In Situ Growth of Cu2O-Coated Cu Aggregates on Wood and Bamboo for Efficient Mold Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
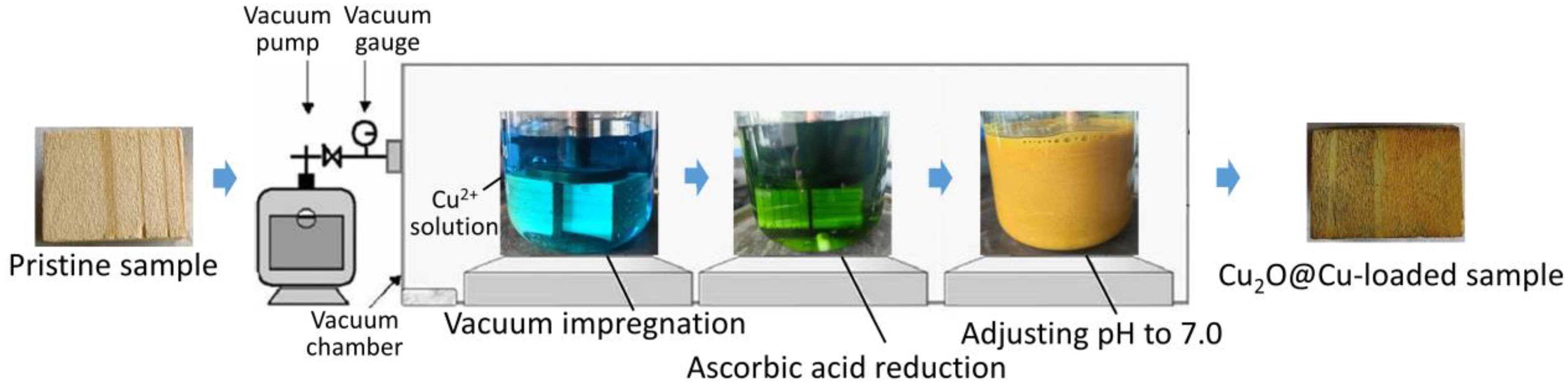
2.2. Preparation of Cu2O@Cu-Loaded Woods and Bamboo
2.3. Characterization
2.4. Mold Resistance Test of Cu2O/Cu-Loaded Woods and Bamboo
2.5. Leaching Evaluation of Cu2O@Cu-Loaded Woods and Bamboo
3. Results and Discussion
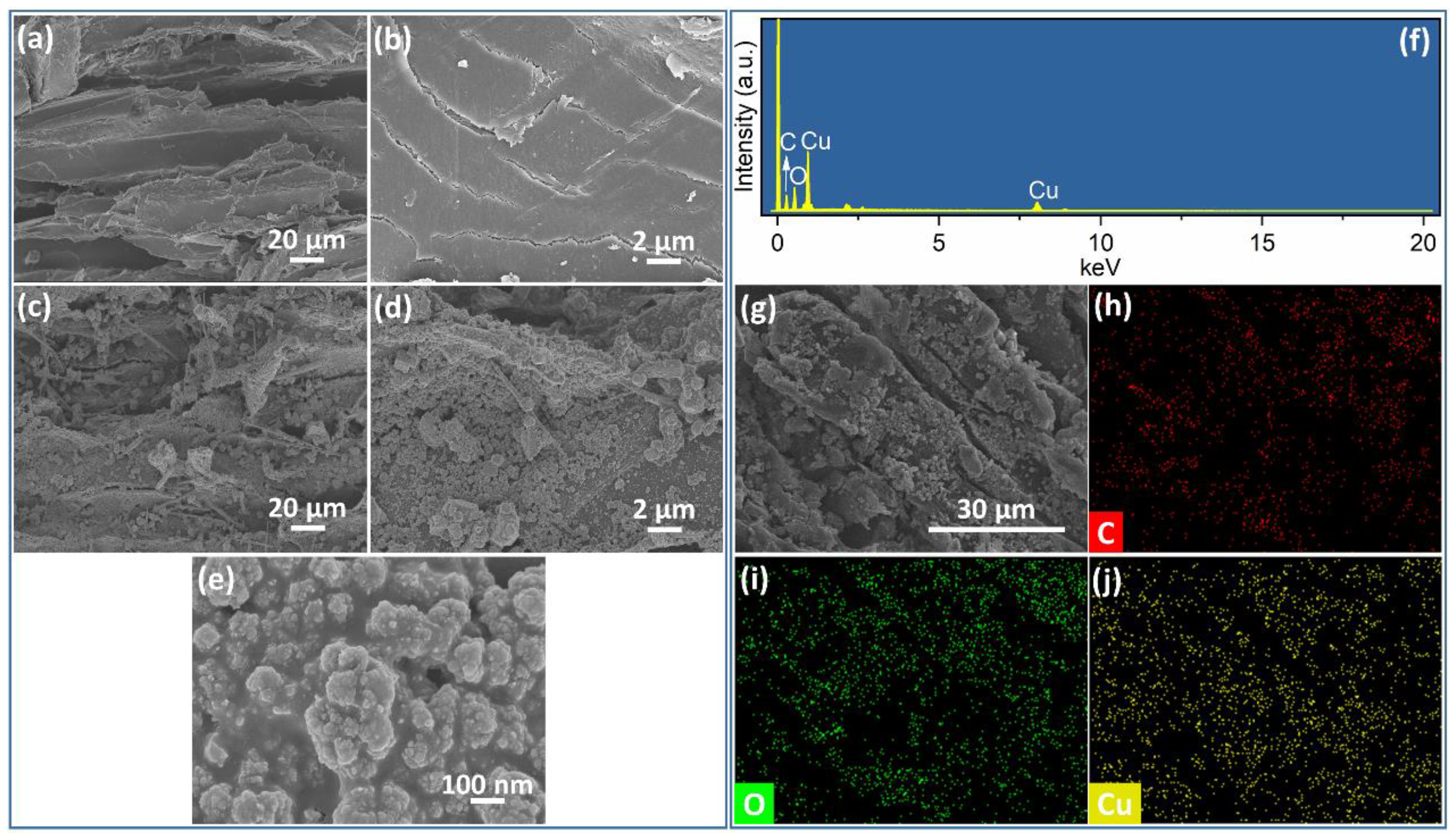
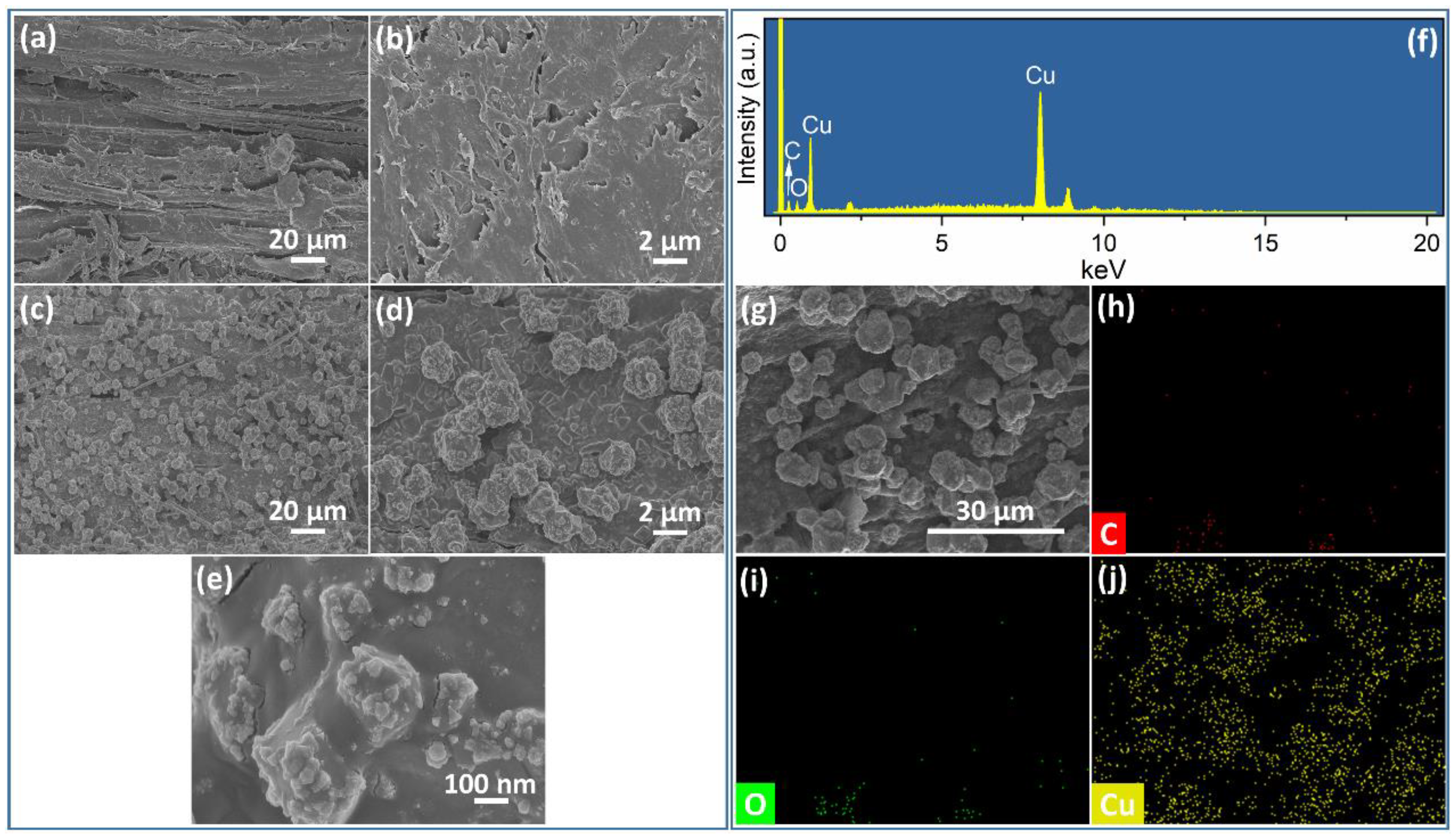
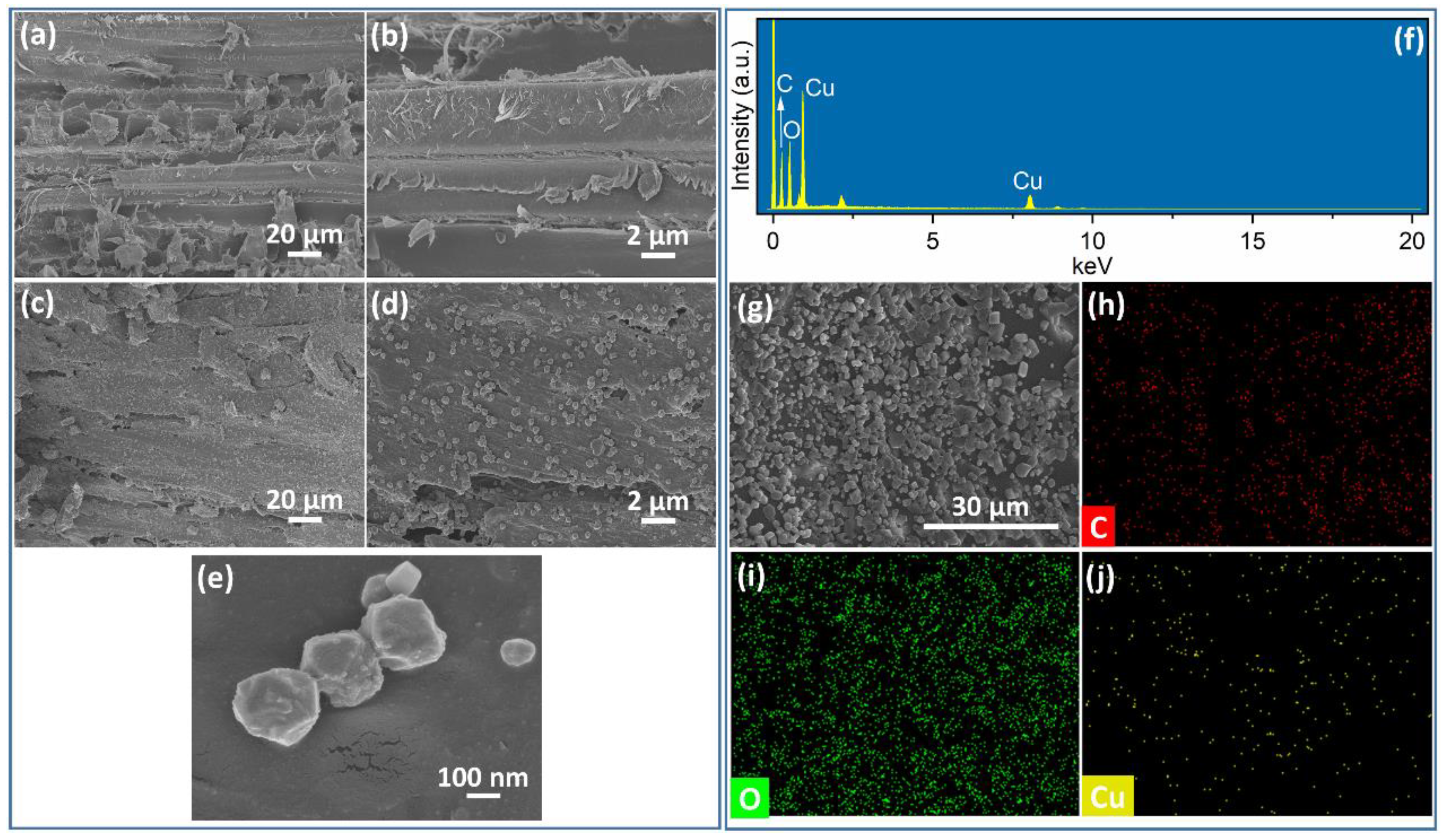
3.1. Characterization of Cu2O@Cu-Loaded Woods and Bamboo
3.2. Anti-Mold Evaluation of Four Cu2O@Cu-Loaded Woods and Bamboo
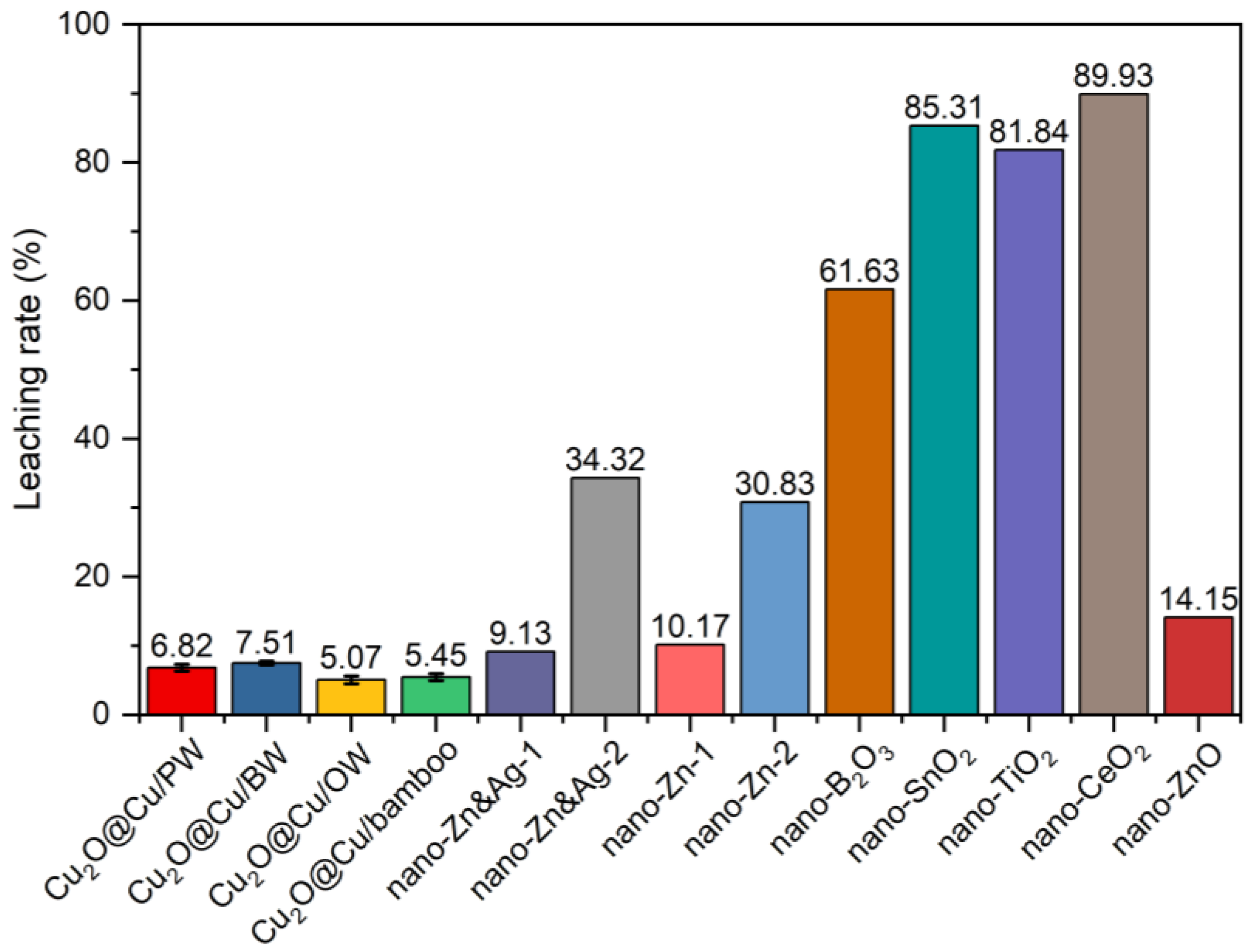
3.3. Leaching Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lao, W.; Hu, J.; Huang, Y.; Li, X. Environmental footprints of wood and bamboo reconstituted materials production in China: A cradle-to-gate life cycle assessment. Ind. Crops. Prod. 2025, 232, 121320. [Google Scholar] [CrossRef]
- Lima, L.; Trindade, E.; Alencar, L.; Alencar, M.; Silva, L. Sustainability in the construction industry: A systematic review of the literature. J. Clean. Prod. 2021, 289, 125730. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, J.; Li, H.; Wei, Y.; Xiong, Z.; Xu, X. Are bamboo construction materials environmentally friendly? A life cycle environmental impact analysis. Environ. Impact Assess. Rev. 2022, 96, 106853. [Google Scholar] [CrossRef]
- Schubert, M.; Panzarasa, G.; Burgert, I. Sustainability in wood products: A new perspective for handling natural diversity. Chem. Rev. 2023, 123, 1889–1924. [Google Scholar] [CrossRef]
- Gregory, S.A.; McGettigan, C.P.; McGuinness, E.K.; Rodin, D.M.; Yee, S.K.; Losego, M.D. Single-cycle atomic layer deposition on bulk wood lumber for managing moisture content, mold growth, and thermal conductivity. Langmuir 2020, 36, 1633–1641. [Google Scholar] [CrossRef]
- Julian, T.C.; Chen, Y.; Wang, Y.; Gao, W.; Sun, Q.; Fukuda, H. Revolutionizing wood construction: Densified integrated wood with voronoi structures. ACS Appl. Eng. Mater. 2023, 1, 3186–3193. [Google Scholar] [CrossRef]
- John, B.; Sahoo, S.K. Development of nanofree, silane-free, and fluorine-free cardanol-based multifunctional, superhydrophobic wood coating. ACS Appl. Polym. Mater. 2024, 6, 9226–9237. [Google Scholar] [CrossRef]
- Ou, J.; Zhao, G.; Wang, F.; Li, W.; Lei, S.; Fang, X.; Siddiqui, A.R.; Xia, Y.; Amirfazli, A. Durable superhydrophobic wood via one-step immersion in composite silane solution. ACS Omega 2021, 6, 7266–7274. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Costas, C.; Palanti, S.; Charpentier, J.P.; Sanromán, M.Á.; Moldes, D. A sustainable treatment for wood preservation: Enzymatic grafting of wood extractives. ACS Sustain. Chem. Eng. 2017, 5, 7557–7567. [Google Scholar] [CrossRef]
- Kumar, R.; Ray, S.; Pandey, K.K.; Muthukumar, A. Potential use of Pterocarpus santalinus bark extracts as UV-protective coats on Hevea brasiliensis wood. ACS Sustain. Chem. Eng. 2023, 11, 9965–9978. [Google Scholar] [CrossRef]
- Chang, M.; Shen, P.; Wang, L.; Ma, Q.; Jia, Z.; Hu, C.; Zhang, X. Research progress of eco-friendly plant-derived biomass-based wood adhesives: A review. Ind. Crop. Prod. 2024, 222, 120093. [Google Scholar] [CrossRef]
- Piao, X.; Zhao, Z.; Guo, H.; Wang, Z.; Jin, C. Improved properties of bamboo by thermal treatment with wood wax oil. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128807. [Google Scholar] [CrossRef]
- Li, X.; Ji, S.; Li, T.; Liu, Z.; Hao, X.; Chen, Z.; Zhong, Y.; Li, X. The physical, mechanical and fire performance of bamboo scrimber processed with thermal-treated bamboo bundles. Ind. Crops. Prod. 2023, 205, 117549. [Google Scholar] [CrossRef]
- Paris, M.J.; Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Postharvest mold growth control in raspberries and blackberries using cinnamon essential oil-loaded alginate beads. ACS Food Sci. Technol. 2025, 5, 127–136. [Google Scholar] [CrossRef]
- Rahmawati, N.; Sumardi, I.; Dungani, R. Isolation and identification of fungi inhabiting rubber impregnated wood, and their role of quality changing the impregnated wood. BioResources 2020, 15, 2839–2849. [Google Scholar] [CrossRef]
- Chaturvedi, K.S.; Henderson, J.P. Pathogenic adaptations to host-derived antibacterial copper. Front. Cell. Infect. Microbiol. 2014, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.C.; Hsiao, C.-D. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Latorre, M.; Troncoso, R.; Uauy, R. Biological Aspects of Copper. In Clinical and Translational Perspectives on Wilson Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 25–31. [Google Scholar]
- Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial properties of copper oxide nanoparticles (review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Baimler, I.V.; Uvarov, O.V.; Smirnova, V.V.; Volkov, M.Y.; Semenova, A.A.; Lisitsyn, A.B. Influence of the concentration of Fe and Cu nanoparticles on the dynamics of the size distribution of nanoparticles. Front. Phys. 2020, 8, 622551. [Google Scholar] [CrossRef]
- Nataraja, S.T.; Vishnu, R.; Anoop, E.V.; Chathoth, A.M.; Valasseri, J.; Babu, S. Zinc and copper nanoparticles for coconut wood preservation. ACS Appl. Nano Mater. 2025, 8, 4946–4957. [Google Scholar] [CrossRef]
- Xie, F.; Pu, Y.; Liang, Y.; Qiu, J.; Zheng, K.; Gao, J. Preparation of highly stable wood with fast curing, ultraviolet, and mildew resistance through copper-montmorillonite nanoparticles hybridized with tung oil. ACS Appl. Mater. Interfaces 2025, 17, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.D.S.M.; Sentena, N.Z.; Sangoi, G.G.; Vizzotto, B.S.; Pinto, E.O.; Pavoski, G.; Espinosa, D.C.R.; Machado, A.K.; Silva, W.L. Copper nanoparticles from acid ascorbic: Biosynthesis, characterization, in vitro safety profile and antimicrobial activity. Mater. Chem. Phys. 2023, 307, 128110. [Google Scholar] [CrossRef]
- Pathak, R.; Punetha, V.D.; Bhatt, S.; Punetha, M. A review on copper-based nanoparticles as a catalyst: Synthesis and applications in coupling reactions. J. Mater. Sci. 2024, 59, 6169–6205. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Khan, M.Q.; Ullah, A.; Bie, X.; Taguchi, G.; Kim, I.S. Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria. Mater. Today Commun. 2020, 25, 101377. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, H.; Nie, C.; Xiao, Y.; Zeng, Q.; Wang, G.; Wang, B.; Lv, H.; Li, Q.; Chen, S. Size-controlled synthesis of Cu2O nanoparticles: Size effect on antibacterial activity and application as a photocatalyst for highly efficient H2O2 evolution. RSC Adv. 2017, 7, 51822–51830. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, X.; Wei, L.; Luo, H.; Liu, Y.; Dong, X.; Yang, D.; Li, Y. Nano-AgCu alloy on wood surface for mold resistance. Nanomaterials 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, H.D.; Bellah, S.M.; Malekfar, R. Surface-enhanced Raman scattering studies of Cu/Cu2O core-shell NPs obtained by laser ablation. Spectrochim. Acta A 2019, 223, 117379. [Google Scholar] [CrossRef]
- Ye, M.; Shao, T.; Liu, J.; Li, C.; Song, B.; Liu, S. Phase engineering of Cu@Cu2O core-shell nanospheres for boosting tandem electrochemical CO2 reduction to C2+ products. Appl. Surf. Sci. 2023, 622, 156981. [Google Scholar] [CrossRef]
- He, S.; Xu, J.; Wu, Z.; Bao, Y.; Yu, H.; Chen, Y. Compare of porous structure of meso bamboo and Pinus sylvestris L. lumber. J. Nanjing For. Univ. 2017, 41, 157–162. [Google Scholar]
- Vitas, S.; Segmehl, J.S.; Burgert, I.; Cabane, E. Porosity and pore size distribution of native and delignified beech wood determined by mercury intrusion porosimetry. Materials 2019, 12, 416. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, H.; Chen, J.; Chen, W.; Zhao, J. Pore structure characterization of Oak via X-ray computed temography. BioResources 2020, 15, 3053–3063. [Google Scholar] [CrossRef]
- Lee, S.; Jang, J.W.; Ryu, Y.B. Surface oxidation of Cu2O nanoparticles by adsorbed ammonia. Nanomaterials 2022, 12, 4242. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Z.; Cao, Y.; Hu, Y.; Li, Y.; Zhang, L.; Cao, X.; Wen, H.; Zhang, Y.; Lv, H.; et al. A Self-amplifying ROS-responsive nanoplatform for simultaneous cuproptosis and cancer immunotherapy. Adv. Sci. 2024, 11, 2401047. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Balasubramanian, S.; Perumal, E. Copper oxide nanoparticles induced reactive oxygen species generation: A systematic review and meta-analysis. Chem. Biol. Interact. 2025, 405, 111311. [Google Scholar] [CrossRef]
- Duan, X.; Peng, D.; Zhang, Y.; Chen, Z.; Huang, Y. Micromolar concentration of copper (ii) ions enhances proliferation in human skin keratinocyte through appropriating generation of reactive oxygen species. J. Army Med. Univ. 2015, 37, 1053–1058. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; ALNadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.N.; Yılgör, N.; Rautkari, L.; Yoshimura, T. Role of various nano-particles in prevention of fungal decay, mold growth and termite attack in wood, and their effect on weathering properties and water repellency. Int. Biodeterior. Biodegrad. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Clausen, C.A.; Kartal, S.N.; Arango, R.A.; Green, F., III. The role of particle size of particulate nano-zinc oxide wood preservatives on termite mortality and leach resistance. Nanoscale Res. Lett. 2011, 6, 427. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F., III.; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Tang, C.; Qin, X.; Huang, W.; Debi, S.; Zhang, Z.; Guo, J.; Liu, W.; Mo, J. Environmental sources, fates, toxicological effects, and health risks of copper pyrithione: An overview. Front. Environ. Sci. Eng. 2024, 18, 132. [Google Scholar] [CrossRef]
- Muhcu, D.; Terzi, E.; Kartal, S.N.; Yoshimura, T. Biological performance, water absorption, and swelling of wood treated with nanoparticles combined with the application of Paraloid B72. J. For. Res. 2017, 28, 381–394. [Google Scholar] [CrossRef]
- Lankone, R.S.; Ruggiero, E.; Goodwin, D.G., Jr.; Vilsmeier, K.; Mueller, P.; Pulbere, S.; Challis, K.; Bi, Y.; Westerhoff, P.; Ranville, J.; et al. Evaluating performance, degradation, and release behavior of a nanoform pigmented coating after natural and accelerated weathering. NanoImpact 2020, 17, 100199. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Oviedo, C.; Reyes, L.; Navarrete, J.; Gómez, L.; Torres, H.; Gaviño, G.; Trollund, E. Radiata pine wood treated with copper nanoparticles: Leaching analysis and fungal degradation. Forests 2021, 12, 1606. [Google Scholar] [CrossRef]
- Mantanis, G.; Terzi, E.; Kartal, S.N.; Papadopoulos, A.N. Evaluation of mold, decay and termite resistance of pine wood treated with zinc- and copper-based nanocompounds. Int. Biodeter. Biodegrad. 2014, 90, 140–144. [Google Scholar] [CrossRef]
- Harandi, D.; Walsh-Korb, Z.; Moradienayat, M. CNCs and CeO2 as organic–inorganic additives to enhance HPC bio-polymer wood coatings against photochemical degradation. J. Coat. Technol. Res. 2025, 22, 691–701. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Wu, Q. Antifungal capability of TiO2 coated film on moist wood. Build. Environ. 2009, 44, 1088–1093. [Google Scholar] [CrossRef]


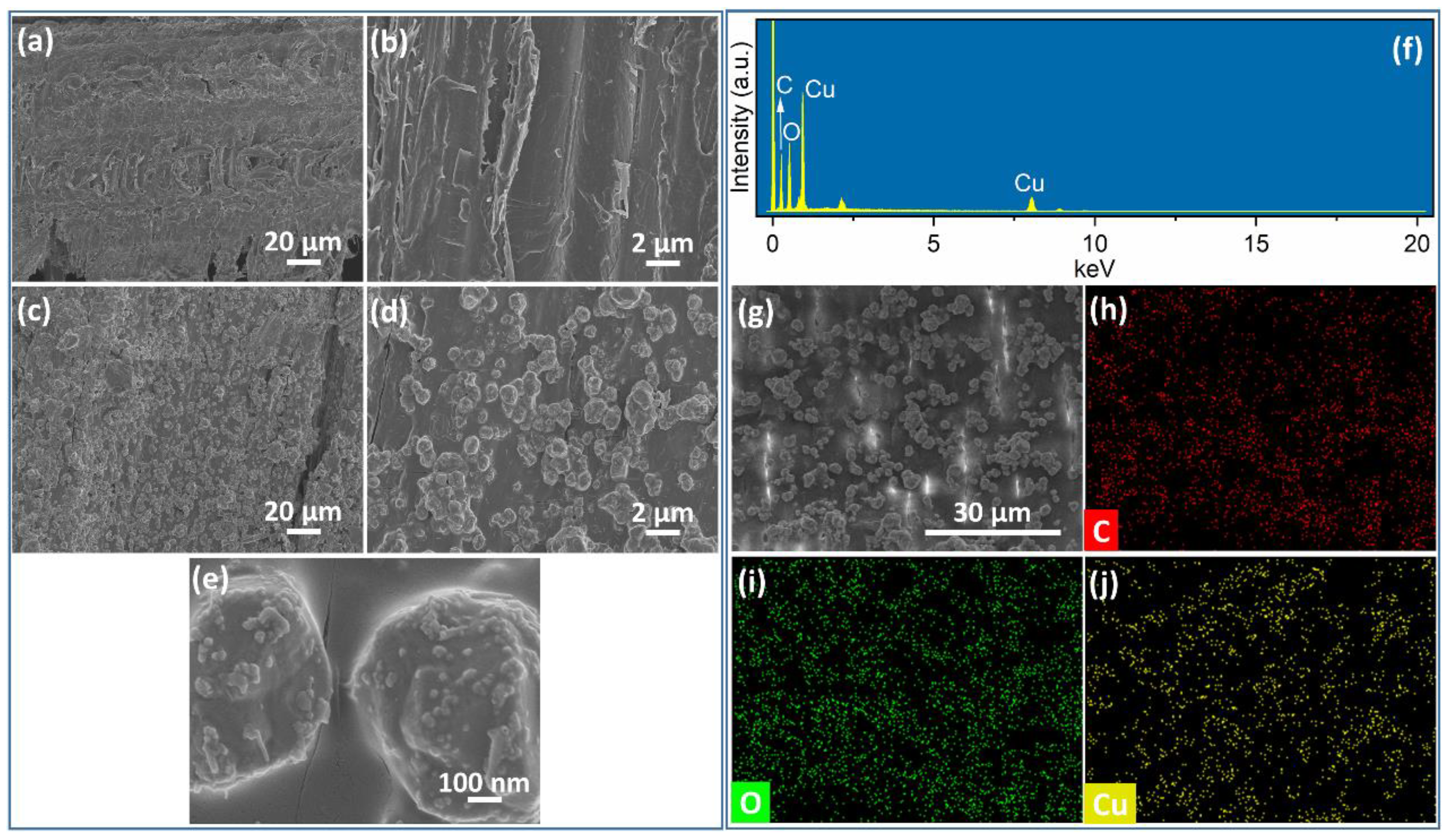

| Sample | C (at. %) | O (at. %) | Cu (at. %) |
|---|---|---|---|
| PW | 63.9 | 36.1 | - |
| BW | 64.8 | 35.2 | - |
| OW | 61.5 | 38.5 | - |
| Bamboo | 62.7 | 37.3 | - |
| Cu2O@Cu/PW | 50.8 | 29.1 | 20.1 |
| Cu2O@Cu/BW | 59.2 | 13.7 | 27.1 |
| Cu2O@Cu/OW | 49.1 | 38.3 | 12.6 |
| Cu2O@Cu/bamboo | 55.2 | 31.8 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Zhang, F.; Chen, M. In Situ Growth of Cu2O-Coated Cu Aggregates on Wood and Bamboo for Efficient Mold Resistance. Surfaces 2025, 8, 66. https://doi.org/10.3390/surfaces8030066
Zhou D, Zhang F, Chen M. In Situ Growth of Cu2O-Coated Cu Aggregates on Wood and Bamboo for Efficient Mold Resistance. Surfaces. 2025; 8(3):66. https://doi.org/10.3390/surfaces8030066
Chicago/Turabian StyleZhou, Dayong, Fuhua Zhang, and Mingli Chen. 2025. "In Situ Growth of Cu2O-Coated Cu Aggregates on Wood and Bamboo for Efficient Mold Resistance" Surfaces 8, no. 3: 66. https://doi.org/10.3390/surfaces8030066
APA StyleZhou, D., Zhang, F., & Chen, M. (2025). In Situ Growth of Cu2O-Coated Cu Aggregates on Wood and Bamboo for Efficient Mold Resistance. Surfaces, 8(3), 66. https://doi.org/10.3390/surfaces8030066





