Contributions
Conceptualization, methodology, writing-original draft: R.E.M.; writing-review, validation, I.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the helpful and insightful comments made by the reviewers and the Academic Editor. They also acknowledge the continued support from the University of Newcastle, the laboratory staff who contributed to the experimental work summarized herein, and the staff at Auchmuty Library.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Richardson, M.G. Fundamentals of Durable Reinforced Concrete; SponPress: London, UK, 2002. [Google Scholar]
- Tuutti, K. Corrosion of Steel in Concrete; Research Report No.4; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Clear, K.C. Time-to-Corrosion for Reinforcing Steel in Concrete Slabs, V. 3: Performance After 830 Daily Salt Applications; FHWA-RD-76-70; Federal Highway Administration: Washington, DC, USA, 1976; 64p.
- Papworth, F.; Andrade, C.; Lollini, F. Selection of a critical chloride level for full probabilistic modelling. Corros. Mater. Degrad. 2025, 6, 21. [Google Scholar] [CrossRef]
- Ang, A.H.-S.; Tang, W.H. Probability Concepts in Engineering Planning and Design, Vol. I-Basic Principles; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel-concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Corros. 2019, 52, 88. [Google Scholar] [CrossRef]
- Friedland, R. Influence of the quality of mortar and concrete upon corrosion of reinforcement. J. ACI 1950, 22, 125–139. [Google Scholar]
- Verbeck, C.J. Mechanism of corrosion of steel in concrete. Spec. Publ. 1975, 49, 21–38. [Google Scholar]
- Shalon, R.; Raphael, M. Influence of seawater of corrosion of reinforcement. J. Allergy Clin. Immunol. 1959, 30, 1251–1268. [Google Scholar]
- Melchers, R.E.; Chaves, I.A. Reinforcement corrosion in marine concretes-1. Initiation. ACI Mater. J. 2019, 116, 57–66. [Google Scholar] [CrossRef]
- Wakeman, C.M.; Dockweiler, E.V.; Stover, H.D.; Whiteneck, L.L. Use of concrete in marine environments. JACI Proc. 1957, 54, 841–856; discussion 1309–1346. [Google Scholar]
- Hadley, H.M. Concrete in seawater: A revised viewpoint needed. Proc. ASCE 1941, 107, 345–358; discussion 359–394. [Google Scholar]
- Beaton, J.L.; Spellman, D.L.; Stratfull, R.F. Corrosion of steel in continuously submerged reinforced concrete piling. Highw. Res. Rec. 1967, 204, 11–21. [Google Scholar]
- Lukas, W. Relationship between chloride content in concrete and corrosion in untensioned reinforcement on Austrian bridges and concrete road surfacings. Betonw. Und Fert.-Tech. 1958, 51, 730–734. [Google Scholar]
- Angst, U.M.; Elsener, B.; Larsen, C.K.; Vennesland, O. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Lau, K.; Sagüés, A.A.; Yao, L.; Powers, R.G. Corrosion performance of concrete cylinder piles. Corrosion 2007, 63, 366–378. [Google Scholar] [CrossRef]
- Melchers, R.E.; Li, C.Q.; Davison, M.A. Observations and analysis of a 63-year old reinforced concrete promenade railing exposed to the North Sea. Mag. Concr. Res. 2009, 61, 233–243. [Google Scholar] [CrossRef]
- Melchers, R.E.; Pape, T.M.; Chaves, I.A.; Heywood, R. Long-term durability of reinforced concrete piles from the Hornibrook Highway bridge. Aust. J. Struct. Eng. 2017, 18, 41–57. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A. A comparative study of chlorides and longer-term reinforcement corrosion. Mater. Corros. 2017, 68, 613–621. [Google Scholar] [CrossRef]
- Melchers, R.E. Modelling durability of reinforced concrete structures. Corros. Eng. Sci. Technol. 2020, 55, 171–181. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term durability of marine reinforced concrete structures. J. Mar. Sci. Eng. 2020, 8, 290. [Google Scholar] [CrossRef]
- Melchers, R.E.; Howlett, C.M. Reinforcement corrosion of the Phoenix caissons after 75 years of marine exposure. Proc. Inst. Civ. Engrs. Marit. Eng. 2020, 174, 19–30. [Google Scholar] [CrossRef]
- Melchers, R.E. Experienced-based physico-chemical models for long-term reinforcement corrosion. Corros. Mater. Degrad. 2021, 2, 100–119. [Google Scholar] [CrossRef]
- Wallbank, E.J. The Performance of Concrete in Bridges: A Survey of 200 Highway Bridges; Department of Transport; HMSO: London, UK, 1989. [Google Scholar]
- Volkswein, A.; Dorner, H. Untersuchungen zur Chloridkorrosion der Bewehrung von Autobahn-Brucken aus Stahl-oder Spannbeton. In Forschung Strassenbau und Strassenverkehrstechnik; Heft 460; Bundesminister fur Verkehr, Abteilung Strassenbau: Bonn-Bad Godesberg, Germany, 1986. [Google Scholar]
- Jellett, J.H. The lay-out, assembly, and behaviour of the breakwaters at Arromanches Harbour (Mulberry B). In The Civil Engineer in War, A Symposium of Papers on War-Time Engineering Problems; vol 2–Docks and Harbours; Institution of Civil Engineers: London, UK, 1948; pp. 291–312. [Google Scholar]
- Godart, B.; de Rooij, M.R. Diagnosis, appraisal, repair and management, Chapter 5. In Alkali-Aggregate Reaction in Concrete: A World Review; Sims, I., Poole, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Melchers, R.E. Mechanisms in long-term marine corrosion of steel reinforcement in concretes. Corrosion 2023, 79, 380–387. [Google Scholar] [CrossRef]
- François, R.; Arliguie, G.; Maso, J.-C. Durabilité du béton armé soumis à l’action des chlorures. Ann. L’institut Tech. Batim. Trav. Publics 1994, 529, 1–48. [Google Scholar]
- Yu, L.; François, R.; Dang, V.H.; l’Hostis, V.; Gagné, R. Development of chloride-induced corrosion in pre-cracked RC beams under sustained loading: Effect of load-induced cracks, concrete cover, and exposure conditions. Cem. Concr. Res. 2015, 67, 246–258. [Google Scholar] [CrossRef]
- Zhu, W.; François, R.; Liu, Y. Propagation of corrosion and corrosion patterns of bars embedded in RC beams stored in chloride environment for various periods. Constr. Build. Mater. 2017, 145, 147–156. [Google Scholar] [CrossRef]
- Boschmann, C.; Angst, U.M.; Aguilar, A.M.; Elsener, B. A systematic data collection on chloride-induced steel corrosion in concrete to improve service life modelling and towards understanding corrosion initiation. Corros. Sci. 2019, 157, 331–336. [Google Scholar] [CrossRef]
- Angst, U.M. A critical review of the science and engineering of cathodic protection of steel in soil and concrete. Corrosion 2019, 75, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Angst, U.M.; Isgor, O.B.; Hansson, C.M.; Sagüés, A.; Gelker, M.R. Beyond the chloride threshold concept for predicting corrosion of steel in concrete. Appl. Phys. Rev. 2020, 9, 011321. [Google Scholar] [CrossRef]
- Johnston, J.; Grove, C. The solubility of calcium hydroxide in aqueous salt solutions. J. Am. Chem. Soc. 1931, 53, 3976–3991. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A. Reinforcement corrosion in marine concretes—2. Long-Tterm effects. ACI Mater. J. 2020, 117, 217–228. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A.; Simundic, G. Effect of coarse calcareous aggregates on corrosion of steel reinforcement in marine concretes. Struct. Infrastruct. Eng. 2024, 1–12. [Google Scholar] [CrossRef]
- Brown, T.L.; Lemay, H.E. Chemistry: The Central Science; Prentice Hall: New York, NY, USA, 1977. [Google Scholar]
- Pourbaix, M. Significance of protection potential in pitting and intergranular corrosion. Corrosion 1970, 26, 431–438. [Google Scholar] [CrossRef]
- Bertolini, C.; Elsener, B.; Pedeferri, P.; Polder, R. Corrosion of Steel in Concrete-Prevention, Diagnosis and Repair; Wiley-VCH: Weinheim, Germany, 2013; Chapter 7. [Google Scholar]
- Pourbaix, M. Recent applications of electrode potential measurements in the thermodynamics and kinetics of corrosion of metals. Corrosion 1969, 25, 267–281. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Heyn, E.; Bauer, O. Ueber den Angriff des Eisens durchWasser und wässerige Losungen. Stahl Eisen 1908, 28, 1564–1573. [Google Scholar]
- Mercer, A.D.; Lumbard, E.A. Corrosion of mild steel in water. Brit. Cor. J. 1995, 30, 43–55. [Google Scholar] [CrossRef]
- Sharland, S.M.; Tasker, P.W. A mathematical model of crevice and pitting corrosion-I. The physical model. Corros. Sci. 1988, 28, 603–620. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M.; Vines, S.P. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. The transition from short- to long-term marine corrosion of carbon steels: 1. Experimental observations. Corrosion 2022, 78, 415–426. [Google Scholar] [CrossRef]
- Pickering, H.W. Important early developments and current understanding of the IR mechanism of localized corrosion. J. Electrochem. Soc. 2003, 150, K1–K13. [Google Scholar] [CrossRef]
- Sulphate -Resisting Cement: Technical Data Sheet, Version 3.3; Hanson Heidelberg Cement: Ketton, UK, 2015.
- Melchers, R.E.; Richardson, P.J. Carbonation, neutralization and reinforcement corrosion for concrete in long-term atmospheric exposures. Corrosion 2023, 79, 395–404. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Alhozaimy, A.; Hussain, R.-R.; Al-Zaid Singh, R.J.K.; Singh, D.D.N. Role of manganese sulfide inclusions in steel rebar in the formation and breakdown of passive films in concrete pore solutions. Corrosion 2014, 70, 74–86. [Google Scholar] [CrossRef]
- Rieders, N.; Nandasiri, M.; Mogk, D.; Avci, R. New insights into sulfide inclusions in 1018 carbon steels. Metals 2021, 11, 428. [Google Scholar] [CrossRef]
- Nawy, E.G. Concrete Construction Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2008; pp. 30–57. [Google Scholar]
- Liu, Z.; Hansen, W.; Meng, B. Characterisation of air-void systems in concrete. Mag. Conc. Res. 2015, 68, 178–186. [Google Scholar] [CrossRef]
- Novak, P.; Mala, R.; Joska, L. Influence of pre-rusting on steel corrosion in concrete. Cem. Conc. Res. 2001, 31, 589–593. [Google Scholar] [CrossRef]
- Boubitsas, D.; Tang, L. The influence of reinforcement steel surface condition on initiation of chloride induced corrosion. Mater. Struct. 2015, 48, 2641–2658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).