In-Situ Evaluation of the Protectivity of Coatings Applied to Metal Cultural Artefacts Using Non-Destructive Electrochemical Measurements
Abstract
1. Introduction
2. Materials and Methods
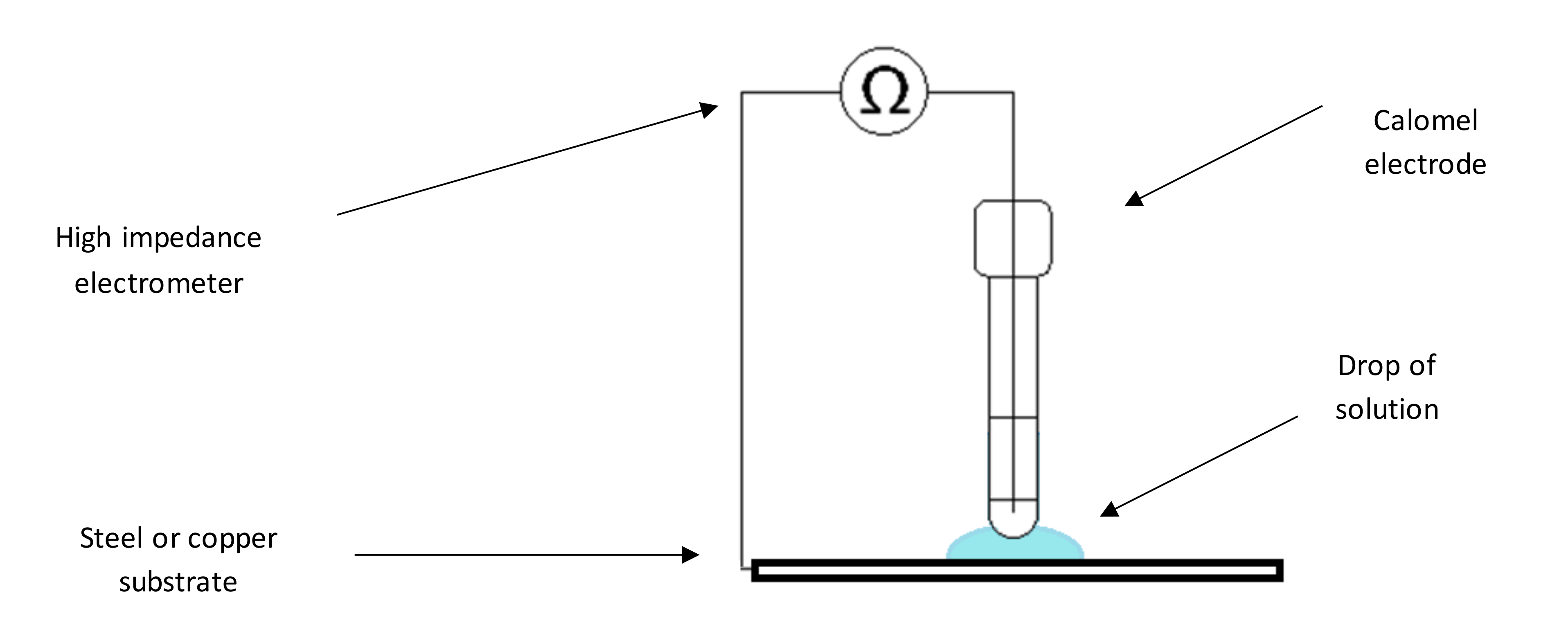
2.1. Experimental
- Paraloid B44 (based on an acrylic polymer)—10% solution in ethanol
- Paraloid B82 (based on an acrylic polymer)—10% solution in acetone
- Winacet (based on polyvinyl acetate)—10% solution in toluene
- McKenic (based on an acrylic polymer)—10% solution in toluene
2.2. Results
2.2.1. Thickness of Coatings
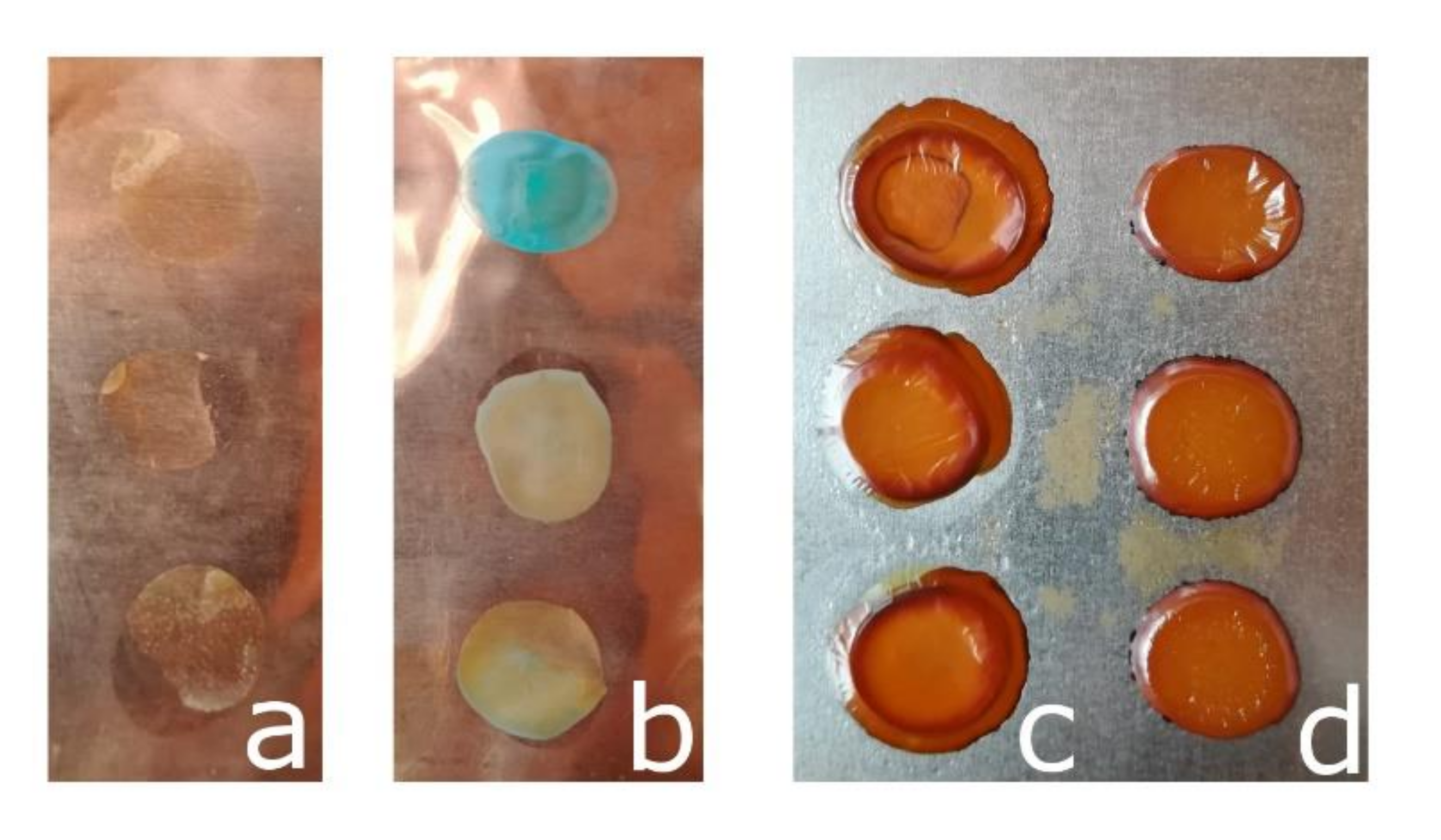
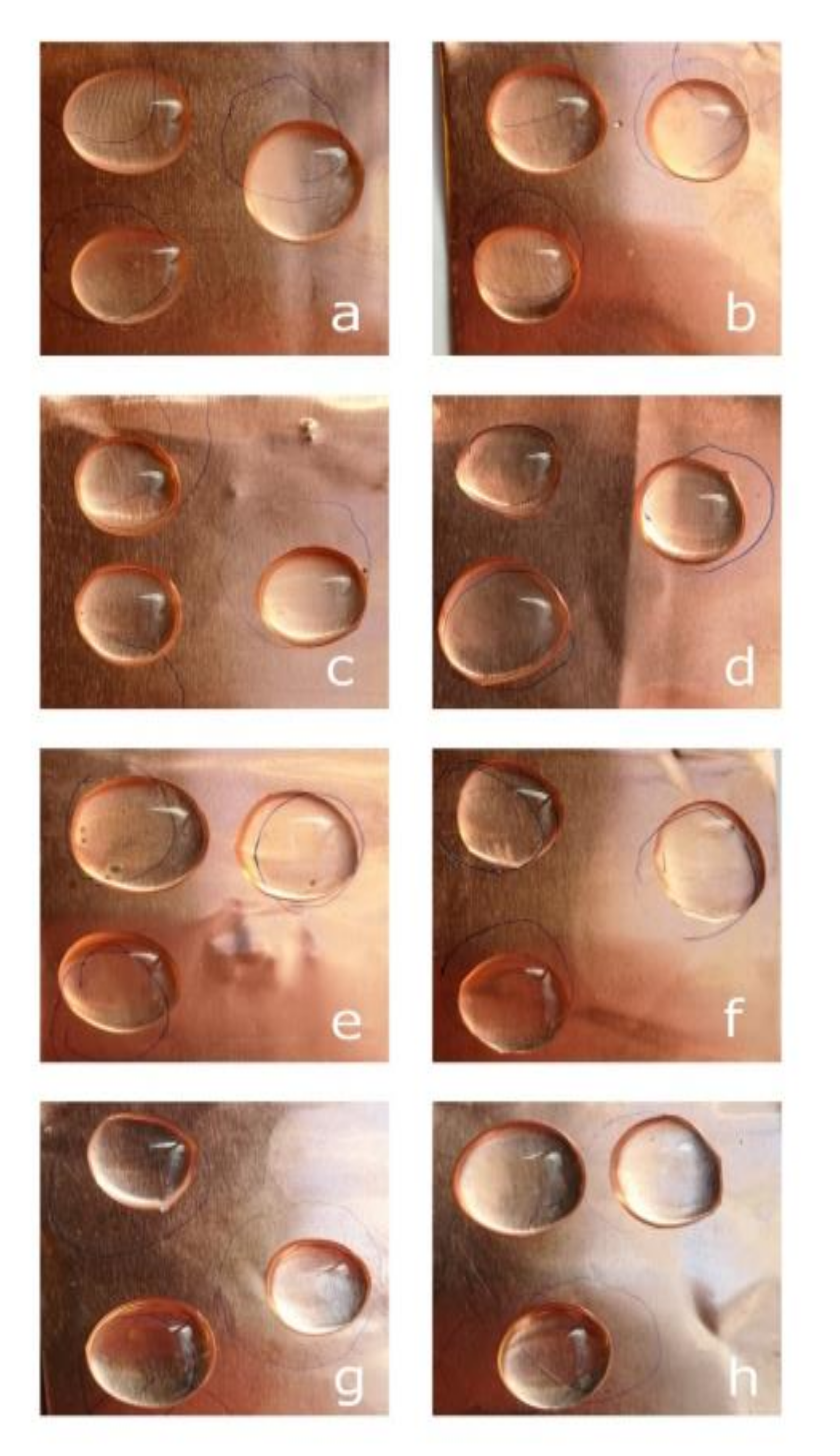
2.2.2. Surface Images
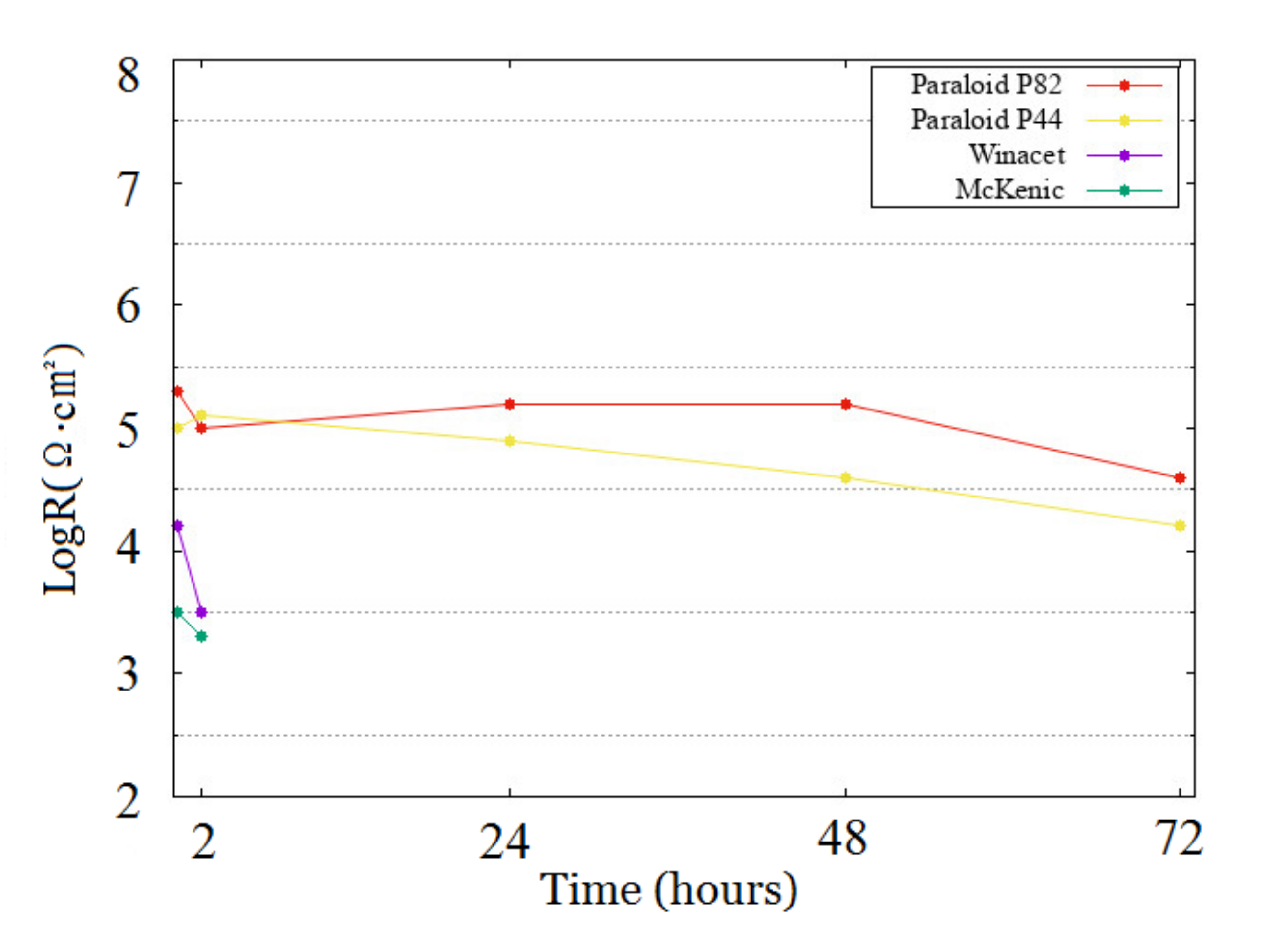
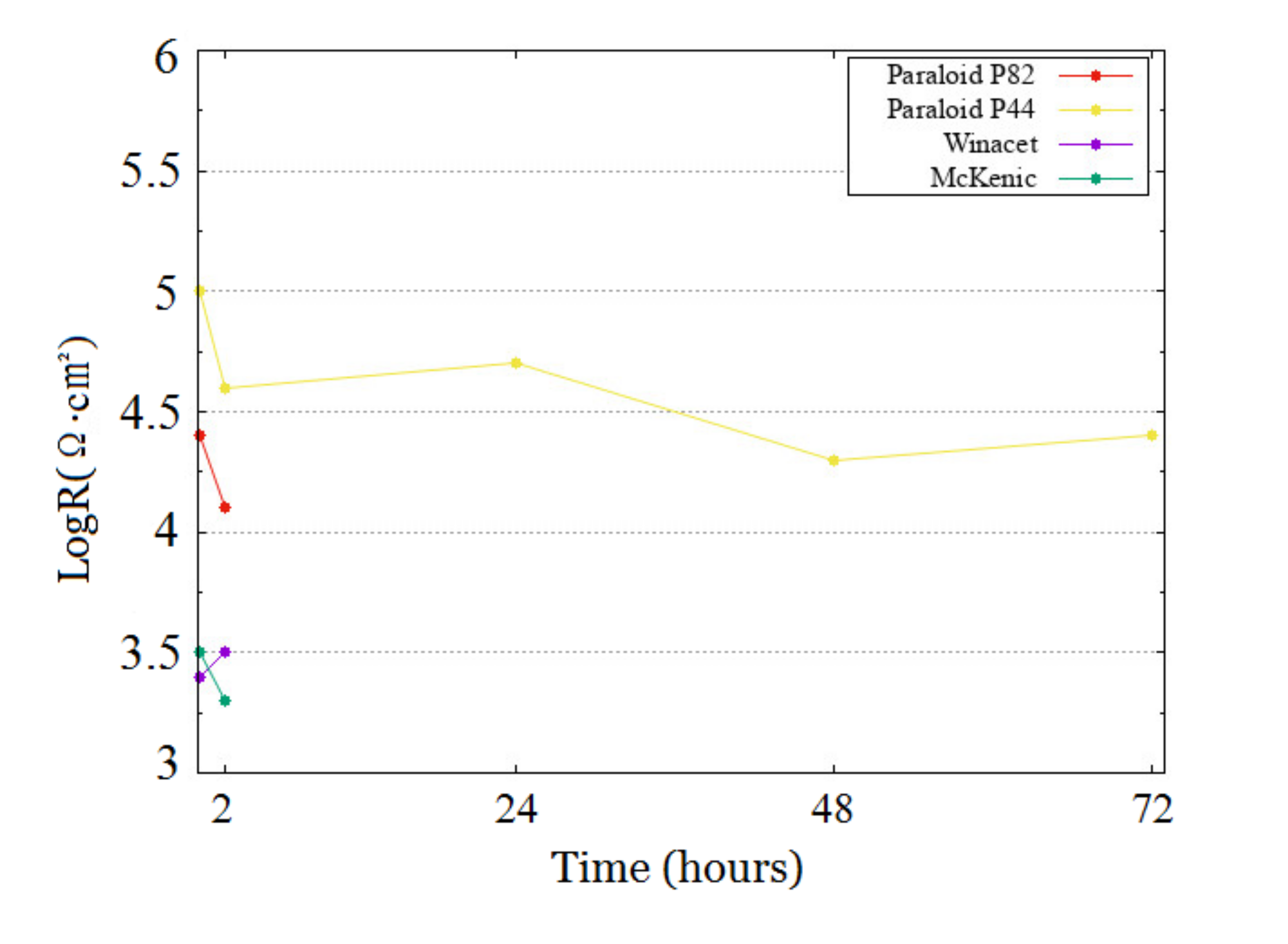
2.2.3. ERM Measurements
2.3. Discussion
2.3.1. Steel
2.3.2. Copper
2.4. Summary
- On Steel: Paraloid B82 = Paraloid B44 >> Winacet > McKenic
- On Copper: Paraloid B44 ≥ Paraloid B82 > McKenic = Winacet
3. Study 2
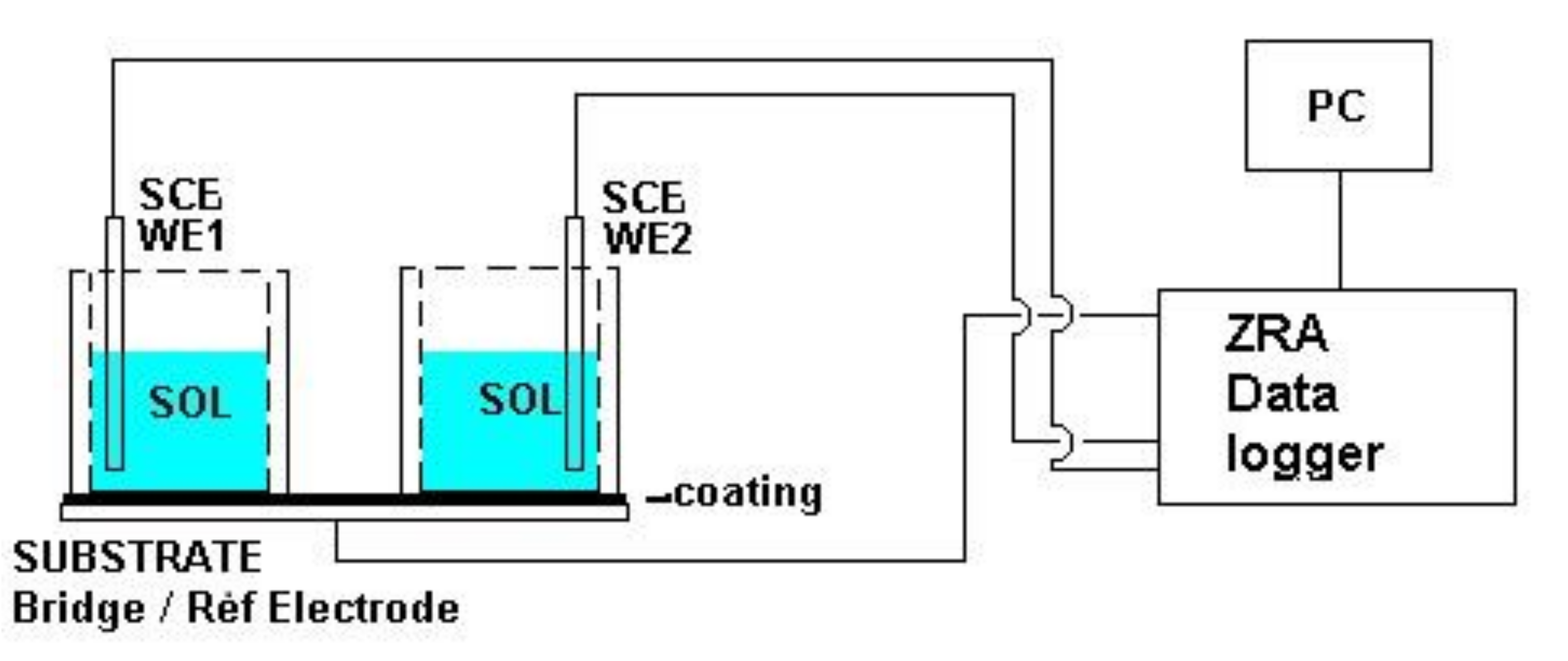
3.1. Electrochemical Noise Measurement (ENM) Using the Single Substrate Arrangement
3.2. Procedure
3.3. Results
3.3.1. Thickness Measurement
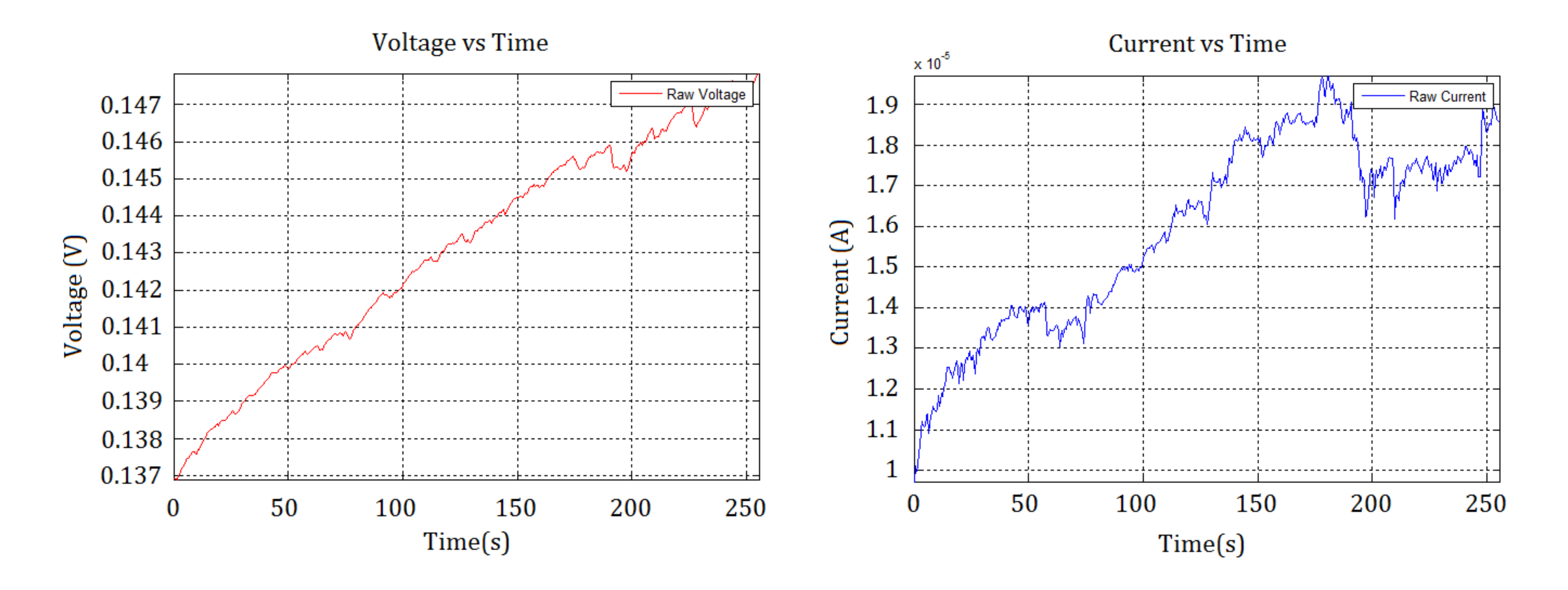
3.3.2. ENM Results
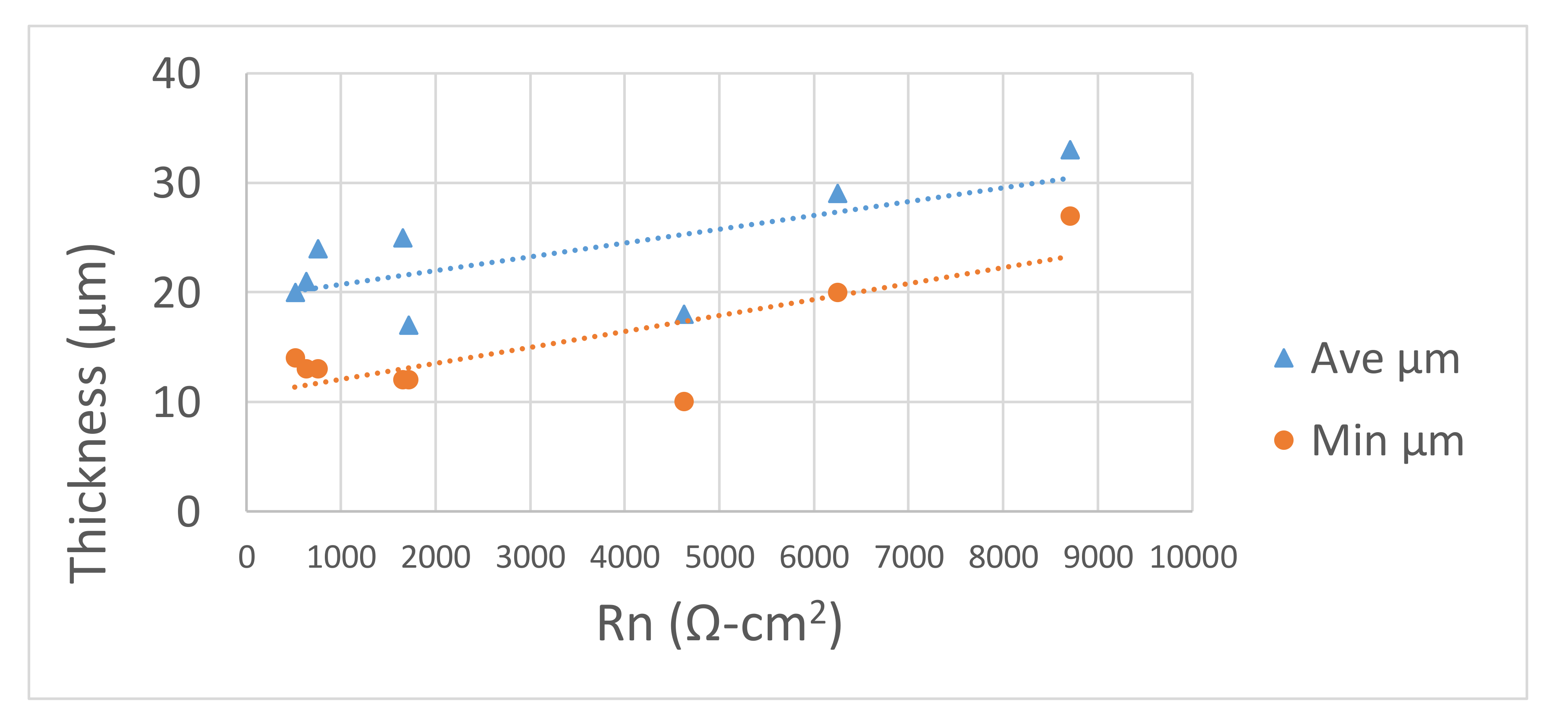
3.3.3. Correlation between Thickness Values and Resistance Noise (Rn) Values of the Bowls
3.4. Discussion of Results from Study 2
3.5. Comparison ENM and ERM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef]
- Cano, E.; Bastidas, D.M.; Argyropoulos, V.; Fajardo, S.; Siatou, A.; Bastidas, J.M.; Degrigny, C. Electrochemical characterization of organic coatings for protection of historic steel artefacts. J. Solid State Electrochem. 2010, 14, 453–463. [Google Scholar] [CrossRef]
- Guilminot, E.; Neff, D.; Remazeilles, C.; Reguer, S.; Kergourlay, F.; Pele, C.; Dillman, P.; Refait, P.; Nicot, F.; Mielcarek, F.; et al. Influence of critical parameters on the dichlorination tertamnet of ferrous objectsfrom seawater. Stud. Conserv. 2012, 57, 227–236. [Google Scholar] [CrossRef]
- Adriaens, A.; Dowsett, M. Time resolved spectroelectrochemistry studies for protection of heritage metals. Surf. Eng. 2008, 24, 84–89. [Google Scholar] [CrossRef]
- Keersmaecker, M.D.; De Wael, K.; Adriaens, A. The use of lead dodecanoate as an environmentally friendly coating to inhibit the corrosion of lead objects: Comparison of three different deposition methods. Prog. Org. Coat. 2012, 74, 1–7. [Google Scholar] [CrossRef][Green Version]
- Jamali, S.S.; Zhao, Y.; Gao, Z.; Li, H.; Hee, A.C. In situ evaluation of corrosion damage using non-destructive electrochemical measurements—A case study. J. Ind. Eng. Chem. 2016. [Google Scholar] [CrossRef]
- Corbellini, S.; Parvis, M.; Grassini, S. Noninvasive solution for Electrochemical Impedance Spectroscopy on metallic works of art. IEEE Trans. Instrum. Meas. 2012, 61, 1193–1200. [Google Scholar] [CrossRef]
- Bacon, R.C.; Smith, J.J.; Rugg, F.M. Electrolytic resistance in evaluating protective merit of coatings on metals. Ind. Eng. Chem. 1948, 40, 161–167. [Google Scholar] [CrossRef]
- Mills, D.J. Comparison of ENM, EIS and DC Resistance for assessing and monitoring anti-corrosive coatings. J. Corros. Sci. Eng. 2004, 8, 12. [Google Scholar]
- Schaefer, K.; Mills, D.J. The application of organic coatings in conservation of archaeological objects excavated from the sea. Progress Org. Coat. 2016, 102, 99–106. [Google Scholar] [CrossRef]
- Wityk, T. Anticorrosive Evaluation of Organic Based Archaeological Coatings Based on DC Resistance Measurements; Company Report (Project); University of Northampton: Northampton, UK, 2018. [Google Scholar]
- Mills, D.J.; Mayne, J.E.O. The inhomogeneous nature of polymer films and its effect on resistance inhibition. In Corrosion Control by Organic Coatings; Leidheiser, H.J., Ed.; NACE International: Houston, TX, USA, 1981; pp. 12–17. [Google Scholar]
- Mabbutt, S.J.; Mills, D.J. Recent UK work investigating anti-corrosive organic coatings using the Electrochemical Noise Method (ENM). Surf. Coat. Int. Part B Coat. Trans. 2001, 84, 277. [Google Scholar] [CrossRef]
- Mills, D.J.; Jamali, S.S. A critical review of Electrochemical Noise Measurement for assessment of organic coatings. Progress Org. Coat. 2016, 95, 26–37. [Google Scholar] [CrossRef]






| Area Number | PB44 1 Layer | PB44 2 Layers | PB82 1 Layer | PB82 2 Layers | Winacet 1 Layer | Winacet 2 Layers | McKenic 1 Layer | McKenic 2 Layers |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.0 | 6.3 | 3.3 | 5.0 | 4.7 | 4.3 | 2.0 | 2.0 |
| 2 | 1.7 | 4.7 | 5.3 | 8.3 | 4.0 | 3.3 | 3.3 | 1.3 |
| 3 | 2.3 | 4.3 | 3.3 | 7.7 | 4.7 | 7.3 | 2.0 | 2.0 |
| Area Number | PB44 1 Layer | PB44 2 Layers | PB82 1 Layer | PB82 2 Layers | Winacet 1 Layer | Winacet 2 Layers | McKenic 1 Layer | McKenic 2 Layers |
|---|---|---|---|---|---|---|---|---|
| 1 | 13.3 | 26.3 | 10.7 | 10.7 | 12.0 | 7.0 | 10.3 | 7.7 |
| 2 | 20.7 | 36.3 | 11.7 | 15.0 | 9.0 | 6.3 | 5.3 | 10.7 |
| 3 | 9.3 | 34.7 | 9.0 | 21.0 | 10.3 | 8.7 | 9.3 | 18.6 |
| Thickness Values from Areas of Bowl 2 Used for ENM Measurements (μm) | ||||||
|---|---|---|---|---|---|---|
| Area No Measured | I | II | III | IV | V | VI |
| Single values | 17 | 49 | 39 | 50 | 26 | 25 |
| 23 | 30 | 32 | 52 | 28 | 31 | |
| 26 | 25 | 41 | 39 | 13 | 33 | |
| 20 | 5 | 40 | 47 | 12 | 31 | |
| 27 | 31 | 36 | 39 | 10 | 41 | |
| Average values | 22.6 | 28 | 37.6 | 45.4 | 17.8 | 32.2 |
| Item | Number | Area | Noise Resistance (Ω cm2) | Comment |
|---|---|---|---|---|
| Otago | 1 | - | 3.80 × 105 | brushed 18–85 |
| 2 | - | 1.59 × 106 | brushed 55–185 | |
| Sword | 1 | - | 3.80 × 101 | brushed 138–212 |
| 2 | - | 3.90 × 101 | brushed 180–260 | |
| Bell | 1 | - | 3.22 × 102 | brushed 0–170 |
| 2 | - | 4.14 × 102 | not brushed | |
| Bowls | 1 | 1 (I-II) | 2.09 × 103 | - |
| 2 (III-IV) | 2.55 × 103 | - | ||
| 3 (V-VI) | 2.50 × 102 | - | ||
| 2 | 1 (I-II) | 1.70 × 102 | - | |
| 2 (III-IV) | 6.00 × 101 | - | ||
| 3 (V-VI) | 2.10 × 102 | - | ||
| 3 | 1 (I-II) | 2.90 × 103 | - | |
| 2 (III-IV) | 1.54 × 103 | - | ||
| 3 (V-VI) | 5.70 × 102 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mills, D.J.; Schaefer, K.; Wityk, T. In-Situ Evaluation of the Protectivity of Coatings Applied to Metal Cultural Artefacts Using Non-Destructive Electrochemical Measurements. Corros. Mater. Degrad. 2021, 2, 120-132. https://doi.org/10.3390/cmd2010007
Mills DJ, Schaefer K, Wityk T. In-Situ Evaluation of the Protectivity of Coatings Applied to Metal Cultural Artefacts Using Non-Destructive Electrochemical Measurements. Corrosion and Materials Degradation. 2021; 2(1):120-132. https://doi.org/10.3390/cmd2010007
Chicago/Turabian StyleMills, Douglas J., Katarzyna Schaefer, and Tomasz Wityk. 2021. "In-Situ Evaluation of the Protectivity of Coatings Applied to Metal Cultural Artefacts Using Non-Destructive Electrochemical Measurements" Corrosion and Materials Degradation 2, no. 1: 120-132. https://doi.org/10.3390/cmd2010007
APA StyleMills, D. J., Schaefer, K., & Wityk, T. (2021). In-Situ Evaluation of the Protectivity of Coatings Applied to Metal Cultural Artefacts Using Non-Destructive Electrochemical Measurements. Corrosion and Materials Degradation, 2(1), 120-132. https://doi.org/10.3390/cmd2010007




