Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst
Abstract
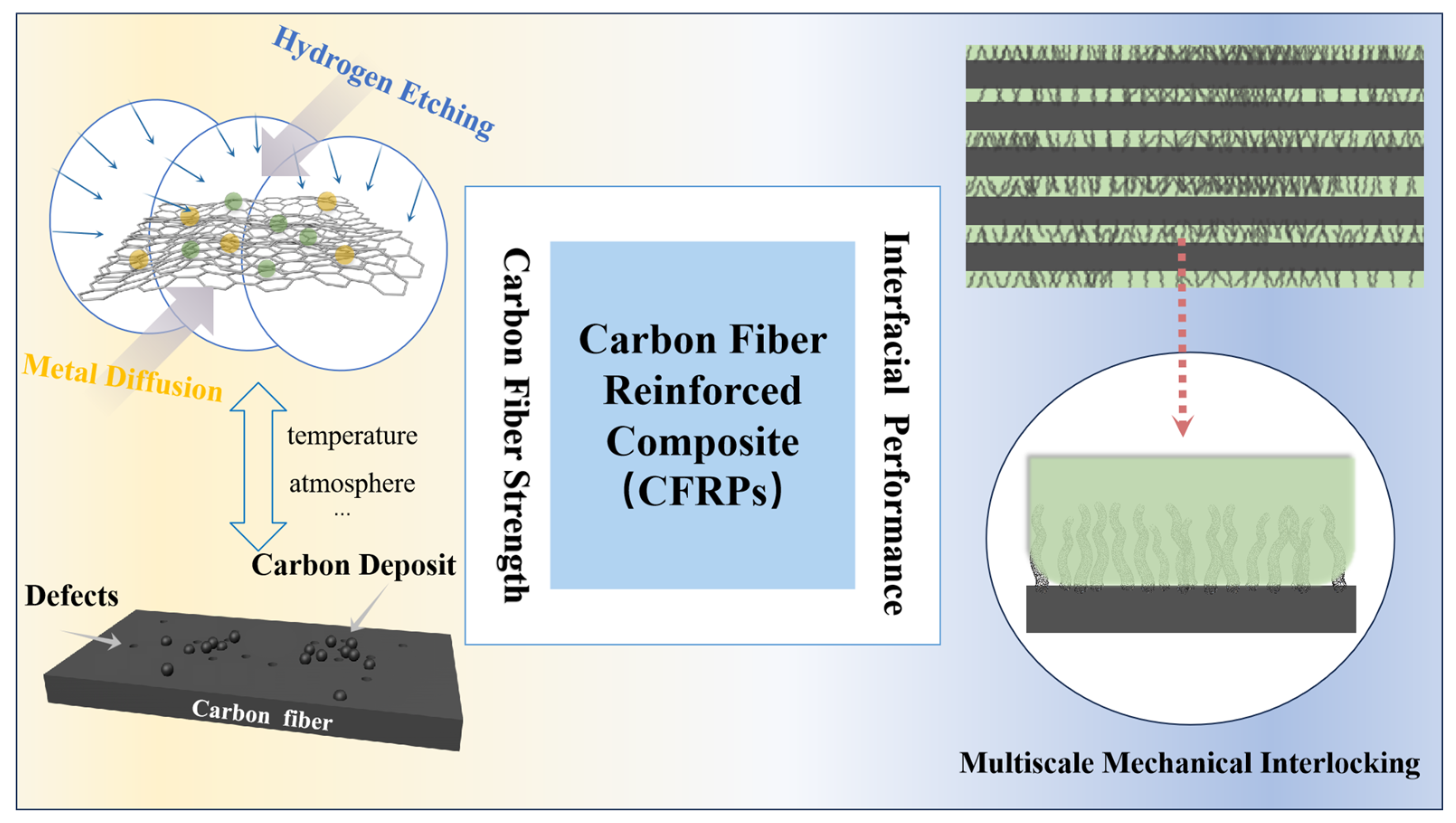
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Continuous Preparation Process of CNTs/CF
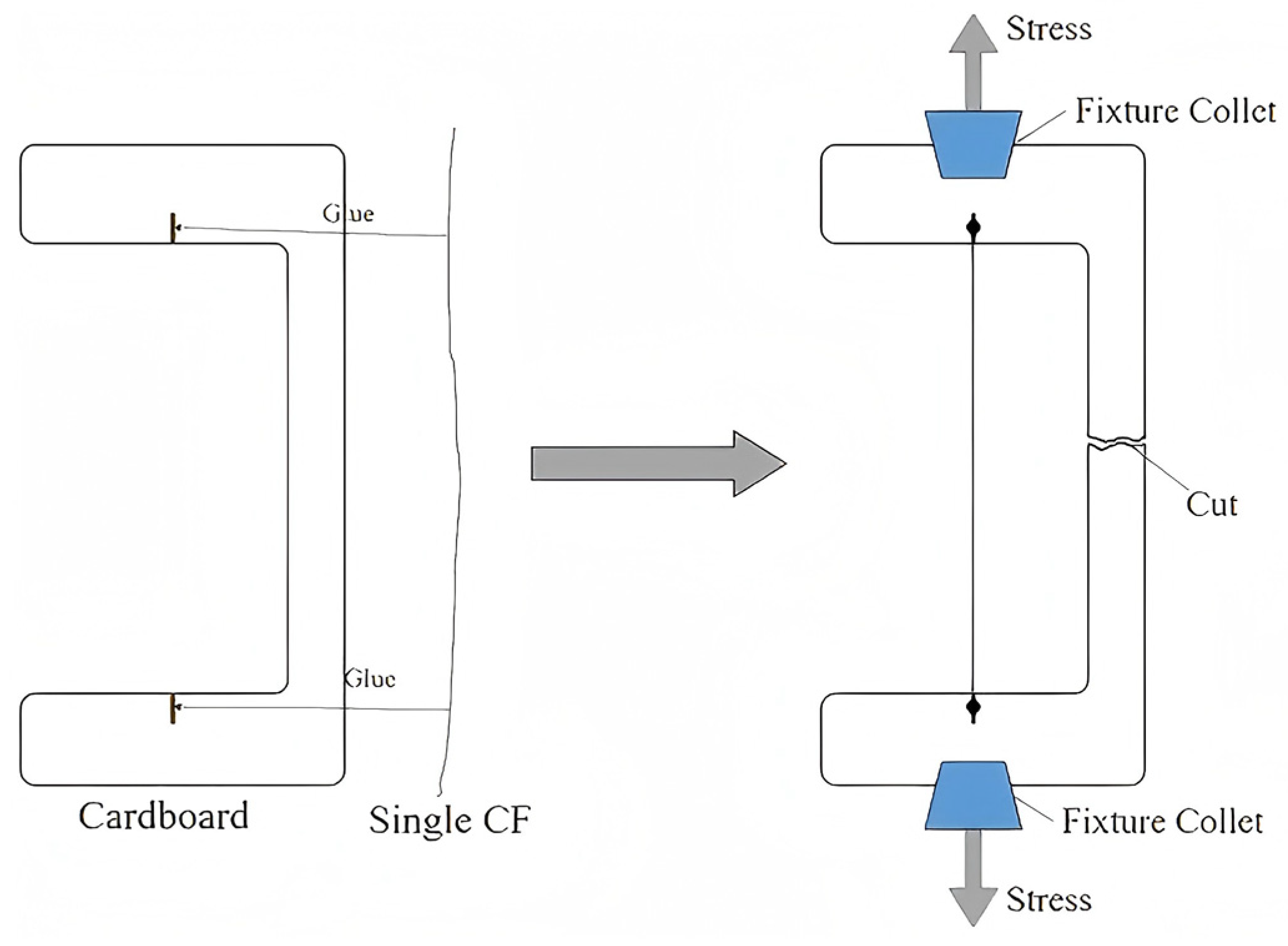
2.3. Characterizations and Testing
3. Results and Discussion
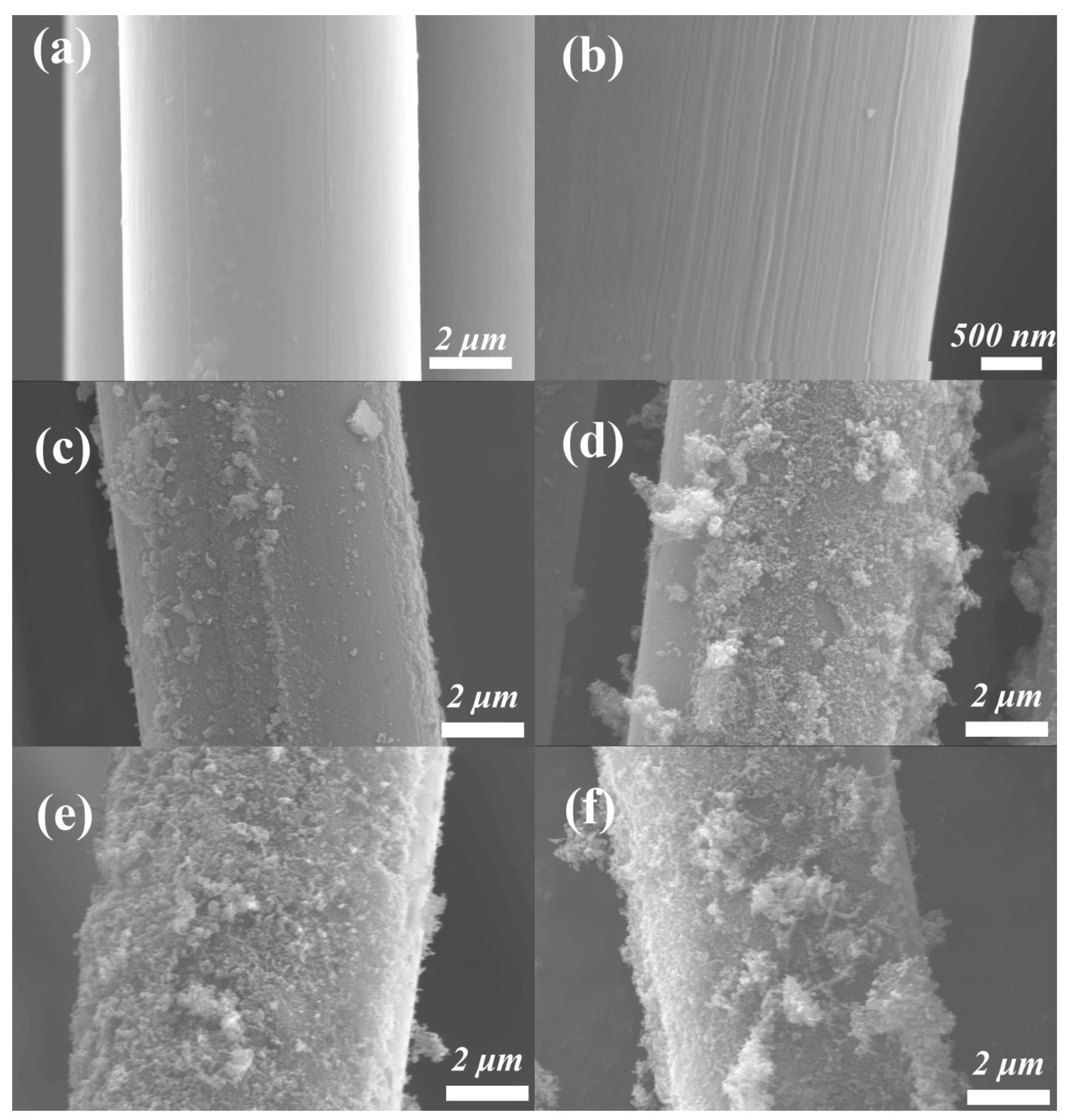
3.1. Influence of Temperature on the Continuous Preparation of CNTs/CF
3.1.1. Morphological Properties
3.1.2. Raman Measurements at Different Growth Temperatures
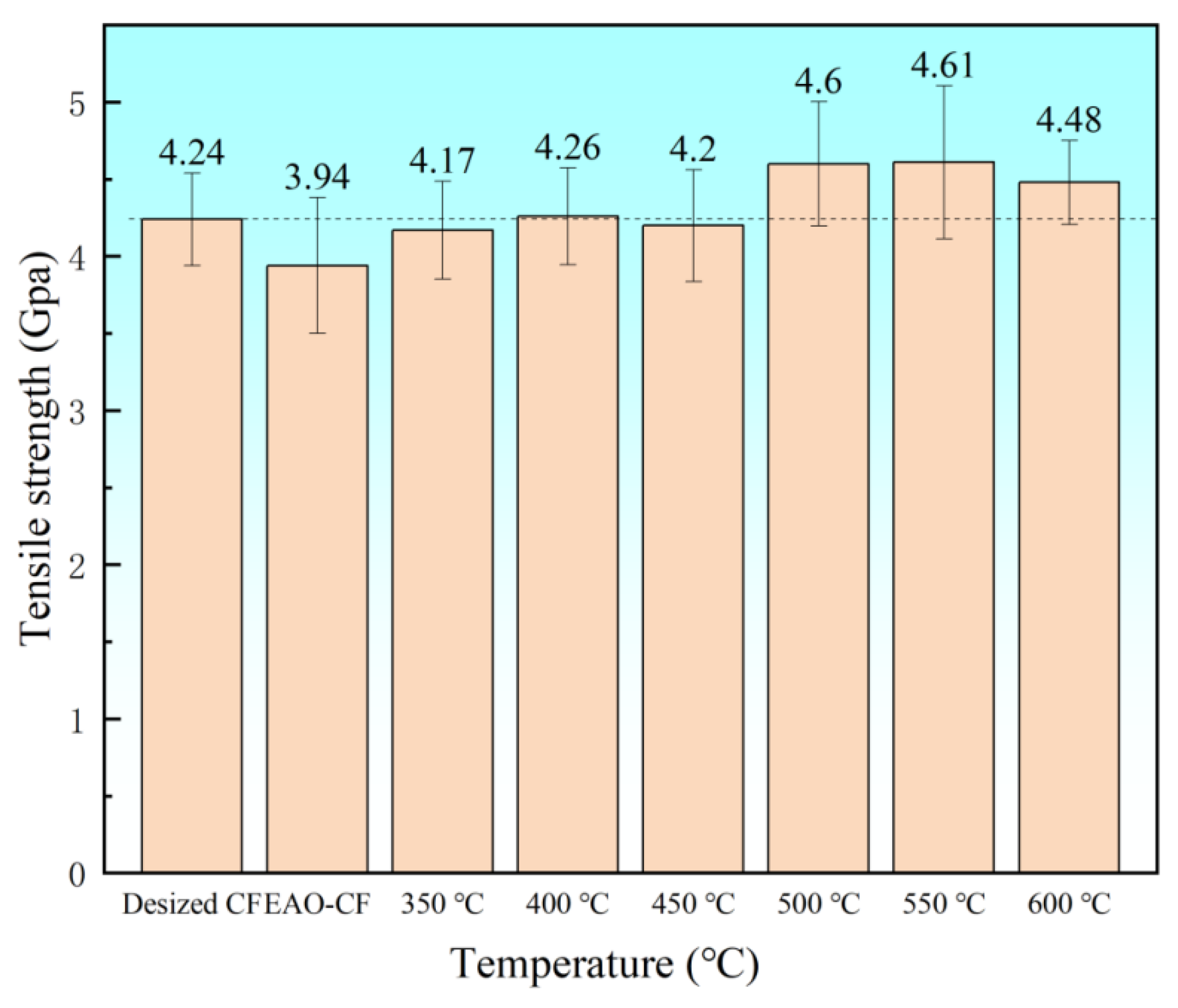
3.1.3. Test of Monofilament Tensile Strength at Different Growth Temperatures
3.2. Influence of H2 Flow Rate on the Continuous Preparation of CNTs/CF
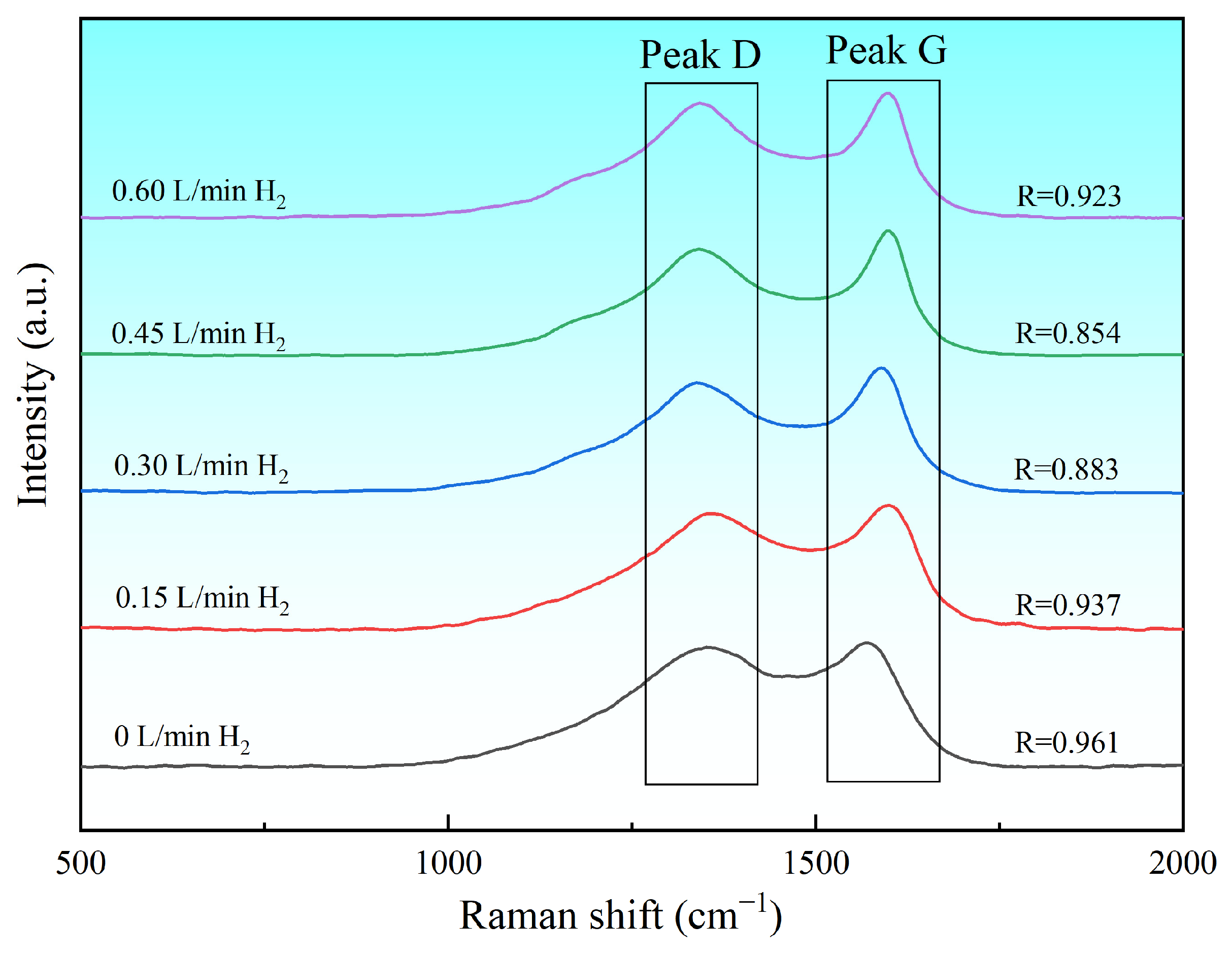
3.2.1. Raman Measurements at Different H2 Flow
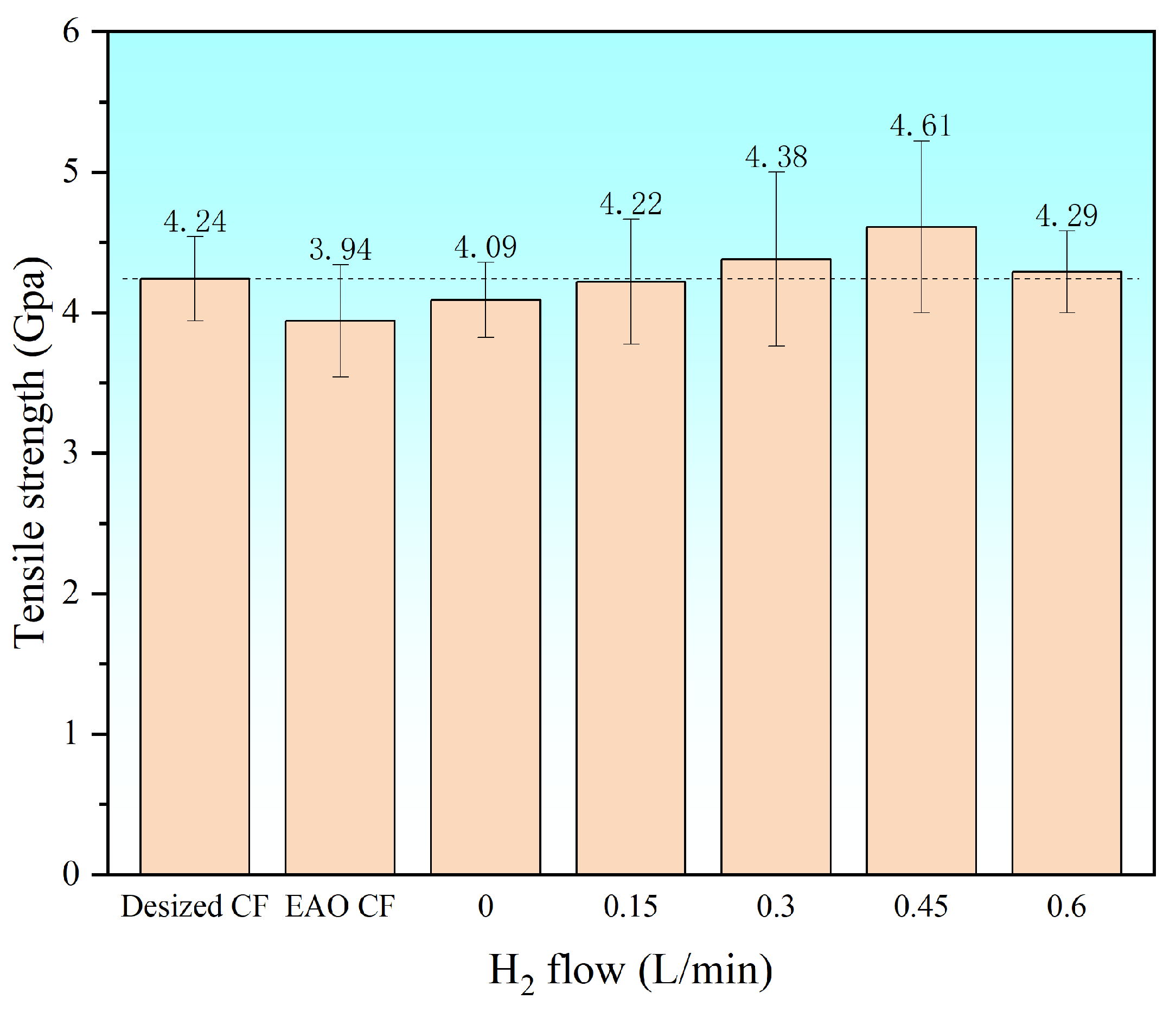
3.2.2. Test of Monofilament Tensile Strength at Different H2 Flow
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; Song, G.; Li, P.; Li, X.; Pan, D.; Huang, Y.; Ma, L.; Guo, Z. Enhancing interfacial performance of epoxy resin composites via in-situ nucleophilic addition polymerization modification of carbon fibers with hyperbranched polyimidazole. Compos. Sci. Technol. 2021, 201, 108522. [Google Scholar] [CrossRef]
- Wu, D.; Yao, Z.; Sun, X.; Liu, X.; Liu, L.; Zhang, R.; Wang, C. Mussel-tailored carbon fiber/carbon nanotubes interface for elevated interfacial properties of carbon fiber/epoxy composites. Chem. Eng. J. 2022, 429, 132449. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, W.; Li, B.; Zhu, J.; Wang, C.; Song, G.; Wu, G.; Yang, X.; Huang, Y.; Ma, L. Recent advances of interphases in carbon fiber-reinforced polymer composites: A review. Compos. Part B Eng. 2022, 233, 109639. [Google Scholar] [CrossRef]
- He, M.; Xu, P.; Zhang, Y.; Liu, K.; Yang, X. Phthalocyanine nanowires@GO/carbon fiber composites with enhanced interfacial properties and electromagnetic interference shielding performance. Chem. Eng. J. 2020, 388, 124255. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, Y.; Feng, P.; Song, G.; Huang, Y.; Liu, H.; Zhang, J.; Fan, J.; Hou, H.; Guo, Z. Reinforcing carbon fiber epoxy composites with triazine derivatives functionalized graphene oxide modified sizing agent. Compos. Part B Eng. 2019, 176, 107078. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, L.; Ma, L.; Ma, L.; Yang, X.; Huang, Y.; Ryu, J.E.; Shankar, A.; Li, T.; Yan, C.; et al. Layer-by-layer grafting CNTs onto carbon fibers surface for enhancing the interfacial properties of epoxy resin composites. Compos. Sci. Technol. 2018, 154, 28–36. [Google Scholar] [CrossRef]
- Lee, S.-B.; Choi, O.; Lee, W.; Yi, J.-W.; Kim, B.-S.; Byun, J.-H.; Yoon, M.-K.; Fong, H.; Thostenson, E.T.; Chou, T.-W. Processing and characterization of multi-scale hybrid composites reinforced with nanoscale carbon reinforcements and carbon fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 337–344. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Liu, J.; Huang, J.; Ji, J.; Wang, T.; Li, T. Enhancing Mechanical Properties of CF/Al Composites through Multi-scale Interfacial Strengthening by Introducing Carbon Nanotube-grafted Carbon Fibers. Surf. Interfaces 2025, 64, 106440. [Google Scholar] [CrossRef]
- Quan, G.; Wu, Y.; Li, W.; Li, D.; Liu, X.; Wang, K.; Dai, S.; Xiao, L.; Ao, Y. Construction of cellulose nanofiber/carbon nanotube synergistic network on carbon fiber surface to enhance mechanical properties and thermal conductivity of composites. Compos. Sci. Technol. 2024, 248, 110454. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Mathur, R.B.; Chatterjee, S.; Singh, B.P. Growth of carbon nanotubes on carbon fibre substrates to produce hybrid/phenolic composites with improved mechanical properties. Compos. Sci. Technol. 2008, 68, 1608–1615. [Google Scholar] [CrossRef]
- Tomie, T.; Inoue, S.; Kohno, M.; Matsumura, Y. Prospective growth region for chemical vapor deposition synthesis of carbon nanotube on C-H-O ternary diagram. Diam. Relat. Mater. 2010, 19, 1401–1404. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Qin, J.; Wang, X.; Lu, R.; Qu, C.; Wang, C. Scalable manufacturing of carbon nanotubes on continuous carbon fibers surface from chemical vapor deposition. Vacuum 2018, 152, 84–90. [Google Scholar] [CrossRef]
- De Greef, N.; Zhang, L.; Magrez, A.; Forro, L.; Locquet, J.-P.; Verpoest, I.; Seo, J.W. Direct growth of carbon nanotubes on carbon fibers: Effect of the CVD parameters on the degradation of mechanical properties of carbon fibers. Diam. Relat. Mater. 2015, 51, 39–48. [Google Scholar] [CrossRef]
- Kim, K.J.; Yu, W.-R.; Youk, J.H.; Lee, J. Degradation and Healing Mechanisms of Carbon Fibers during the Catalytic Growth of Carbon Nanotubes on Their Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, C.; Yao, Z.; Ma, Z.; Cui, X.; Gao, Q.; Wang, Y.; Wang, Q.; Wei, H. Mechanical property deterioration and defect repair factors of carbon fibers during the continuous growth of carbon nanotubes by chemical vapor deposition. Ceram. Int. 2021, 47, 19213–19219. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Sager, R.; Dai, L.; Baur, J. Hierarchical composites of carbon nanotubes on carbon fiber: Influence of growth condition on fiber tensile properties. Compos. Sci. Technol. 2009, 69, 594–601. [Google Scholar] [CrossRef]
- Liu, L.; Jia, C.; He, J.; Zhao, F.; Fan, D.; Xing, L.; Wang, M.; Wang, F.; Jiang, Z.; Huang, Y. Interfacial characterization, control and modification of carbon fiber reinforced polymer composites. Compos. Sci. Technol. 2015, 121, 56–72. [Google Scholar] [CrossRef]
- Khavrus, V.O.; Lemesh, N.V.; Gordijchuk, S.V.; Tripolsky, A.I.; Ivashchenko, T.S.; Biliy, M.M.; Strizhak, P.E. Chemical catalytic vapor deposition (CCVD) synthesis of carbon nanotubes by decomposition of ethylene on metal (Ni, Co, Fe) nanoparticles. React. Kinet. Catal. Lett. 2008, 93, 295–303. [Google Scholar] [CrossRef]
- Cui, B.; Wang, C.; Wang, Y.; Wang, C.; Jiang, H.; Li, M.; Xu, Z. In-situ growth of bamboo-like carbon nanotubes from Cu catalyst on continuous carbon fibre for interfacial enhancement. Compos. Sci. Technol. 2023, 234, 109933. [Google Scholar] [CrossRef]
- Chen, Y.; Ciuparu, D.; Lim, S.Y.; Yang, Y.H.; Haller, G.L.; Pfefferle, L. Synthesis of uniform diameter single-wall carbon nanotubes in Co-MCM-41: Effects of the catalyst prereduction and nanotube growth temperatures. J. Catal. 2004, 225, 453–465. [Google Scholar] [CrossRef]
- Saputri, D.D.; Jan’ah, A.M.; Saraswati, T.E. Synthesis of Carbon Nanotubes (CNT) by Chemical Vapor Deposition (CVD) Using a Biogas-Based Carbon Precursor: A Review, Proceedings of the 15th Joint Conference on Chemistry (JCC), Salatiga, Indonesia, 9 September 2020; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Chiang, W.-H.; Sakr, M.; Gao, X.P.A.; Sankaran, R.M. Nanoengineering NixFe1−x Catalysts for Gas-Phase, Selective Synthesis of Semiconducting Single-Walled Carbon Nanotubes. ACS Nano 2009, 3, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Liao, Y.; Hussain, A.; Zhang, Q.; Ding, E.-X.; Jiang, H.; Kauppinen, E.I. Systematic investigation of the catalyst composition effects on single-walled carbon nanotubes synthesis in floating-catalyst CVD. Carbon 2019, 149, 318–327. [Google Scholar] [CrossRef]
- Cui, B.; Wang, C.; Wang, Y.; Wang, C.; Jiang, H.; Li, M.; Tan, H.; Xu, Z. One-step scheme in continuous carbon nanofilament-carbon fiber multi-scale reinforcement fabrication at ultra-low temperature with simultaneous tensile and interfacial improvement. Chem. Eng. J. 2023, 478, 147447. [Google Scholar] [CrossRef]
- Lee, G.; Youk, J.H.; Lee, J.; Sul, I.H.; Yu, W.-R. Low-temperature grafting of carbon nanotubes on carbon fibers using a bimetallic floating catalyst. Diam. Relat. Mater. 2016, 68, 118–126. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C.; Qin, J.; Su, S.; Wang, Y.; Wang, Q.; Yu, M.; Wei, H. Interfacial improvement of carbon fiber/epoxy composites using one-step method for grafting carbon nanotubes on the fibers at ultra-low temperatures. Carbon 2020, 164, 133–142. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, Y.; Yan, C.; Liu, D.; Xu, H. Electrochemically oxidized carbon fiber surfaces: Mechanism-driven models for enhanced surface engineering. Mater. Today Chem. 2025, 46, 102708. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, P.; Liu, Z.; Sun, M.; Liu, H.; Sun, J.; Li, B.; Zhao, Y.; Bao, J. Effect of electrochemical anodic oxidation modification on the interfacial properties of carbon fiber reinforced polyimide composites. Polym. Compos. 2025, 46, 2390–2403. [Google Scholar] [CrossRef]
- Gakis, G.P.; Termine, S.; Trompeta, A.-F.A.; Aviziotis, I.G.; Charitidis, C.A. Unraveling the mechanisms of carbon nanotube growth by chemical vapor deposition. Chem. Eng. J. 2022, 445, 136807. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of carbon nanotube array growth kinetics during chemical vapor deposition. Appl. Phys. A Mater. Sci. Process. 2005, 81, 223–240. [Google Scholar] [CrossRef]
- Ma, Y.; Dichiara, A.B.; He, D.; Zimmer, L.; Bai, J. Control of product nature and morphology by adjusting the hydrogen content in a continuous chemical vapor deposition process for carbon nanotube synthesis. Carbon 2016, 107, 171–179. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, Y.; Zhang, J.; Guo, J.; Li, Y.; Xin, S.; Xu, Z.; Yuan, Y.; Zhang, D. Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst. Surfaces 2025, 8, 60. https://doi.org/10.3390/surfaces8030060
Zhu Y, Wang Y, Zhang J, Guo J, Li Y, Xin S, Xu Z, Yuan Y, Zhang D. Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst. Surfaces. 2025; 8(3):60. https://doi.org/10.3390/surfaces8030060
Chicago/Turabian StyleZhu, Yanying, Yanxiang Wang, Jianwei Zhang, Jinghe Guo, Yingfan Li, Siao Xin, Ziyi Xu, Yanru Yuan, and Dong Zhang. 2025. "Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst" Surfaces 8, no. 3: 60. https://doi.org/10.3390/surfaces8030060
APA StyleZhu, Y., Wang, Y., Zhang, J., Guo, J., Li, Y., Xin, S., Xu, Z., Yuan, Y., & Zhang, D. (2025). Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst. Surfaces, 8(3), 60. https://doi.org/10.3390/surfaces8030060






