Glucose-Mediated Microstructure Refinement of Electroless Silver Coatings on Atomized Fe Particles
Abstract
1. Introduction
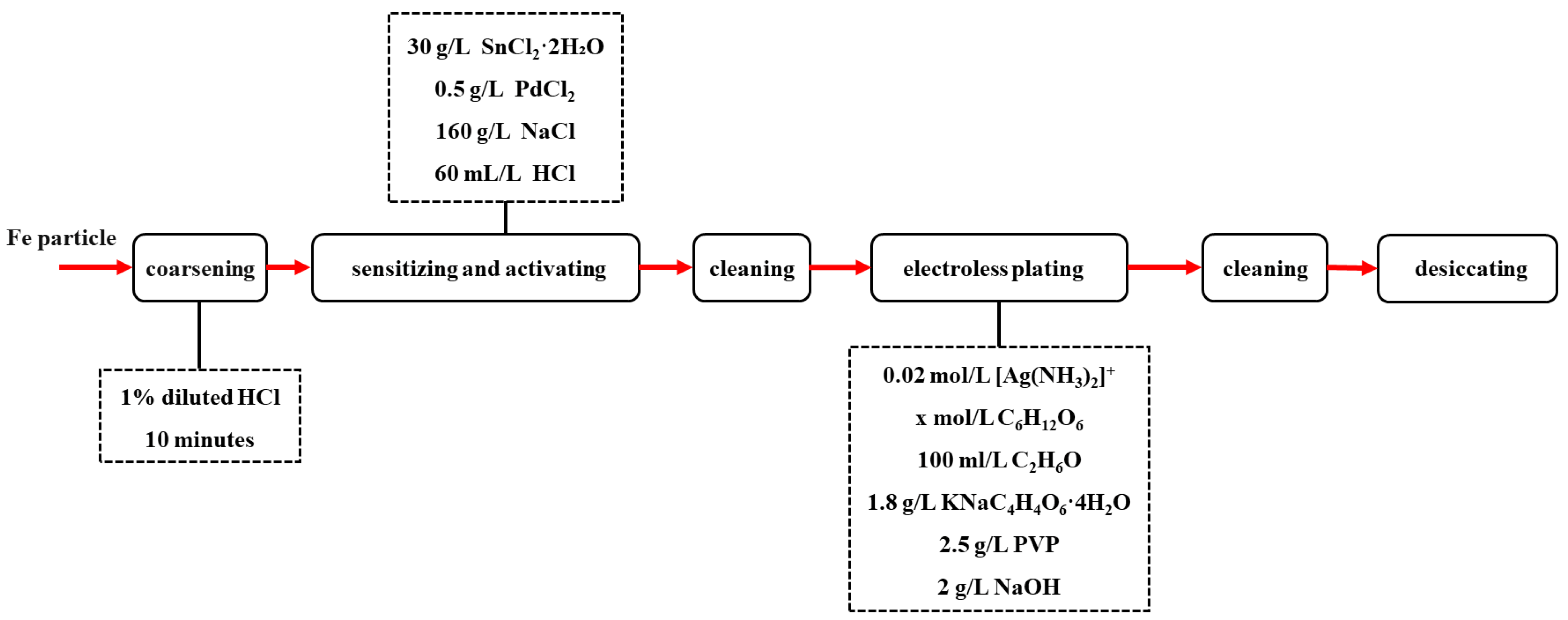
2. Materials and Methods
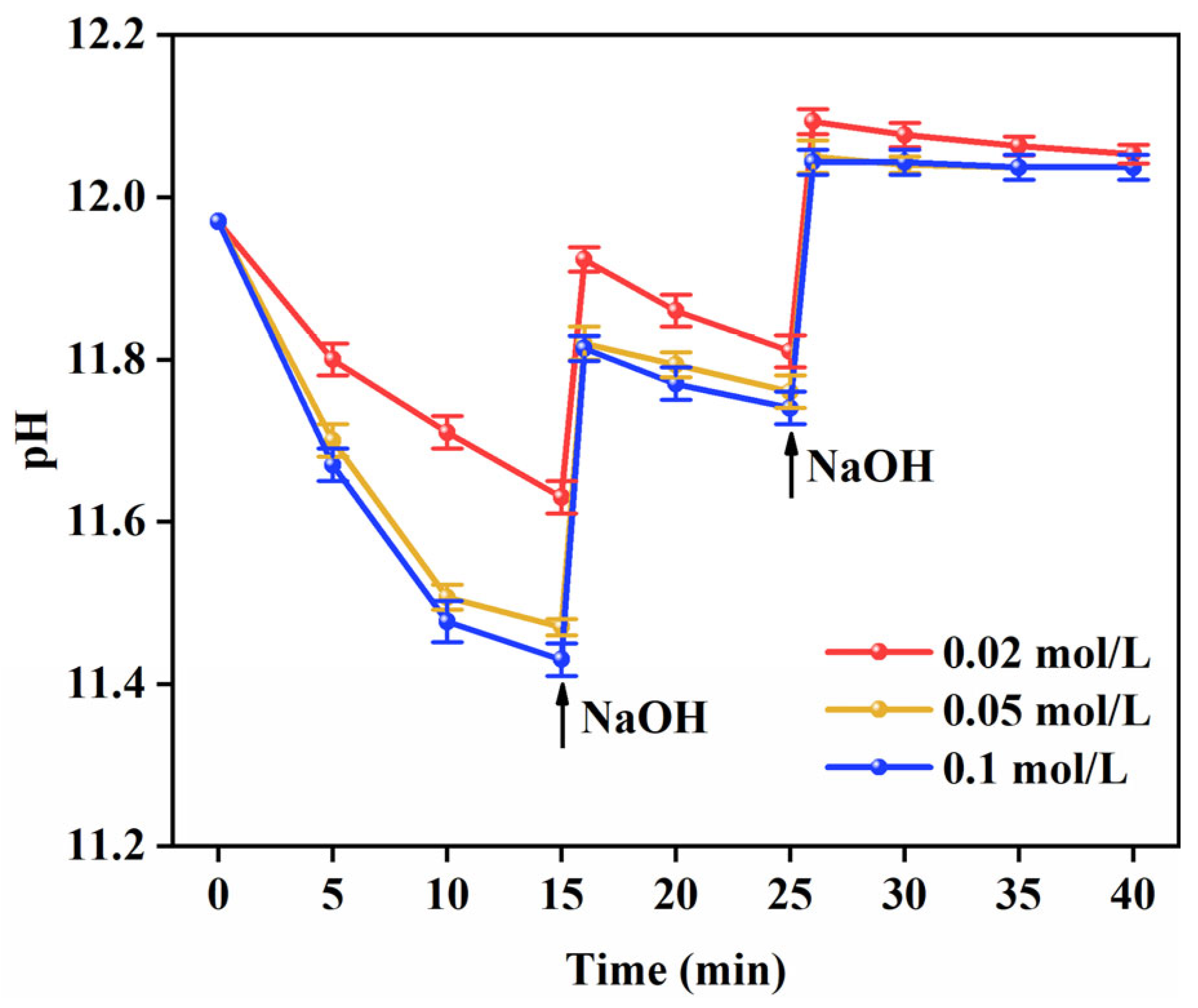
2.1. Electroless Silver Plating
2.2. Microstructure and Performance Characterization
3. Results
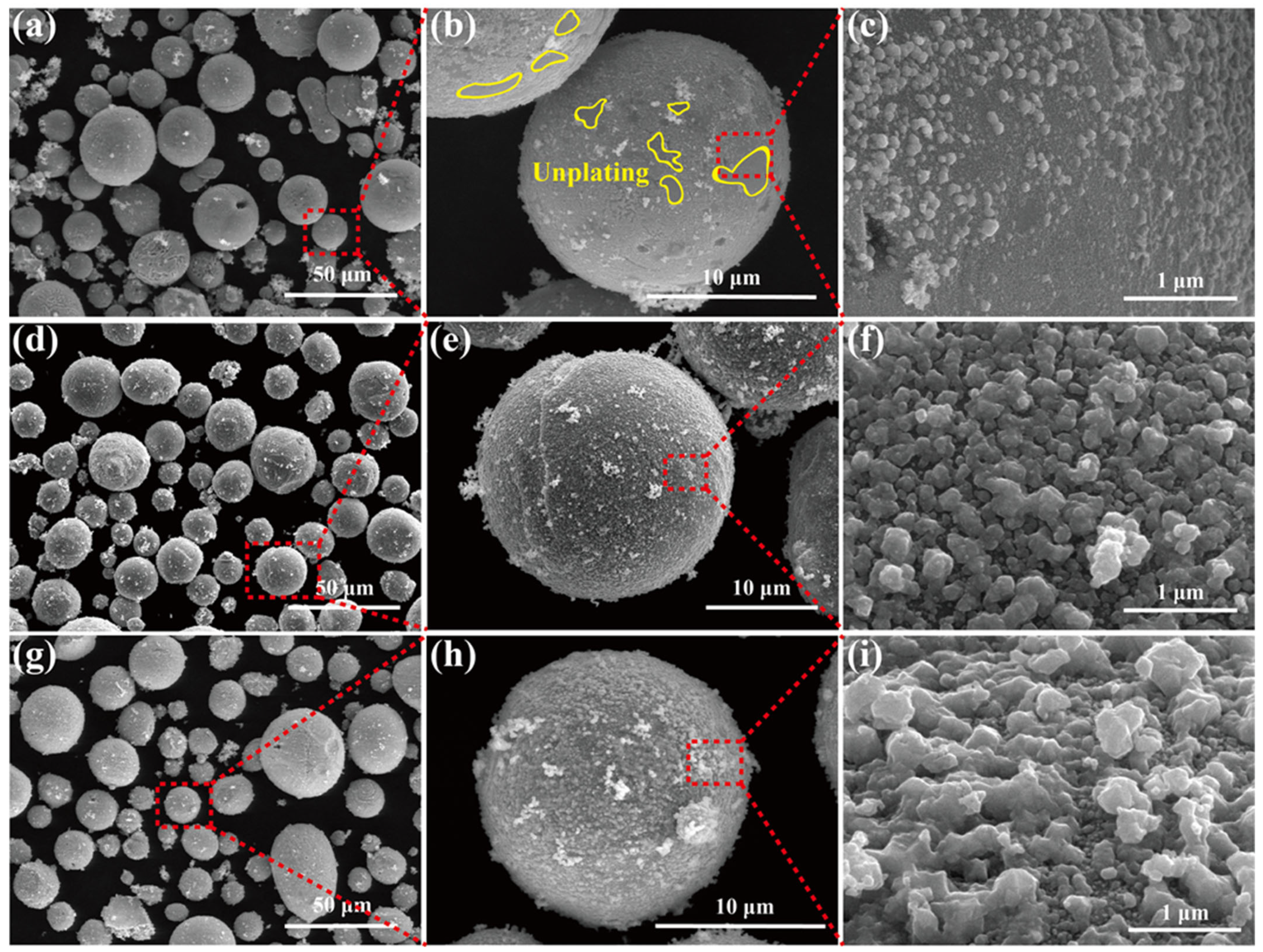
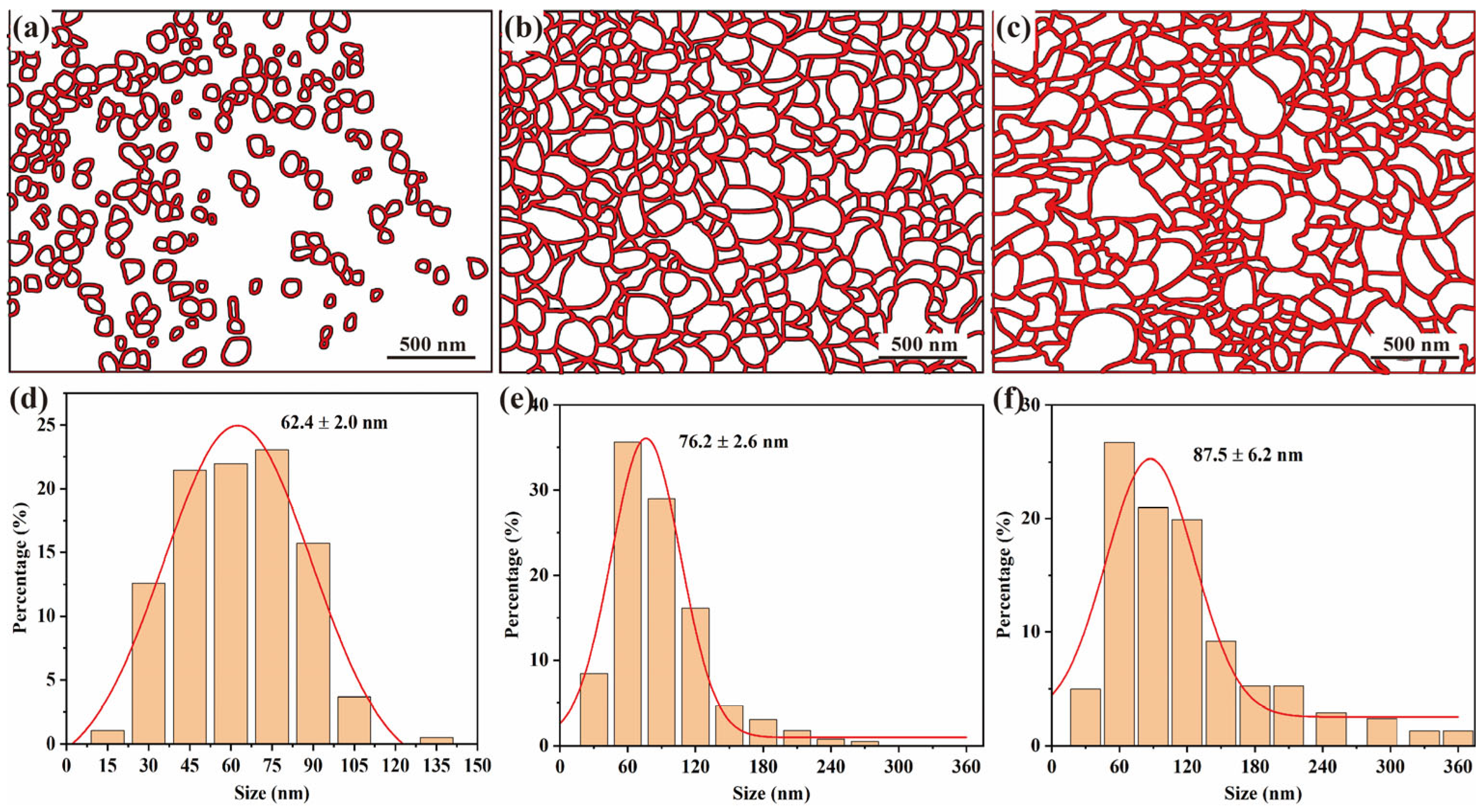
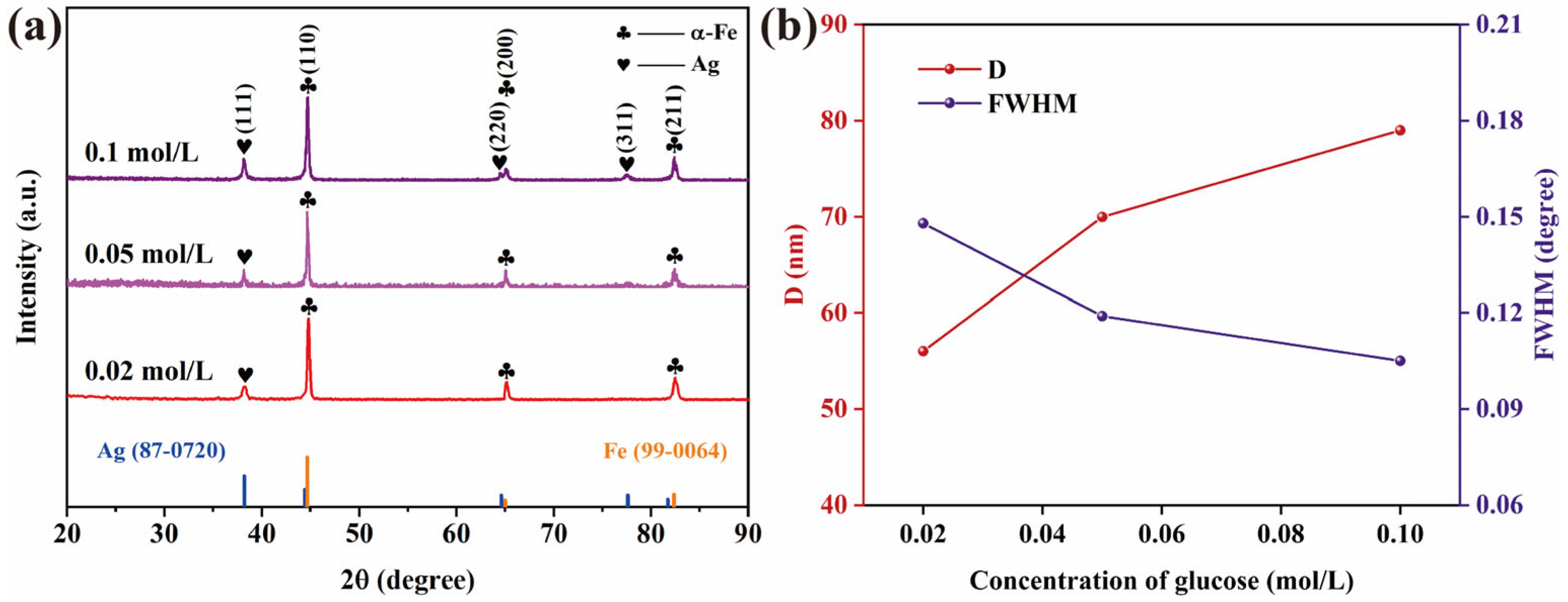
3.1. Microstructure
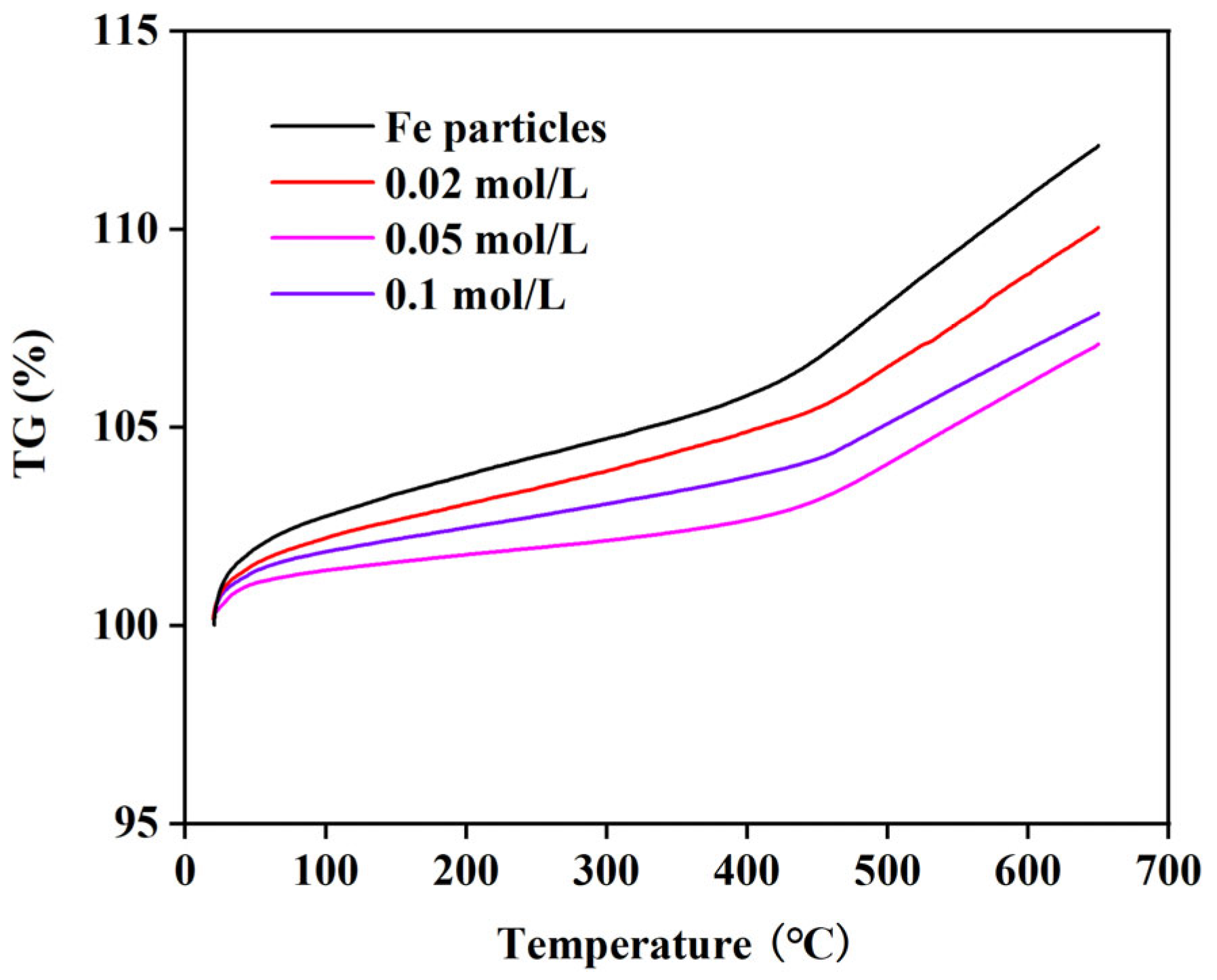
3.2. Oxidation Resistance
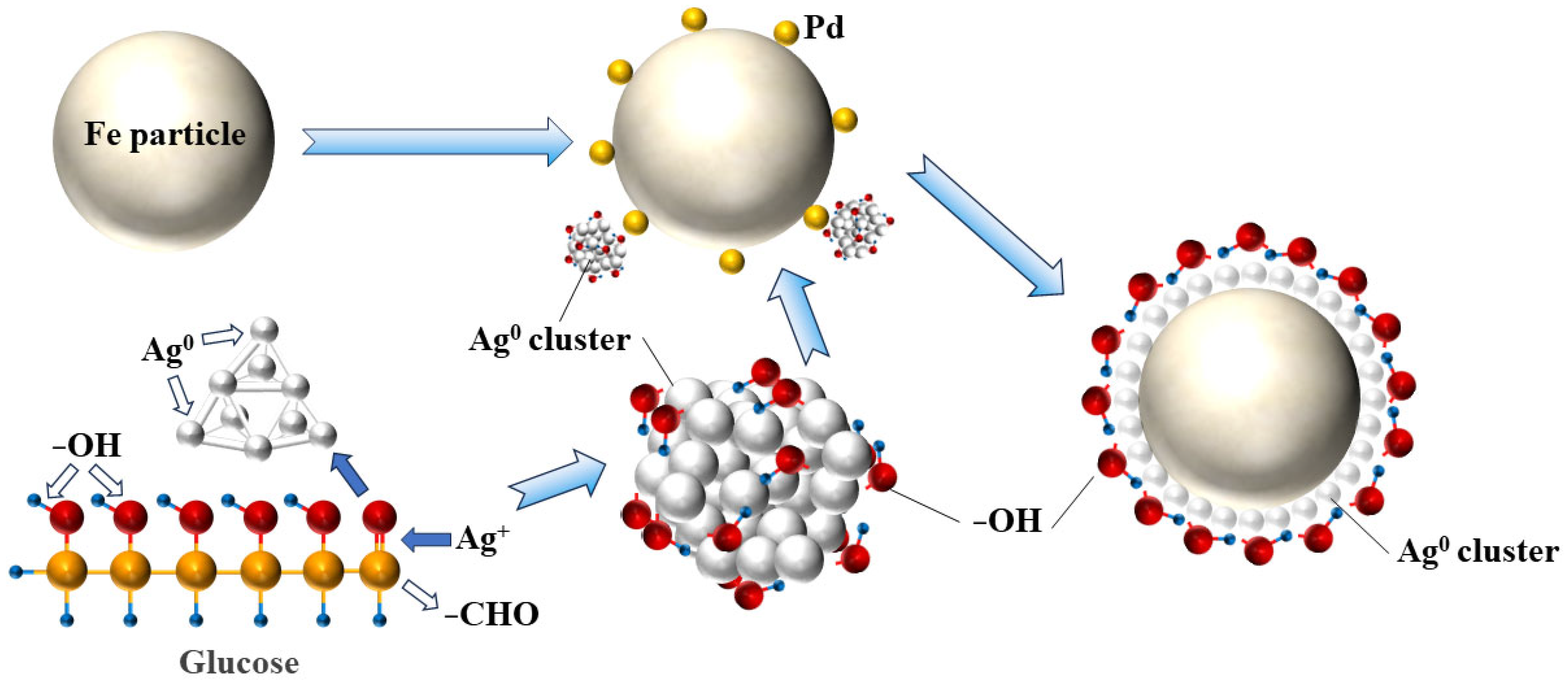
4. Discussion
5. Conclusions
- The grain size of silver coatings exhibits a strong dependence on glucose concentration, with higher concentrations leading to coarser Ag grains. The optimal glucose concentration of 0.05 mol/L enables the formation of a dense and well-covered silver layer on Fe particles, featuring an average Ag grain size of approximately 76 nm.
- Thermogravimetric analysis reveals that silver-plated Fe particles prepared with 0.05 mol/L of glucose demonstrate superior oxidation resistance, showing only 107.1% weight gain at 650 °C. In contrast, coatings with coarse Ag microstructures exhibit significantly inferior oxidation resistance compared to their completely coated fine-grained counterparts.
- Glucose serves dual functions in the plating process: as a reducing agent through its aldehyde group for Ag+ reduction and as a grain growth barrier via the selective adsorption of hydroxyl groups on Ag {100} facets to inhibit grain growth.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seshimo, M.; Hirai, T.; Rahman, M.M.; Ozawa, M.; Sone, M.; Sakurai, M.; Higo, Y.; Kameyama, H. Functionally graded Pd/γ-alumina composite membrane fabricated by electroless plating with emulsion of supercritical CO2. J. Membrane. Sci. 2009, 342, 321–326. [Google Scholar] [CrossRef]
- Ai, N.; Chen, K.; Jiang, S.; Lü, Z.; Su, W. Vacuum-assisted electroless copper plating on Ni/(Sm,Ce)O2 anodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen. Energ. 2011, 36, 7661–7669. [Google Scholar] [CrossRef]
- Dai, X.; Nan, J.; Cheng, Q. Enhanced thermoelectric and mechanical properties of p-type Bi0. 5Sb1. 5Te3 bulk alloys by composite electroless plating with Ni&Cu. J. Wuhan. Univ. Technol. 2022, 37, 1009–1013. [Google Scholar]
- Yin, H.; Yang, W.; Zhao, L.; Hu, X.; Liu, S.; Cui, C.; Wang, X. Fabrication and mechanical property of three-dimensional carbon fiber reinforced Mg-based bulk metallic glass matrix composite. Mat. Sci. Eng. A. 2022, 839, 142853. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhang, J.; Gong, P.; Yang, W.; Zhao, L.; Wang, X. Design optimization and mechanical properties of SiC particle reinforced Ti-based metallic glass matrix composite. Materials 2023, 16, 5323. [Google Scholar] [CrossRef]
- Ma, X.; Lun, N.; Wen, S. Formation of gold nanoparticles supported on carbon nanotubes by using an electroless plating method. Diam. Relat. Mater. 2005, 14, 68–73. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, H. Preparation of silver-coated copper powder and its oxidation resistance research. Powder. Technol. 2012, 226, 53–56. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Takahashi, J.; Wan, Y.; Li, M.; Xiao, B.; Zhang, Y.; He, X. Investigation of modification of Cu-Ni-graphite composite by silver. Mater. Chem. Phys. 2020, 239, 121990. [Google Scholar] [CrossRef]
- Wang, B.; Saka, N. Spark erosion behavior of silver-based particulate composites. Wear 1996, 195, 133–147. [Google Scholar] [CrossRef]
- Lermusiaux, L.; Roach, L.; Lehtihet, M.; Plissonneau, M.; Bertry, L.; Buissette, V.; Mercier, T.L.; Duguet, E.; Drisko, G.L.; Leng, J.; et al. Silver nanoshells with optimized infrared optical response: Synthesis for thin-shell formation, and optical/thermal properties after embedding in polymeric films. Nanomaterials 2023, 13, 614. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Yuan, W.; Li, H.; Huang, X.; Zhang, Z.; Sheng, Y.; Li, Z.; Dong, K.; Chen, W.; et al. Alternative coatings to cyanide silver coatings with low infrared emissivity for tokamak components. Nucl. Mater. Energy 2024, 39, 101664. [Google Scholar] [CrossRef]
- Li, K.; Liang, L.; Du, P.; Cai, Z.; Xiang, T.; Kanetaka, H.; Wu, H.; Xie, G. Mechanical properties and corrosion resistance of powder metallurgical Mg-Zn-Ca/Fe bulk metal glass composites for biomedical application. J. Mater. Sci. Technol. 2022, 103, 73–83. [Google Scholar] [CrossRef]
- Shao, Y.; Zheng, W.; Guo, W.; Lü, S.; Wu, S. In situ Fe-rich particle reinforced Mg-based metallic glass matrix composites via dealloying in metallic melt. Mater. Lett. 2021, 285, 129165. [Google Scholar] [CrossRef]
- Wang, J.F.; Huang, S.; Wei, Y.Y.; Guo, S.F.; Pan, F.S. Enhanced mechanical properties and corrosion resistance of a Mg-Zn-Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013, 91, 311–314. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Li, Y.; Wei, Y.; Guo, S.; Pan, F. Ultrahigh strength MgZnCa eutectic alloy/Fe particle composites with excellent plasticity. Mater. Lett. 2014, 137, 139–142. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L.; Tong, Z.; Xie, M.; Yi, J. A study on the uniformity of silver coatings on nanometer SnO2 particles through chemical plating. Int. J. Mater. Prod. Tec. 2015, 51, 241–247. [Google Scholar] [CrossRef]
- Maw, S.S.; Watanabe, T.; Miyahara, M.T.; Watanabe, S. Seedless and electroless plating of silver on silica particles by using microreactor. Adv. Powder. Technol. 2024, 35, 104414. [Google Scholar] [CrossRef]
- Güler, O.; Varol, T.; Alver, Ü.; Canakci, V. Effect of Al2O3 content and milling time on the properties of silver coated Cu matrix composites fabricated by electroless plating and hot pressing. Mater. Today. Commun. 2020, 24, 101153. [Google Scholar] [CrossRef]
- Güler, O.; Alver, Ü.; Varol, T. Fabrication and characterization of novel layered materials produced by electroless plating and hot pressing. J. Alloy. Compd. 2020, 835, 155278. [Google Scholar] [CrossRef]
- Varol, T.; Güler, O.; Akçay, S.B.; Aksa, H.C. The effect of silver coated copper particle content on the properties of novel Cu-Ag alloys prepared by hot pressing method. Powder. Technol. 2021, 384, 236–246. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, L.; Mao, H.; Ren, Y.; Yang, H. High performance low cost silver coated copper paste for silicon heterojunction solar cells. J. Mater. Sci-Mater. El. 2025, 36, 267. [Google Scholar] [CrossRef]
- Hsueh, Y.; Cheng, C.; Chien, H.; Huang, X.; Huang, C.; Wu, C.; Chen, S.; Ou, S. Synergistic effects of collagen and silver on the deposition characteristics, antibacterial ability, and cytocompatibility of a collagen/silver coating on titanium. J. Alloy. Compd. 2020, 830, 154490. [Google Scholar] [CrossRef]
- Baksi, A.; Gandi, M.; Chaudhari, S.; Bag, S.; Gupta, S.S.; Pradeep, T. Extraction of Silver by Glucose. Angew. Chem. Int. Ed. 2016, 55, 7777–7781. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Whitten, J.L. Adsorption of O, H, OH, and H2O on Ag(100). J. Phys. Chem. B 2005, 109, 8852–8856. [Google Scholar] [CrossRef]
- Romero-Romero, M.; Domínguez-Ríos, C.; Torres-Sánchez, R.; Aguilar-Elguezabal, A. Electroless Ni-B coating onto TiH2 powder: An approach for a simplified surface preparation. Surf. Coat. Tech. 2017, 315, 181–187. [Google Scholar] [CrossRef]
- Tang, P.; Jiang, S.; Yan, J.; Lin, H.; Shi, F.; Liu, J. Role of various pretreatment processes in electroless nickel deposition on TiH2 particles with a simple plating bath. J. Alloy. Compd. 2020, 825, 154037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Wang, T.; Zhao, L.; Gong, P.; Wang, X. Glucose-Mediated Microstructure Refinement of Electroless Silver Coatings on Atomized Fe Particles. Surfaces 2025, 8, 44. https://doi.org/10.3390/surfaces8030044
Song D, Wang T, Zhao L, Gong P, Wang X. Glucose-Mediated Microstructure Refinement of Electroless Silver Coatings on Atomized Fe Particles. Surfaces. 2025; 8(3):44. https://doi.org/10.3390/surfaces8030044
Chicago/Turabian StyleSong, Dehou, Tiebao Wang, Lichen Zhao, Pan Gong, and Xin Wang. 2025. "Glucose-Mediated Microstructure Refinement of Electroless Silver Coatings on Atomized Fe Particles" Surfaces 8, no. 3: 44. https://doi.org/10.3390/surfaces8030044
APA StyleSong, D., Wang, T., Zhao, L., Gong, P., & Wang, X. (2025). Glucose-Mediated Microstructure Refinement of Electroless Silver Coatings on Atomized Fe Particles. Surfaces, 8(3), 44. https://doi.org/10.3390/surfaces8030044








