Layered Structures Based on Ga2O3/GaS0.98Se0.02 for Gas Sensor Applications
Abstract
1. Introduction
2. Materials and Methods
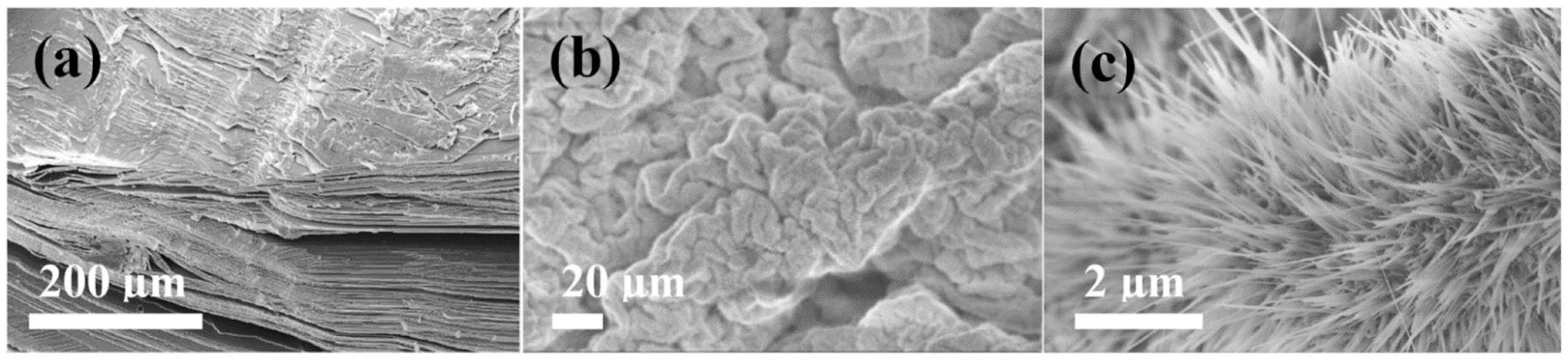
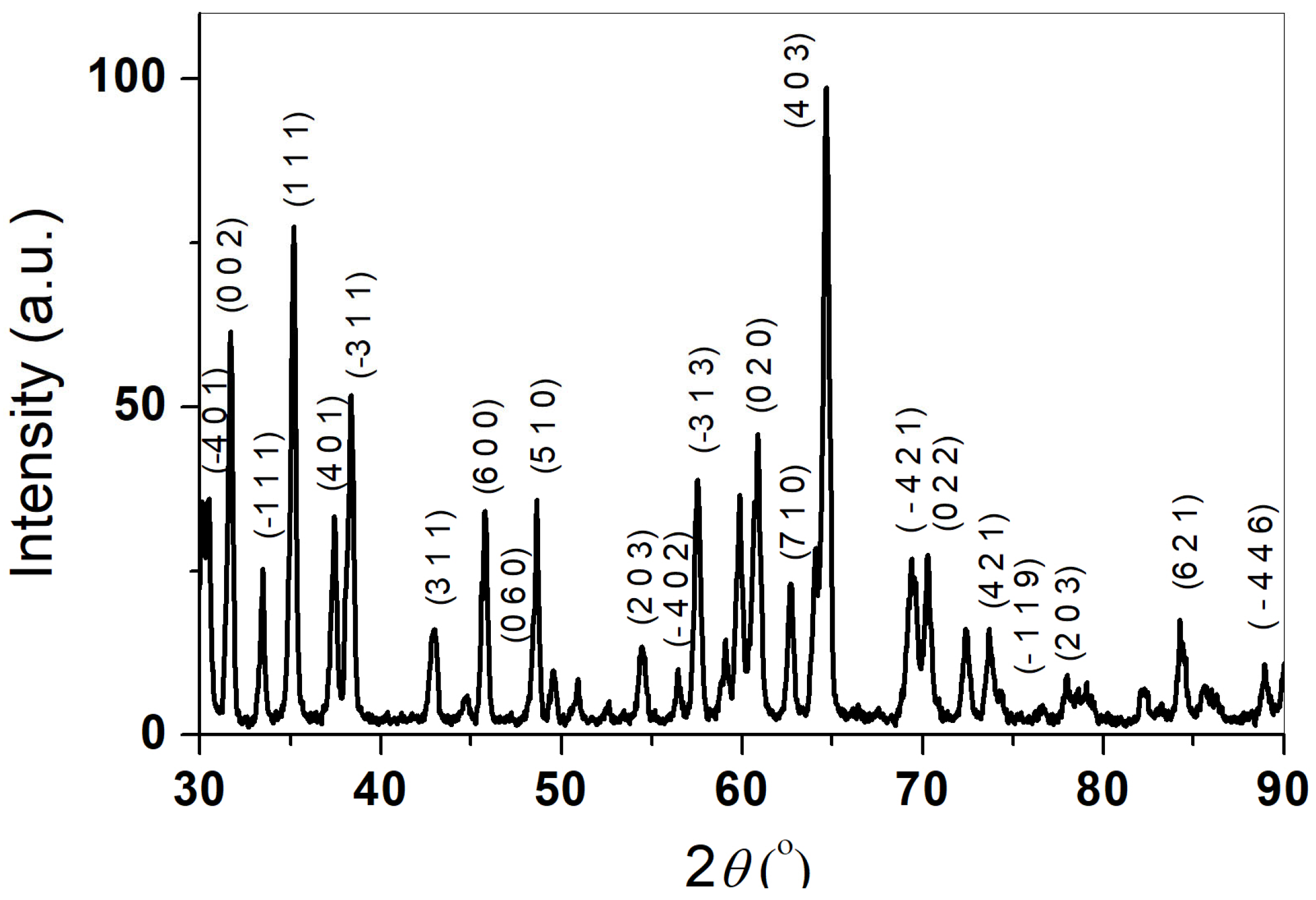
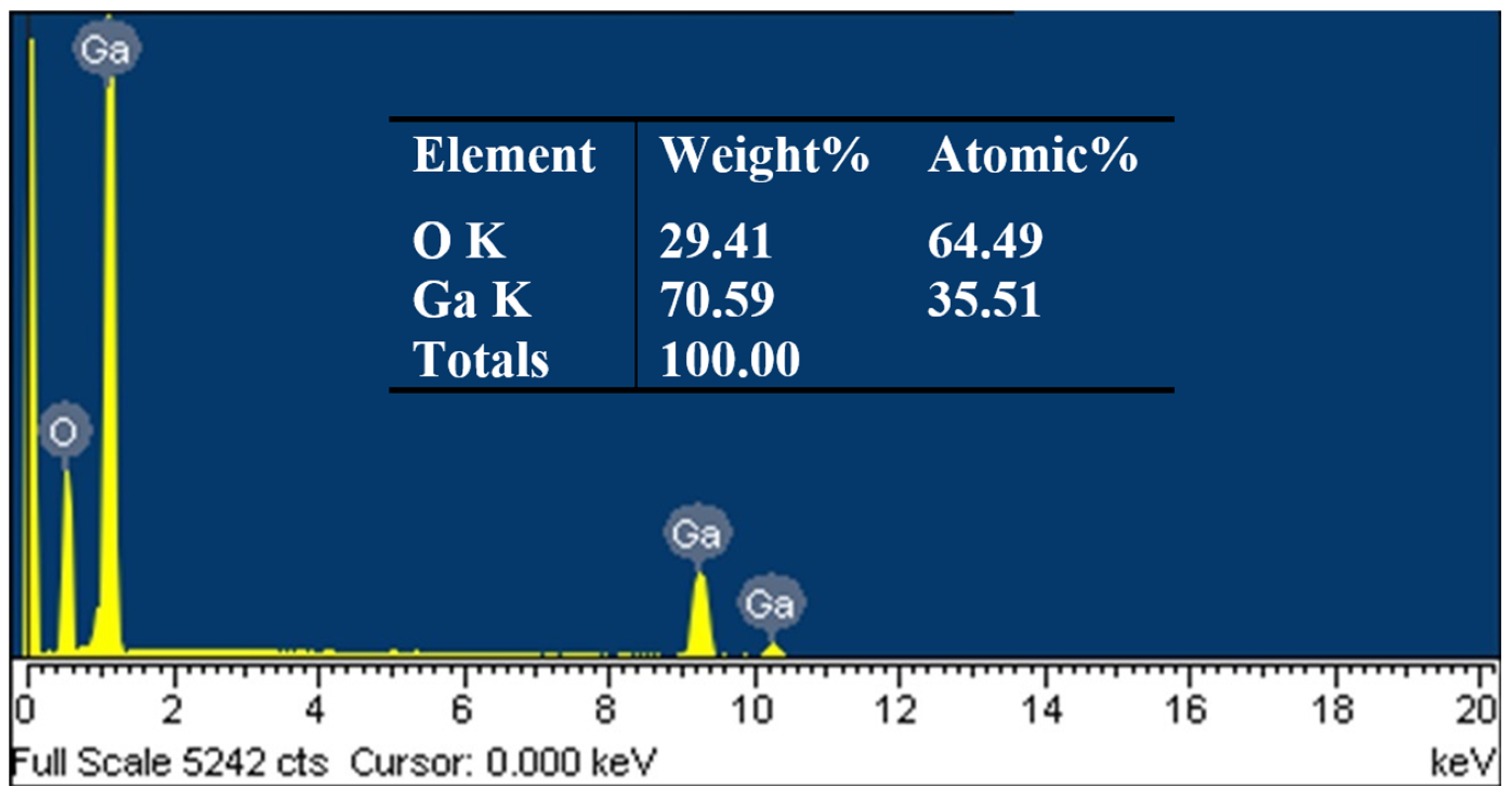
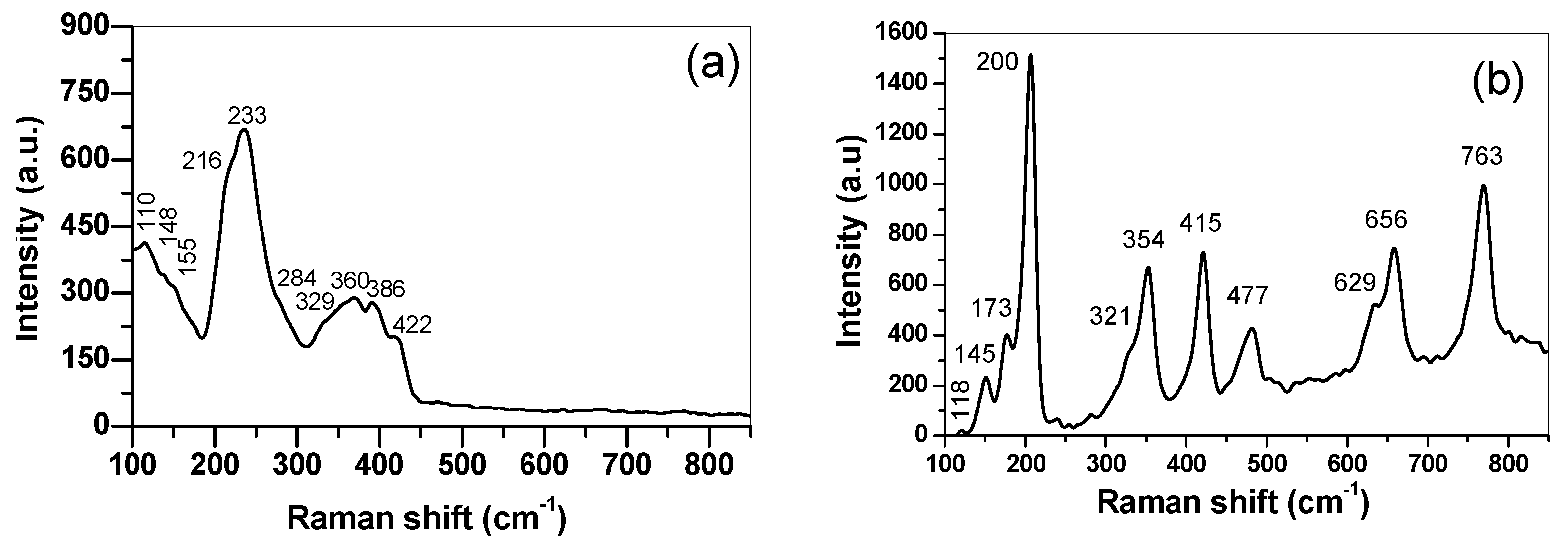
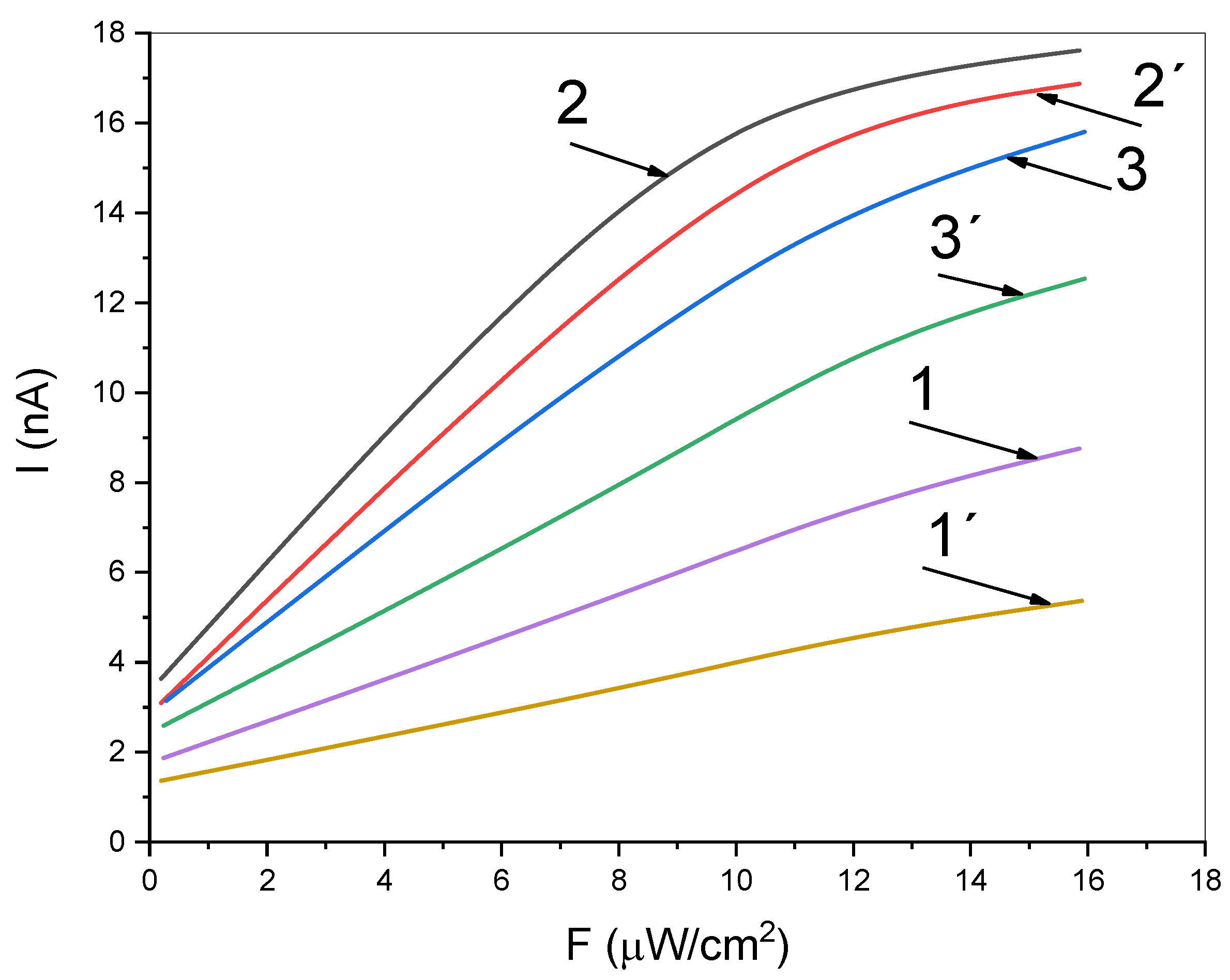
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, T.; Li, W.; Chen, S.; Jiang, K.; Zhu, J.; Hu, Z.; Huang, Z.; Chu, J.; Kokh, K.A.; Andreev, Y.M. Effects of S-doping on the electronic transition, band gap, and optical absorption of GaSe1−xSx single crystals. J. Alloys Compd. 2017, 721, 164–171. [Google Scholar] [CrossRef]
- Budiman, M.; Okamoto, T.; Yamada, A.; Konagai, M. Epitaxial growth and characterization of GaSxSe1−x layered compound semiconductor by molecular beam epitaxy. Appl. Surf. Sci. 1997, 117–118, 518–522. [Google Scholar] [CrossRef]
- Jellinek, F.; Hahn, H. Zur polytypie des galliummonoselenids, gase. Z. Naturforsch. B 1961, 16, 713–715. [Google Scholar] [CrossRef]
- Kokh, K.A.; Molloy, J.; Naftaly, M.; Andreev, Y.; Svetlichnyi, V.; Lanskii, G.; Lapin, I.; Izaak, T.; Kokh, A. Growth and optical properties of solid solution crystals GaSe1−xSx. Mater. Chem. Phys. 2015, 154, 152–157. [Google Scholar] [CrossRef]
- Ho, C.; Wang, S.; Huang, Y.; Tiong, K.-K. Structural and luminescent property of gallium chalcogenides GaSe1−xSx layer compounds. J. Mater. Sci. Mater. Electron. 2009, 20, 207–210. [Google Scholar] [CrossRef]
- Mercier, A.; Voitchovsky, J.-P. Donor-acceptor pair recombination and phonon replica in GaSxSe1−x. J. Phys. Chem. Solids 1975, 36, 1411–1417. [Google Scholar] [CrossRef]
- Siciliano, T.; Tepore, M.; Genga, A.; Micocci, G.; Siciliano, M.; Tepore, A. Thermal oxidation of amorphous GaSe thin films. Vacuum 2013, 92, 65–69. [Google Scholar] [CrossRef]
- Filippo, E.; Siciliano, T.; Genga, A.; Micocci, G.; Siciliano, M.; Tepore, A. Phase and morphological transformations of GaS single crystal surface by thermal treatment. Appl. Surf. Sci. 2012, 261, 454–457. [Google Scholar] [CrossRef]
- D’Olimpio, G.; Nappini, S.; Vorokhta, M.; Lozzi, L.; Genuzio, F.; Menteş, T.O.; Paolucci, V.; Gürbulak, B.; Duman, S.; Ottaviano, L.; et al. Enhanced electrocatalytic activity in GaSe and InSe nanosheets: The role of surface oxides. Adv. Funct. Mater. 2020, 30, 2005466. [Google Scholar] [CrossRef]
- Bletskan, D.; Kabatsii, V.; Kranjčec, M. Photoelectric properties of ordered-vacancy Ga2Se3 single crystals. Inorg. Mater. 2010, 46, 1290–1295. [Google Scholar] [CrossRef]
- Zervos, M.; Othonos, A.; Gianneta, V.; Travlos, A.; Nassiopoulou, A. Sn doped β-Ga2O3 and β-Ga2S3 nanowires with red emission for solar energy spectral shifting. J. Appl. Phys. 2015, 118, 194302. [Google Scholar] [CrossRef]
- Ho, C.-H.; Lin, M.-H.; Wang, Y.-P.; Huang, Y.-S. Synthesis of In2S3 and Ga2S3 crystals for oxygen sensing and UV photodetection. Sens. Actuators A Phys. 2016, 245, 119–126. [Google Scholar] [CrossRef]
- Tippins, H. Optical absorption and photoconductivity in the band edge of β-Ga2O3. Phys. Rev. 1965, 140, A316. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Saito, K.; Tanaka, T.; Nishio, M.; Guo, Q. The impact of growth temperature on the structural and optical properties of catalyst-free β-Ga2O3 nanostructures. Mater. Res. Express 2016, 3, 025003. [Google Scholar] [CrossRef]
- Alhalaili, B.; Vidu, R.; Islam, M.S. The growth of Ga2O3 nanowires on silicon for ultraviolet photodetector. Sensors 2019, 19, 5301. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, X.; Jiang, H.; Zou, X.; Lau, K.M.; Wang, G. Vertical β-Ga2O3 Schottky barrier diodes with enhanced breakdown voltage and high switching performance. Phys. Status Solidi (A) 2020, 217, 1900497. [Google Scholar] [CrossRef]
- Ito, R.; Akatsuka, M.; Ozawa, A.; Kato, Y.; Kawaguchi, Y.; Yamamoto, M.; Tanabe, T.; Yoshida, T. Photocatalytic activity of Ga2O3 supported on Al2O3 for water splitting and CO2 reduction. ACS Omega 2019, 4, 5451–5458. [Google Scholar] [CrossRef]
- Lee, J.-H.; Doan, T.A.; Park, Y.J.; Hoa, H.T.M.; Phuong, P.H.; Le, D.T.; Hung, N.H.; Tran, Q.T.; Lee, H.-S.; Ryu, J.H.; et al. Synthesis and photocatalytic activity of β-Ga2O3 nanostructures for decomposition of formaldehyde under deep ultraviolet irradiation. Catalysts 2020, 10, 1105. [Google Scholar] [CrossRef]
- Fleischer, M.; Seth, M.; Kohl, C.-D.; Meixner, H. A study of surface modification at semiconducting Ga2O3 thin film sensors for enhancement of the sensitivity and selectivity. Sens. Actuators B Chem. 1996, 36, 290–296. [Google Scholar] [CrossRef]
- Baban, C.; Toyoda, Y.; Ogita, M. Oxygen sensor based on Ga2O3 films operating at high temperature. J. Optoelectron. Adv. Mater. 2005, 7, 891–896. [Google Scholar]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mangalaraj, D. Morphology controllable synthesis of parallely arranged single-crystalline β-Ga2O3 nanorods for photocatalytic and antimicrobial activities. Chem. Eng. J. 2014, 236, 181–190. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Van de Walle, C.G. Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- Ueda, N.; Hosono, H.; Waseda, R.; Kawazoe, H. Synthesis and control of conductivity of ultraviolet transmitting β-Ga2O3 single crystals. Appl. Phys. Lett. 1997, 70, 3561–3563. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra and Molecular Structure: Diatomic Molecules; Prentice-Hall: Upper Saddle River, NJ, USA, 1939; Volume 1. [Google Scholar]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Yin, Y.; Liao, Y.; Mo, H.; Qian, Q.; Guo, Y.; Chen, X.; Zhang, Z.; Hua, M. Two-dimensional gallium oxide monolayer for gas-sensing application. J. Phys. Chem. Lett. 2021, 12, 5813–5820. [Google Scholar] [CrossRef]
- Wei, Z.; Akbari, M.K.; Hai, Z.; Ramachandran, R.K.; Detavernier, C.; Verpoort, F.; Kats, E.; Xu, H.; Hu, J.; Zhuiykov, S. Ultra-thin sub-10 nm Ga2O3-WO3 heterostructures developed by atomic layer deposition for sensitive and selective C2H5OH detection on ppm level. Sens. Actuators B Chem. 2019, 287, 147–156. [Google Scholar] [CrossRef]
- Calestani, D.; Alabi, A.B.; Coppedè, N.; Villani, M.; Lazzarini, L.; Fabbri, F.; Salviati, G.; Zappettini, A. Growth and characterization of β-Ga2O3 nanowires obtained on not-catalyzed and Au/Pt catalyzed substrates. J. Cryst. Growth 2017, 457, 255–261. [Google Scholar] [CrossRef]
- Xiao, H.-D.; Ma, H.-L.; Xue, C.-S.; Zhuang, H.-Z.; Ma, J.; Zong, F.-J.; Zhang, X.-J. Synthesis and structural properties of beta-gallium oxide particles from gallium nitride powder. Mater. Chem. Phys. 2007, 101, 99–102. [Google Scholar] [CrossRef]
- Jangir, R.; Ganguli, T.; Tiwari, P.; Porwal, S.; Srivastava, H.; Rai, S.; Khattak, B.; Oak, S. Synthesis and characterization of β-Ga2O3 nanostructures grown on GaAs substrates. Appl. Surf. Sci. 2011, 257, 9323–9328. [Google Scholar] [CrossRef]
- Jouanne, M.; Julien, C.; Balkanski, M. Polarization studies of polar phonons of InSe. Phys. Status Solidi B Basic Res. 1987, 144, K147–K150. [Google Scholar] [CrossRef]
- Wu, W.; Huang, H.; Wang, Y.; Yin, H.; Han, K.; Zhao, X.; Feng, X.; Zeng, Y.; Zou, Y.; Hou, X. Structure engineering of Ga2O3 photodetectors: A review. J. Phys. D Appl. Phys. 2025, 58, 063003. [Google Scholar]
- Xu, K.; Wang, R.; Wang, Y.; Wang, J.; Zhi, T.; Yang, G.; Xue, J.; Chen, D.; Zhang, R. Advances and prospects in Ga2O3/GaN heterojunctions: From fabrication to high-performance devices. Mater. Sci. Semicond. Process. 2025, 185, 108874. [Google Scholar] [CrossRef]
- Kumarbekov, K.K.; Kakimov, A.B.; Karipbayev, Z.T.; Kassymzhanov, M.T.; Brik, M.G.; Ma, C.; Piasecki, M.; Suchikova, Y.; Kemere, M.; Konuhova, M. Temperature-dependent luminescence of europium-doped Ga2O3 ceramics. Opt. Mater. X 2025, 25, 100392. [Google Scholar]
- Suchikova, Y.; Nazarovets, S.; Popov, A.I. Ga2O3 solar-blind photodetectors: From civilian applications to missile detection and research agenda. Opt. Mater. 2024, 157, 116397. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef]
- Shin, J.; Yoo, H. Photogating Effect-Driven Photodetectors and Their Emerging Applications. Nanomaterials 2023, 13, 882. [Google Scholar] [CrossRef]
- Zhai, H.; Wu, Z.; Fang, Z. Recent progress of Ga2O3-based gas sensors. Ceram. Int. 2022, 48, 24213–24233. [Google Scholar] [CrossRef]
- Ogita, M.; Kobayashi, K.; Yamada, Y.; Nakanishi, Y.; Hatanaka, Y. Properties of gallium oxide thin film sputtered from powder target for high temperature oxygen sensor. In Proceedings of the IECON’01 27th Annual Conference of the IEEE Industrial Electronics Society (Cat. No. 37243), Denver, CO, USA, 29 November–2 December 2001; pp. 137–140. [Google Scholar]
- Nigar, H.; Navascués, N.; De la Iglesia, O.; Mallada, R.; Santamaría, J. Removal of VOCs at trace concentration levels from humid air by Microwave Swing Adsorption, kinetics and proper sorbent selection. Sep. Purif. Technol. 2015, 151, 193–200. [Google Scholar] [CrossRef]
- Juan, Y.; Chang, S.-J.; Hsueh, H.; Wang, S.; Weng, W.; Cheng, T.; Wu, C.-L. Effects of humidity and ultraviolet characteristics on β-Ga2O3 nanowire sensor. RSC Adv. 2015, 5, 84776–84781. [Google Scholar] [CrossRef]
- Siciliano, T.; Di Giulio, M.; Tepore, M.; Genga, A.; Micocci, G.; Tepore, A. In2O3 films prepared by thermal oxidation of amorphous InSe thin films. Thin Solid Films 2012, 520, 2455–2460. [Google Scholar] [CrossRef]
- Novkovski, N.; Tanuševski, A. Origin of the optical absorption of In2O3 thin films in the visible range. Semicond. Sci. Technol. 2008, 23, 095012. [Google Scholar] [CrossRef]
- Williams, R.; McEvoy, A. Electron emission studies from GaSe surfaces. J. Vac. Sci. Technol. 1972, 9, 867–870. [Google Scholar] [CrossRef]
- Beechem, T.E.; Kowalski, B.M.; Brumbach, M.T.; McDonald, A.E.; Spataru, C.D.; Howell, S.W.; Ohta, T.; Pask, J.A.; Kalugin, N.G. Oxidation of ultrathin GaSe. Appl. Phys. Lett. 2015, 107, 173103. [Google Scholar] [CrossRef]
- George, K.; de Groot, C.H.; Gurnani, C.; Hector, A.L.; Huang, R.; Jura, M.; Levason, W.; Reid, G. Telluroether and selenoether complexes as single source reagents for low pressure chemical vapor deposition of crystalline Ga2Te3 and Ga2Se3 thin films. Chem. Mater. 2013, 25, 1829–1836. [Google Scholar] [CrossRef]
- Kanaya, K.; Okayama, S. Penetration and energy-loss theory of electrons in solid targets. J. Phys. D Appl. Phys. 1972, 5, 43–58. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Nanofunctional gallium oxide (Ga2O3) nanowires/nanostructures and their applications in nanodevices. Phys. Status Solidi–Rapid Res. Lett. 2013, 7, 781–792. [Google Scholar] [CrossRef]
- Liu, H.; Antwi, K.A.; Yakovlev, N.; Tan, H.; Ong, L.; Chua, S.; Chi, D. Synthesis and phase evolutions in layered structure of Ga2S3 semiconductor thin films on epiready GaAs (111) substrates. ACS Appl. Mater. Interfaces 2014, 6, 3501–3507. [Google Scholar] [CrossRef]
- Lucazeau, G.; Leroy, J. Etude vibrationnelle de α-Ga2S3. Spectrochim. Acta Part A Mol. Spectrosc. 1978, 34, 29–32. [Google Scholar] [CrossRef]
- Julien, C.; Barnier, S.; Ivanov, I.; Guittard, M.; Pardo, M.; Chilouet, A. Vibrational studies of copper thiogallate solid solutions. Mater. Sci. Eng. B 1999, 57, 102–109. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman spectra and valence force field of single-crystalline β-Ga2O3. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Finkman, E.; Tauc, J.; Kershaw, R.; Wold, A. Lattice dynamics of tetrahedrally bonded semiconductors containing ordered vacant sites. Phys. Rev. B 1975, 11, 3785–3793. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G. Valence force field and raman spectra of β-Ga2O3. J. Mol. Struct. 1982, 79, 419–422. [Google Scholar] [CrossRef]
- Gao, Y.; Bando, Y.; Sato, T.; Zhang, Y.; Gao, X. Synthesis, Raman scattering and defects of β-Ga2O3 nanorods. Appl. Phys. Lett. 2002, 81, 2267–2269. [Google Scholar] [CrossRef]
- Chun, H.J.; Choi, Y.S.; Bae, S.Y.; Seo, H.W.; Hong, S.J.; Park, J.; Yang, H. Controlled structure of gallium oxide nanowires. J. Phys. Chem. B 2003, 107, 9042–9046. [Google Scholar] [CrossRef]
- Filippo, E.; Siciliano, M.; Genga, A.; Micocci, G.; Tepore, A.; Siciliano, T. Single crystalline β-Ga2O3 nanowires synthesized by thermal oxidation of GaSe layer. Mater. Res. Bull. 2013, 48, 1741–1744. [Google Scholar] [CrossRef]
- Kowalski, B.M.; Manz, N.; Bethke, D.; Shaner, E.A.; Serov, A.; Kalugin, N.G. Role of humidity in oxidation of ultrathin GaSe. Mater. Res. Express 2019, 6, 085907. [Google Scholar] [CrossRef]
- Yao, Y.; Davis, R.F.; Porter, L.M. Investigation of different metals as ohmic contacts to β-Ga2O3: Comparison and analysis of electrical behavior, morphology, and other physical properties. J. Electron. Mater. 2017, 46, 2053–2060. [Google Scholar] [CrossRef]
- Oshima, T.; Kaminaga, K.; Mashiko, H.; Mukai, A.; Sasaki, K.; Masui, T.; Kuramata, A.; Yamakoshi, S.; Ohtomo, A. β-Ga2O3 single crystal as a photoelectrode for water splitting. Jpn. J. Appl. Phys. 2013, 52, 111102. [Google Scholar] [CrossRef]
- Yatskiv, R.; Vorochta, M.; Basinova, N.; Dinhova, T.N.; Maixner, J.; Grym, J. Low-temperature gas sensing mechanism in β-Ga2O3 nanostructures revealed by near-ambient pressure XPS. Appl. Surf. Sci. 2024, 663, 160155. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Huang, P.; Li, M.; Lu, Y.; Homewood, K.P.; Chang, G.; Chen, H.; He, Y. Influence of growth temperature on the characteristics of β-Ga2O3 epitaxial films and related solar-blind photodetectors. Appl. Surf. Sci. 2019, 489, 101–109. [Google Scholar] [CrossRef]
- Chen, X.; Mi, W.; Wu, J.; Yang, Z.; Zhang, K.; Zhao, J.; Luan, C.; Wei, Y. A solar-blind photodetector based on β-Ga2O3 film deposited on MgO (100) substrates by RF magnetron sputtering. Vacuum 2020, 180, 109632. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, W.; Zhu, F.; Yu, T.; Zhang, X. UV-enhanced O2 sensing using β-Ga2O3 nanowires at room temperature. Sens. Actuators A Phys. 2024, 379, 115997. [Google Scholar] [CrossRef]
- Cui, S.-J.; Mei, Z.-X.; Hou, Y.-N.; Chen, Q.-S.; Liang, H.-L.; Zhang, Y.-H.; Huo, W.-X.; Du, X.-L. Enhanced photoresponse performance in Ga/Ga2O3 nanocomposite solar-blind ultraviolet photodetectors. Chin. Phys. B 2018, 27, 067301. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Wang, Z.; Zeng, X.; Ungar, G.; Guo, D.; Shen, J.; Li, P.; Liu, A.; Li, C.; et al. In situ synthesis of monoclinic β-Ga2O3 nanowires on flexible substrate and solar-blind photodetector. J. Alloys Compd. 2019, 787, 133–139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprincean, V.; Caraman, M.; Braniste, T.; Tiginyanu, I. Layered Structures Based on Ga2O3/GaS0.98Se0.02 for Gas Sensor Applications. Surfaces 2025, 8, 53. https://doi.org/10.3390/surfaces8030053
Sprincean V, Caraman M, Braniste T, Tiginyanu I. Layered Structures Based on Ga2O3/GaS0.98Se0.02 for Gas Sensor Applications. Surfaces. 2025; 8(3):53. https://doi.org/10.3390/surfaces8030053
Chicago/Turabian StyleSprincean, Veaceslav, Mihail Caraman, Tudor Braniste, and Ion Tiginyanu. 2025. "Layered Structures Based on Ga2O3/GaS0.98Se0.02 for Gas Sensor Applications" Surfaces 8, no. 3: 53. https://doi.org/10.3390/surfaces8030053
APA StyleSprincean, V., Caraman, M., Braniste, T., & Tiginyanu, I. (2025). Layered Structures Based on Ga2O3/GaS0.98Se0.02 for Gas Sensor Applications. Surfaces, 8(3), 53. https://doi.org/10.3390/surfaces8030053






