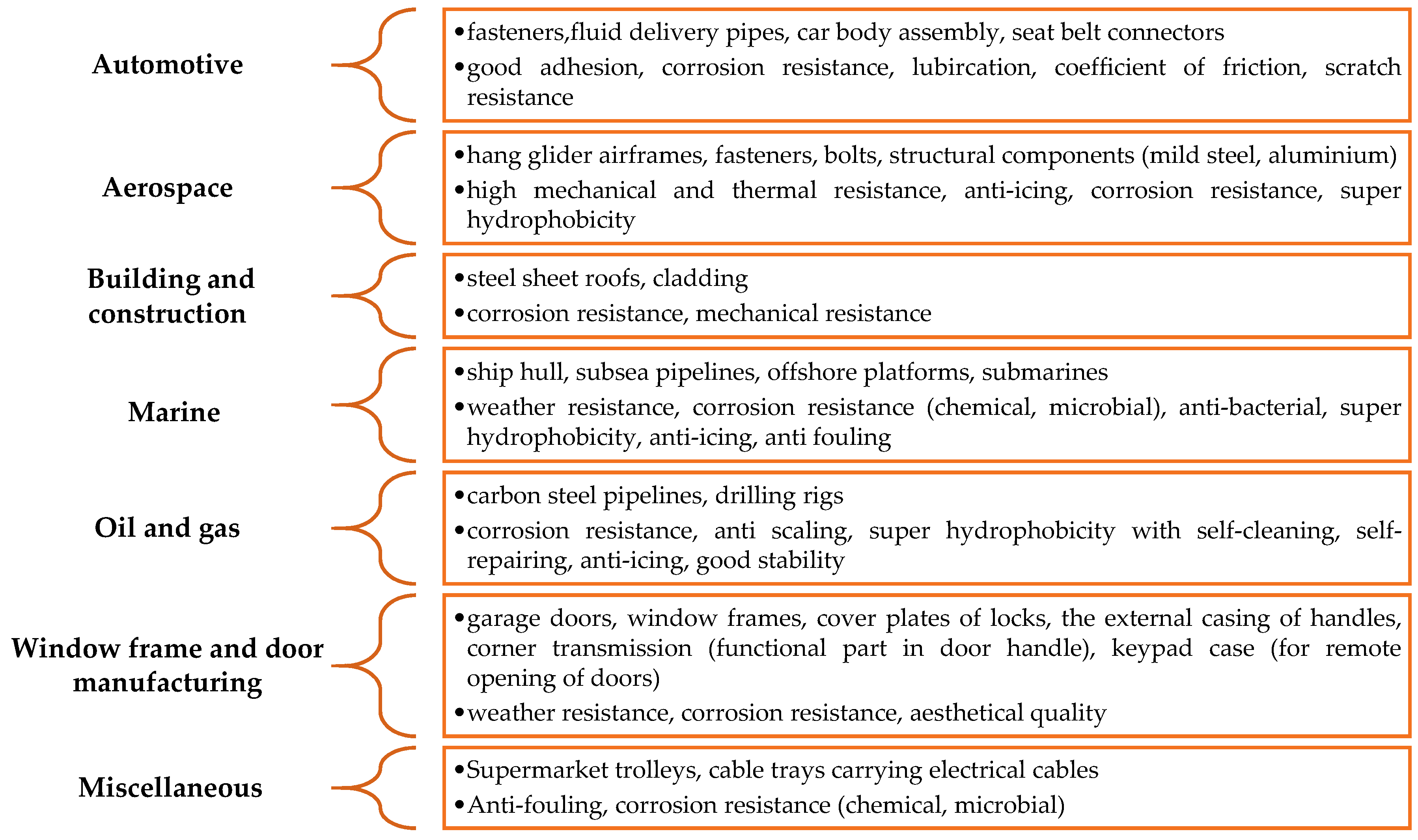
Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment
Abstract
1. Introduction
2. Corrosion Performance of Zinc and Zinc–Alloy Coatings
2.1. Zn Coatings
- texture
- composition
- morphology
- grain size
| System | Substrate | Additive | Functional Role 1 | References |
|---|---|---|---|---|
| Alkaline zincate | mild steel | Poly vinyl alcohol (PVA) | Texture | [37] |
| Alkaline zincate | mild steel | (PVA) + piperonal | grain refiner | [37] |
| Acidic sulphate | steel sheet | Gelatin | grain refiner, lowering the surface roughness | [49] |
| Acidic sulphate | steel sheet | polyethylene glycol (PEG) | grain refiner, lowering the surface roughness | [49] |
| Acidic sulphate | steel sheet | Saccharin | grain refiner, lowering the surface roughness | [49] |
| Acidic sulphate | steel sheet | tetrabutylammonium chloride | grain refiner, lowering the surface roughness | [49] |
| Acidic sulphate | steel sheet | sodium lauryl sulfate | grain refiner, lowering the surface roughness | [49] |
| Acidic sulphate | mild steel | cetyltrimethyl ammonium bromide (CTAB) + ethyl vanillin | grain refiner | [50] |
| Acidic chloride | carbon steel | Sodium benzoate | grain refiner | [51] |
| Alkaline zincate | carbon steel | trisodium nitrilotriacetic (NTA) | complexing agent | [52] |
| Acidic sulphate | mild steel | (CTAB) + veratraldehyde (VV) | grain refiner, texture, morphology | [48] |
| Acidic sulphate | glassy carbon | [3-(2-furyl) acrolein] | grain refiner | [29] |
| Acidic sulphate | mild steel | PEG | grain refiner, texture | [31] |
| Acidic sulphate | mild steel | CTAB | grain refiner, texture | [31] |
| Acidic sulphate | mild steel | Thiourea | grain refiner, texture | [31] |
| Acidic sulphate + gluconate | mild steel | PEG | grain refiner, texture | [31] |
| Acidic sulphate + gluconate | mild steel | CTAB | grain refiner, texture | [31] |
| Acidic sulphate + gluconate | mild steel | Thiourea | grain refiner, texture | [31] |
| Acidic sulphate | mild steel | Polyacrylamide | grain refiner | [36] |
| Acidic chloride | mild steel | (PEG) and syringaldehyde (SGA) | grain refiner, texture | [28] |
| Acidic chloride | carbon steel | Formic acid (FA) + cyclohexylamine (CHA) | Texture | [45] |
2.2. Zn-Alloy Coatings
2.3. Zn and Zn–Alloy Composite Coatings
| Zn/Zn-X | Second Phase | Substrate | System | Mode of Deposition | ECorr, V (SCE) | iCorr, µA cm−2 | References |
|---|---|---|---|---|---|---|---|
| Zn | CeO2 | mild steel | chloride | direct current | −1.127 | 3.56 | [110] |
| pulse current | −1.147 | 0.69 | |||||
| Zn | TiO2 | steel | sulfate | direct current | −1.052 | 2.7 | [111] |
| pulse current | −1.118 | 15.1 | |||||
| Zn | SiO2 | mild steel | chloride | galvanostatic | −1.127 | ~1 | [100] |
| Zn | Al2O3 | mild steel | chloride | galvanostatic | −1.282 | ~1 | [100] |
| Zn | ZrO2 | mild steel | sulfate | direct current | −1.034 | 4.45 | [91] |
| Zn | SiC | mild steel | sulfate | direct current | −1.100 | 2.090 | [112] |
| Zn | graphene oxide | mild steel | sulfate | direct current | −1.131 | 4.1 | [113] |
| Zn–Ni | TiO2 | steel | citrate | galvanostatic | −0.90 | 176 | [82] |
| Zn–Ni | Fe2O3 | mild steel | sulfate | direct current | −1.1991 | 0.682 | [114] |
| Zn–Ni | CeO2 | mild steel | chloride | reverse pulse current | −0.78 | 28 | [115] |
| Zn–Fe | graphene | mild steel | sulfate | direct current | −1.087 | 19.20 | [103] |
| Zn–Co | CNTs | mild steel | sulfate | direct current | −0.901 | 0.156 | [102] |
3. Recent Developments
3.1. Zn and Zn–Alloy Deposition in Ionic Liquids
| System | Coating | Substrate | Mode of Deposition | Corrosion Test Method | ECorr, V (vs Pt/SCE) | ICorr, µA cm−2 | References |
|---|---|---|---|---|---|---|---|
| ChCl–Urea | Zn | Carbon steel | Potentiostatic | LPP | −0.289 1 | 0.68 | [129] |
| Zn–Mn (0.4–0.7) 3 | Copper | Potentiostatic | LPP, EIS | −1.021 1 | 1.075 | [70] | |
| Zn–Mn (0.4–1.0) 3 | Copper | Potentiostatic | LPP, EIS | −1.054 1 | 0.917 | ||
| Zn–Mn (0.4–1.4) 3 | Copper | Potentiostatic | LPP, EIS | −1.098 1 | 1.175 | ||
| Zn–Mn (0.4–0.7) 3 | Copper | Potentiostatic | LPP, EIS | −1.062 1 | 0.989 | ||
| Zn–Mn (0.4–1.0) 3 | Copper | Potentiostatic | LPP, EIS | −1.079 1 | 0.875 | ||
| Zn–Mn (0.4–1.4) 3 | Copper | Potentiostatic | LPP, EIS | −1.109 1 | 1.251 | ||
| ChCl–Urea (1 wt.% H2O) | Zn–Ni | Carbon steel | Potentiostatic | LPP | −0.414 1 | 0.82 | [129] |
| ChCl–Urea (3 wt.% H2O) | Zn–Ni | Carbon steel | Potentiostatic | LPP | −0.478 1 | 1.3 | |
| ChCl–Urea (5 wt.% H2O) | Zn–Ni | Carbon steel | Potentiostatic | LPP | −0.801 1 | 2.1 | |
| ChCl–Urea (7 wt.% H2O) | Zn–Ni | Carbon steel | Potentiostatic | LPP | −0.931 1 | 5.6 | |
| ChCl –EG | Zn | Mild steel (AISI 304) | Potentiostatic | LPP, EIS | −1.040 1 | 6.57 | [130] |
| [EMIm][Tf2N]- Zn[Tf2N] | Zn–Mn | DP-1000 steel | Potentiostatic | LPP | −1.016 1 | 0.0119 | [131] |
| Zn–Mn | DP-1000 steel | Potentiostatic | LPP | −0.776 1 | 0.0112 | [131] | |
| ChCl–Urea | Zn | WE43-T6 Mg alloy | galvanostatic | LPP | −1.420 1 | 38.68 | [132] |
| ChCl–Urea | Zn–Mn (1–1) 4 | Steel | galvanostatic | LPP | 1.110 | 1.06 | [128] |
| ChCl–Urea | Zn–Mn (1–1) 4 | Steel | galvanostatic | LPP | 1.040 | 3.2 | [128] |
| ChCl–Urea | Zn–Mn (1–1) 4 | Steel | galvanostatic | LPP | 1.045 | 3.6 | [128] |
| ChCl–Urea | Zn–Mn (1–3) 4 | Steel | galvanostatic | LPP | 1.130 | 0.90 | [128] |
| ChCl–Urea | Zn–Mn (1–3) 4 | Steel | galvanostatic | LPP | 1.040 | 0.82 | [128] |
| ChCl–Urea | Zn–Mn (1–3) 4 | Steel | galvanostatic | LPP | 1.046 | 5.3 | [128] |
| ChCl –EG | Zn | Copper | galvanostatic | LPP | −1.197 2 | 7.987 | [133] |
| NaOAc: EG2 | Zn | Mild steel | galvanostatic | LPP | −1.066 2 | 1.01 | [134] |
3.2. Superhydrophobic Zn and Zn–Alloy Coatings
| Coating | Substrate | System/Bath | Surface Energy Reducer Agent | CA° | Reference |
|---|---|---|---|---|---|
| Zn | steel | chloride | vulcanized silicone polymer | 155 ± 1 | [142] |
| Zn | X65 steel | sulphate | stearic acid | 158.4 ± 1.5 | [143] |
| Zn | X90 steel | sulphate | perfluoro octanoic acid | 154.21 | [144] |
| Zn | carbon steel | Sulfate-acetate | stearic acid | 153 | [145] |
| Zn | carbon steel | alkaline | stearic acid | 158.7 | [146] |
| Zn | copper | DES 1 | stearic acid | 164.8 ± 0.6 | [147] |
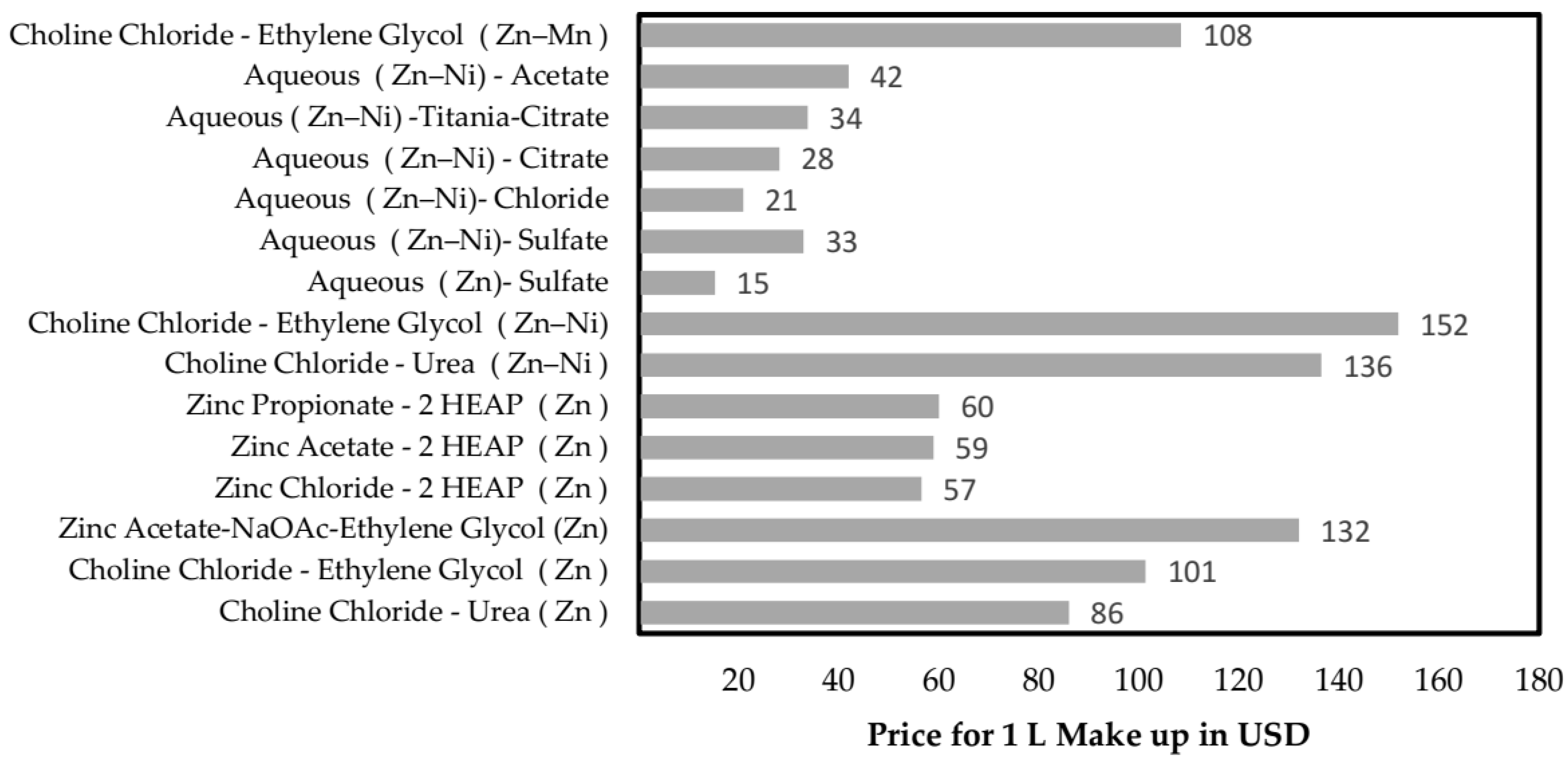
4. Cost Considerations and Future Challenges
4.1. Economic Aspects
4.2. Future Challenges
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.-Q.; Zhang, Q.; Tu, S.-S.; Wang, Y.; Li, Y.-M.; Huang, Y. A study on time-variant corrosion model for immersed steel plate elements considering the effect of mechanical stress. Ocean Eng. 2016, 125, 134–146. [Google Scholar] [CrossRef]
- Deepa, M.J.; Arunima, S.R.; Riswana, G.; Riyas, A.H.; Sha, M.A.; Suneesh, C.V.; Shibli, S.M.A. Exploration of Mo incorporated TiO2 composite for sustained biocorrosion control on zinc coating. Appl. Surf. Sci. 2019, 494, 361–376. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Meena, B.N.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Winand, R. Electrodeposition of Zinc and Zinc Alloys. In Modern Electroplating, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 285–307. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Afroukhteh, S. A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J. Nat. Gas Sci. Eng. 2019, 71, 102971. [Google Scholar] [CrossRef]
- Weng, T.Y.; Kong, G.; Che, C.S.; Wang, Y.Q. Corrosion behaviour of a Zn/Zn-Al double coating in 5% NaCl solution. Int. J. Electrochem. Sci. 2018, 13, 11882–11894. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Corrosion behavior of mild steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2008, 3, 806–818. [Google Scholar]
- Kinugasa, J.; Yuse, F.; Tsunezawa, M.; Nakaya, M. Effect of Corrosion Resistance and Rust Characterization for Hydrogen Absorption into Steel under an Atmospheric Corrosion Condition. ISIJ Int. 2016, 56, 459–464. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Tsoutsouva, M.G. Cathodic electrolytic deposition of ZnO on mild steel. Corros. Eng. Sci. Technol. 2011, 46, 513–516. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Strauss, H.W.; Brahimi, S.; Chromik, R.R.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribological behavior of electrodeposited Zn, Zn-Ni, Cd and Cd-Ti coatings on low carbon steel substrates. Tribol. Int. 2012, 56, 107–120. [Google Scholar] [CrossRef]
- Abioye, O.P.; Musa, A.J.; Loto, C.A.; Fayomi, O.S.I.; Gaiya, G.P. Evaluation of Corrosive Behavior of Zinc Composite Coating on Mild Steel for Marine Applications. J. Phys. Conf. Ser. 2019, 1378. [Google Scholar] [CrossRef]
- Lopez-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of protective coatings for offshore applications. Corrosion and tribocorrosion behavior in synthetic seawater. Surf. Coat. Technol. 2018, 349, 1083–1097. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Doerre, M.; Hibbitts, L.; Patrick, G.; Akafuah, N.K. Advances in automotive conversion coatings during pretreatment of the body structure: A review. Coatings 2018, 8, 405. [Google Scholar] [CrossRef]
- Sorour, N.; Zhang, W.; Ghali, E.; Houlachi, G. A review of organic additives in zinc electrodeposition process (performance and evaluation). Hydrometallurgy 2017, 171, 320–332. [Google Scholar] [CrossRef]
- International Zinc Association. Available online: https://www.zinc.org/ (accessed on 18 January 2021).
- Chatterjee, B. Science and Industry of Processes for Zinc-based Coatings with Improved Properties. Jahrb. Oberfl. 2017, 72, 1–34. [Google Scholar]
- Pushpavanam, M. Critical review on alloy plating: A viable alternative to conventional plating. Bull. Electrochem. 2000, 16, 559–566. [Google Scholar]
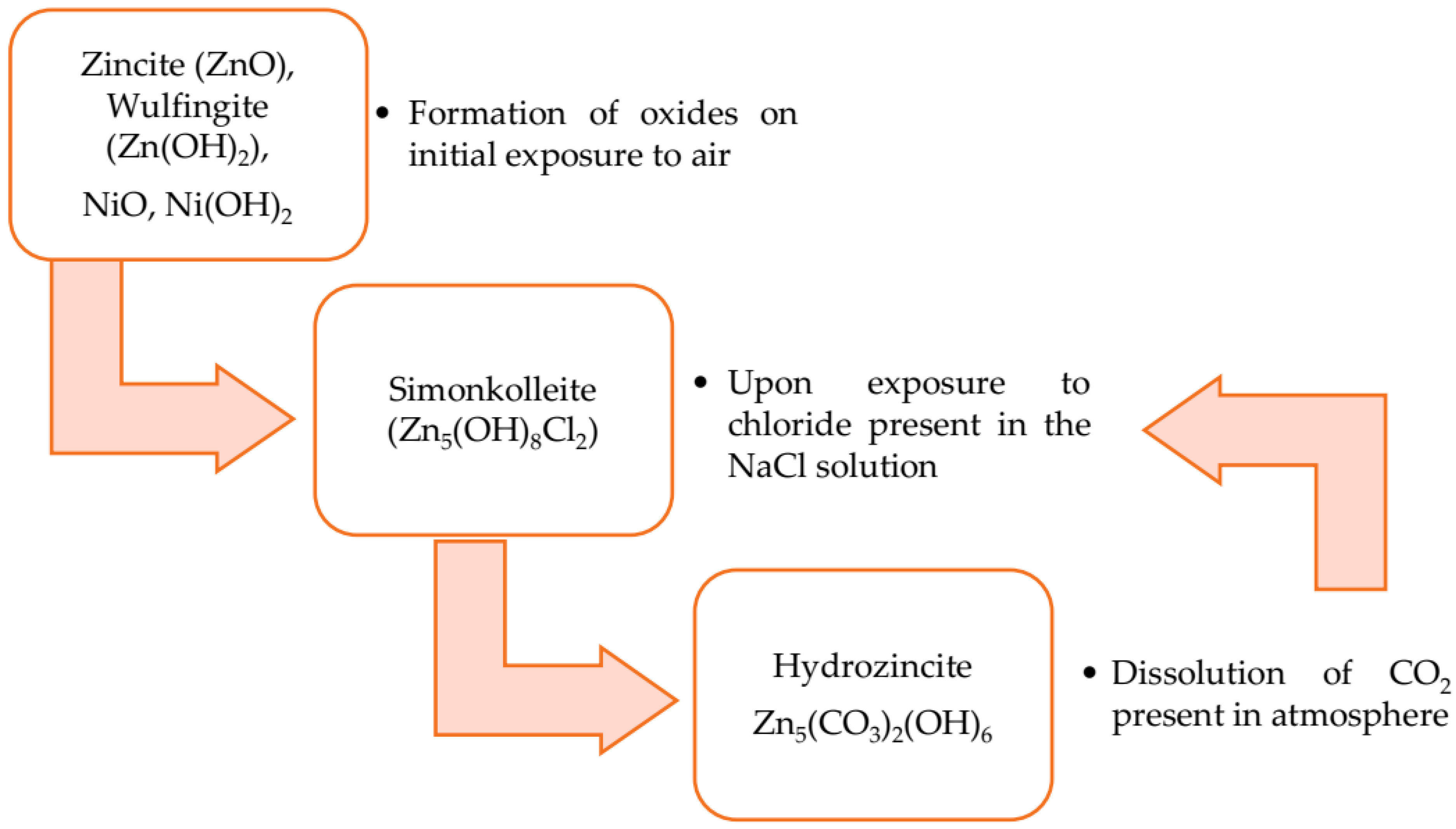
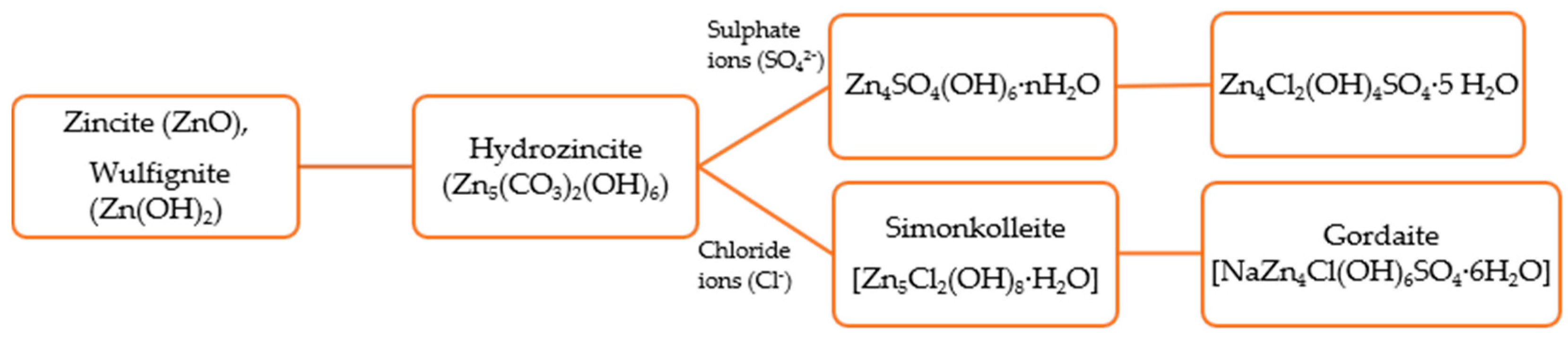
- De la Fuente, D.; Castaño, J.G.; Morcillo, M. Long-term atmospheric corrosion of zinc. Corros. Sci. 2007, 49, 1420–1436. [Google Scholar] [CrossRef]
- Narkevicius, A.; Bučinskienė, D.; Ručinskienė, A.; Pakštas, V.; Bikulčius, G. Study on long term atmospheric corrosion of electrodeposited zinc and zinc alloys. Trans. IMF 2013, 91, 68–73. [Google Scholar] [CrossRef]
- Odnevall, W.I.; Leygraf, C. A critical review on corrosion and runoff from zinc and zinc-based alloys in atmospheric environments. Corrosion 2017, 73, 1016–1077. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K.; Kumar, M.K.P. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- American Tinning & Galvanizing Company. Corrosion Control & Metal Finishing. Available online: http://www.galvanizeit.com/ (accessed on 11 April 2021).
- Chung, P.P.; Wang, J.; Durandet, Y. Deposition processes and properties of coatings on steel fasteners—A review. Friction 2019, 7, 389–416. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X.; Dong, T.G.; Zhou, D.G. Effects of temperature and current density on zinc electrodeposition from acidic sulfate electrolyte with [BMIM]HSO4 as additive. J. Appl. Electrochem. 2009, 39, 1207–1216. [Google Scholar] [CrossRef]
- Shivakumara, S.; Manohar, U.; Arthoba Naik, Y.; Venkatesha, T.V. Effect of condensation product on electrodeposition of zinc on mild steel. Bull. Mater. Sci. 2007, 30, 463–468. [Google Scholar] [CrossRef][Green Version]
- Tuaweri, T.J.; Adigio, E.M.; Jombo, P.P. A Study of Process Parameters for Zinc Electrodeposition from a Sulphate Bath. Int. J. Eng. Sci. 2013, 2, 2319–6734. [Google Scholar]
- Onkarappa, N.K.; Adarakatti, P.S.; Malingappa, P. A Study on the Effect of Additive Combination on Improving Anticorrosion Property of Zinc Electrodeposit from Acid Chloride Bath. Ind. Eng. Chem. Res. 2017, 56, 5284–5295. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Yu, X.; Huang, C.; Chen, S.; Chen, G.; Wu, Q.H. Effect of additive on zinc electrodeposition in acidic bath. Surf. Eng. 2015, 31, 446–451. [Google Scholar] [CrossRef]
- Achary, G.; Sachin, H.P.; Naik, Y.A.; Venkatesha, T.V. Effect of a new condensation product on electrodeposition of zinc from a non-cyanide bath. Bull. Mater. Sci. 2007, 30, 219–224. [Google Scholar] [CrossRef]
- Esfahani, M.; Zhang, J.; Durandet, Y.; Wang, J.; Wong, Y.C. Electrodeposition of Nanocrystalline Zinc from Sulfate and Sulfate-Gluconate Electrolytes in the Presence of Additives. J. Electrochem. Soc. 2016, 163, D476–D484. [Google Scholar] [CrossRef]
- Scott, M.; Moats, M. Optimizing Additive Ratios in Alkaline Zincate Electrodeposition. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 123–131. [Google Scholar]
- Selvaraju, V.; Thangaraj, V. Influence of γ-phase on corrosion resistance of Zn–Ni alloy electrodeposition from acetate electrolytic bath. Mater. Res. Express 2018, 5, 056502. [Google Scholar] [CrossRef]
- Onkarappa, N.K.; Satyanarayana, J.C.A.; Suresh, H.; Malingappa, P. Influence of additives on morphology, orientation and anti-corrosion property of bright zinc electrodeposit. Surf. Coat. Technol. 2020, 397, 126062. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Jyothirmayi, A.; Hebalkar, N.; Sundararajan, G. Influence of pulsed current on the aqueous corrosion resistance of electrodeposited zinc. Surf. Coat. Technol. 2015, 272, 373–379. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Cui, J.; An, M.; Li, D. Electrodeposition of nanocrystalline zinc on steel for enhanced resistance to corrosive wear. Surf. Coat. Technol. 2016, 304, 567–573. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Malathy, P. Synergetic effects of pulse constraints and additives in electrodeposition of nanocrystalline zinc: Corrosion, structural and textural characterization. Mater. Chem. Phys. 2010, 124, 516–528. [Google Scholar] [CrossRef]
- Raeissi, K.; Saatchi, A.; Golozar, M.A.; Szpunar, J.A. Texture and surface morphology in zinc electrodeposits. J. Appl. Electrochem. 2004, 34, 1249–1258. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Golozar, M.A. An investigation on the role of texture and surface morphology in the corrosion resistance of zinc electrodeposits. Corros. Sci. 2011, 53, 2676–2678. [Google Scholar] [CrossRef]
- Jantaping, N.; Schuh, C.A.; Boonyongmaneerat, Y. Influences of crystallographic texture and nanostructural features on corrosion properties of electrogalvanized and chromate conversion coatings. Surf. Coat. Technol. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Park, H.; Szpunar, J.A. The role of texture and morphology in optimizing the corrosion resistance of zinc-based electrogalvanized coatings. Corros. Sci. 1998, 40, 525–545. [Google Scholar] [CrossRef]
- Zor, S.; Erten, Ü.; Bingöl, D. Investigation of the effect of physical conditions of a coating bath on the corrosion behavior of zinc coating using response surface methodology. Prot. Met. Phys. Chem. Surf. 2015, 51, 304–309. [Google Scholar] [CrossRef]
- Mouanga, M.; Ricq, L.; Douglade, J.; Berçot, P. Effects of some additives on the corrosion behaviour and preferred orientations of zinc obtained by continuous current deposition. J. Appl. Electrochem. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- Ballesteros, J.C.; Díaz-Arista, P.; Meas, Y.; Ortega, R.; Trejo, G. Zinc electrodeposition in the presence of polyethylene glycol 20,000. Electrochim. Acta 2007, 52, 3686–3696. [Google Scholar] [CrossRef]
- Da Lopes, C.S.; de Santana, P.M.; Rocha, C.L.F.; de Souza, C.A.C. Evaluation of Formic Acid and Cyclohexylamine as Additives in Electrodeposition of Zn Coating. Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Youssef, K.M.; Koch, C.C.; Fedkiw, P.S. Influence of pulse plating parameters on the synthesis and preferred orientation of nanocrystalline zinc from zinc sulfate electrolytes. Electrochim. Acta 2008, 54, 677–683. [Google Scholar] [CrossRef]
- De Jesus Almeida, M.D.; Rovere, C.A.D.; de AndradeLima, L.R.P.; Ribeiro, D.V.; De Souza, C.A.C. Glycerol effect on the corrosion resistance and electrodeposition conditions in a zinc electroplating process. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Nayana, K.O.; Venkatesha, T.V. Bright zinc electrodeposition and study of influence of synergistic interaction of additives on coating properties. J. Ind. Eng. Chem. 2015, 26, 107–115. [Google Scholar] [CrossRef]
- Nakano, H.; Ura, T.; Oue, S.; Kobayashi, S. Effect of preadsorption of organic additives on the appearance and morphology of electrogalvanized steel sheets. ISIJ Int. 2014, 54, 1653–1660. [Google Scholar] [CrossRef]
- Nayana, K.O.; Venkatesha, T.V.; Praveen, B.M.; Vathsala, K. Synergistic effect of additives on bright nanocrystalline zinc electrodeposition. J. Appl. Electrochem. 2011, 41, 39–49. [Google Scholar] [CrossRef]
- Mo, Y.; Huang, Q.; Li, W.; Hu, S.; Huang, M.; Huang, Y. Effect of sodium benzoate on zinc electrodeposition in chloride solution. J. Appl. Electrochem. 2011, 41, 859–865. [Google Scholar] [CrossRef]
- De Carvalho, M.F.; Carlos, I.A. Zinc electrodeposition from alkaline solution containing trisodium nitrilotriacetic added. Electrochim. Acta 2013, 113, 229–239. [Google Scholar] [CrossRef]
- Lotfi, N.; Aliofkhazraei, M.; Rahmani, H.; Darband, G.B. Zinc–Nickel Alloy Electrodeposition: Characterization, Properties, Multilayers and Composites. Prot. Met. Phys. Chem. Surf. 2018, 54, 1102–1140. [Google Scholar] [CrossRef]
- Sironi, L. Plating of Zn-Ni Alloy from Acidic Electrolytes for Corrosion Protection. Master’s Thesis, Polytechnic University of Milan, Milan, Italy, 2016. Available online: https://www.lagalvanotecnica.com/images/ZnNi_tesi.pdf (accessed on 20 January 2021).
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Characterization of corrosion resistance of electrodeposited Zn-Ni Zn and Cd coatings. Electrochim. Acta 2013, 105, 314–323. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shet, V.B. Development and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co coatings. Surf. Eng. 2020, 36, 429–437. [Google Scholar] [CrossRef]
- Ferreira Fernandes, M.; dos Santos, J.R.M.; de Oliveira Velloso, V.M.; Voorwald, H.J.C. AISI 4140 Steel Fatigue Performance: Cd Replacement by Electroplated Zn-Ni Alloy Coating. J. Mater. Eng. Perform. 2020, 29, 1567–1578. [Google Scholar] [CrossRef]
- Faid, H.; Mentar, L.; Khelladi, M.R.; Azizi, A. Deposition potential effect on surface properties of Zn–Ni coatings. Surf. Eng. 2017, 33, 529–535. [Google Scholar] [CrossRef]
- Shanmugasigamani, S.; Pushpavanam, M. Bright zinc-nickel alloy deposition from alkaline non-cyanide bath. Trans. Inst. Met. Finish. 2008, 86, 122–128. [Google Scholar] [CrossRef]
- Conde, A.; Arenas, M.A.; de Damborenea, J.J. Electrodeposition of Zn-Ni coatings as Cd replacement for corrosion protection of high strength steel. Corros. Sci. 2011, 53, 1489–1497. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. Electrodeposition of single gamma phased Zn-Ni alloy coatings from additive-free acidic bath. Appl. Surf. Sci. 2014, 311, 635–642. [Google Scholar] [CrossRef]
- Mohan, G.N.N. Influence of Deposition Temperature on the Corrosion Resistance of Electrodeposited Zinc-Nickel Alloy Coatings. Bachelor’s Thesis, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 2017. Available online: http://utpedia.utp.edu.my/17908/ (accessed on 25 December 2020).
- Fashu, S.; Khan, R. Recent work on electrochemical deposition of Zn-Ni (-X) alloys for corrosion protection of steel. Anti-Corros. Methods Mater. 2019, 66, 45–60. [Google Scholar] [CrossRef]
- Loukil, N.; Feki, M. Review—Zn–Mn Electrodeposition: A Literature Review. J. Electrochem. Soc. 2020, 167, 022503. [Google Scholar] [CrossRef]
- Zhai, X.; Ren, Y.; Wang, N.; Guan, F.; Agievich, M.; Duan, J.; Hou, B. Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings. Molecules 2019, 24, 1974. [Google Scholar] [CrossRef]
- Loukil, N.; Feki, M. Zn–Mn alloy coatings from acidic chloride bath: Effect of deposition conditions on the Zn–Mn electrodeposition-morphological and structural characterization. Appl. Surf. Sci. 2017, 410, 574–584. [Google Scholar] [CrossRef]
- Cavallotti, P.L.; Nobili, L.; Vicenzo, A. Phase structure of electrodeposited alloys. Electrochim. Acta 2005, 50, 4557–4565. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Kovacheva, D.; Vitkova, S.; Nemska, S. Influence of the alloying component on the protective ability of some zinc galvanic coatings. Electrochim. Acta 2005, 51, 77–84. [Google Scholar] [CrossRef]
- Ballote, L.D.; Ramanauskas, R.; Bartolo-Perez, P. Mn Oxide Film As Corrosion Inhibitor of Zn-Mn Coatings. Corros. Rev. 2000, 18, 41–52. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Zheng, H.; Wang, X.L.; Tu, J.P. Electrodeposition, Morphology, Composition, and Corrosion Performance of Zn-Mn Coatings from a Deep Eutectic Solvent. J. Mater. Eng. Perform. 2015, 24, 434–444. [Google Scholar] [CrossRef]
- Claudel, F.; Stein, N.; Allain, N.; Tidu, A.; Hajczak, N.; Lallement, R.; Close, D. Pulse electrodeposition and characterization of Zn–Mn coatings deposited from additive-free chloride electrolytes. J. Appl. Electrochem. 2019, 49, 399–411. [Google Scholar] [CrossRef]
- Bucko, M.; Rogan, J.; Stevanovic, S.I.; Stankovic, S.; Bajat, J.B. The influence of anion type in electrolyte on the properties of electrodeposited Zn Mn alloy coatings. Surf. Coat. Technol. 2013, 228, 221–228. [Google Scholar] [CrossRef]
- Beheshti, M.; Ismail, M.C.; Kakooei, S.; Shahrestani, S.; Mohan, G.; Zabihiazadboni, M. Influence of deposition temperature on the corrosion resistance of electrodeposited zinc-nickel alloy coatings. Materwiss. Werksttech. 2018, 49, 472–482. [Google Scholar] [CrossRef]
- Azizi, F.; Kahoul, A. Electrodeposition and corrosion behaviour of Zn–Co coating produced from a sulphate bath. Trans. IMF 2016, 94, 43–48. [Google Scholar] [CrossRef]
- Garcia, J.R.; do Lago, D.C.B.; Cesar, D.V.; Senna, L.F. Pulsed cobalt-rich Zn–Co alloy coatings produced from citrate baths. Surf. Coat. Technol. 2016, 306, 462–472. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Sohi, M.H. Electrochemical studies of zinc–cobalt alloy coatings deposited from alkaline baths containing glycine as complexing agent. J. Appl. Electrochem. 2010, 40, 1563–1570. [Google Scholar] [CrossRef]
- Yogesha, S.; Bhat, K.U.; Hegde, A.C. Effect of Current Density on Deposit Characters of Zn-Co Alloy and their Corrosion Behaviors. Synth. React. Inorg. Met. Nano-Metal Chem. 2011, 41, 405–411. [Google Scholar] [CrossRef]
- Selvaraju, V.; Thangaraj, V. Corrosion properties of mild steel surface modified by bright Zn-Co alloy electrodeposit from acetate electrolytic bath. Mater. Res. Express 2018, 6, 026501. [Google Scholar] [CrossRef]
- Muresan, L.M. Electrodeposited Zn-Nanoparticles Composite Coatings for Corrosion Protection of Steel. In Handbook of Nanoelectrochemistry; Springer International Publishing: Cham, Switzerland, 2016; pp. 333–353. [Google Scholar]
- Popoola, P.A.I.; Malatji, N.; Fayomi, O.S. Fabrication and Properties of Zinc Composite Coatings for Mitigation of Corrosion in Coastal and Marine Zone. In Applied Studies of Coastal and Marine Environments; InTech: London, UK, 2016; Volume 32, pp. 137–144. [Google Scholar]
- Anwar, S.; Khan, F.; Zhang, Y.; Caines, S. Zn composite corrosion resistance coatings: What works and what does not work? J. Loss Prev. Process Ind. 2021, 69. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y. Corrosion behaviour of Zn-Ni alloy and Zn-Ni-nano-TiO2 composite coatings electrodeposited from ammonium citrate baths. Process Saf. Environ. Prot. 2020, 141, 366–379. [Google Scholar] [CrossRef]
- Ataie, S.A.; Zakeri, A. RSM optimization of pulse electrodeposition of Zn-Ni-Al2O3 nanocomposites under ultrasound irradiation. Surf. Coat. Technol. 2019, 359, 206–215. [Google Scholar] [CrossRef]
- Li, X.; Liang, M.; Zhou, H.; Huang, Q.; Lv, D.; Li, W. Composite of indium and polysorbate 20 as inhibitor for zinc corrosion in alkaline solution. Bull. Korean Chem. Soc. 2012, 33, 1566–1570. [Google Scholar] [CrossRef]
- Utu, I.D.; Muntean, R.; Mitelea, I. Corrosion and Wear Properties of Zn-Based Composite Coatings. J. Mater. Eng. Perform. 2020, 29, 5360–5365. [Google Scholar] [CrossRef]
- Alipour, K.; Nasirpouri, F. Effect of Morphology and Surface Modification of Silica Nanoparticles on the Electrodeposition and Corrosion Behavior of Zinc-Based Nanocomposite Coatings. J. Electrochem. Soc. 2019, 166, D1–D9. [Google Scholar] [CrossRef]
- Boshkova, N.; Kamburova, K.; Koprinarov, N.; Konstantinova, M.; Boshkov, N.; Radeva, T. Obtaining and Corrosion Performance of Composite Zinc Coatings with Incorporated Carbon Spheres. Coatings 2020, 10, 665. [Google Scholar] [CrossRef]
- Tuaweri, T.J. Influence of Process Parameters on the Cathode Current Efficiency of Zn/SiO2 Electrodeposition. Int. J. Mech. Eng. Appl. 2013, 1, 93. [Google Scholar] [CrossRef]
- Tuaweri, T.J.; Wilcox, G.D. Behaviour of Zn-SiO2 electrodeposition in the presence of N, N-dimethyldodecylamine. Surf. Coat. Technol. 2006, 200, 5921–5930. [Google Scholar] [CrossRef]
- Ohgai, T.; Ogushi, K.; Takao, K. Morphology Control of Zn-SiO2 Composite Films Electrodeposited from Aqueous Solution Containing Quaternary Ammonium Cations. J. Phys. Conf. Ser. 2013, 417, 012006. [Google Scholar] [CrossRef]
- Vathsala, K.; Venkatesha, T.V. Zn–ZrO2 nanocomposite coatings: Elecrodeposition and evaluation of corrosion resistance. Appl. Surf. Sci. 2011, 257, 8929–8936. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Mozafari, A.; Omidvar, H.; Javanbakht, M. Preparation and corrosion resistance of pulse electrodeposited Zn and Zn–SiC nanocomposite coatings. Appl. Surf. Sci. 2014, 300, 1–7. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Szymkiewicz, K.; Rogal, Ł.; Gileadi, E.; Eliaz, N. Direct Current Electrodeposition of Zn-SiC Nanocomposite Coatings from Citrate Bath. J. Electrochem. Soc. 2018, 165, D526–D535. [Google Scholar] [CrossRef]
- Punith Kumar, M.K.; Srivastava, C. Morphological and electrochemical characterization of electrodeposited Zn–Ag nanoparticle composite coatings. Mater. Charact. 2013, 85, 82–91. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V. Generation and corrosion behavior of Zn-nano sized carbon black composite coating. Int. J. Electrochem. Sci. 2009, 4, 258–266. [Google Scholar]
- Punith Kumar, M.K.; Singh, M.P.; Srivastava, C. Electrochemical behavior of Zn–graphene composite coatings. RSC Adv. 2015, 5, 25603–25608. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Srivastava, C. Microstructure and corrosion properties of zinc-graphene oxide composite coatings. Corros. Sci. 2019, 152, 234–248. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Lau, H.C.; Ramakrishna, S. Nanocomposite coatings on steel for enhancing the corrosion resistance: A review. J. Compos. Mater. 2020, 54, 681–701. [Google Scholar] [CrossRef]
- Setiawan, A.R.; Noorprajuda, M.; Ramelan, A.; Suratman, R. Preparation of Zn-ZrO2 Nanocomposite Coating by DC and Pulsed Current Electrodeposition Technique with Low Current Density. Mater. Sci. Forum 2015, 827, 332–337. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Fayomi, O.S.I.; Loto, C.A. Multifaceted incorporation of Zn-Al2O3/Cr2O3/SiO2 nanocomposite coatings: Anti-corrosion, tribological, and thermal stability. Int. J. Adv. Manuf. Technol. 2016, 82, 1335–1341. [Google Scholar] [CrossRef]
- Boshkov, N. Influence of Organic Additives and of Stabilized Polymeric Micelles on the Metalographic Structure of Nanocomposite Zn and Zn-Co Coatings. Port. Electrochim. Acta 2017, 35, 53–63. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, B.; Srivastava, C. ZnCo-carbon nanotube composite coating with enhanced corrosion resistance behavior. Surf. Coat. Technol. 2020, 398, 126083. [Google Scholar] [CrossRef]
- Punith Kumar, M.K.; Rekha, M.Y.; Agrawal, J.; Agarwal, T.M.; Srivastava, C. Microstructure, morphology and electrochemical properties of ZnFe-Graphene composite coatings. J. Alloys Compd. 2019, 783, 820–827. [Google Scholar] [CrossRef]
- Roventi, G. Electrodeposition of Zn-Ni-ZrO2, Zn-Ni-Al2O3 and Zn-Ni-SiC Nanocomposite Coatings from an Alkaline Bath. Int. J. Electrochem. Sci. 2017, 663–678. [Google Scholar] [CrossRef]
- Hammami, O.; Dhouibi, L.; Berçot, P.; Rezrazi, E.M.; Triki, E. Study of Zn-Ni Alloy Coatings Modified by Nano-SiO2 Particles Incorporation. Int. J. Corros. 2012, 2012, 301392. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. The effect of pulse electroplating on Zn–Ni alloy and Zn–Ni–Al2O3 composite coatings. J. Alloys Compd. 2015, 622, 918–924. [Google Scholar] [CrossRef]
- Blejan, D.; Muresan, L.M. Corrosion behavior of Zn-Ni-Al2O3 nanocomposite coatings obtained by electrodeposition from alkaline electrolytes. Mater. Corros. 2013, 64, 433–438. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; An, M.-Z. Electrodeposition of Zn–Ni–Al2O3 nanocomposite coatings under ultrasound conditions. J. Alloys Compd. 2008, 459, 548–552. [Google Scholar] [CrossRef]
- Conrad, H.; Golden, T.D. Electrodeposited Zinc-Nickel Nanocomposite Coatings. In Nanocomposites—Recent Evolutions; IntechOpen: London, UK, 2019; Volume 32, pp. 137–144. [Google Scholar]
- Nemes, P.I.; Lekka, M.; Fedrizzi, L.; Muresan, L.M. Influence of the electrodeposition current regime on the corrosion resistance of Zn–CeO2 nanocomposite coatings. Surf. Coat. Technol. 2014, 252, 102–107. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Ghorbani, M.; Afshar, A. Microstructure-corrosion resistance relationship of direct and pulse current electrodeposited Zn-TiO2 nanocomposite coatings. Ceram. Int. 2015, 41, 217–224. [Google Scholar] [CrossRef]
- Al-Dhire, T.M.; Zuhailawati, H.; Anasyida, A.S. Effect of current density on corrosion and mechanical properties of Zn-SiC composite coating. Mater. Today Proc. 2019, 17, 664–671. [Google Scholar] [CrossRef]
- Karimi Azar, M.M.; Shooshtari Gugtapeh, H.; Rezaei, M. Evaluation of corrosion protection performance of electroplated zinc and zinc-graphene oxide nanocomposite coatings in air saturated 3.5wt.% NaCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125051. [Google Scholar] [CrossRef]
- PraveenKumar, C.M.; Venkatesha, T.V.; Vathsala, K.; Nayana, K.O. Electrodeposition and corrosion behavior of Zn–Ni and Zn–Ni–Fe2O3 coatings. J. Coat. Technol. Res. 2012, 9, 71–77. [Google Scholar] [CrossRef]
- Exbrayat, L.; Rébéré, C.; Ndong Eyame, R.; Steyer, P.; Creus, J. Corrosion behaviour in saline solution of pulsed-electrodeposited zinc-nickel-ceria nanocomposite coatings. Mater. Corros. 2017, 68, 1129–1142. [Google Scholar] [CrossRef]
- Roventi, G.; Fratesi, R.; Della Guardia, R.A.; Barucca, G. Normal and anomalous codeposition of Zn-Ni alloys from chloride bath. J. Appl. Electrochem. 2000, 30, 173–179. [Google Scholar] [CrossRef]
- Tome, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of metals and alloys from ionic liquids. J. Alloys Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Abbott, A.P.; Dalrymple, I.; Endres, F.; Macfarlane, D.R. Why use Ionic Liquids for Electrodeposition? In Electrodeposition from Ionic Liquids; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–13. [Google Scholar]
- Marcus, Y. Application of Deep Eutectic Solvents. In Deep Eutectic Solvents; Springer Nature: Cham, Switzerland, 2019; pp. 111–151. [Google Scholar] [CrossRef]
- Bernasconi, R.; Panzeri, G.; Accogli, A.; Liberale, F.; Nobili, L.; Magagnin, L. Electrodeposition from Deep Eutectic Solvents. In Progress and Developments in Ionic Liquids; InTech: London, UK, 2017. [Google Scholar]
- An, M.-Z.; Yang, P.-X.; Su, C.-N.; Nishikata, A.; Tsuru, T. Electrodeposition of Cobalt in an Ionic Liquid Electrolyte at Ambient Temperature. Chin. J. Chem. 2008, 26, 1219–1224. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Wang, X.L.; Tu, J.P. Effect of EDTA and NH4Cl additives on electrodeposition of Zn-Ni films from choline chloride-based ionic liquid. Trans. Nonferrous Met. Soc. China 2015, 25, 2054–2064. [Google Scholar] [CrossRef]
- Bernasconi, R.; Panzeri, G.; Firtin, G.; Kahyaoglu, B.; Nobili, L.; Magagnin, L. Electrodeposition of ZnNi Alloys from Choline Chloride/Ethylene Glycol Deep Eutectic Solvent and Pure Ethylene Glycol for Corrosion Protection. J. Phys. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Alesary, H.F.; Khan, F.; Abbott, A.P.; Ryder, K.S. Gamma-phase Zn-Ni alloy deposition by pulse-electroplating from a modified deep eutectic solution. Surf. Coat. Technol. 2020, 403, 126434. [Google Scholar] [CrossRef]
- Bucko, M.; Tomic, M.V.; Maksimovic, M.; Bajat, J.B. The importance of using hydrogen evolution inhibitor during the Zn and Zn–Mn electrodeposition from ethaline. J. Serbian Chem. Soc. 2019, 84, 1221–1234. [Google Scholar] [CrossRef]
- Bucko, M.; Stevanovic, S.; Bajat, J. Tailoring the corrosion resistance of Zn-Mn coating by electrodeposition from deep eutectic solvents. Zast. Mater. 2018, 59, 173–181. [Google Scholar] [CrossRef]
- Li, R.; Dong, Q.; Xia, J.; Luo, C.; Sheng, L.; Cheng, F.; Liang, J. Electrodeposition of composition controllable Zn–Ni coating from water modified deep eutectic solvent. Surf. Coat. Technol. 2019, 366, 138–145. [Google Scholar] [CrossRef]
- Pereira Oliveira, N.M. Metal Electrodeposition from Deep Eutectic Solvents. Ph.D. Thesis, Universidade Do Porto, Porto, Portugal, 2018. Available online: https://repositorio-aberto.up.pt/bitstream/10216/110784/2/252422.pdf (accessed on 20 January 2021).
- Marín-Sánchez, M.; Ocón, P.; Conde, A.; García, I. Electrodeposition of Zn-Mn coatings on steel from 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide ionic liquid. Surf. Coat. Technol. 2014, 258, 871–877. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Electrodeposition onto magnesium in air and water stable ionic liquids: From corrosion to successful plating. Electrochem. Commun. 2007, 9, 2428–2435. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, K.; Shi, W.; Yang, X.; Zhang, Y. Corrosion behavior of zinc coating electroplated at high current from a deep eutectic solvent. Mater. Res. Express 2018, 6, 016402. [Google Scholar] [CrossRef]
- Panzeri, G.; Muller, D.; Accogli, A.; Gibertini, E.; Mauri, E.; Rossi, F.; Nobili, L.; Magagnin, L. Zinc electrodeposition from a chloride-free non-aqueous solution based on ethylene glycol and acetate salts. Electrochim. Acta 2019, 296, 465–472. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z. Effect of different liquid–solid contact models on the corrosion resistance of superhydrophobic magnesium surfaces. Corros. Sci. 2014, 87, 452–459. [Google Scholar] [CrossRef]
- Qian, H.; Xu, D.; Du, C.; Zhang, D.; Li, X.; Huang, L.; Deng, L.; Tu, Y.; Mol, J.M.C.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef]
- Liu, J.; Fang, X.; Zhu, C.; Xing, X.; Cui, G.; Li, Z. Fabrication of superhydrophobic coatings for corrosion protection by electrodeposition: A comprehensive review. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125498. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Cirisano, F. Superhydrophobic coatings from recyclable materials for protection in a real sea environment. Coatings 2019, 9, 303. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Hou, B. Super-hydrophobic film prepared on zinc as corrosion barrier. Corros. Sci. 2011, 53, 2080–2086. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J.; Audibert-Hayet, A.; Melot, D. Nano-micro structured superhydrophobic zinc coating on steel for prevention of corrosion and ice adhesion. J. Colloid Interface Sci. 2015, 447, 240–247. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Frank Cheng, Y. Stearic acid modified zinc nano-coatings with superhydrophobicity and enhanced antifouling performance. Surf. Coat. Technol. 2018, 340, 55–65. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X.; Zhang, S.; Zhao, Y. A simple method for fabrication of bionic superhydrophobic zinc coating with crater-like structures on steel substrate. J. Bionic Eng. 2016, 13, 622–630. [Google Scholar] [CrossRef]
- Polyakov, N.A.; Botryakova, I.G.; Glukhov, V.G.; Red’kina, G.V.; Kuznetsov, Y.I. Formation and anticorrosion properties of superhydrophobic zinc coatings on steel. Chem. Eng. J. 2020, 127775. [Google Scholar] [CrossRef]
- Hu, C.; Xie, X.; Zheng, H.; Qing, Y.; Ren, K. Facile fabrication of superhydrophobic zinc coatings with corrosion resistanceviaan electrodeposition process. New J. Chem. 2020, 44, 8890–8901. [Google Scholar] [CrossRef]
- Li, R.; Gao, Q.; Dong, Q.; Luo, C.; Sheng, L.; Liang, J. Template-free electrodeposition of ultra-high adhesive superhydrophobic Zn/Zn stearate coating with ordered hierarchical structure from deep eutectic solvent. Surf. Coat. Technol. 2020, 403, 126267. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wu, J.; Wan, Y. Super-hydrophobic film prepared on zinc and its effect on corrosion in simulated marine atmosphere. Corros. Sci. 2013, 69, 23–30. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Santini, E.; Ravera, F.; Liggieri, L.; Guzman, E.; Cirisano, F. Biofouling control by superhydrophobic surfaces in shallow euphotic seawater. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 369–375. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Aigbodion, V.S.; Fayomi, O.S.I. Surface characterization, mechanical properties and corrosion behaviour of ternary based Zn–ZnO–SiO2 composite coating of mild steel. J. Alloys Compd. 2016, 654, 561–566. [Google Scholar] [CrossRef]
- Chu, Q.; Liang, J.; Hao, J. Facile fabrication of a robust super-hydrophobic surface on magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 118–122. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Jiang, W.; Fan, M.; Liu, D.; Zhao, J. Superhydrophobic nickel coatings fabricated by scanning electrodeposition on stainless steel formed by selective laser melting. Surf. Coat. Technol. 2019, 377, 124886. [Google Scholar] [CrossRef]
- Rivas-Esquivel, F.M.; Brisard, G.M.; Ortega-Borges, R.; Trejo, G.; Meas, Y. Zinc electrochemical deposition from ionic liquids and aqueous solutions onto indium tin oxide. Int. J. Electrochem. Sci. 2017, 12, 2026–2041. [Google Scholar] [CrossRef]
- Prado, R.; Weber, C.C. Applications of Ionic Liquids. In Application, Purification, and Recovery of Ionic Liquids; Kuzmina, O., Hallett, J.P., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–58. [Google Scholar] [CrossRef]
- Odnevall, I.; Leygraf, C. The formation of Zn4Cl2(OH)4SO4·5H2O in an urban and an industrial atmosphere. Corros. Sci. 1994, 36, 1551–1559. [Google Scholar] [CrossRef]
- Ivan, C.C. Recent progress and required developments in atmospheric corrosion of galvanised steel and zinc. Materials 2017, 10, 1288. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniam, K.K.; Paul, S. Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment. Corros. Mater. Degrad. 2021, 2, 163-189. https://doi.org/10.3390/cmd2020010
Maniam KK, Paul S. Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment. Corrosion and Materials Degradation. 2021; 2(2):163-189. https://doi.org/10.3390/cmd2020010
Chicago/Turabian StyleManiam, Kranthi Kumar, and Shiladitya Paul. 2021. "Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment" Corrosion and Materials Degradation 2, no. 2: 163-189. https://doi.org/10.3390/cmd2020010
APA StyleManiam, K. K., & Paul, S. (2021). Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment. Corrosion and Materials Degradation, 2(2), 163-189. https://doi.org/10.3390/cmd2020010







