Abstract
In this study, polymer films based on the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl with repellent N,N-diethyl-3-methylbenzamide were prepared and used as functional textile coatings. The high sorption activity of oxides with respect to N,N-diethyl-3-methylbenzamide (63–239 mg/g) allows for the use of these compounds as repellent carrier materials, and their mixture with polyacrylates allows for the formation of functional coatings–polymer films. Scanning electron microscopy and Fourier transform infrared spectroscopy results revealed that the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl were successfully anchored in the polyacrylate structure, and the FTIR spectra confirmed the presence of repellent molecules. The thermal diffusion parameters of N,N-diethyl-3-methylbenzamide were also calculated via thermogravimetric analysis and high-performance liquid chromatography with diode array detection. The highest thermal diffusion rates and concentrations were observed for the material with Al2O3 (up to 148.3∙10−9 mol at 200 °C), and lower values for ZrO2 and SiO2-phenyl (up to 15.2∙10−9 mol and 34.3∙10−9 mol at 200 °C, respectively). The heat flux parameter Jf was also calculated according to Onsager’s theory and Fourier’s law. The release of repellent from polymeric materials can be achieved by applying less heat than that required to reach the boiling point of N,N-diethyl-3-methylbenzamide.
1. Introduction
Functional coatings based on polyacrylate compounds are a class of polymeric materials that can be used on their own or can be applied to textiles [1,2]. Polyacrylates are widely used in the textile industry and other fields due to their properties such as high mechanical strength, chemical and biological inertness, and resistance to thermal and ultraviolet degradation [3,4,5,6]. The properties of polyacrylates can be varied by incorporating additives into the polymeric structure. Thus, the incorporation of inorganic oxides of silica, alumina and zirconia or zinc oxide and titanium dioxide leads to an increase in the mechanical strength of the polymer structure and in the photocatalytic activity of the materials and allows for the introduction of carbon nanotubes to improve the electrical conductivity of polymers [3]. In addition, polyacrylates and their copolymers are widely used in agriculture as pesticide carriers in the form of microcapsules or heterogeneous dispersions that can deliver the active ingredient in a targeted manner [5,6,7]. This approach not only reduces the toxicological impact of pesticides on the environment but also ensures their long-term effectiveness.
Functional polymer coatings are particularly important in the development of materials to protect against insect attack. Insects are a major source of dangerous infectious diseases [8]. Previous studies have shown that the polymeric binding of some pyrethroids to polyacrylates thermally fixed to textile materials results in prolonged activity against blood-sucking insects. Another advantage of polyacrylate coatings is their high resistance to environmental factors and to repeated washing [9].
However, the use of pyrethroids in textiles is limited by their high toxicity. Polymeric coatings based on repellents—compounds of synthetic and natural origin that can repel insects while maintaining the protective functions of textiles—can be an alternative approach [4,10,11,12,13,14,15,16]. These compounds were shown to be effective against blood-sucking insects as early as the mid-20th century [17] and are still widely used in various formulations, but there are a number of difficulties with their application and fixation to the surface of textiles, as these compounds are oily liquids that are poorly soluble in water. There are few technologies available to produce functional coatings with repellent properties. Therefore, to fix the repellent N,N-diethyl-3-methylbenzamide (DEET), electrospinning, which is based on the action of a high electric potential on a polymeric solution, is possible [11,12,15,18]. As a result of Coulomb forces, the electrostatically charged polymer is pulled towards the surface of the material, exceeding the critical speed of the liquid and resulting in the solidification of superstrong structures. This approach provides a long-lasting repellent effect of textiles against blood-sucking insects, but special laboratory equipment is required to obtain materials with a repellent coating [12]. In addition, the production of protective clothing from such textiles requires the scaling up of such technologies, and it is not clear whether the process can be replicated on an industrial scale.
Another approach to functional coatings with repellent properties is the sequential synthesis of repellent conjugates with dye molecules for subsequent application to textile fibers [19,20]. Owing to the -N=N- bonds, the modified dye molecule is formed, whereas the -N=C=O bond of the DEET molecule, which is responsible for the repellent activity, remains unchanged. However, the synthesis of derivatives requires the introduction of an amino group to form a conjugate. The effect of this modification on the repellent activity of the substance has also not been investigated. The production of safe functional repellent coatings for protective clothing against attacks by dangerous blood-sucking insects on humans therefore remains an urgent task.
The purpose of this study is to investigate the properties of functional textile coatings with repellent effects containing N,N-diethyl-3-methylbenzamide. First, polymeric materials containing inorganic sorbents such as zirconia, alumina, and silica with grafted phenyl groups were obtained. Previously, we studied and described the sorption properties of these oxides and demonstrated their high sorption capacity towards synthetic repellents, particularly DEET [21]. In this work, repellent-containing sorbents were fixed on a polyacrylate film. The structure of the films obtained was studied by scanning electron microscopy and FTIR spectroscopy, and the process of thermal diffusion of the repellent was also investigated. The resulting composition was applied to the textile, after which the material structure was examined by scanning electron microscopy, and the repellency was determined via high-performance liquid chromatography with diode array detection. The results obtained open new possibilities for the development of protective materials with reduced toxicity against attacks by blood-sucking insects with controlled release of repellents.
2. Materials and Methods
2.1. Materials
N,N-diethyl-3-methylbenzamide (DEET) was purchased from Merck (Darmstadt, Germany); acetonitrile was purchased from Honeywell (Charlotte, NC, USA); aluminum nitrate nonahydrate, zirconyl nitrate dihydrate, ammonium nitrate, citric acid hydrate, ethylene glycol and ethyl acetate were purchased from JSC LenReactiv (St. Petersburg, Russia); polyacrylic acid copolymer consisting of a mixture of 2-(perfluorodecyl)ethyl acrylate and stearyl acrylate was purchased from Zhangzhou F&S New Material Co., Ltd. (Zhangzhou, Fujian, China); and the Silasorb phenyl (SiO2-phenyl) used was from Elsico (Moscow, Russia) without further purification. Water was purified via a Direct-Q system (Millipore, Darmstadt, Germany).
2.2. Methods
2.2.1. Synthesis of the Zirconia and Alumina Sorbents
The synthesis of zirconia and alumina, which are sorbents for the DEET repellent, has been described in detail previously [21]. The sol–gel method for the preparation of these oxides involves the interaction of zirconyl nitrate (or aluminum nitrate) with ammonium nitrate in the presence of citric acid and ethylene glycol. The mixture was then heated at 95 °C until evaporation occurred and subsequently ignited. The resulting product was purified from residual carbon at 800 °C for 2 h.
2.2.2. Static Sorption of DEET
A detailed description of the sorption of the repellent DEET on inorganic oxides has also been described in a previous paper [21]. When sorption was complete, the oxides were quantitatively removed from the solution and dried for 2 h using an IKA RV digital 10 V rotary evaporator (Staufen, Germany) to remove residual acetonitrile.
2.2.3. Preparation of Polymeric Films with DEET-Containing Inorganic Sorbents
In total, 2 g of sorbent (Al2O3, ZrO2, SiO2-phenyl) containing the DEET repellent was mixed with 7 g of polyacrylic acid copolymer in a 50 mL glass beaker and stirred for 10 min at room temperature. Then, 0.25 mL of the obtained mixture was placed in a mold (15 mm diameter, 0.5 mm height) and centrifuged using an EZ4 spin coater (Lebo Science, Beijing, China) for 5 min at 300 rpm. The polymeric opaque film (15 mm diameter, 0.5 mm thickness) was then removed from the mold and dried at 30 °C for 3 h.
2.2.4. Scanning Electron Microscopy (SEM)
The morphology of the samples was examined on a MIRA3 autoemission scanning electron microscope (TESCAN, Brno, Czech Republic) with an SE detector at an acceleration voltage of 1 kV.
2.2.5. FTIR Spectroscopy
The FTIR spectra were recorded on an InfraLUM FT-08 FTIR spectrometer in ATR FTIR mode (Lumex, St. Petersburg, Russia) via a KBr beam splitter in the spectral range of 600–3000 cm−1. In OMNIC data acquisition software (OMNIC 8.3, Thermo Fisher Scientific, Waltham, MA, USA) of the FTIR spectrometer, the resolution was set at 4 cm−1, and optical aperture as “open”, number of points—3662 and 3527 for DEET in liquid film and polymer films, respectively.
2.2.6. Thermogravimetric Analysis of Polymer Films Containing DEET
Thermogravimetric analysis (TGA) (Thermal Analyser—STA 449 F5 Jupiter, Netzsch, Selb, Germany) was performed for samples (10 ± 2 mg weight) with different sorbents and for a sample of polyacrylate film (15 mm diameter, 0.5 mm thickness) without sorbents. The samples were heated to 800 °C under nitrogen at a heating rate of 10 °C/min.
2.2.7. Thermal Diffusion Parameters of DEET from Polymer Films
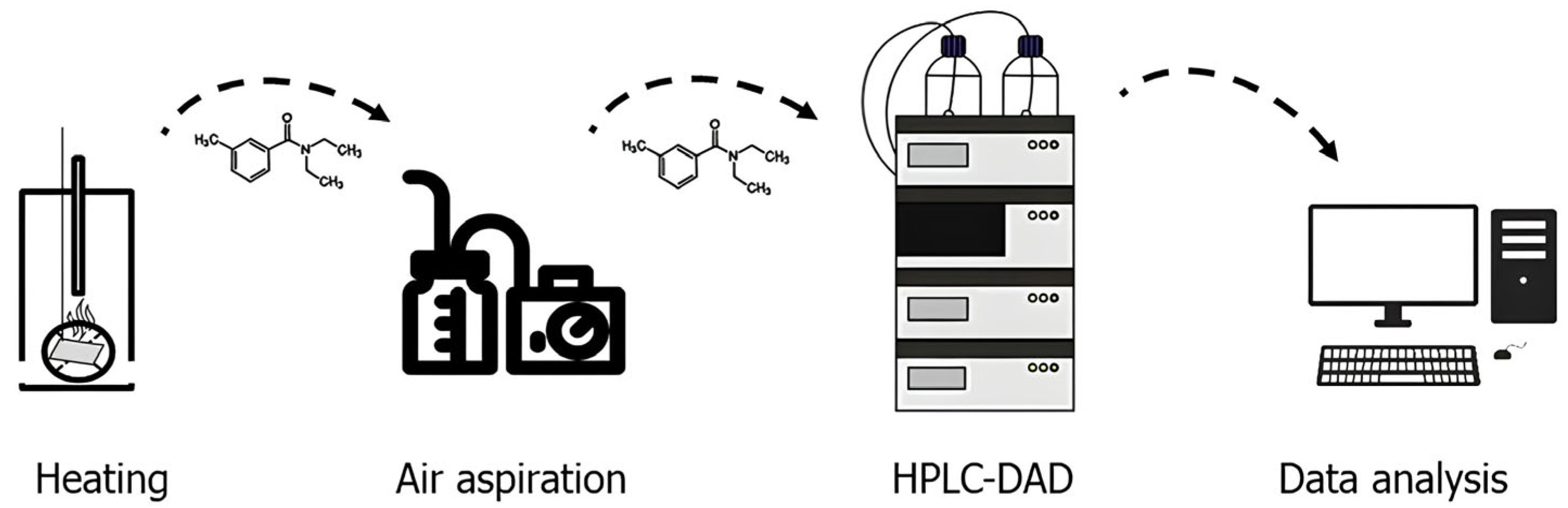
The polymeric film (15 mm diameter, 0.5 mm thickness) containing DEET immobilized on the sorbent was placed on a glass substrate and heated to 50, 100, 150 and 200 °C (Figure 1). After the material was held at a given temperature for 1, 5, 10, 15 and 30 min, an air sample was taken via a UOPV 4–40 aspirator (Niki-Mlt, St. Petersburg, Russia) with two Petri absorbers connected in series, each filled with 10 mL of acetonitrile. The air sample was taken at a rate of 2 L∙min−1 for 5 min. The solvent was then evaporated, and the sample was diluted in 5 mL of acetonitrile. The DEET content of the samples was determined via high-performance liquid chromatography with diode array detection (DAD) on an Ultimate 3000 HPLC-system (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of DEET in the samples was calculated via absolute calibration at a wavelength of 210 nm.
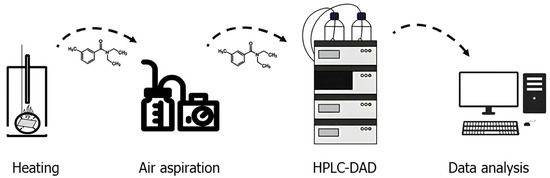
Figure 1.
Scheme of the experiment for determining the diffusion flux of N,N-diethyl-3-methylbenzamide from a polymeric film material.
From the data obtained, DEET concentration values (mol, 10−9) were calculated and plotted as a function of the flux Jf (W∙m−1∙K−1), which was calculated via the Fourier equation [22] (1)
Q—heat supplied to the polymeric material, W;
Δx—thickness of the polymeric material layer, m;
ε—an arbitrary number smaller but close to 1;
T1—material heating temperature, K;
T2—material surface temperature, K;
K—thermal conductivity constant of the polymeric film material, W∙m−1∙s−1∙K−1.
For each of the polymeric film formulations, 10 replicates were performed.
2.2.8. Coating of Textile Fibers with DEET-Containing Polymer Films
A 5 × 5 cm polyester textile sample (density of 160 g/m2) was immersed in a solution of the copolymer of polyacrylic acid with the inorganic sorbents, obtained according to point 2.2.3 of the Methods Section, and kept for 3 min. The sample was then wiped out and dried at 170 °C for 3 min. The samples were then stored in heat-sealed polyester bags to prevent the loss of repellency.
2.2.9. Determination of DEET Concentration in Textile Samples
The textile samples obtained according to Section 2.2.8 in the Methods Section were cut into small pieces and placed in a 100 mL round-bottom flask to which 50 mL of ethyl acetate was added. The solution was refluxed for 5 h with stirring, the pieces of textile were removed, the contents of the flask were evaporated (IKA RV digital 10 V, Staufen, Germany), and the sample was diluted in 5 mL of acetonitrile. The DEET content was determined under the conditions described in the Methods Section 2.2.7. Five replicates were performed for each textile sample.
2.2.10. Data Analysis
Chromatographic data were collected and processed via Chromeleon 7 software (Thermo Fischer Scientific, Waltham, MA, USA). Excel 2019 (Microsoft Corporation, Redmond, WA, USA) and OriginPro 2018 version b9.5.1.195 (Origin Corp., Northampton, MA, USA) were used for detailed calculations and plotting. Correlation between the values was determined using Spearman’s criteria [23]. Significance was set at p = 0.05 for all calculations.
3. Results and Discussion
3.1. Polymer Films Containing DEET
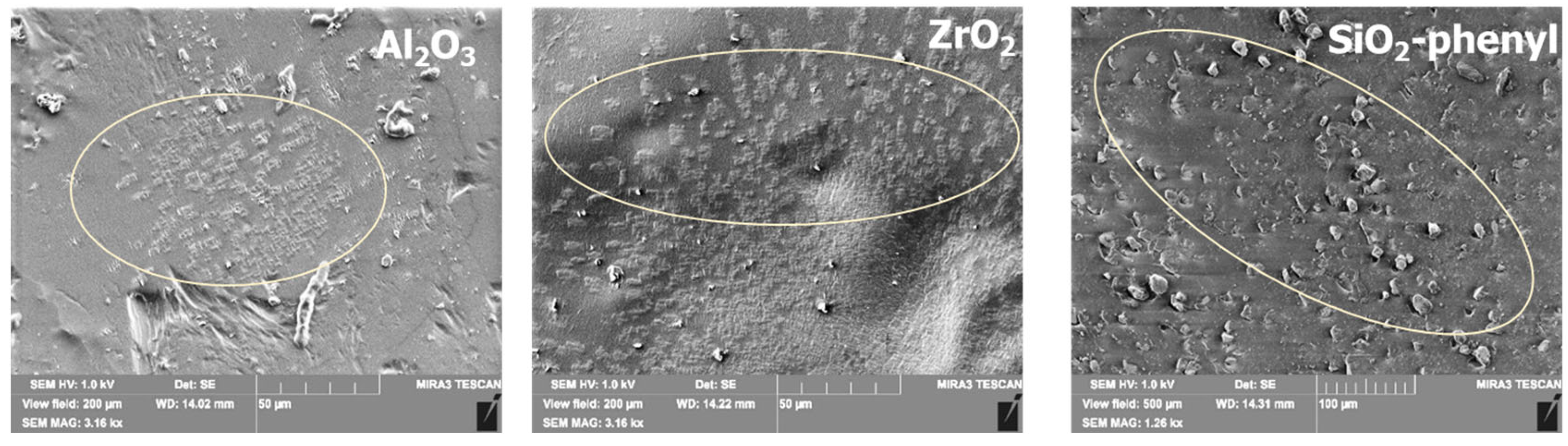
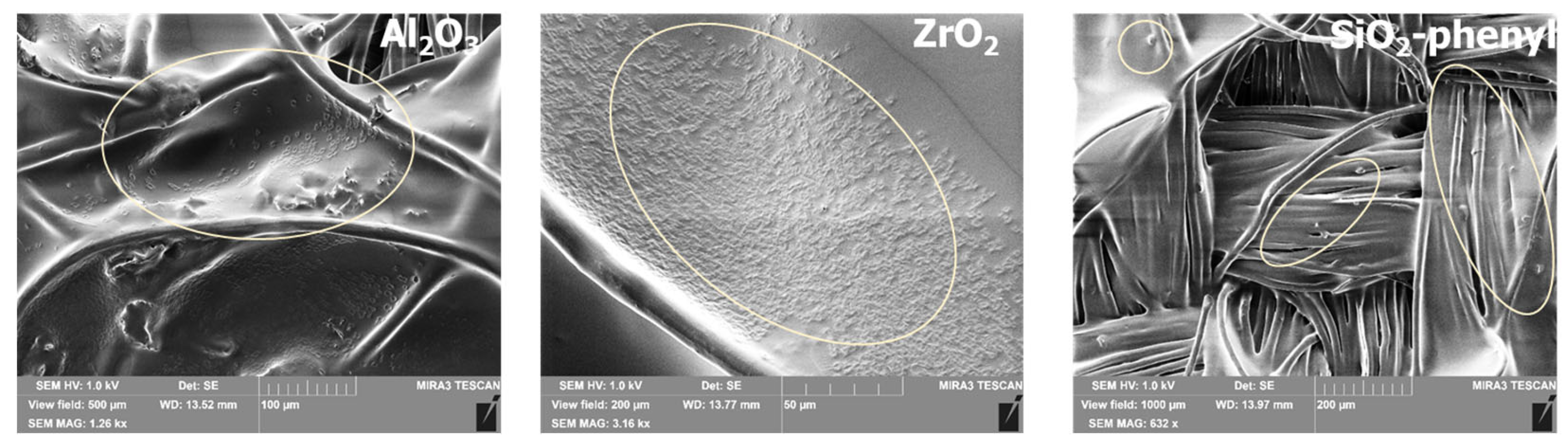
The inorganic oxides Al2O3, ZrO2 and SiO2-phenyl were selected as sorbents for the DEET repellent according to the results of previous studies [21], which showed pronounced sorption capacities around 63, 119 and 239 mg/g, respectively. To potentially exploit the sorption properties of the sorbents, inorganic substances were incorporated into a polyacrylate formulation based on 2-(perfluorodecyl)ethyl acrylate and stearyl acrylate. The choice of polyacrylates was due to their widespread use in the textile industry for fixing substances on the surface of fabrics and their ability to retain active substances on their surface when exposed to external influences. However, DEET is poorly soluble in water when mixed with selected polyacrylates; so, alternative solutions are needed to obtain functional coatings with repellent properties. It was possible to fix solid oxide particles in the polymeric layer when the sorbents were mixed with polyacrylates (Figure 2). Scanning electron microscopy results revealed that the sorbent particles were successfully incorporated into the polymer layer in all the cases. The particle size and shape of the inorganic sorbents are in agreement with the SEM data obtained in previous studies [21].
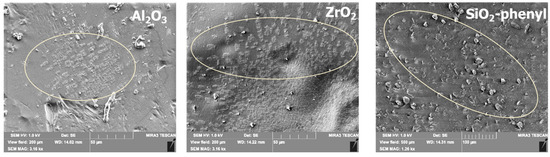
Figure 2.
Scanning electron microscopy images of polymer films with embedded inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl containing DEET.
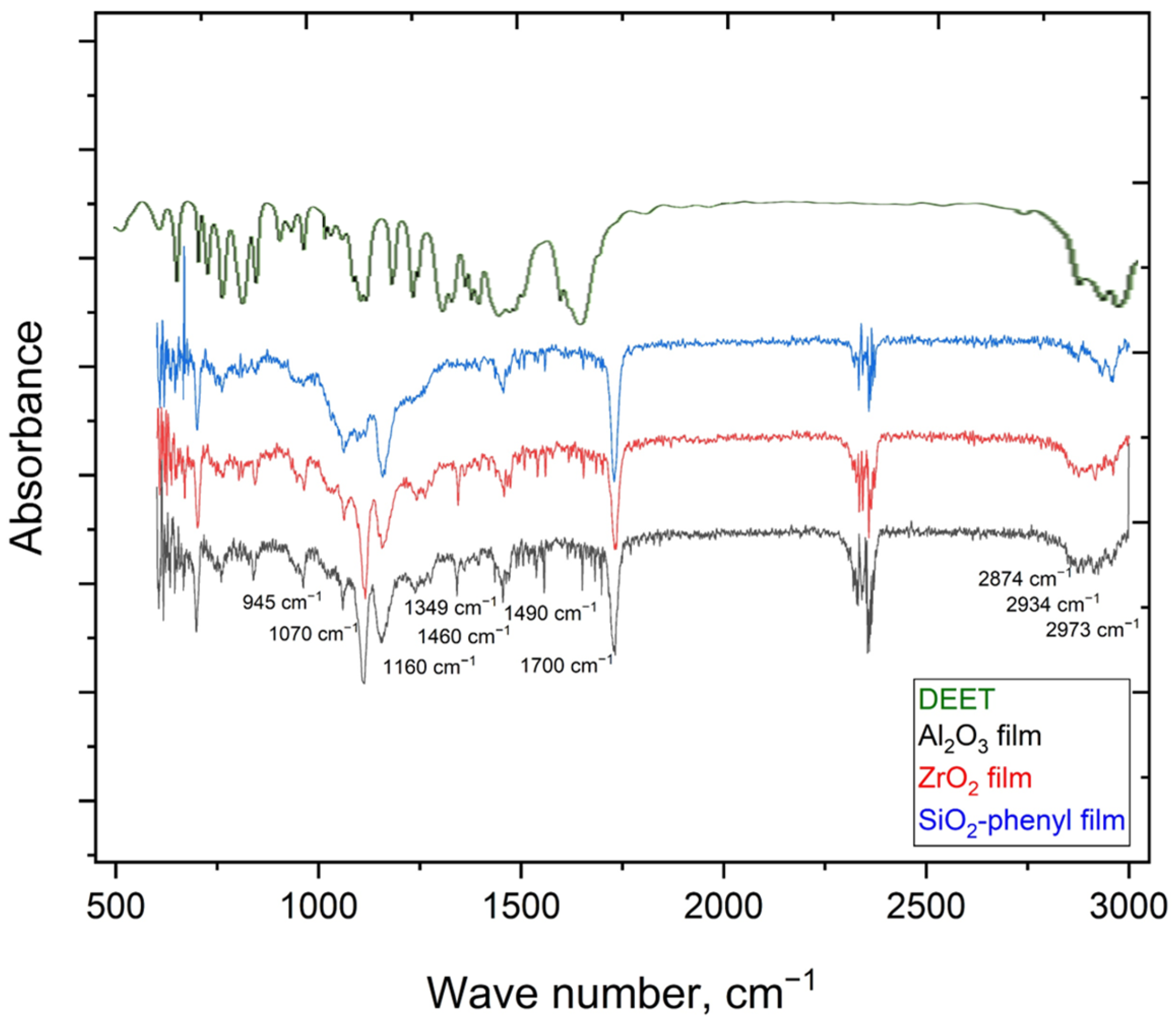
The presence of the DEET repellent in the polymeric materials containing the Al2O3, ZrO2 and SiO2 phenyl sorbents was confirmed by FTIR spectroscopy, as signals characteristic of N,N-diethyl-3-methylbenzamide (945, 1070, 1160, 1349, 1460, 1460, 1490, 1700, 2874, 2934, 2973 cm−1) were detected (Figure 3), indicating that DEET was successfully incorporated into the polymeric materials in all the cases.
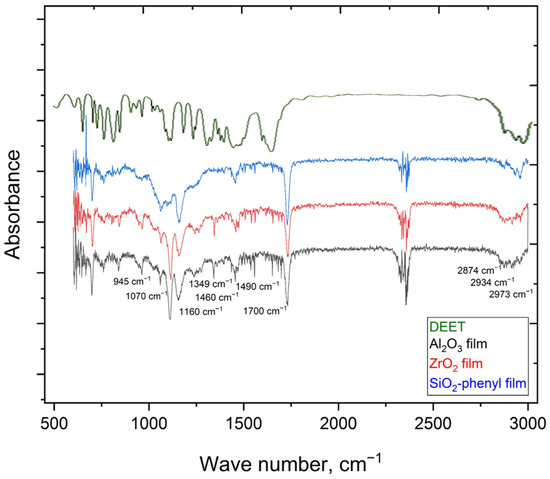
Figure 3.
FTIR spectra of DEET in liquid film and polymer films with the incorporated inorganic sorbents such as Al2O3, ZrO2 and SiO2-phenyl.
3.2. Determination of the Thermal Diffusion Parameters of DEET from Polymer Films
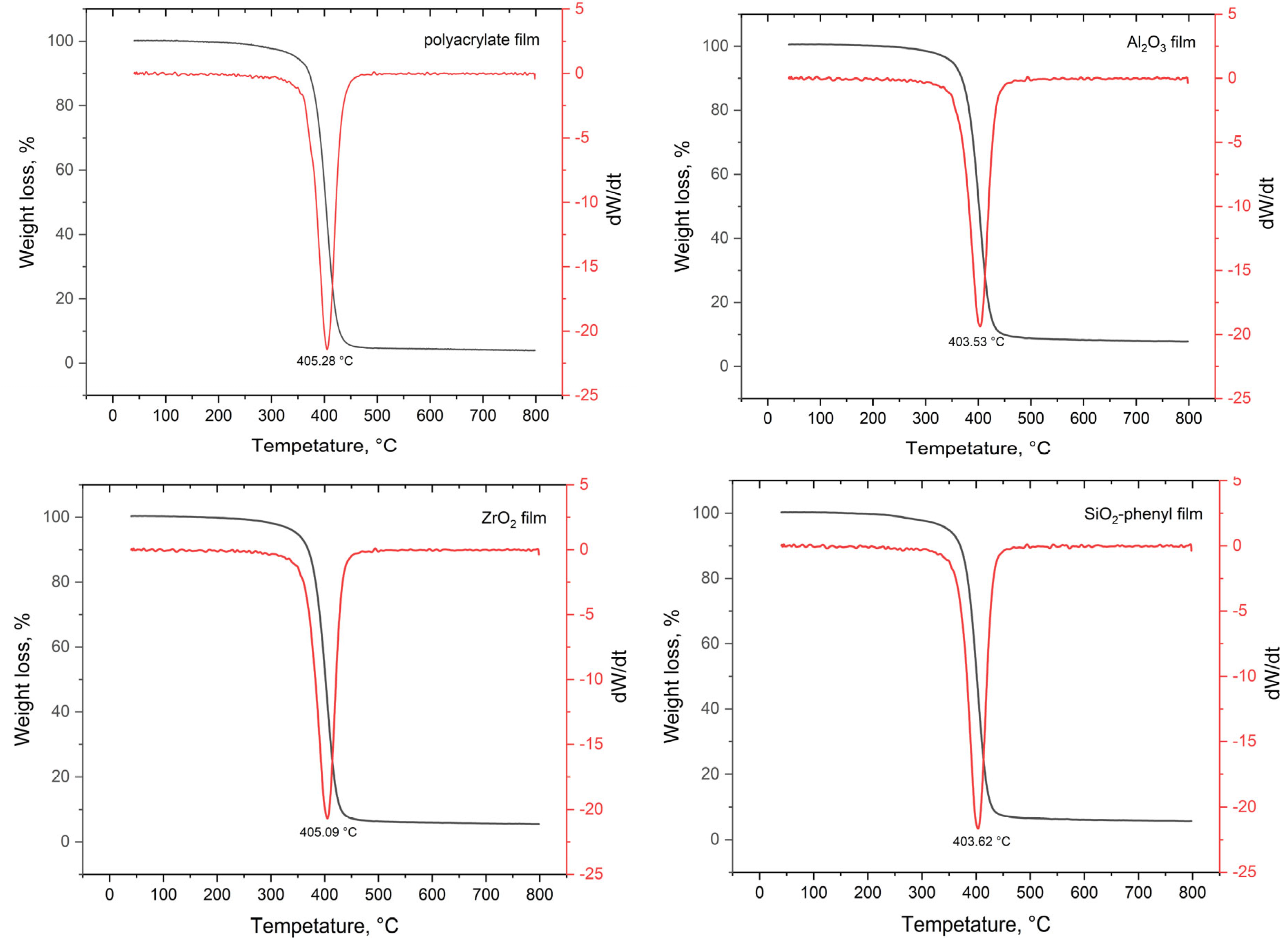
To determine the thermal diffusion parameters of DEET from polymeric materials with embedded inorganic sorbents, TGA studies were performed, and their derivatives were plotted (Figure 4).
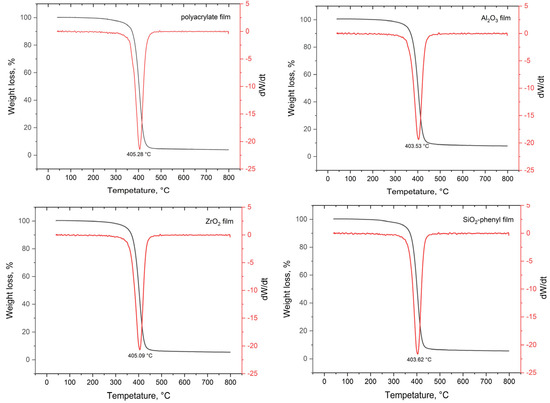
Figure 4.
TGA curves (black line) and their first derivative (red line) of polymer films with the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl containing the DEET repellent.
The decomposition temperatures of all the polymeric materials ranged from 403.5 to 405.3 °C, which is the same as the decomposition temperature of the polyacrylate copolymer. In the 40–400 °C section of the TGA curves (Figure 4), other volatile components of the system are indicated, i.e., water, which is a component of the polyacrylate copolymer, and the DEET repellent, which has a boiling point of 288 °C. However, according to the TGA curves obtained, the study of the release rate of the repellent was complicated by the low amount of repellent fixed in the polymer material; so, it was not possible to determine the optimum energy for the controlled release of DEET from the polymer surface.
To calculate the thermal diffusion parameters of DEET, an experiment was carried out in which polymer films with the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl were heated, followed by air sampling over the surface of the materials (Figure 1). This approach made it possible to study the rate of release of the repellent during successive heating of the polymeric film material. DEET concentration versus temperature was plotted for all the polymeric materials (Figure 5).
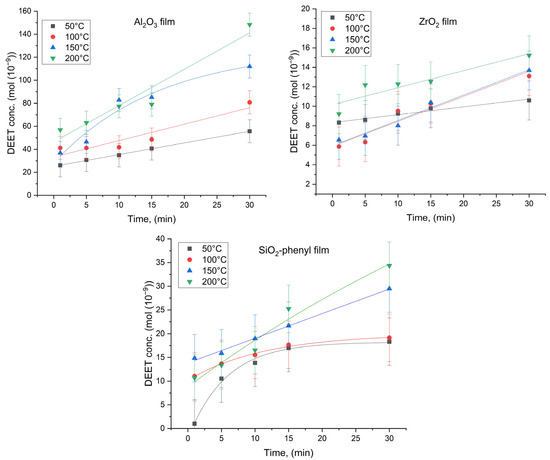
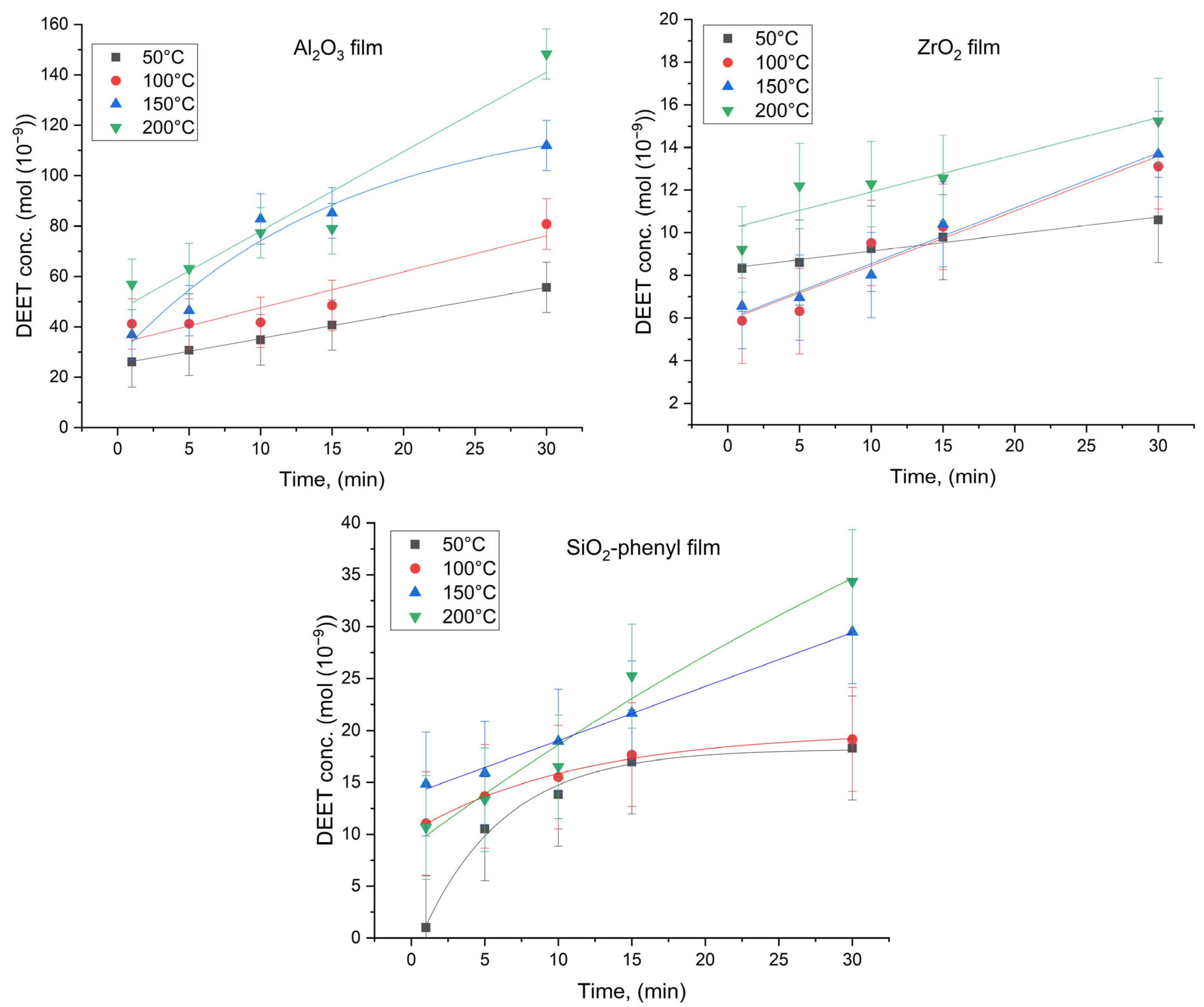
Figure 5.
Concentration of DEET (mol) formed by thermal diffusion on the surface of polymer films containing the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl as a function of time (min) (n = 10).
The plots obtained show that the concentration of repellent gradually increased with increasing exposure time of the polymeric materials under the given conditions. Moreover, significantly higher DEET concentrations were observed for the Al2O3 sorbent-based materials (up to 148.3∙10−9 mol at 200 °C) and, to a lesser extent, for the ZrO2 and SiO2-phenyl materials (up to 15.2∙10−9 mol and 34.3∙10−9 mol at 200 °C, respectively). This could be due to the porosity of these inorganic sorbents, with the mean pore size of Al2O3 being the smallest (3.84 nm—Al2O3, 9.85 nm—SiO2-phenyl, 46.0 nm—ZrO2) [21]. Analysis of the 200 °C DEET concentration data and sorbent average pore size values revealed a rank correlation of these parameters with a coefficient of +1 by Spearman’s test. It can be assumed that DEET thermal diffusion is significantly influenced by sorbent pore size. Moreover, we were not able to determine a single dependence that would describe the rate of release of the repellent for all the sorbents, because the shapes of the Al2O3, ZrO2 and SiO2-phenyl particles were different according to the SEM data. Notably, despite the greater sorption capacity of inorganic SiO2-phenyl oxide (239 mg/g) at the considered DEET ratio compared to those of Al2O3 (119 mg/g) and ZrO2 (63 mg/g) oxides, the amount of repellent diffusing from the surface of the polymeric film within 30 min was greater for the material containing the Al2O3 sorbent. Obviously, such materials would be able to repel faster, but SiO2-phenyl-based materials could last longer.
In addition, the data show that less heat than that required to reach the boiling point of DEET itself (288 °C) can be used to release the repellent from the material. In this way, the targeting efficiency of DEET-based materials can be enhanced by thermal diffusion processes that allow the repellent concentration to be controlled and fully predictable.
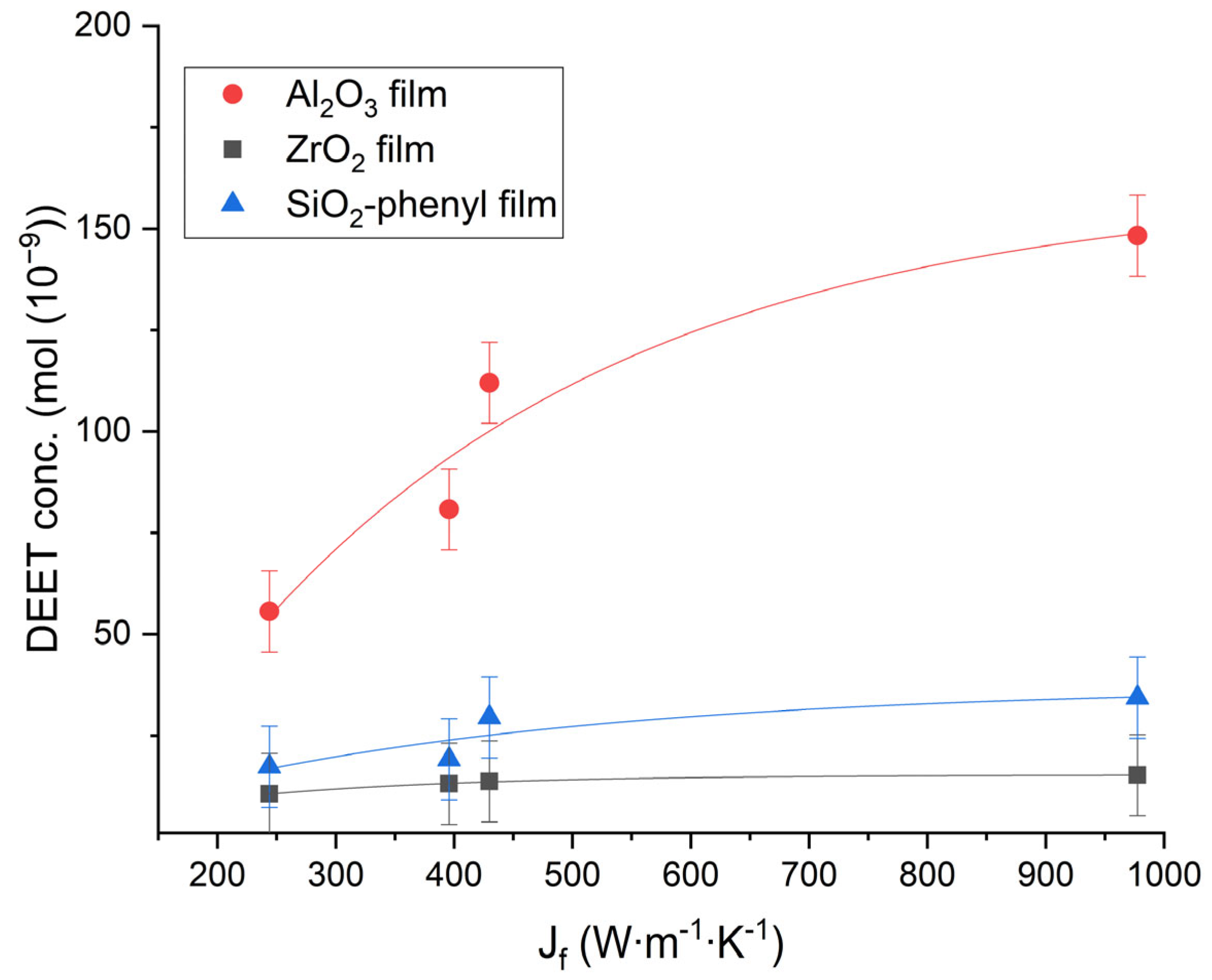
To determine the parameters of the total flux of matter, the values of the heat flux Jf (W∙m−1∙K−1) were calculated for each polymeric material on the basis of the provisions of the Onsager theory and the Fourier law [22]. From the data obtained, the DEET repellent concentration determined by thermal diffusion was plotted against the heat flux acting on the materials after 30 min (Figure 6).
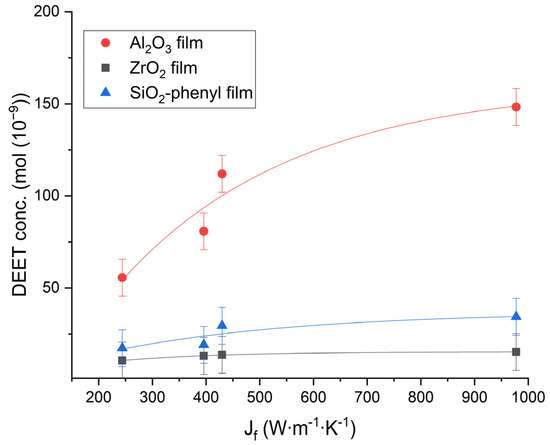
Figure 6.
The dependence of the concentration of DEET (mol) as a result of thermal diffusion over the surface of polymer films containing the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl on the heat flux Jf (W∙m−1∙K−1) at 30 min (n = 10).
The heat flow on the polymer films containing Al2O3 could lead to DEET diffusion in air faster and more efficiently than when applied to the films containing the ZrO2 or SiO2 phenyl sorbents. Moreover, the repellent concentrations obtained from the polymer materials of different composition in this study showed only basic differences and could be increased by using more sorbent in the polyacrylate composition.
3.3. Study of Polymer Films Containing the Sorbents with the DEET Repellent, Applied to Textile Materials
The obtained polymer films containing Al2O3, ZrO2 and SiO2-phenyl with the DEET repellent, were coated on textiles via thermal polymerization of the polyacrylate copolymer at 170 °C. This approach not only allowed the repellent to be successfully incorporated into the surface of the textile but also resulted in materials that were resistant to environmental factors.
The structure of the samples obtained was examined by scanning electron microscopy to confirm that the inorganic sorbents with the DEET repellent successfully coated the textiles fibers (Figure 7). The resulting images showed that Al2O3, ZrO2 and SiO2-phenyl were all attached to the surface of the polymerized polyacrylate layer on the textile fibers, providing a repellent effect.
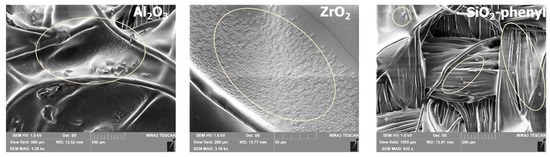
Figure 7.
Scanning electron microscopy images of textile materials with incorporated Al2O3, ZrO2 and SiO2-phenyl inorganic sorbents containing DEET.
The concentration of DEET in the obtained textile samples was determined using HPLC- DAD after extraction of the repellent in ethyl acetate for 5 h. The experimental time was optimized by repeating the extraction under the same conditions. The results are summarized in Table 1.

Table 1.
Results of the determination of the DEET repellent content in textile materials.
The results obtained fully correlated with the sorption capacity data of the sorbents themselves, with the highest DEET repellent content being a feature of SiO2-phenyl oxide. A similar relationship was found for the sorption capacity of the textile materials containing Al2O3 and ZrO2 oxides. The suitability of this method for the determination of repellent content in textile samples is shown by the DEET content on re-extraction under the given conditions (<0.12) and the extraction rate of this method (over 99%). It was shown that by creating functional polyacrylate-based coatings, it was possible to successfully deliver the difficult-to-dissolve repellent DEET to textiles, opening new possibilities for creating controlled-release repellent materials.
4. Conclusions
In this study, polymer films based on polyacrylate (2-(perfluorodecyl)ethyl acrylate and stearyl acrylate) containing the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl with the DEET repellent were prepared. The high sorption capacity of oxides allows them to be used as carrier materials for repellents in functional coatings for the targeted delivery of repellents to a variety of surfaces, such as textiles. The obtained scanning electron microscopy images and FTIR spectroscopy data confirmed that this approach can be used to obtain polymer films with repellent properties.
The thermal diffusion of DEET from the surface of polymeric materials was also the subject of detailed investigations. According to the results obtained, materials containing the Al2O3 sorbent are able to thermally diffuse the DEET repellent from their surface faster and at higher concentrations than materials containing the other ZrO2 and SiO2-phenyl oxides, despite the greater sorption capacity of phenyl group-modified silica. Release of the repellent from polymeric materials can also be achieved by applying less heat than is required to reach the boiling point of DEET. In this way, the resulting materials can be acted upon in a controlled manner by means of thermal diffusion processes, which helps to improve their target efficiency.
The possibility of using these polymer materials as functional coatings on textiles was demonstrated in the final step of the research. The SEM images and HPLC data obtained show that a polyacrylate formulation containing the inorganic sorbents Al2O3, ZrO2 and SiO2-phenyl with the DEET repellent was successfully applied to textiles, opening new possibilities for developing controlled-release materials.
Author Contributions
Conceptualization, S.Z. and S.A.; methodology, S.A.; software, S.Z. and M.R.; formal analysis, S.Z.; investigation, S.Z., EA. and V.M.; resources, S.Z., V.V. and M.R.; data curation, S.Z. and E.A.; writing—original draft preparation, S.Z.; writing—review and editing, S.A. and V.V.; visualization, S.Z.; supervision, S.A. and V.V.; project administration, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to the staff of the MIREA—Russian Technological University (M.V. Lomonosov Institute of Fine Chemical Technologies) and Gubkin University for their assistance in conducting physicochemical studies.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DEET | N,N-diethyl-3-methylbenzamide |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| ATR | Attenuated total reflectance |
| TGA | Thermogravimetric analysis |
| HPLC | High-performance liquid chromatography |
| DAD | Diode array detection |
References
- Tiwari, I.; Mahanwar, P.A. Polyacrylate/silica hybrid materials: A step towards multifunctional properties. J. Dispers. Sci. Technol. 2019, 40, 925–957. [Google Scholar] [CrossRef]
- Shi, H.; Hong, L.; Pan, K.; Wei, W.; Liu, X.; Li, X. Biodegradable polyacrylate copolymer coating for bio-functional magnesium alloy. Prog. Org. Coat. 2021, 159, 106422. [Google Scholar] [CrossRef]
- Wu, L.; Baghdachi, J. Functional Polymer Coatings: Principles, Methods, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–369. [Google Scholar]
- Coetzee, D.; Militky, J.; Venkataraman, M. Functional coatings by natural and synthetic agents for insect control and their applications. Coatings 2022, 12, 476. [Google Scholar] [CrossRef]
- Sikder, A.; Pearce, A.K.; Parkinson, S.J.; Napier, R.; O’Reilly, R.K. Recent trends in advanced polymer materials in agriculture related applications. ACS Appl. Polym. Mater. 2021, 3, 1203–1217. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in agriculture: A review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Focke, W.W.; Tewo, R.K.; Androsch, R.; Kruger, T. Mosquito-repellent controlled-release formulations for fighting infectious diseases. Malar. J. 2021, 20, 165. [Google Scholar] [CrossRef]
- Zverev, S.A.; Andreev, S.V.; Sakharov, K.A.; Akhmetshina, M.B.; Istomina, L.I.; Verzhutskaya, Y.A.; Shashina, N.I. Evaluation of the efficacy of permethrin-and cypermethrin-based textile against taiga tick, Ixodes persulcatus. Exp. Appl. Acarol. 2023, 89, 275–286. [Google Scholar] [CrossRef]
- Duan, J.; Xu, Y.; Lei, Y.; Cheng, Y.; Tan, Y.; Zhu, S.; Xia, H.; Li, S.; Shi, P.; Tang, J. Preparation and properties of water-soluble coatings resistant to scratching on the surface of nuclear cladding. Nucl. Eng. Des. 2024, 417, 112831. [Google Scholar] [CrossRef]
- Elsayed, G.A.; Hassabo, A.G. Insect repellent of cellulosic fabrics (a review). Lett. Appl. NanoBioScience 2022, 11, 3181–3190. [Google Scholar] [CrossRef]
- Mapossa, A.B.; da Silva Júnior, A.H.; Mhike, W.; Sundararaj, U.; Silva de Oliveira, C.R. Electrospun Polymeric Nanofibers for Malaria Control: Advances in Slow-Release Mosquito Repellent Technology. Macromol. Mater. Eng. 2024, 309, 2400130. [Google Scholar] [CrossRef]
- Fulton, A.C.; Thum, M.D.; Jimenez, J.; Camarella, G.; Cilek, J.E.; Lundin, J.G. Long-Term Insect Repellent Electrospun Microfibers from Recycled Poly (ethylene terephthalate). ACS Appl. Mater. Interfaces 2023, 15, 44722–44730. [Google Scholar] [CrossRef] [PubMed]
- Chatha, S.A.S.; Asgher, M.; Asgher, R.; Hussain, A.I.; Iqbal, Y.; Hussain, S.M.; Bilal, M.; Saleem, F.; Iqbal, H.M. Environmentally responsive and anti-bugs textile finishes–Recent trends, challenges, and future perspectives. Sci. Total Environ. 2019, 690, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Peila, R.; Scordino, P.; Shanko, D.B.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of β-cyclodextrin nanosponges for N, N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94. [Google Scholar] [CrossRef]
- Bonadies, I.; Longo, A.; Androsch, R.; Jehnichen, D.; Göbel, M.; Di Lorenzo, M.L. Biodegradable electrospun PLLA fibers containing the mosquito-repellent DEET. Eur. Polym. J. 2019, 113, 377–384. [Google Scholar] [CrossRef]
- Ap dos Santos, D.; Oliveira, A.M.; Cerize, N.N.; Manhani, K.C.; Santos, D.D.S.; de Araujo, H.C.; Costa, S.A.; Costa, S.M. Synthesis of polymeric particles with insect repellent for potential application on textile substrates. Mater. Chem. Phys. 2022, 280, 125662. [Google Scholar] [CrossRef]
- Gilbert, I.H.; Gouck, H.K. Evaluation of repellents against mosquitoes in Panama. Fla. Entomol. 1955, 38, 153–163. [Google Scholar] [CrossRef]
- Xiang, C.; Etrick, N.R.; Frey, M.W.; Norris, E.J.; Coats, J.R. Structure and properties of polyamide fabrics with insect-repellent functionality by electrospinning and oxygen plasma-treated surface coating. Polymers 2020, 12, 2196. [Google Scholar] [CrossRef]
- Singh, A.; Sheikh, J. Development of multifunctional polyester using disperse dyes based through a combination of mosquito repellents. J. Mol. Struct. 2021, 1232, 129988. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Mokhtari, J.; Kolkoohi, S.; Amin Sarli, M. Imparting insect repellency to nylon 6 fibers by means of a novel MCT reactive dye. J. Appl. Polym. Sci. 2012, 126, 1097–1104. [Google Scholar] [CrossRef]
- Zverev, S.A.; Vinogradova, Y.V.; Selivanova, A.A.; Solovov, R.D.; Sakharov, K.A.; Ischenko, A.A.; Andreev, S.V. Study of sorption properties of zirconia, alumina, and silica in relation to repellents. Colloid Polym Sci. 2024, 302, 1259–1268. [Google Scholar] [CrossRef]
- Sher, E.; Moshkovich-Makarenko, I.; Moshkovich, Y.; Cukurel, B. Implementation of the Onsager theorem to evaluate the speed of the deflagration wave. Entropy 2020, 22, 1011. [Google Scholar] [CrossRef]
- Spearman, C. Correlation calculated from faulty data. Br. J. Psychol. 1910, 3, 271–295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).