Biodegradation and Microbial Contamination of Limestone Surfaces: An Experimental Study from Batalha Monastery, Portugal
Abstract
1. Introduction
2. Materials and Methods
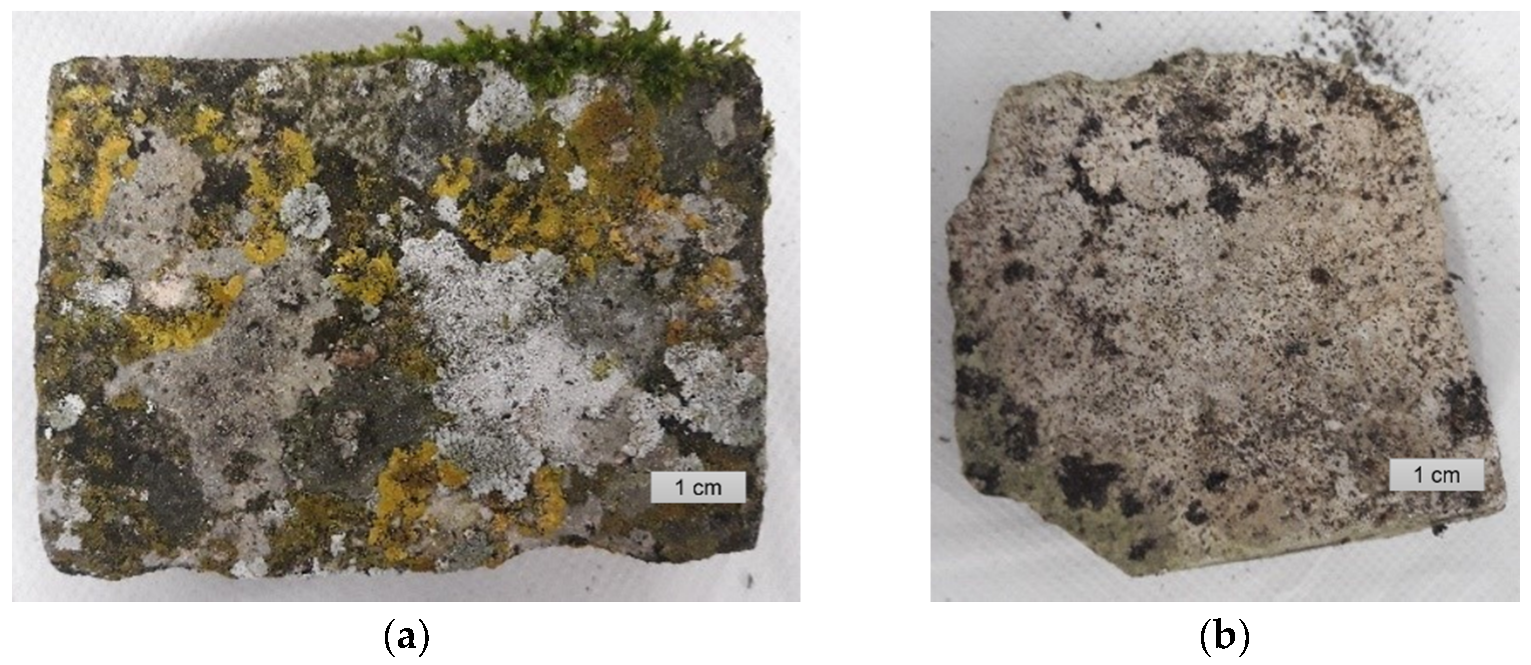
2.1. Location and Sampling
2.2. Air Monitoring
2.3. Molecular Analysis of Bacterial Communities
2.4. Morphology Observation, Mineralogical, and Chemical Characterization of the Bio-Deteriorated Stone
2.4.1. Optical Microscopy (OM)
2.4.2. X-Ray Micro-Diffractometry (µ-XRD)
2.4.3. Low-Vacuum Scanning Electron Microscopy Coupled with Energy-Dispersive Spectrometry (LV-SEM + EDS)
3. Results
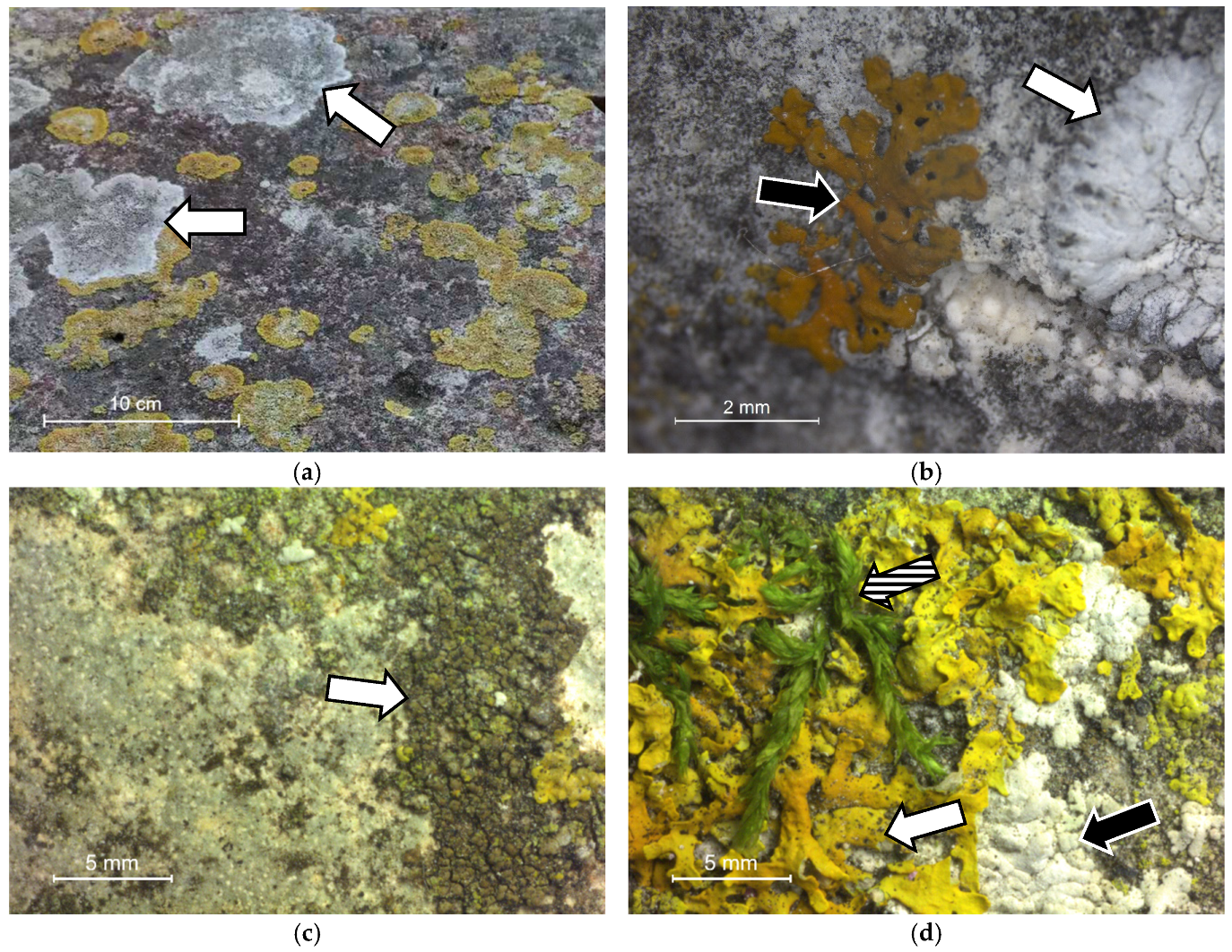
3.1. Optical Microscope Observation
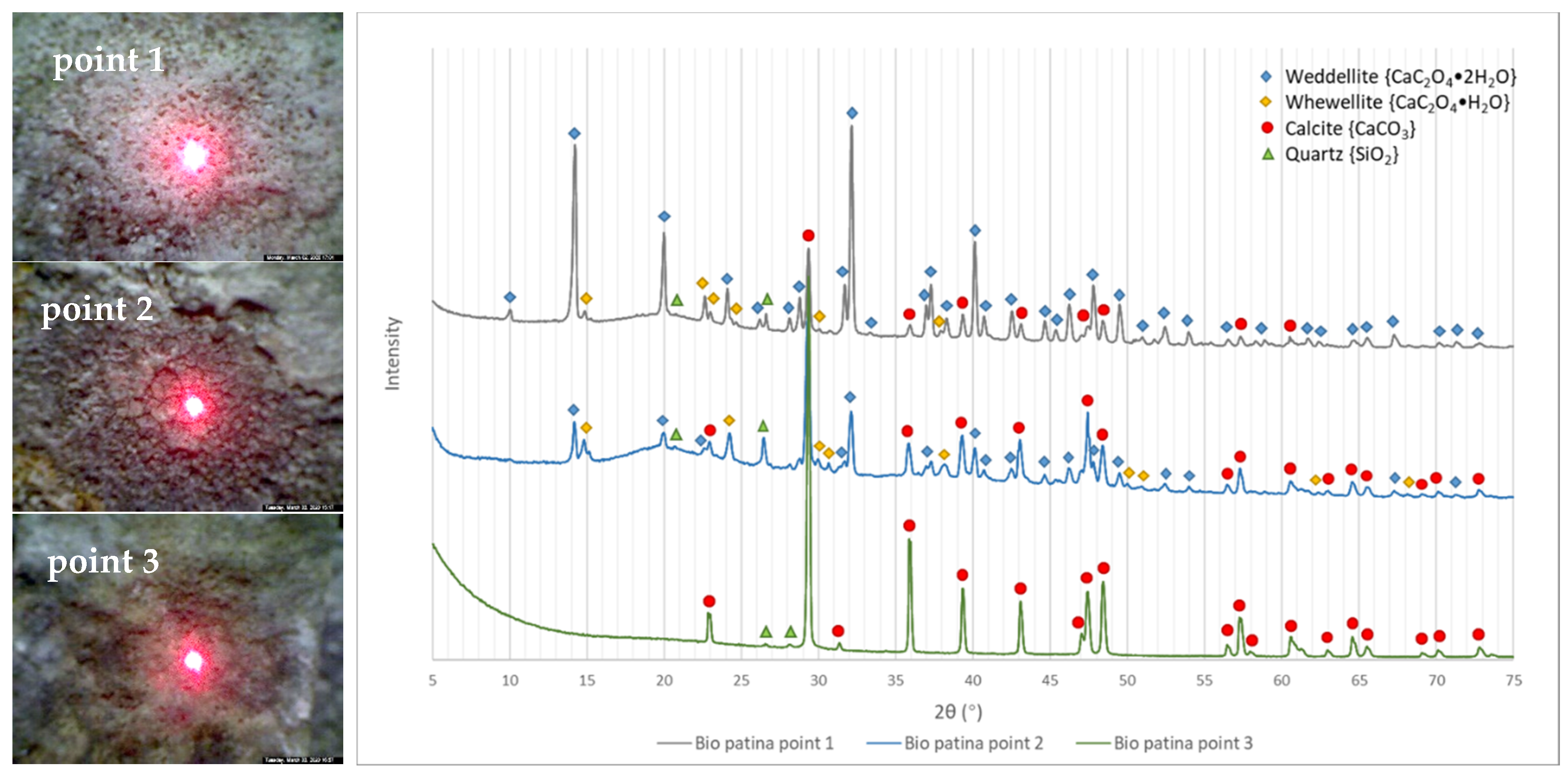
3.2. XRD Results
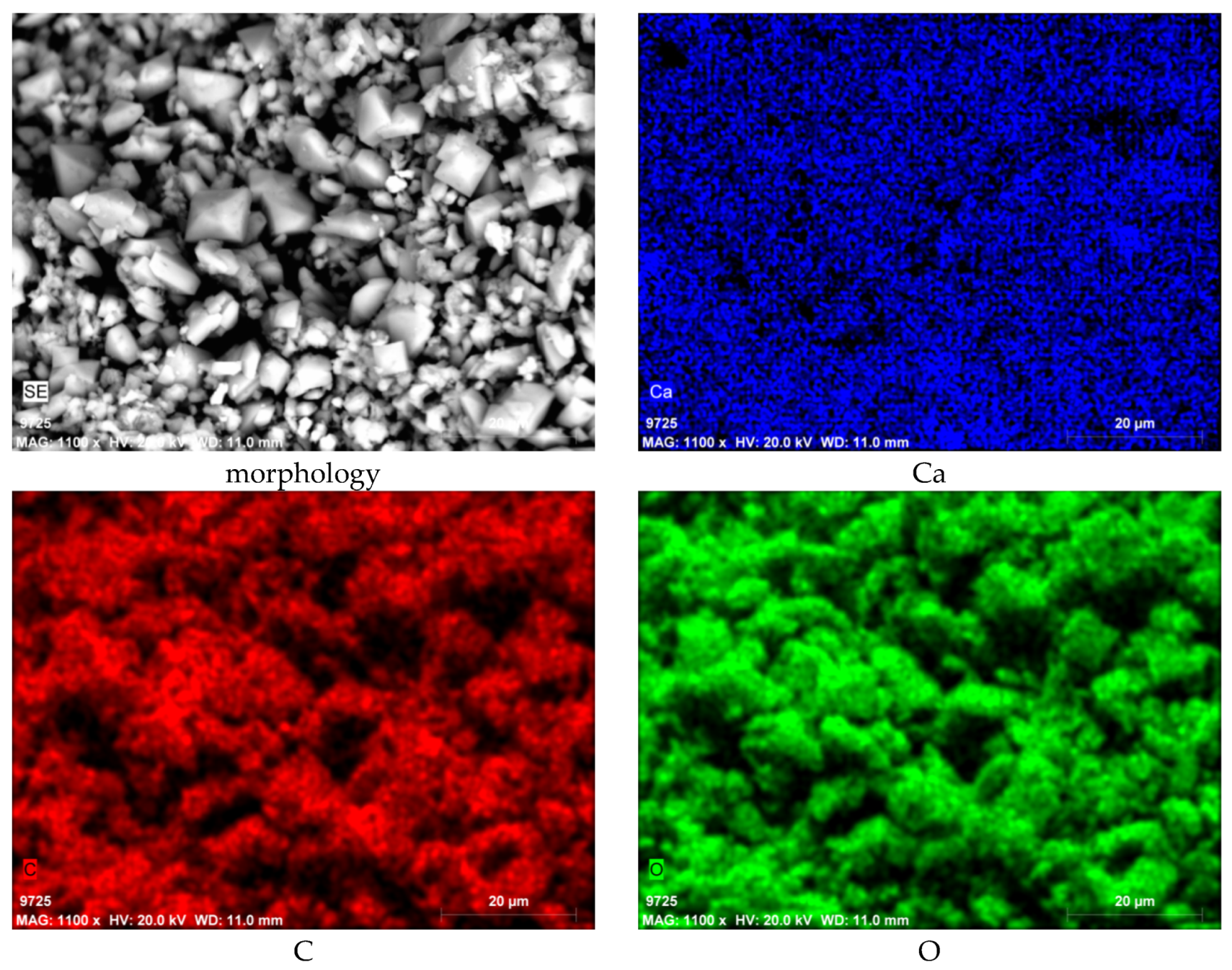
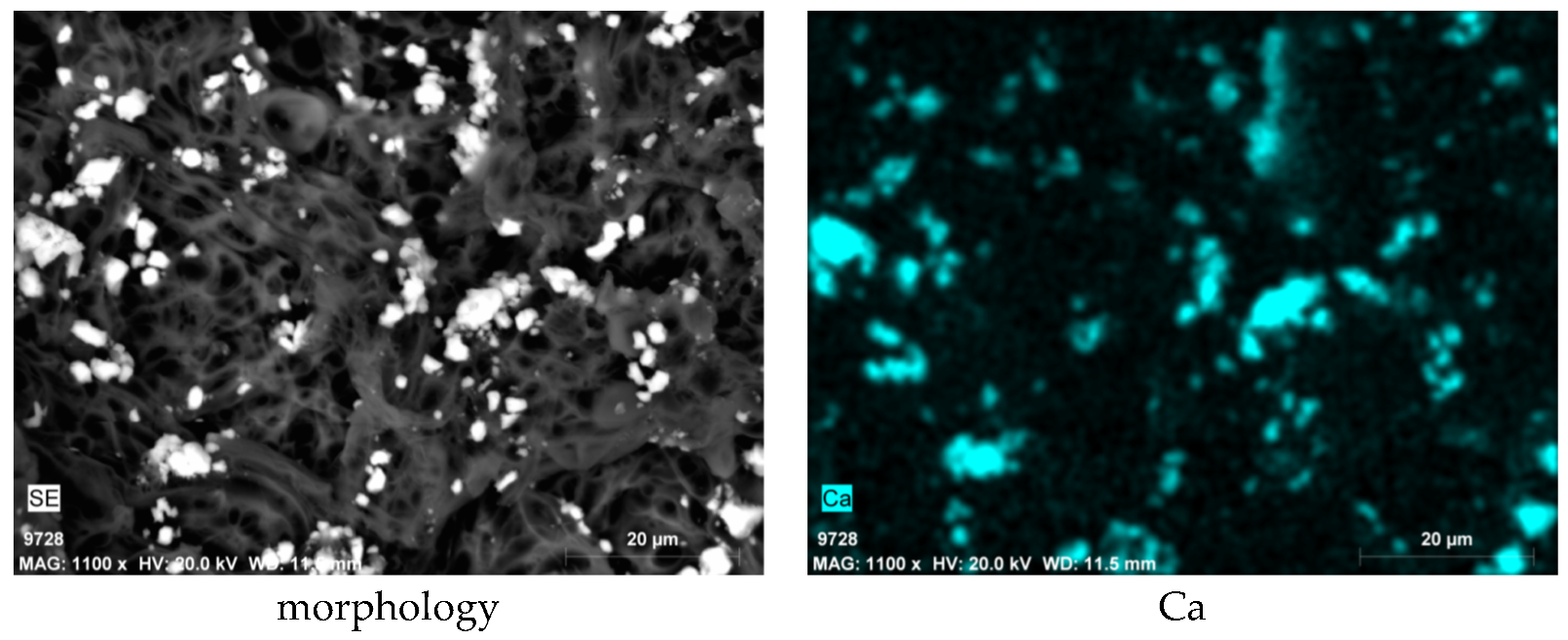
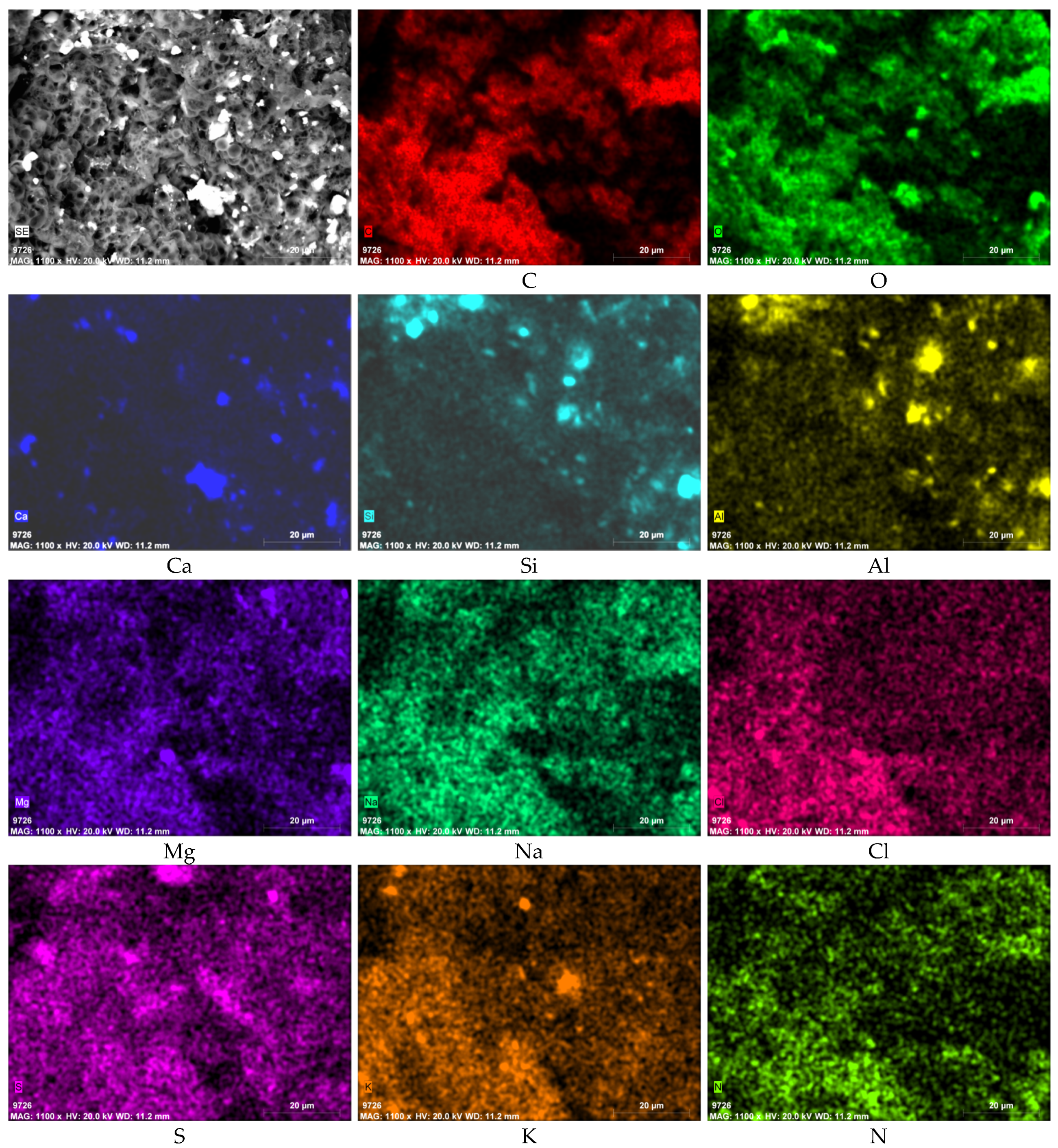
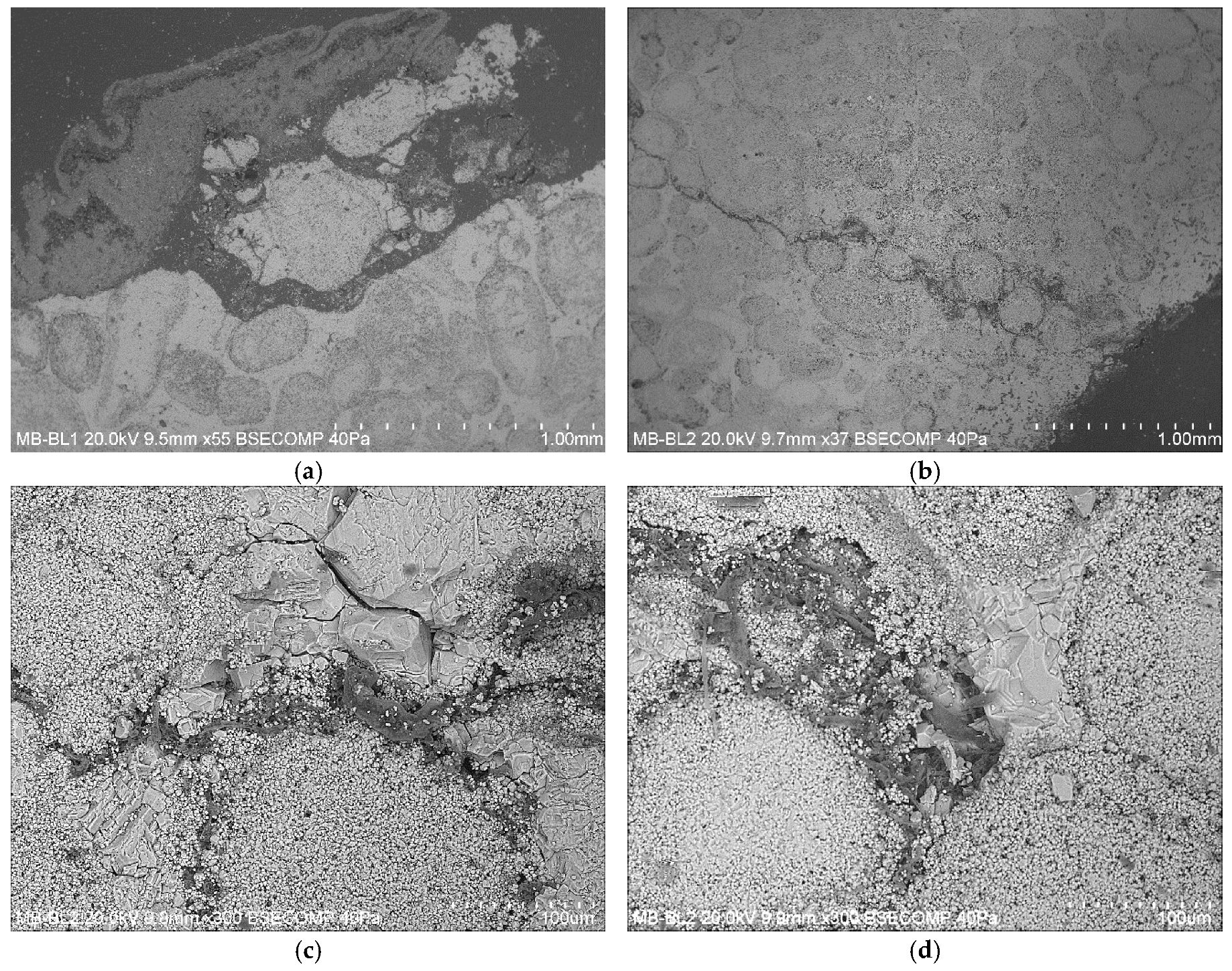
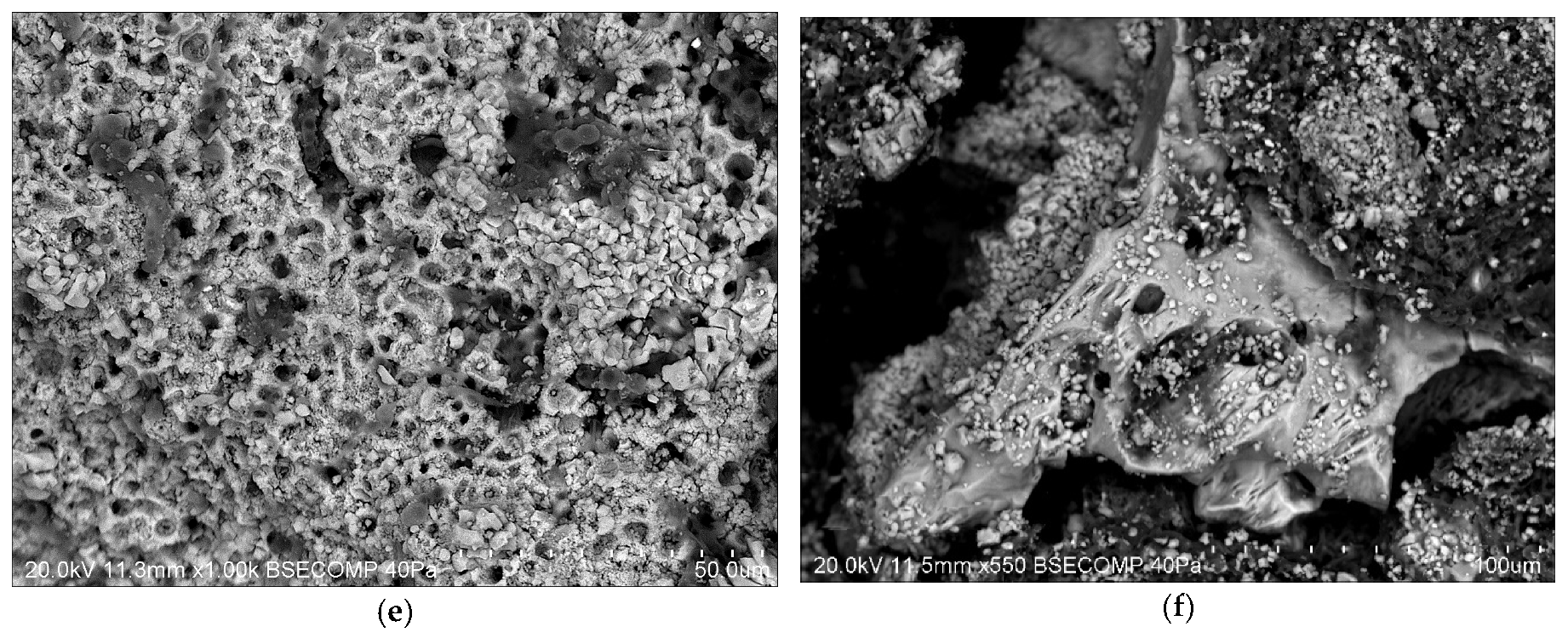
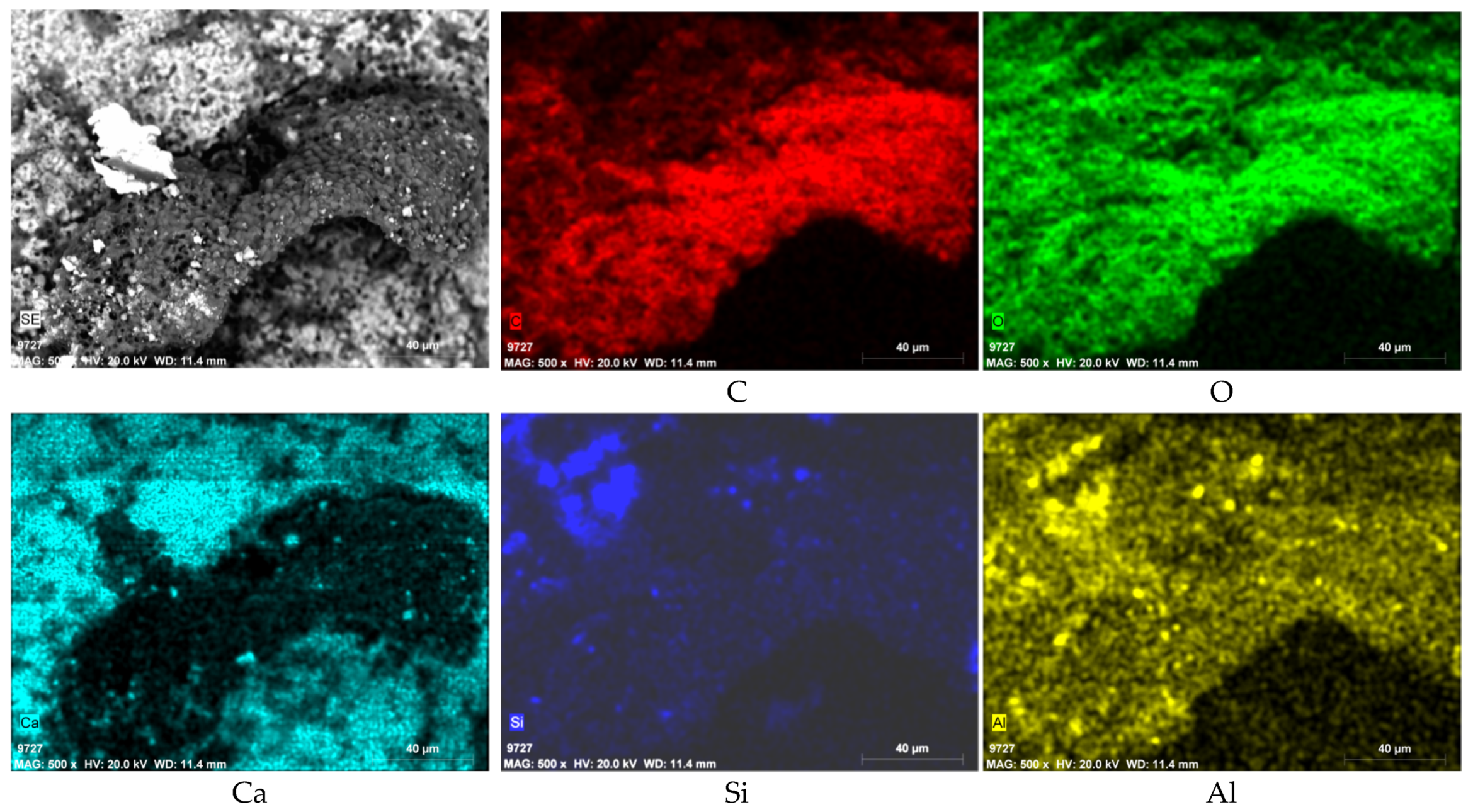
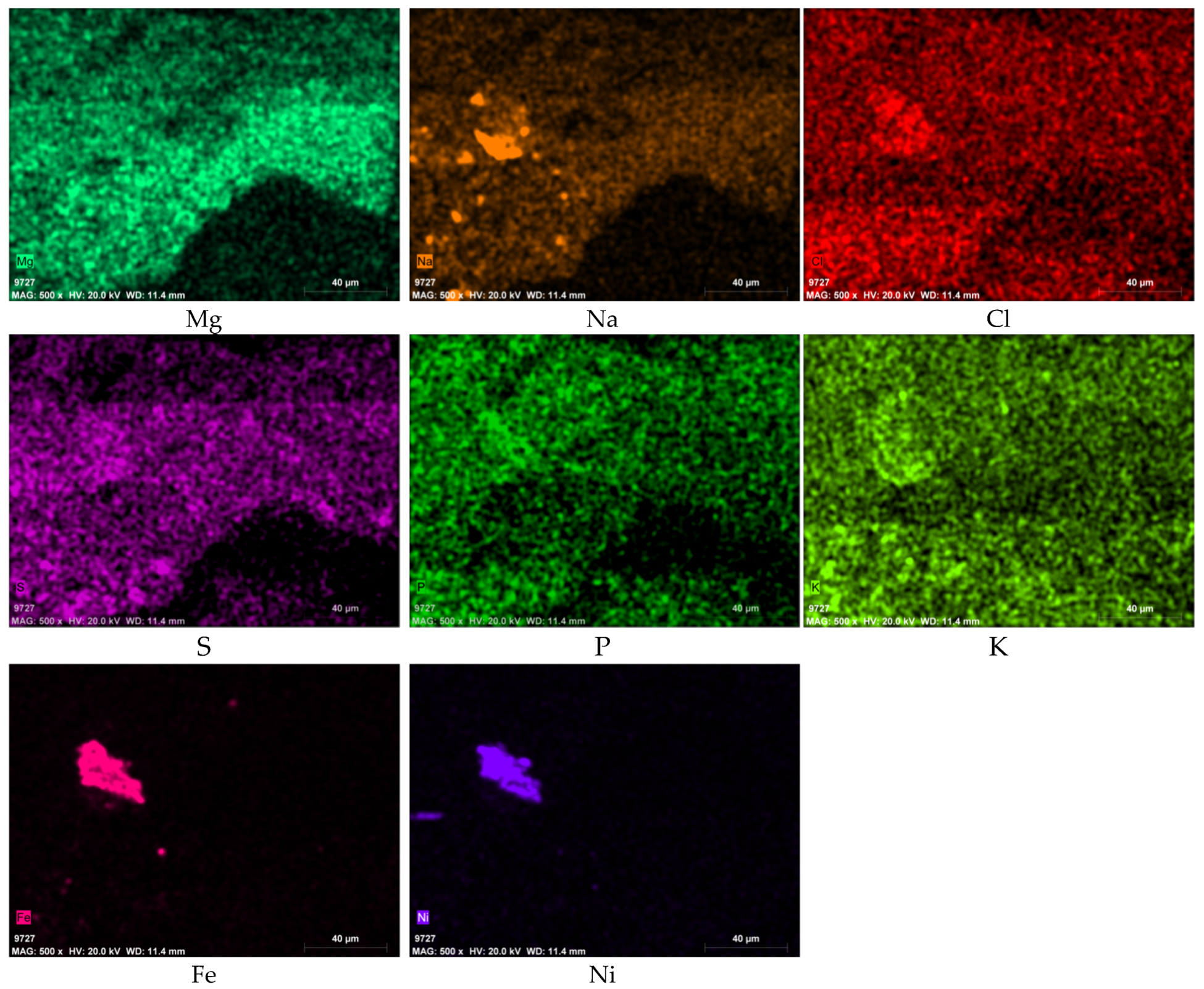
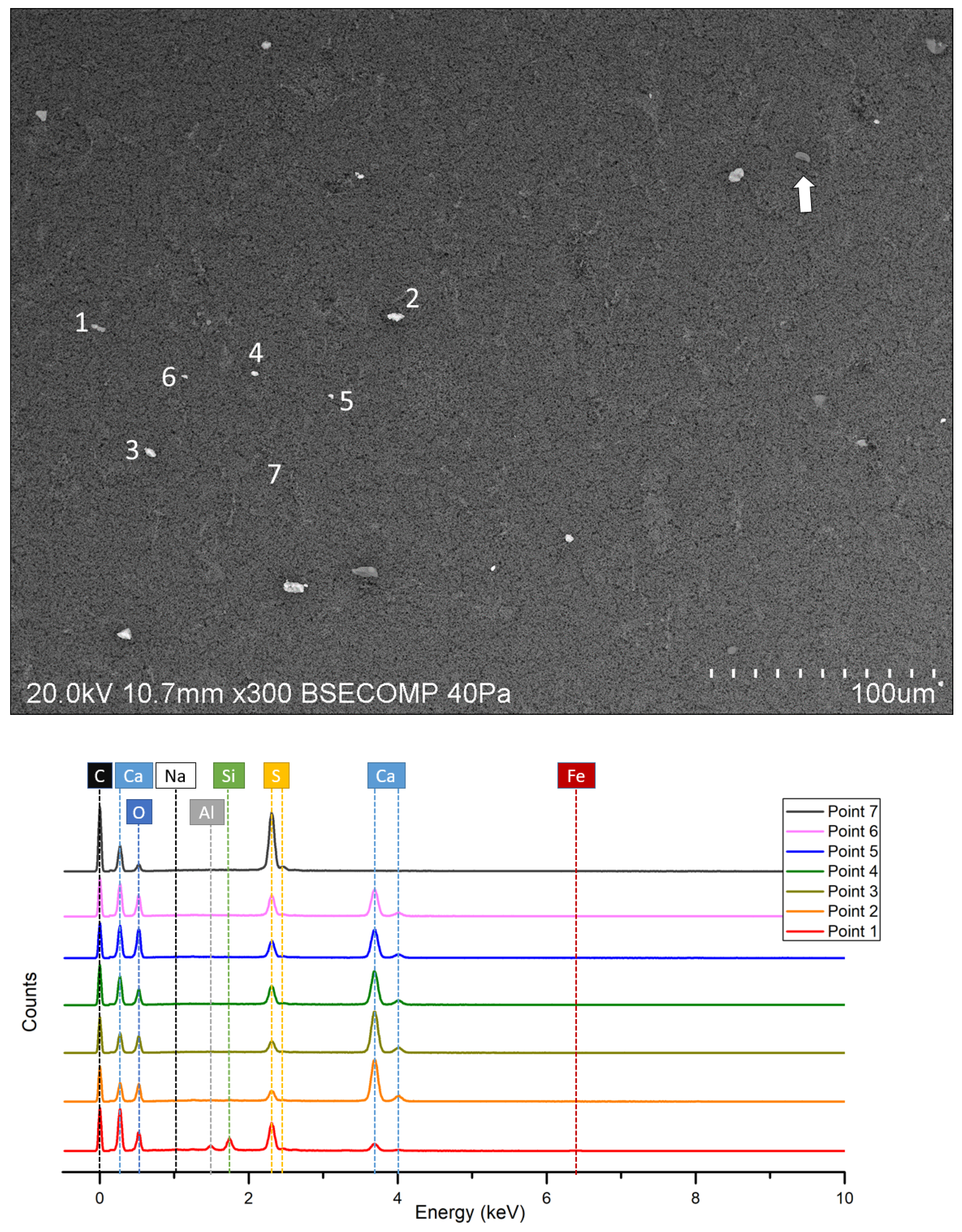
3.3. SEM-EDS Results
3.4. High-Throughput Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ahmadjian, V. Resynthesis of lichens. In The Lichens; Elsevier Inc.: Cambridge, MA, USA; Academic Press: Cambridge, MA, USA, 1973; pp. 565–579. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring functional contexts of symbiotic sustain within lichen-associated bacteria by comparative omics. ISME J. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Trovão, J.; Portugal, A.; Soares, F.; Paiva, D.S.; Mesquita, N.; Coelho, C.; Pinheiro, A.C.; Catarino, L.; Gil, F.; Tiago, I. Fungal diversity and distribution across distinct biodeterioration phenomena in limestone walls of the old cathedral of Coimbra, UNESCO World Heritage Site. Int. Biodeterior. Biodegrad. 2019, 142, 91–102. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Mesquita, N.; Trovão, J.; Soares, F.; Tiago, I.; Coelho, C.; Carvalho, H.P.; Gil, F.; Catarino, L.; Piñar, G.; et al. Limestone biodeterioration: A review on the Portuguese cultural heritage scenario. J. Cult. Herit. 2019, 36, 275–285. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef] [PubMed]
- Prada, J.L.; Pugés, M.; Rocabayera, R. Estudio de las patologias del Retablo del Altar Mayor de la Sé da Guarda. In Património Estudos; Instituto Português do Património Arquitectónico: Lisbon, Portugal, 2002; p. 70. [Google Scholar]
- Ascaso, C.; Wierzchos, J.; Delgado Rodrigues, J.; Aires-Barros, L.; Henriques, F.; Charola, A.E. Endolithic microorganisms in the biodeterioration of the tower of Belem. Int. Z. FürBauinstandsetz. 1998, 4, 627–640. [Google Scholar]
- Ascaso, C.; Wierzchos, J.; Souza-Egipsy, V.; De los Rıos, A.; Rodrigues, J.D. In situ evaluation of the biodeteriorating action of microorganisms and the effects of biocides on carbonate rock of the Jeronimos Monastery (Lisbon). Int. Biodeterior. Biodegrad. 2002, 49, 1–12. [Google Scholar] [CrossRef]
- Mateus, D.M.; Silva, R.B.; Costa, F.M.; Coroado, J.P. Diversidade microbiológica do edifício da Sacristia Incompleta do Convento de Cristo, em Tomar, e avaliação do seu controlo por biocidas. Conserv. Património 2013, 17, 11–17. [Google Scholar] [CrossRef]
- Schiavon, N.; Chiavari, G.; Schiavon, G.; Fabbri, D. Nature and decay effects of urban soiling on granitic building stones. Sci. Total Environ. 1995, 167, 87–101. [Google Scholar] [CrossRef]
- Schiavon, N. Biodeterioration of calcareous and granitic building stones in urban environments. Geol. Soc. Lond. Spec. Publ. 2002, 205, 195–205. [Google Scholar] [CrossRef]
- Ortega-Morales, O.; Montero-Muñoz, J.L.; Neto, J.A.B.; Beech, I.B.; Sunner, J.; Gaylarde, C. Deterioration and microbial colonization of cultural heritage stone buildings in polluted and unpolluted tropical and subtropical climates: A meta-analysis. Int. Biodeterior. Biodegrad. 2019, 143, 104734. [Google Scholar] [CrossRef]
- Ding, Y.; Mirão, J.; Redol, P.; Dias, L.; Moita, P.; Angelini, E.; Grassini, S.; Schiavon, N. A combined petrographic and geochemical metrological approach to assess the provenance of the building limestone used in the Batalha Monastery (Portugal). In Metrology for Archaeology and Cultural Heritage; Battisti, V., Gallo, V., Eds.; IMEKO: Florence, Italy, 2019; p. 338. [Google Scholar]
- Dias, L.; Rosado, T.; Coelho, A.; Barrulas, P.; Lopes, L.; Moita, P.; Candeias, A.; Mirão, J.; Caldeira, A.T. Natural limestone discolouration triggered by microbial activity—A contribution. AIMS Microbiol. 2018, 4, 594. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Dias, L.; Lança, M.; Nogueira, C.; Santos, R.; Martins, M.R.; Candeias, A.; Mirão, J.; Caldeira, A.T. Assessment of microbiota present on a Portuguese historical stone convent using high-throughput sequencing approaches. MicrobiologyOpen 2020, 9, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Rosado, T.; Candeias, A.; Mirão, J.; Caldeira, A.T. A change in composition, a change in colour: The case of limestone sculptures from the Portuguese National Museum of Ancient Art. J. Cult. Herit. 2020, 42, 255–262. [Google Scholar] [CrossRef]
- Mifsud, S.; Lanfranco, E.; Fiorentino, J.; Mifsud, S.D. An Updated Flora of Selmunett (St. Paul’s Island) including Mosses and Lichens. XJENZA 2016, 4, 142–159. [Google Scholar]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and higher plants on stone: A review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Sayer, J.A.; Kierans, M.; Gadd, G.M. Solubilisation of some naturally occurring metal-bearing minerals, limescale and lead phosphate by Aspergillus niger. FEMS Microbiol. Lett. 1997, 154, 29–35. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Sayer, J.A.; Gadd, G.M. Solubilization of natural gypsum (CaSO4.2H2O) and the formation of calcium oxalate by Aspergillus niger and Serpula himantioides. Mycol. Res. 1998, 102, 825–830. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—an updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Shi, J.; Wang, N.; Gao, H.; Baker, A.; Yao, X.; Zhang, D. Phosphorus solubility in aerosol particles related to particle sources and atmospheric acidification in Asian continental outflow. Atmos. Chem. Phys. 2019, 19, 847–860. [Google Scholar] [CrossRef]
- Leiva, D.; Clavero-León, C.; Carú, M.; Orlando, J. Intrinsic factors of Peltigera lichens influence the structure of the associated soil bacterial microbiota. FEMS Microbiol. Ecol. 2016, 92, fiw178. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.T.; Cropsey, G.W.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial communities associated with the lichen symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, T.A.; Dedysh, S.N.; Zavarzin, G.A. The leading role of actinobacteria in aerobic cellulose degradation in Sphagnum peat bogs. Dokl. Biol. Sci. 2006, 410, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.A.; Danko, D.C.; Sandoval, T.A.; Pishchany, G.; Moncada, B.; Kolter, R.; Mason, C.E.; Zambrano, M.M. The microbiomes of seven lichen genera reveal host specificity, a reduced core community and potential as source of antimicrobials. Front. Microbiol. 2020, 11, 398. [Google Scholar] [CrossRef]
- Bjelland, T.; Grube, M.; Hoem, S.; Jorgensen, S.L.; Daae, F.L.; Thorseth, I.H.; Øvreås, L. Microbial metacommunities in the lichen–rock habitat. Environ. Microbiol. Rep. 2011, 3, 434–442. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, E.H.; Lee, H.K.; Hong, S.G. Biodiversity and physiological characteristics of Antarctic and Arctic lichens-associated bacteria. World J. Microbiol. Biotechnol. 2014, 30, 2711–2721. [Google Scholar] [CrossRef]
- Özvan, A.; Dinçer, İ.; Akın, M.; Oyan, V.; Tapan, M. Experimental studies on ignimbrite and the effect of lichens and capillarity on the deterioration of Seljuk Gravestones. Eng. Geol. 2015, 185, 81–95. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Salvador, C.S.C.; Caldeira, A.T.; Angelini, E.; Schiavon, N. Biodegradation and Microbial Contamination of Limestone Surfaces: An Experimental Study from Batalha Monastery, Portugal. Corros. Mater. Degrad. 2021, 2, 31-45. https://doi.org/10.3390/cmd2010002
Ding Y, Salvador CSC, Caldeira AT, Angelini E, Schiavon N. Biodegradation and Microbial Contamination of Limestone Surfaces: An Experimental Study from Batalha Monastery, Portugal. Corrosion and Materials Degradation. 2021; 2(1):31-45. https://doi.org/10.3390/cmd2010002
Chicago/Turabian StyleDing, Yufan, Catia Sofia Clemente Salvador, Ana Teresa Caldeira, Emma Angelini, and Nick Schiavon. 2021. "Biodegradation and Microbial Contamination of Limestone Surfaces: An Experimental Study from Batalha Monastery, Portugal" Corrosion and Materials Degradation 2, no. 1: 31-45. https://doi.org/10.3390/cmd2010002
APA StyleDing, Y., Salvador, C. S. C., Caldeira, A. T., Angelini, E., & Schiavon, N. (2021). Biodegradation and Microbial Contamination of Limestone Surfaces: An Experimental Study from Batalha Monastery, Portugal. Corrosion and Materials Degradation, 2(1), 31-45. https://doi.org/10.3390/cmd2010002






