The Intention to React to Sounds Induces Sleep Disturbances and Alters Brain Responses to Sounds during Sleep: A Pilot Study
Abstract
1. Introduction
2. Results
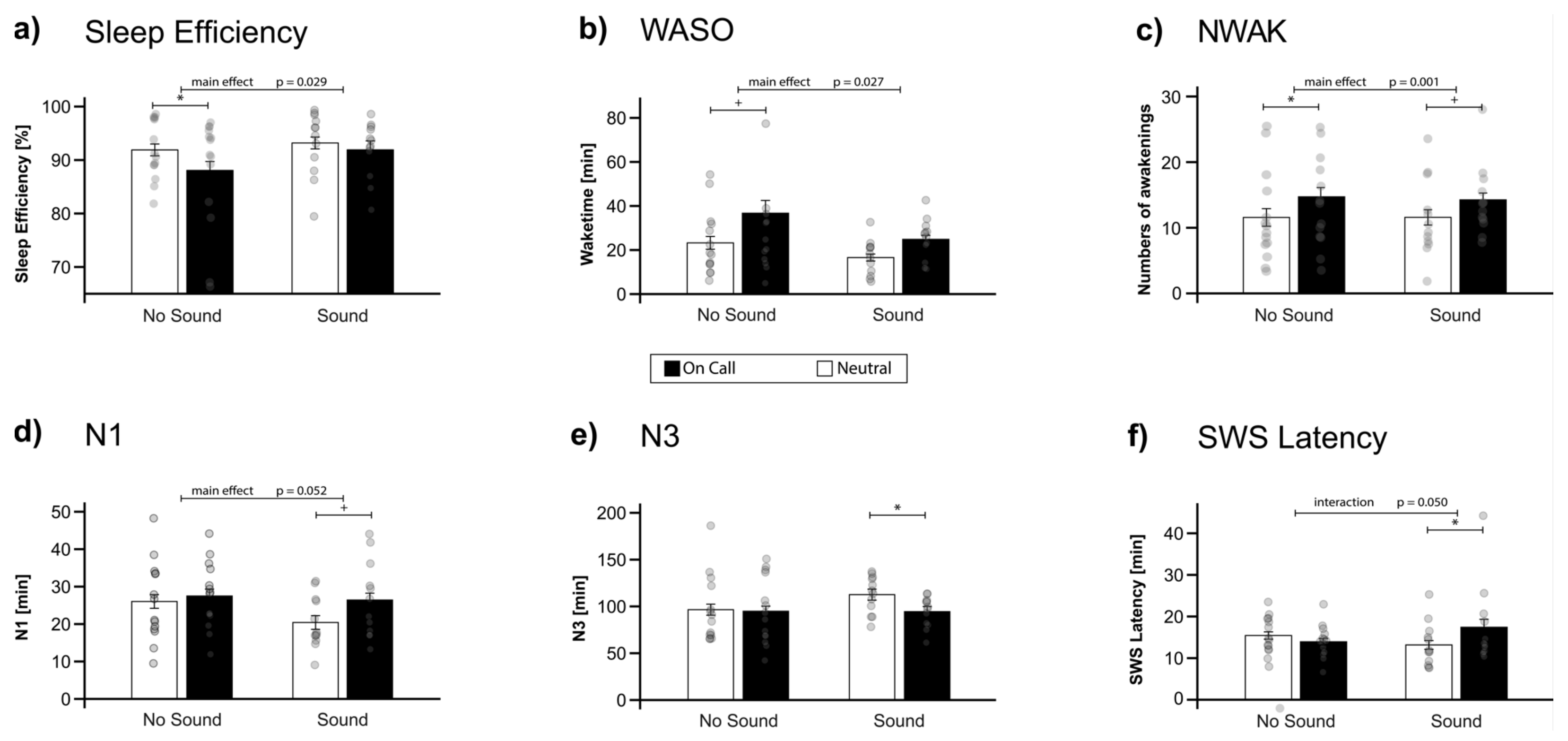
2.1. Objective and Subjective Sleep Parameters
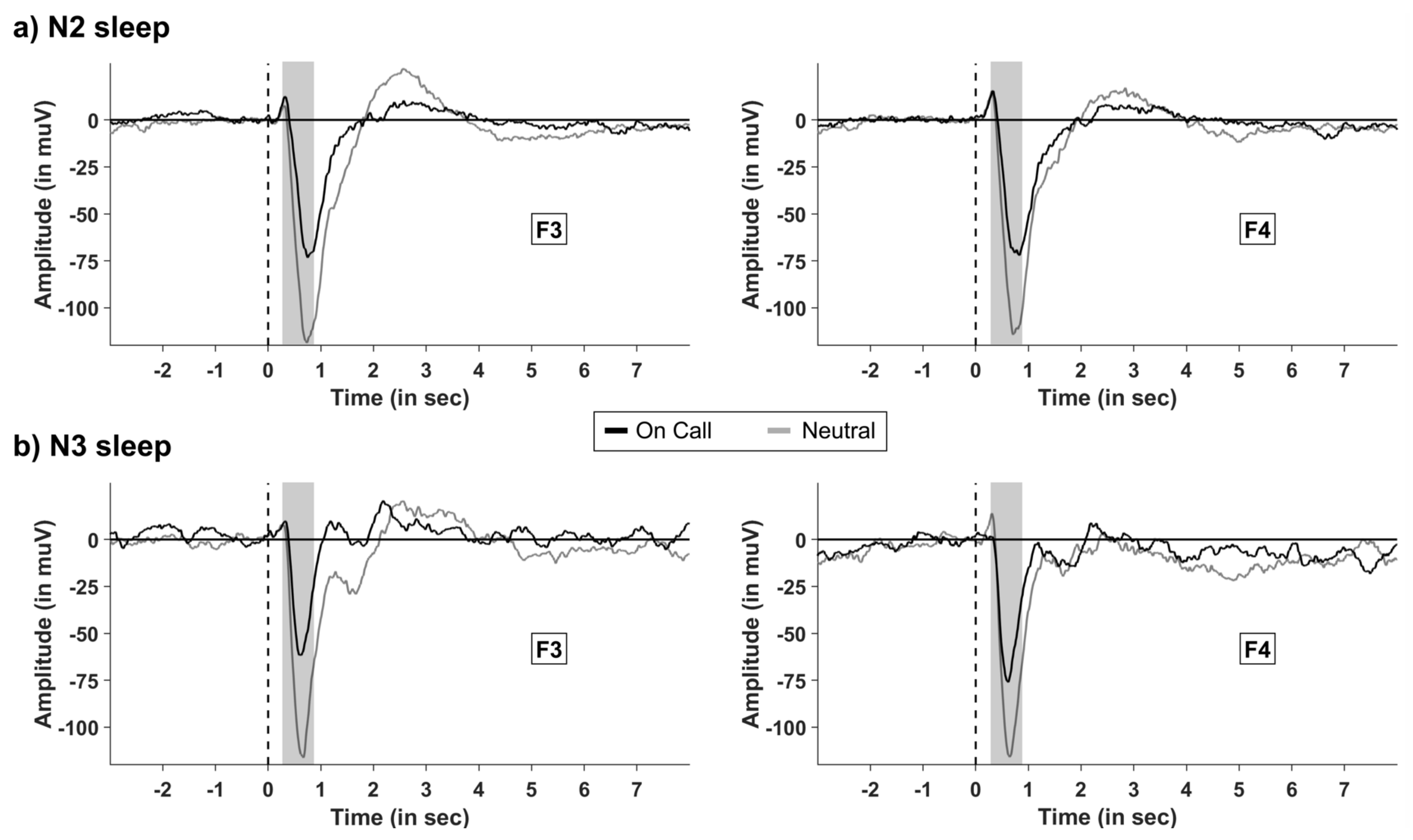
2.2. The Effect of Instruction on Event-Related Potentials (ERP) during Sleep
2.3. EEG Power Analysis
2.4. Heart Rate and Heart Rate Variability
2.5. Memory Consolidation and Vigilance
3. Discussion
3.1. Limitation
3.2. Outlook
4. Materials and Methods
4.1. Methods
4.1.1. Participants
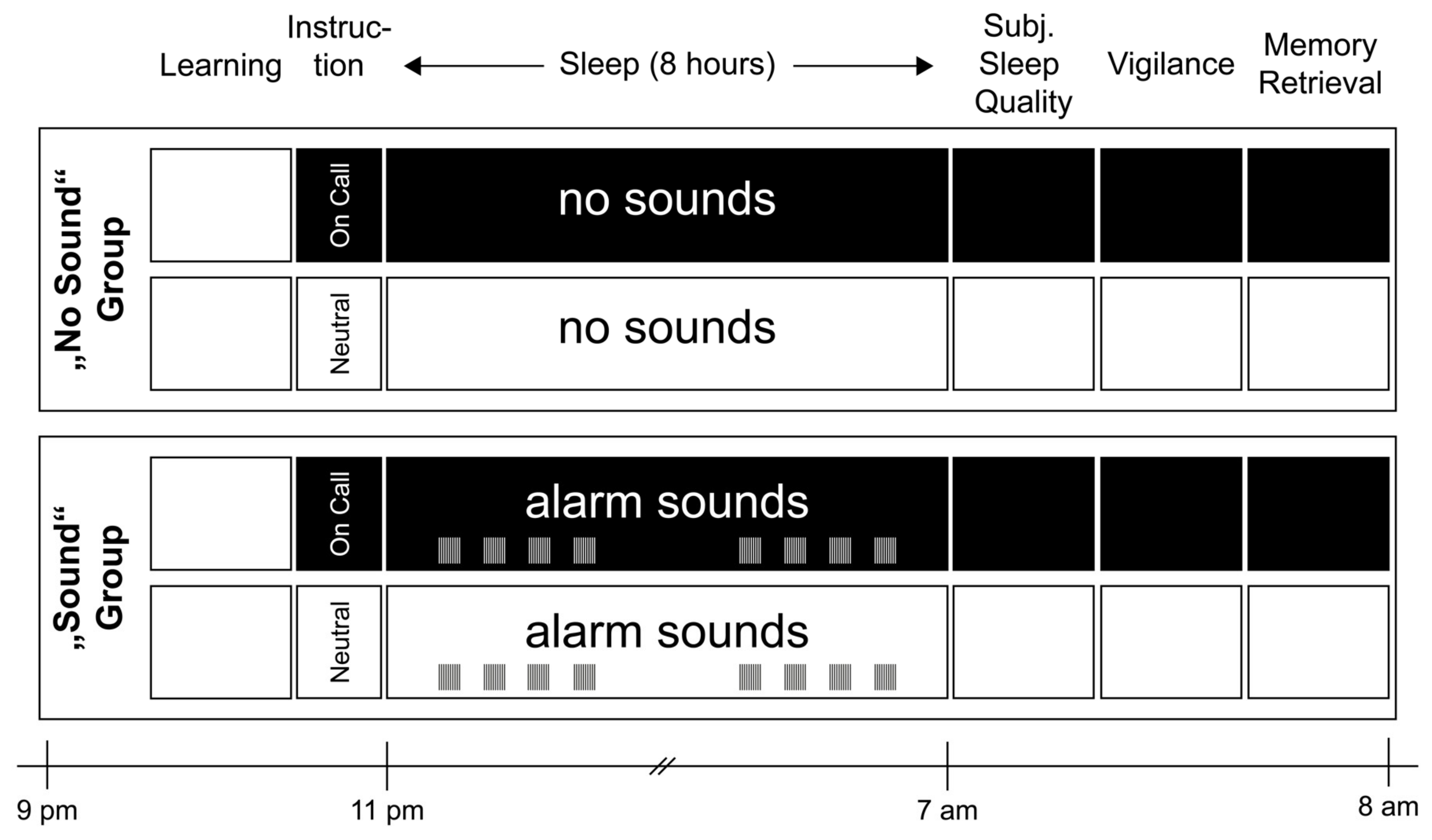
4.1.2. Experimental Design and Procedure
4.2. Material
4.2.1. On-Call Instruction
4.2.2. Sound Presentation during the Night
4.2.3. Sleep Setting
4.2.4. Polysomnographic Recordings and Sleep Analysis
4.2.5. Analysis of EEG Data
4.2.6. EEG Power Analysis
4.2.7. Event-Related Potentials (ERP) Analysis during Sleep
4.2.8. Analysis of ECG and Physiological Arousal
4.2.9. Questionnaires
4.2.10. Memory Measurement
4.2.11. Psychomotor Vigilance Test (PVT)
4.2.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordi, M.J.; Ackermann, S.; Rasch, B. Effects of relaxing music on healthy sleep. Sci. Rep. 2019, 9, 9079. [Google Scholar] [CrossRef] [PubMed]
- Kaneita, Y.; Yokoyama, E.; Harano, S.; Tamaki, T.; Suzuki, H.; Munezawa, T.; Nakajama, H.; Asai, T.; Ohida, T. Associations between sleep disturbance and mental health status: A longitudinal study of Japanese junior high school students. Sleep Med. 2009, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Dimitrov, S.; Born, J. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 2010, 1193, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Born, J. About Sleep’s Role in Memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Nir, Y.; Massimini, M.; Boly, M.; Tononi, G. Sleep and Consciousness. In Neuroimaging of Consciousness; Cavanna, A.E., Nani, A., Blumenfeld, H., Laureys, S., Eds.; Springer: Berlin Heidelberg, Germany, 2013; pp. 133–182. [Google Scholar]
- Hugdahl, K. Fifty years of dichotic listening research—Still going and going and…. Brain Cogn. 2011, 76, 211–213. [Google Scholar] [CrossRef]
- Posner, M.I. Attention: The mechanisms of consciousness. Proc. Natl. Acad. Sci. USA 1994, 91, 7398–7403. [Google Scholar] [CrossRef]
- Westerhausen, R.; Kompus, K. How to get a left-ear advantage: A technical review of assessing brain asymmetry with dichotic listening. Scand. J. Psychol. 2018, 59, 66–73. [Google Scholar] [CrossRef]
- Ceklic, T.; Bastien, C.H. Information processing during NREM sleep and sleep quality in insomnia. Int. J. Psychophysiol. 2015, 98, 460–9469. [Google Scholar] [CrossRef]
- Loewy, D.H.; Campbell, K.B.; de Lugt, D.R.; Elton, M.; Kok, A. The mismatch negativity during natural sleep: Intensity deviants. Clin. Neurophysiol. 2000, 111, 863–872. [Google Scholar] [CrossRef]
- Sabri, M.; Campbell, K.B. The effects of digital filtering on mismatch negativity in wakefulness and slow-wave sleep. J. Sleep Res. 2002, 11, 123–127. [Google Scholar] [CrossRef]
- Cottrell, L.; Khan, A. Impact of childhood epilepsy on maternal sleep and socioemotional functioning. Clin. Pediatr. 2005, 44, 613–616. [Google Scholar] [CrossRef]
- McCann, D.; Bull, R.; Winzenberg, T. Sleep deprivation in parents caring for children with complex needs at home: A mixed methods systematic review. J. Fam. Nurs. 2015, 21, 86–118. [Google Scholar] [CrossRef]
- Gaba, D.M.; Howard, S.K. Fatigue among clinicians and the safety of patients. N. Engl. J. Med. 2002, 347, 1249–1255. [Google Scholar] [CrossRef]
- Smithers, F. The pattern and effect of on call work in transplant co-ordinators in the United Kingdom. Int. J. Nurs. Stud. 1995, 32, 469–483. [Google Scholar] [CrossRef]
- Finnerty, J.D. How often will the firemen get their sleep? Manag. Sci. 1977, 23, 1169–1173. [Google Scholar] [CrossRef]
- Paterson, J.L.; Aisbett, B.; Ferguson, S.A. Sound the alarm: Health and safety risks associated with alarm response for salaried and retained metropolitan firefighters. Saf. Sci. 2016, 82, 174–181. [Google Scholar] [CrossRef]
- Pilcher, J.J.; Coplen, M.K. Work/rest cycles in railroad operations: Effects of shorter than 24-h shift work schedules and on-call schedules on sleep. Ergonomics 2000, 43, 573–588. [Google Scholar] [CrossRef]
- Torsvall, L.; Akerstedt, T. Disturbed sleep while being on-call: An EEG study of ships’ engineers. Sleep 1988, 11, 35–38. [Google Scholar] [CrossRef]
- Wuyts, J.; De Valck, E.; Vandekerckhove, M.; Pattyn, N.; Exadaktylos, V.; Haex, B.; Maes, J.; Verbraecken, J.; Cluydts, R. Effects of pre-sleep simulated on-call instructions on subsequent sleep. Biol. Psychol. 2012, 91, 383–388. [Google Scholar] [CrossRef]
- Görtelmeyer, R. SF-A/R und SF-B/R: Schlaffragebogen A und B; Hogrefe: Bern, Switzerland, 2011. [Google Scholar]
- Tarvainen, M.P.; Lipponen, J.; Niskanen, J.-P.; Ranta-aho, P.O. Kubios HRV Version 3.3: User’s Guide; Kubios Oy: Kuopio, Finland, 2019; 40p. [Google Scholar]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sus-tained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Hall, S.J.; Ferguson, S.A.; Turner, A.I.; Robertson, S.J.; Vincent, G.E.; Aisbett, B. The effect of working on-call on stress physiology and sleep: A systematic review. Sleep Med. Rev. 2017, 33, 79–87. [Google Scholar] [CrossRef]
- Berntson, G.G.; Thomas Bigger, J., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, P.J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Arnetz, B.B.; Anderzén, I. Physicians during and following night call duty—41 h ambulatory recording of sleep. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 193–196. [Google Scholar] [CrossRef]
- Bamberg, E.; Dettmers, J.; Funck, H.; Krähe, B.; Vahle-Hinz, T. Effects of on-call work on well-being: Results of a daily survey. Appl. Psychol. Health Well Being 2012, 4, 299–320. [Google Scholar] [CrossRef]
- Hall, S.J.; Turner, A.I.; Robertson, S.J.; Ferguson, S.A.; Aisbett, B. Salivary cortisol profiles of on-call from home fire and emergency service personnel. Stress 2019, 22, 436–445. [Google Scholar] [CrossRef]
- Cash, S.S.; Halgren, E.; Dehghani, N.; Rossetti, A.O.; Thesen, T.; Wang, C.; Devinsky, O.; Kuzniecky, R.; Doyle, W.; Madsen, J.R.; et al. The Human K-Complex Represents an Isolated Cortical Donw-State. Science 2009, 324, 1084–1088. [Google Scholar] [CrossRef]
- Einstein, G.O.; McDaniel, M.A. Prospective Memory: Multiple retrieval processes. Curr. Dir. Psychol. Sci. 2005, 14, 286–291. [Google Scholar] [CrossRef]
- Rusted, J.M.; Sawyer, R.; Jones, C.; Trawley, S.L.; Marchant, N.L. Positive effects of nicotine on cognition: The de-ployment of attention for prospective memory. Psychopharmacology 2009, 202, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Loretz, E.; Rasch, B. Exposure to relaxing words during sleep promotes slow-wave sleep and subjective sleep quality. Sleep 2021, 44, zsab148. [Google Scholar] [CrossRef] [PubMed]
- Schechtman, E. Revealing the cognitive contents of sleep to improve diagnosis and research. Sleep 2022. ahead of print. [Google Scholar] [CrossRef]
- Torsvall, L.; Castenfors, K.; Åkerstedt, T.; Fröberg, J. Sleep at sea: A diary study of the effects of unattended machinery space watch duty. Ergonomics 1987, 30, 1335–1340. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Rasch, B.; Born, J.; Gais, S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J. Cogn. Neurosci. 2006, 18, 793–802. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associates Events: Rules, Terminology and Technical Specifications—Version 1, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Atienza, M.; Cantero, J.L.; Escera, C. Auditory information processing during human sleep as revealed by event-related brain potentials. Clin. Neurophysiol. 2001, 112, 2031–2045. [Google Scholar] [CrossRef]
- Yang, C.-M.; Lo, H.-S. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep 2007, 30, 585–592. [Google Scholar] [CrossRef]
- Ackermann, S.; Hartmann, F.; Papassotiropoulos, A.; de Quervain, D.J.F.; Rasch, B. No associations between interin-dividual differences in sleep parameters and episodic memory consolidation. Sleep 2015, 38, 951–959. [Google Scholar]
- Kogler, L.; Müller, V.I.; Chang, A.; Eickhoff, S.B.; Fox, P.T.; Gur, R.C.; Derntl, B. Psychosocial versus physiological stress—Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage 2015, 119, 235–251. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Griefahn, B.; Kunemund, C.; Brode, P.; Mehnert, P. Zur Validitat der deutschen Ubersetzung des Morningness-Eveningness-Questionnaires von Horne und Ostberg. The Validity of a German Version of the Morning-ness-Eveningness-Questionnaire Developed by Horne and Ostberg. Somnologie 2021, 5, 71–80. [Google Scholar] [CrossRef]
- Steyer, R.; Schwenkmezger, P.; Notz, P.; Eid, M. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF): Handanweisung [The Multidimensional Affect Rating Scale (MDBF): Manual]; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Leiner, D.J. SoSci Survey (Version 3.1.06); SoSci Survey GmbH: Munich, Germany, 2018. [Google Scholar]
- Feld, G.B.; Weis, P.P.; Born, J. The limited capacity of sleep-dependent memory consolidation. Front. Psychol. 2016, 7, 1368. [Google Scholar] [CrossRef] [PubMed]
| “No Sound” Group | “Sound” Group | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | On Call | Neutral | On Call | Pre-Sleep Intention | Sound Group | Interaction | ||||||||||||
| Mean | ± | SEM | Mean | ± | SEM | Mean | ± | SEM | Mean | ± | SEM | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Objective Sleep Parameters | ||||||||||||||||||
| Sleep Efficiency (%) | 91.90 | ± | 1.11 | 88.06 | ± | 1.68 | 93.20 | ± | 1.11 | 91.91 | ± | 1.68 | 5.42 | 0.029 | 0.98 | 0.333 | 1.24 | 0.276 |
| SOL (min) | 22.82 | ± | 4.36 | 22.11 | ± | 3.50 | 21.96 | ± | 4.80 | 21.96 | ± | 4.54 | 0.01 | 0.905 | 0.00 | 0.950 | 0.01 | 0.912 |
| WASO (min) | 23.25 | ± | 2.89 | 36.71 | ± | 5.80 | 16.58 | ± | 1.59 | 24.83 | ± | 1.83 | 5.55 | 0.027 | 2.96 | 0.098 | 0.31 | 0.585 |
| NWAK | 11.57 | ± | 1.34 | 14.71 | ± | 1.41 | 11.58 | ± | 1.17 | 14.25 | ± | 1.04 | 14.32 | 0.001 * | 0.01 | 0.926 | 0.09 | 0.761 |
| N1 (min) | 26.04 | ± | 2.07 | 27.50 | ± | 1.75 | 20.42 | ± | 1.33 | 26.42 | ± | 2.00 | 4.16 | 0.052 | 1.09 | 0.307 | 1.68 | 0.207 |
| N2 (min) | 212.71 | ± | 6.51 | 203.71 | ± | 7.37 | 213.08 | ± | 4.91 | 219.42 | ± | 6.25 | 0.14 | 0.716 | 0.47 | 0.948 | 2.15 | 0.156 |
| N3 (min) | 96.50 | ± | 7.02 | 94.86 | ± | 6.94 | 112.58 | ± | 3.91 | 94.46 | ± | 3.20 | 3.39 | 0.078 | 0.59 | 0.450 | 2.68 | 0.115 |
| REM (min) | 100.50 | ± | 3.91 | 93.36 | ± | 3.80 | 95.17 | ± | 2.84 | 92.88 | ± | 4.21 | 2.10 | 0.160 | 0.19 | 0.668 | 0.51 | 0.481 |
| SWS Latency (min) | 15.43 | ± | 0.88 | 13.93 | ± | 0.78 | 13.17 | ± | 1.05 | 17.42 | ± | 1.91 | 1.40 | 0.248 | 0.08 | 0.784 | 8.64 | 0.007 |
| REM Latency (min) | 86.46 | ± | 4.81 | 84.21 | ± | 4.95 | 99.88 | ± | 8.43 | 78.88 | ± | 5.10 | 2.00 | 0.170 | 0.20 | 0.659 | 1.47 | 0.237 |
| TST (min) | 459.00 | ± | 5.99 | 456.18 | ± | 3.48 | 457.83 | ± | 4.75 | 458.04 | ± | 4.46 | 0.12 | 0.730 | 0.00 | 0.960 | 0.14 | 0.710 |
| N1 (%) | 5.69 | ± | 0.46 | 6.02 | ± | 0.37 | 4.48 | ± | 0.30 | 5.77 | ± | 0.42 | 4.25 | 0.050 | 1.11 | 0.303 | 1.63 | 0.214 |
| N2 (%) | 46.49 | ± | 1.51 | 44.55 | ± | 1.50 | 46.50 | ± | 0.86 | 47.84 | ± | 1.15 | 0.13 | 0.723 | 0.50 | 0.485 | 1.92 | 0.179 |
| N3 (%) | 20.96 | ± | 1.46 | 20.78 | ± | 1.51 | 24.59 | ± | 0.82 | 20.70 | ± | 0.75 | 2.93 | 0.100 | 0.67 | 0.421 | 2.81 | 0.107 |
| REM (%) | 21.86 | ± | 0.76 | 20.47 | ± | 0.81 | 20.76 | ± | 0.54 | 20.28 | ± | 0.90 | 1.56 | 0.223 | 0.24 | 0.630 | 0.35 | 0.561 |
| WASO (%) | 5.02 | ± | 0.60 | 8.18 | ± | 1.34 | 3.68 | ± | 0.38 | 5.43 | ± | 0.42 | 5.85 | 0.024 | 0.67 | 0.115 | 0.46 | 0.505 |
| Subjective Sleep Parameters | ||||||||||||||||||
| Sleep Quality | 3.30 | ± | 0.16 | 3.34 | ± | 0.12 | 3.74 | ± | 0.16 | 3.19 | ± | 0.12 | 1.71 | 0.204 | 0.41 | 0.528 | 2.65 | 0.117 |
| SOL (min) | 22.14 | ± | 2.54 | 20.00 | ± | 2.21 | 17.58 | ± | 2.64 | 17.92 | ± | 2.85 | 0.17 | 0.685 | 0.54 | 0.468 | 0.26 | 0.617 |
| WASO (min) | 11.00 | ± | 2.28 | 15.93 | ± | 3.44 | 10.83 | ± | 2.97 | 13.58 | ± | 3.29 | 1.61 | 0.216 | 0.06 | 0.811 | 0.12 | 0.728 |
| NWAK | 2.36 | ± | 0.27 | 2.21 | ± | 0.27 | 2.25 | ± | 0.31 | 3.17 | ± | 0.20 | 1.03 | 0.320 | 1.04 | 0.318 | 2.40 | 0.135 |
| Sleep Depth | 3.14 | ± | 0.22 | 3.14 | ± | 0.23 | 3.75 | ± | 0.22 | 3.33 | ± | 0.21 | 0.54 | 0.470 | 1.27 | 0.271 | 0.63 | 0.436 |
| Memory | ||||||||||||||||||
| Learning | 55.36 | ± | 3.35 | 54.64 | ± | 2.75 | 58.75 | ± | 2.60 | 59.67 | ± | 2.82 | 0.00 | 0.981 | 0.56 | 0.461 | 0.27 | 0.608 |
| Recall | 53.71 | ± | 3.36 | 52.36 | ± | 3.04 | 57.58 | ± | 2.49 | 58.58 | ± | 3.02 | 0.03 | 0.864 | 0.75 | 0.395 | 0.57 | 0.459 |
| Consolidation (%) | 97.05 | ± | 0.95 | 94.92 | ± | 1.14 | 98.21 | ± | 0.83 | 97.71 | ± | 0.95 | 1.47 | 0.230 | 1.52 | 0.229 | 0.51 | 0.483 |
| Vigilance | ||||||||||||||||||
| Mean RT (ms) | 328.73 | ± | 6.96 | 332.84 | ± | 5.90 | 327.82 | ± | 5.92 | 326.83 | ± | 6.61 | 0.11 | 0.738 | 0.09 | 0.771 | 0.27 | 0.609 |
| Errors | 0.77 | ± | 0.15 | 1.08 | ± | 0.33 | 0.67 | ± | 0.16 | 1.33 | ± | 0.37 | 1.59 | 0.220 | 0.04 | 0.840 | 0.22 | 0.642 |
| Reactions | 76.69 | ± | 0.39 | 77.54 | ± | 0.66 | 76.75 | ± | 0.65 | 76.58 | ± | 0.67 | 0.18 | 0.675 | 0.28 | 0.604 | 0.36 | 0.556 |
| Physiological Arousal | ||||||||||||||||||
| PNS index | 1.30 | ± | 0.40 | 1.25 | ± | 0.40 | 1.60 | ± | 0.30 | 1.34 | ± | 0.34 | 0.68 | 0.418 | 0.11 | 0.741 | 0.32 | 0.577 |
| SNS index | −0.51 | ± | 0.24 | −0.45 | ± | 0.21 | −0.48 | ± | 0.33 | −0.43 | ± | 0.27 | 0.06 | 0.809 | 0.00 | 0.958 | 0.00 | 0.986 |
| HRV triangular index | 11.45 | ± | 0.90 | 11.94 | ± | 0.98 | 13.21 | ± | 1.04 | 12.18 | ± | 1.00 | 0.29 | 0.593 | 0.45 | 0.507 | 2.34 | 0.139 |
| “No Sound” Group | “Sound” Group | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | On Call | Neutral | On Call | Pre-Sleep Intention | Sound Group | Interaction | ||||||||||||
| Mean | ± | SEM | Mean | ± | SEM | Mean | ± | SEM | Mean | ± | SEM | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Left | ||||||||||||||||||
| Delta | 92.36 | ± | 0.89 | 92.53 | ± | 0.91 | 93.19 | ± | 0.60 | 93.53 | ± | 0.68 | 0.50 | 0.485 | 0.71 | 0.409 | 0.06 | 0.814 |
| Theta | 4.49 | 0.39 | 4.54 | 0.42 | 4.42 | 0.52 | 3.87 | 0.45 | 1.29 | 0.266 | 0.39 | 0.538 | 1.92 | 0.179 | ||||
| Alpha | 1.77 | ± | 0.18 | 1.75 | ± | 0.20 | 1.83 | ± | 0.21 | 1.73 | ± | 0.27 | 0.37 | 0.549 | 0.01 | 0.934 | 0.18 | 0.675 |
| Slow Spindles | 1.12 | ± | 0.20 | 0.94 | ± | 0.15 | 0.80 | ± | 0.10 | 0.77 | ± | 0.10 | 1.61 | 0.216 | 1.53 | 0.228 | 0.74 | 0.397 |
| Fast Spindles | 1.39 | ± | 0.23 | 1.35 | ± | 0.22 | 0.94 | ± | 0.10 | 0.88 | ± | 0.12 | 0.21 | 0.650 | 3.84 | 0.062 | 0.00 | 0.951 |
| Beta | 0.59 | ± | 0.08 | 0.60 | ± | 0.09 | 0.49 | ± | 0.06 | 0.48 | ± | 0.06 | 0.02 | 0.894 | 1.15 | 0.295 | 0.15 | 0.705 |
| Right | ||||||||||||||||||
| Delta | 92.50 | ± | 0.96 | 93.32 | ± | 0.83 | 92.88 | ± | 0.51 | 93.31 | ± | 0.59 | 1.73 | 0.201 | 0.03 | 0.856 | 0.16 | 0.691 |
| Theta | 4.50 | ± | 0.41 | 4.46 | ± | 0.43 | 0.49 | ± | 0.49 | 3.95 | ± | 0.42 | 1.65 | 0.211 | 0.22 | 0.647 | 1.14 | 0.297 |
| Alpha | 1.77 | ± | 0.19 | 1.72 | ± | 0.22 | 0.20 | ± | 0.20 | 1.74 | ± | 0.26 | 0.42 | 0.525 | 0.01 | 0.917 | 0.03 | 0.859 |
| Slow Spindles | 0.94 | ± | 0.16 | 0.92 | ± | 0.16 | 0.82 | ± | 0.09 | 0.79 | ± | 0.09 | 0.20 | 0.659 | 0.47 | 0.501 | 0.00 | 0.967 |
| Fast Spindles | 1.20 | ± | 0.23 | 1.12 | ± | 0.19 | 0.86 | ± | 0.08 | 0.81 | ± | 0.11 | 0.43 | 0.517 | 2.18 | 0.152 | 0.02 | 0.895 |
| Beta | 0.58 | ± | 0.09 | 0.57 | ± | 0.08 | 0.48 | ± | 0.05 | 0.48 | ± | 0.05 | 0.06 | 0.815 | 0.98 | 0.332 | 0.01 | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combertaldi, S.L.; Wick, A.Z.; Rasch, B. The Intention to React to Sounds Induces Sleep Disturbances and Alters Brain Responses to Sounds during Sleep: A Pilot Study. Clocks & Sleep 2022, 4, 561-576. https://doi.org/10.3390/clockssleep4040044
Combertaldi SL, Wick AZ, Rasch B. The Intention to React to Sounds Induces Sleep Disturbances and Alters Brain Responses to Sounds during Sleep: A Pilot Study. Clocks & Sleep. 2022; 4(4):561-576. https://doi.org/10.3390/clockssleep4040044
Chicago/Turabian StyleCombertaldi, Selina Ladina, Anna Zoé Wick, and Björn Rasch. 2022. "The Intention to React to Sounds Induces Sleep Disturbances and Alters Brain Responses to Sounds during Sleep: A Pilot Study" Clocks & Sleep 4, no. 4: 561-576. https://doi.org/10.3390/clockssleep4040044
APA StyleCombertaldi, S. L., Wick, A. Z., & Rasch, B. (2022). The Intention to React to Sounds Induces Sleep Disturbances and Alters Brain Responses to Sounds during Sleep: A Pilot Study. Clocks & Sleep, 4(4), 561-576. https://doi.org/10.3390/clockssleep4040044







