Kelvin Probe Force Microscopy, Current Mapping, and Optical Properties of Hybrid ZnO Nanorods/Ag Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. ZnO Nanorods and Ag Nanoparticles Synthesis
2.2. Deposition and Characterization Methods
3. Results and Discussion
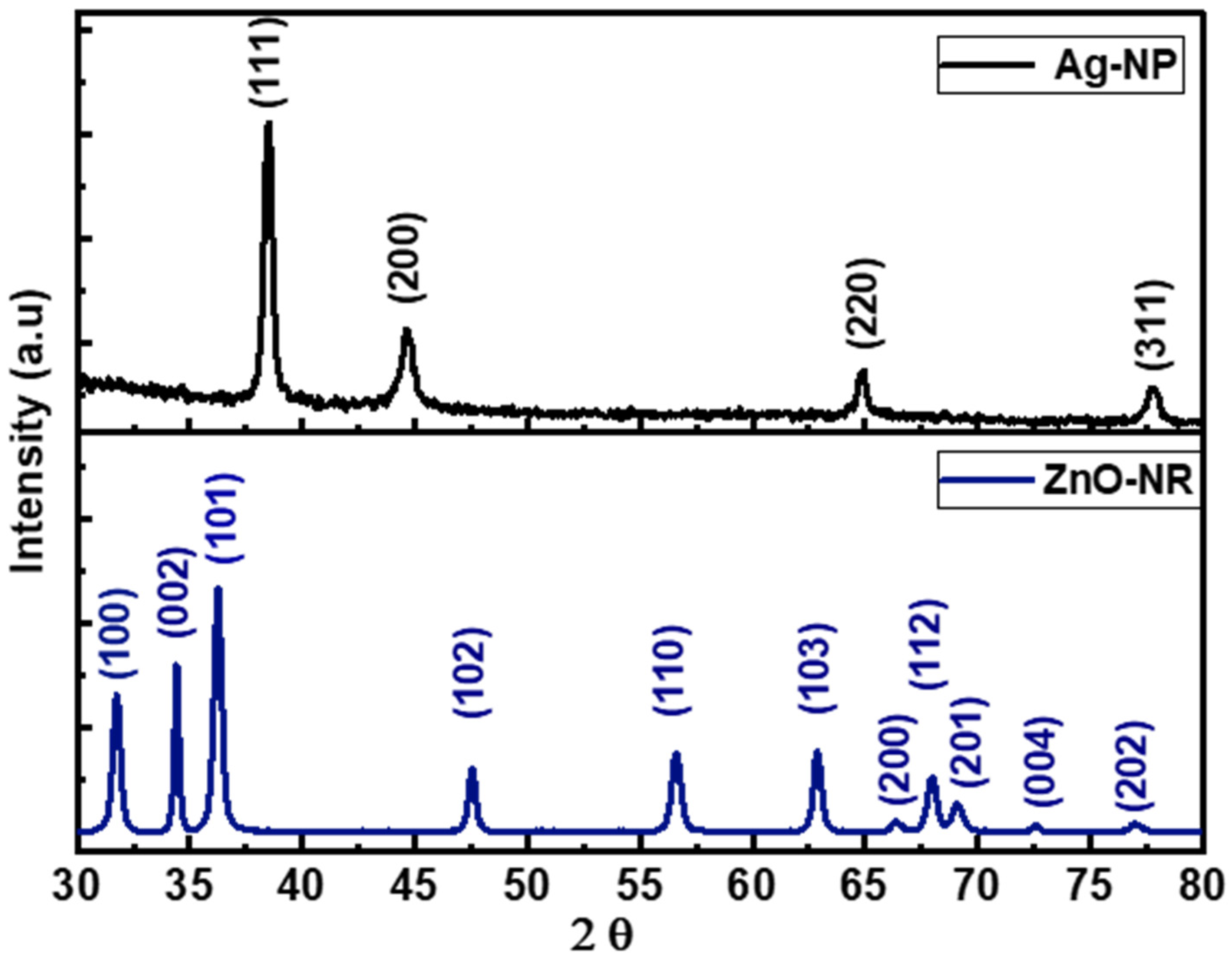
3.1. Morphology and Structure of ZnO-NRs and Ag-NPs
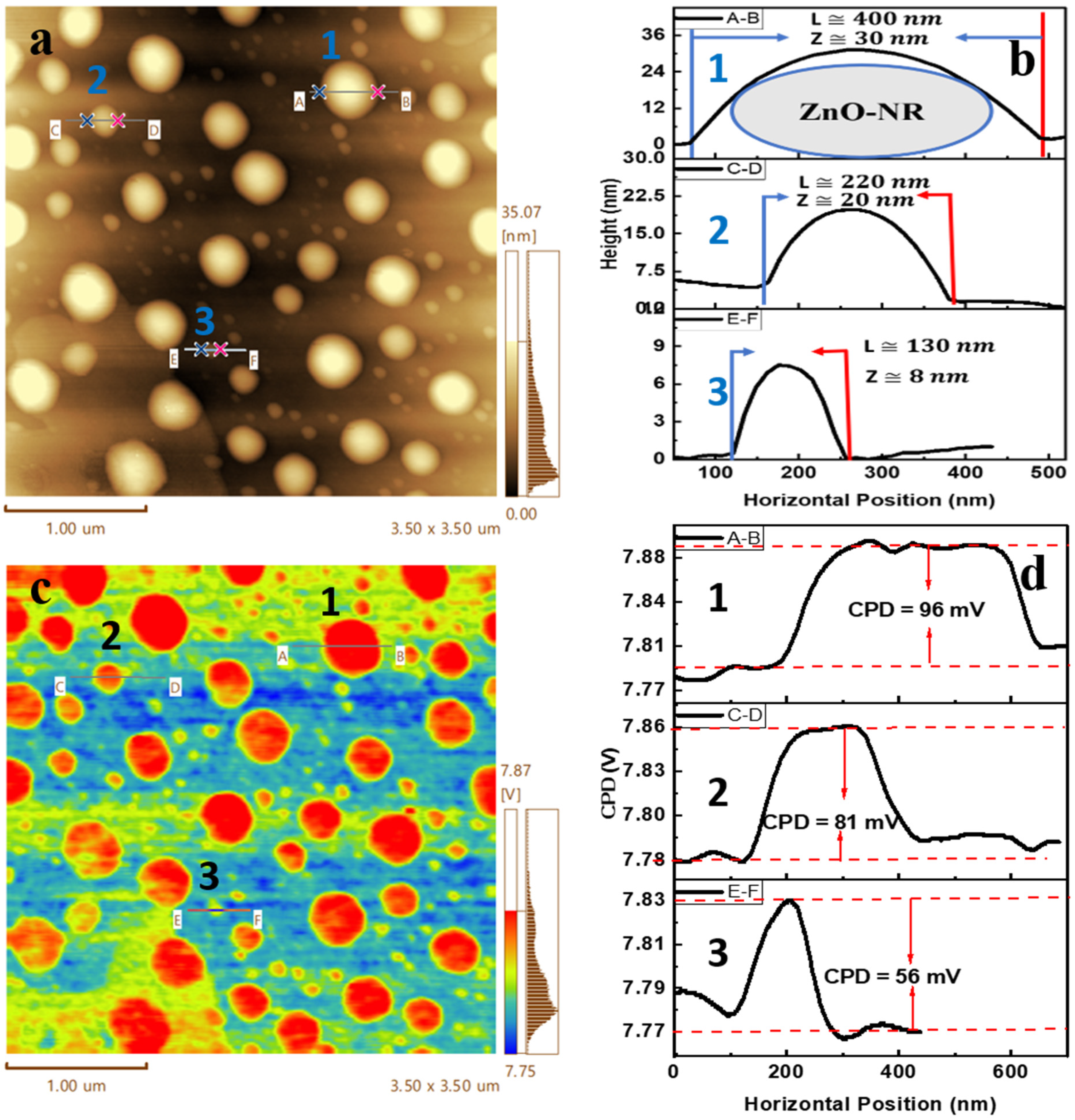
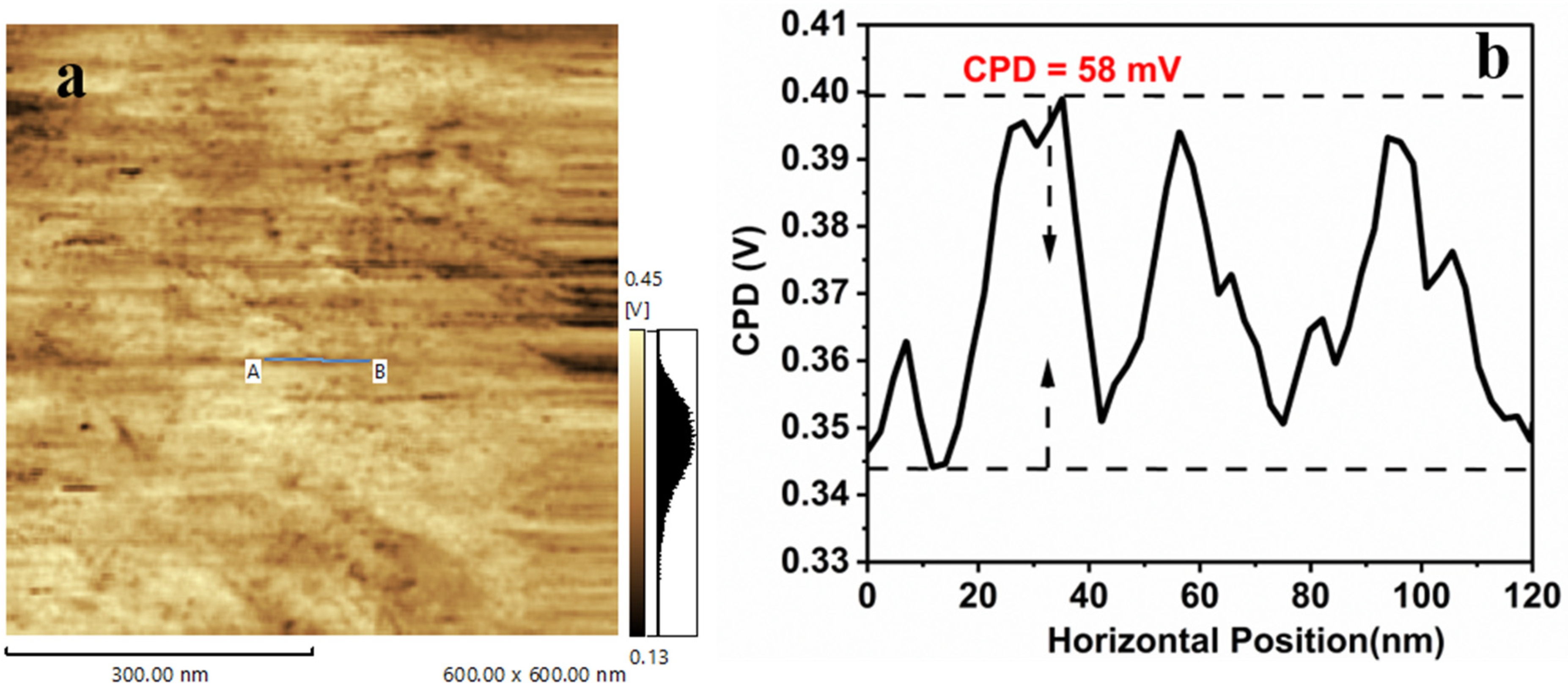
3.2. Kelvin Probe Force Microscopy of ZnO-NRs and AgNPs
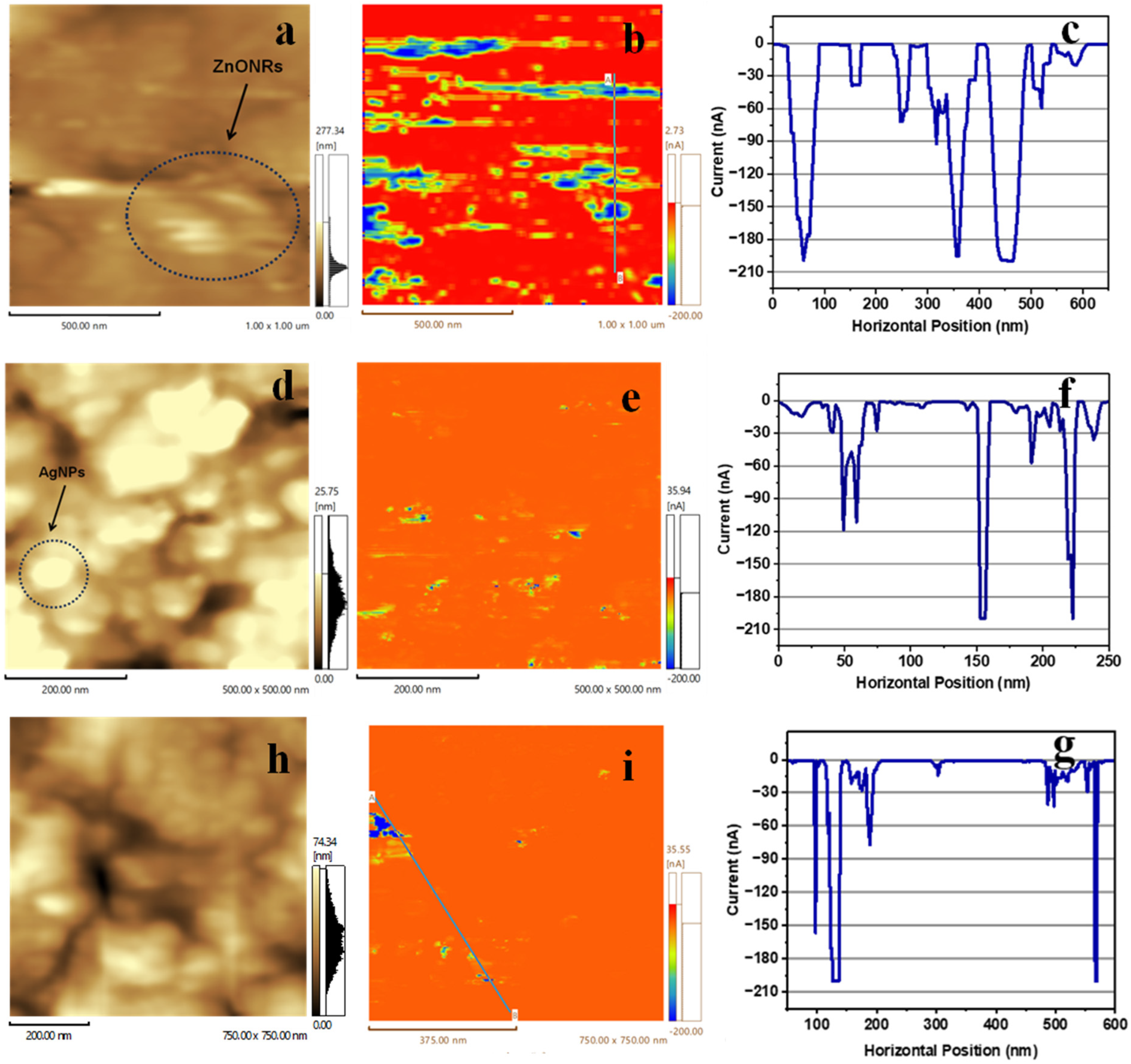
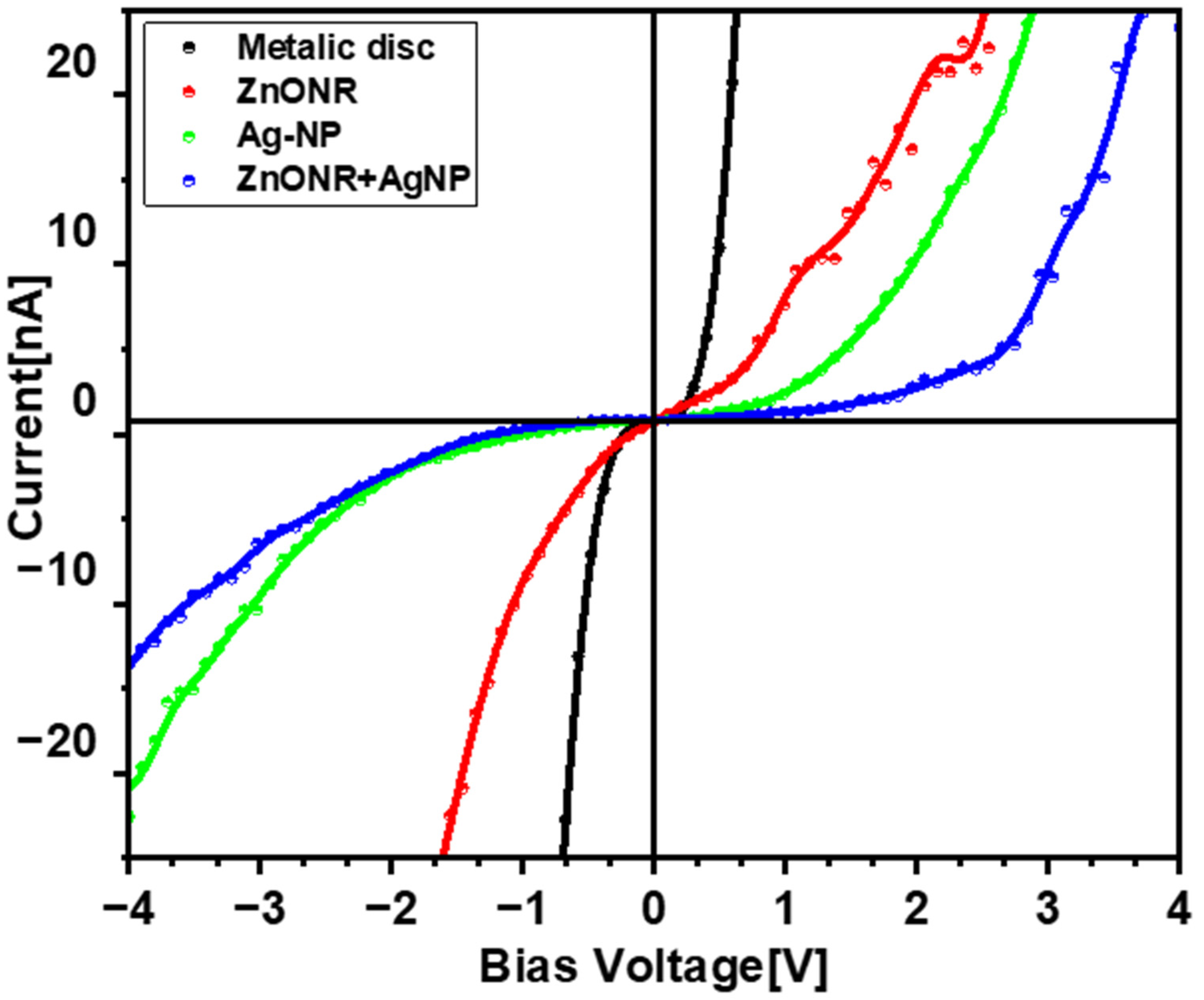
3.3. Current Mapping and I-V Measurements
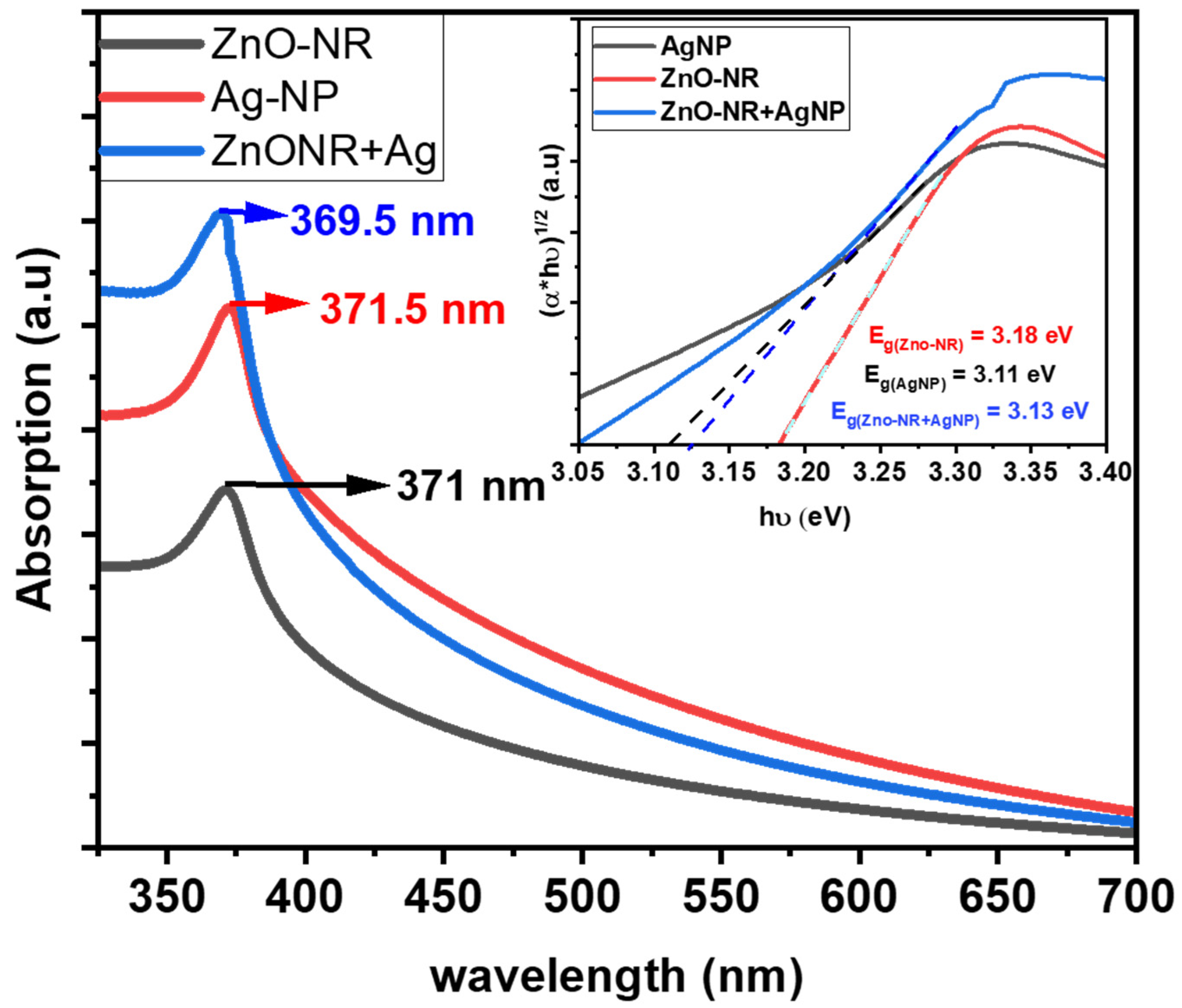
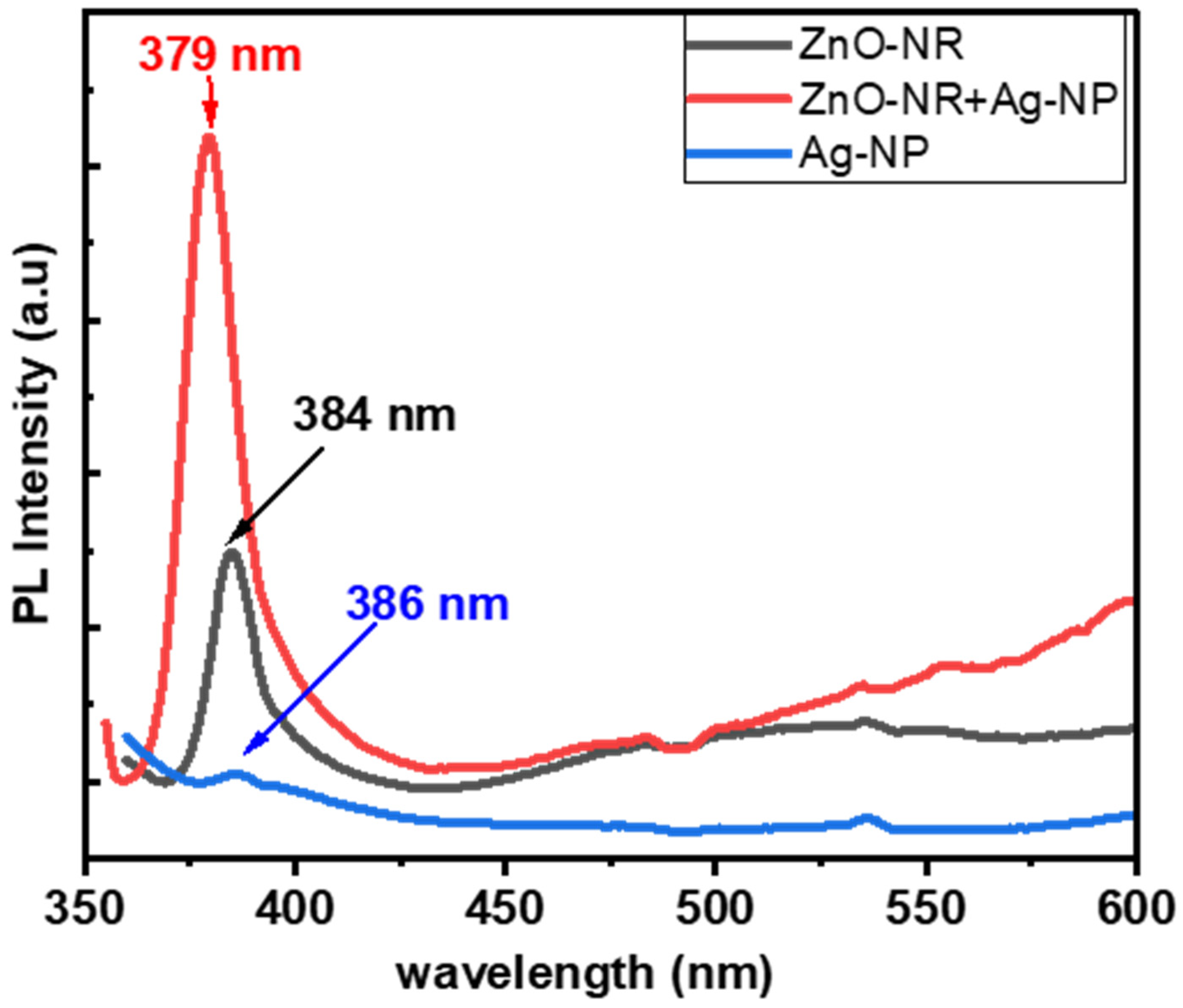
3.4. Optical Properties of Hybrid of ZnO-NRs and Ag-NPs
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Que, M.; Lin, C.; Sun, J.; Chen, L.; Sun, X.; Sun, Y. Progress in ZnO Nanosensors. Sensors 2021, 21, 5502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, Y.; Zheng, H.; Ramakrishna, S. Piezoelectric Materials for Flexible and Wearable Electronics: A Review. Mater. Des. 2021, 211, 110164. [Google Scholar] [CrossRef]
- Putri, A.E.; Roza, L.; Budi, S.; Ali Umar, A.; Fauzia, V. Tuning the Photocatalytic Activity of Nanocomposite ZnO Nanorods by Shape-Controlling the Bimetallic AuAg Nanoparticles. Appl. Surf. Sci. 2021, 536, 147847. [Google Scholar] [CrossRef]
- Lefatshe, K.; Dube, P.; Sebuso, D.; Madhuku, M.; Muiva, C. Optical Dispersion Analysis of Template Assisted 1D-ZnO Nanorods for Optoelectronic Applications. Ceram. Int. 2021, 47, 7407–7415. [Google Scholar] [CrossRef]
- Onyszko, M.; Zywicka, A.; Wenelska, K.; Mijowska, E. Revealing the Influence of the Shape, Size, and Aspect Ratio of ZnO Nanoparticles on Antibacterial and Mechanical Performance of Cellulose Fibers Based Paper. Part. Part. Syst. Charact. 2022, 39, 2200014. [Google Scholar] [CrossRef]
- Boulahlib, S.; Dib, K.; Özacar, M.; Bessekhouad, Y. Optical, Dielectric, and Transport Properties of Ag-Doped ZnO Prepared by Aloe Vera Assisted Method. Opt. Mater. 2021, 113, 110889. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Rajeh, A. Influence of ZnO/Ag Nanoparticles Doping on the Structural, Thermal, Optical and Electrical Properties of PAM/PEO Composite. Phys. B Condens. Matter 2020, 578, 411796. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, D.; Okram, G.S. Influence of Surfactant, Particle Size and Dispersion Medium on Surface Plasmon Resonance of Silver Nanoparticles. J. Phys. Condens. Matter 2020, 32, 145302. [Google Scholar] [CrossRef]
- Mehata, M.S. Green Route Synthesis of Silver Nanoparticles Using Plants/Ginger Extracts with Enhanced Surface Plasmon Resonance and Degradation of Textile Dye. Mater. Sci. Eng. B 2021, 273, 115418. [Google Scholar] [CrossRef]
- Alzoubi, F.Y.; Ahmad, A.A.; Aljarrah, I.A.; Migdadi, A.B.; Al-Bataineh, Q.M. Localize Surface Plasmon Resonance of Silver Nanoparticles Using Mie Theory. J. Mater. Sci. Mater. Electron. 2023, 34, 2128. [Google Scholar] [CrossRef]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized Surface Plasmon Resonance Sensing of Trenbolone Acetate Dopant using Silver Nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef]
- Checa, M.; Neumayer, S.M.; Tsai, W.-Y.; Collins, L. Advanced Modes of Electrostatic and Kelvin Probe Force Microscopy for Energy Applications. In Atomic Force Microscopy for Energy Research; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-317404-2. [Google Scholar]
- Wang, J.; Zhang, H.; Cao, G.; Xie, L.; Huang, W. Injection and Retention Characterization of Trapped Charges in Electret Films by Electrostatic Force Microscopy and Kelvin Probe Force Microscopy. Phys. Status Solidi A 2020, 217, 2000190. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Liu, M.; Sun, L.; Qiu, X. Electrical Characteristics of a Carbon Nanotube-Functionalized Probe for Kelvin Probe Force Microscopy. J. Phys. Chem. C 2020, 124, 28261–28266. [Google Scholar] [CrossRef]
- Jakob, D.S.; Li, N.; Zhou, H.; Xu, X.G. Integrated Tapping Mode Kelvin Probe Force Microscopy with Photoinduced Force Microscopy for Correlative Chemical and Surface Potential Mapping. Small 2021, 17, 2102495. [Google Scholar] [CrossRef]
- Hackl, T.; Poik, M.; Schitter, G. Heterodyne AC Kelvin Probe Force Microscopy for Nanoscale Surface Potential Imaging in Liquids. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Grévin, B.; Husainy, F.; Aldakov, D.; Aumaître, C. Dual-Heterodyne Kelvin Probe Force Microscopy. Beilstein J. Nanotechnol. 2023, 14, 1068–1084. [Google Scholar] [CrossRef]
- Maryon, O.O.; Efaw, C.M.; DelRio, F.W.; Graugnard, E.; Hurley, M.F.; Davis, P.H. Co-Localizing Kelvin Probe Force Microscopy with Other Microscopies and Spectroscopies: Selected Applications in Corrosion Characterization of Alloys. J. Vis. Exp. 2022, 184, 64102. [Google Scholar] [CrossRef]
- Lee, H.; Lee, W.; Lee, J.H.; Yoon, D.S. Surface Potential Analysis of Nanoscale Biomaterials and Devices Using Kelvin Probe Force Microscopy. J. Nanomater. 2016, 2016, 4209130. [Google Scholar] [CrossRef]
- Beinik, I.; Kratzer, M.; Wachauer, A.; Wang, L.; Lechner, R.T.; Teichert, C.; Motz, C.; Anwand, W.; Brauer, G.; Chen, X.Y.; et al. Electrical Properties of ZnO Nanorods Studied by Conductive Atomic Force Microscopy. J. Appl. Phys. 2011, 110, 052005. [Google Scholar] [CrossRef]
- Fernando, P.S.; Mativetsky, J.M. Unambiguous Measurement of Local Hole Current in Organic Semiconductors Using Conductive Atomic Force Microscopy. J. Phys. Chem. C 2023, 127, 9903–9910. [Google Scholar] [CrossRef]
- Caballero-Quintana, I.; Amargós-Reyes, O.; Maldonado, J.-L.; Nicasio-Collazo, J.; Romero-Borja, D.; Barreiro-Argüelles, D.; Molnár, G.; Bousseksou, A. Scanning Probe Microscopy Analysis of Nonfullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 29520–29527. [Google Scholar] [CrossRef]
- Khoury, R.; Alvarez, J.; Ohashi, T.; Martín, I.; Ortega, P.; Lopez, G.; Jin, C.; Li, Z.; Rusli; Bulkin, P.; et al. Observation of Photovoltaic Effect within Locally Doped Silicon Nanojunctions Using Conductive Probe AFM. Nano Energy 2020, 76, 105072. [Google Scholar] [CrossRef]
- Barrigón, E.; Hrachowina, L.; Borgström, M.T. Light Current-Voltage Measurements of Single, as-Grown, Nanowire Solar Cells Standing Vertically on a Substrate. Nano Energy 2020, 78, 105191. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Mahmoud, S.T. Synthesis and Length Dependent Photoluminescence Property of Zinc Oxide Nanorods. Results Phys. 2017, 7, 3552–3556. [Google Scholar] [CrossRef]
- Musa, I.; Mousa, R. Synthesis and Characterization of Variable-Sized Silver Nanoparticles Using Pistacia Palaestina Leaf Extract. Plasmonics 2024, 1–9. [Google Scholar] [CrossRef]
- Meena, P.L.; Bhardwaj, P.; Kumar, Y.; Singh, S.P. Synthesis and Characterization of One Dimensional ZnO Nanorods. In Proceedings of the AIP Conference Proceedings, Longowal, India, 9–11 November 2021; Volume 2352, p. 040043. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N. Study of optical energy gap and quantum confinment effects in zinc oxide nanoparticles and nanorods. Dig. J. Nanomater. Biostruct. 2019, 14, 119–125. [Google Scholar]
- Ali, H.; Azad, A.K.; Khan, K.A.; Rahman, O.; Chakma, U.; Kumer, A. Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques. ACS Omega 2023, 8, 28133–28142. [Google Scholar] [CrossRef]
- Glatzel, T.; Gysin, U.; Meyer, E. Kelvin Probe Force Microscopy for Material Characterization. Microscopy 2022, 71, i165–i173. [Google Scholar] [CrossRef] [PubMed]
- Musa, I.; Faqi, R. Structural, Electrostatic Force Microscopy, Work Function, and Optical Characterization of Pure and Al-Doped ZnO Nanoparticles. Results Mater. 2024, 22, 100570. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Chakravadhanula, V.S.K.; Hrkac, V.; Jebril, S.; Agarwal, D.C.; Mohapatra, S.; Avasthi, D.K.; Kienle, L.; Adelung, R. Crystal Growth Behaviour in Au-ZnO Nanocomposite under Different Annealing Environments and Photoswitchability. J. Appl. Phys. 2012, 112, 064308. [Google Scholar] [CrossRef]
- Sahana, M.B.; Sudakar, C.; Dixit, A.; Thakur, J.S.; Naik, R.; Naik, V.M. Quantum Confinement Effects and Band Gap Engineering of SnO2 Nanocrystals in a MgO Matrix. Acta Mater. 2012, 60, 1072–1078. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Mahmoud, S.T. Ag Nanocluster Production through DC Magnetron Sputtering and Inert Gas Condensation: A Study of Structural, Kelvin Probe Force Microscopy, and Optical Properties. Nanomaterials 2023, 13, 2758. [Google Scholar] [CrossRef]
- Makov, G.; Nitzan, A.; Brus, L.E. On the Ionization Potential of Small Metal and Dielectric Particles. J. Chem. Phys. 1988, 88, 5076–5085. [Google Scholar] [CrossRef]
- Musa, I.; Ghabboun, J. Work Function, Electrostatic Force Microscopy, Tunable Photoluminescence of Gold Nanoparticles, and Plasmonic Interaction of Gold Nanoparticles/Rhodamine 6G Nanocomposite. Plasmonics 2024, 1–10. [Google Scholar] [CrossRef]
- Thappily, P.; Mandin, P.; Sauvage, T.; Sandhya, K. Enhancement of Charge Transport Properties of a Novel Rubbery Semiconductor via Silver Nanocomplexing. Mater. Sci. Semicond. Process. 2021, 131, 105854. [Google Scholar] [CrossRef]
- Kumar, A.; Moradpour, M.; Losito, M.; Franke, W.-T.; Ramasamy, S.; Baccoli, R.; Gatto, G. Wide Band Gap Devices and Their Application in Power Electronics. Energies 2022, 15, 9172. [Google Scholar] [CrossRef]
- Abbas, Y.; Rezk, A.; Anwer, S.; Saadat, I.; Nayfeh, A.; Rezeq, M. Improved Figures of Merit of Nano-Schottky Diode by Embedding and Characterizing Individual Gold Nanoparticles on n-Si Substrates. Nanotechnology 2020, 31, 125708. [Google Scholar] [CrossRef]
- Saha, S.K.; Pal, A.J. Schottky Diodes between Bi2S3 Nanorods and Metal Nanoparticles in a Polymer Matrix as Hybrid Bulk-Heterojunction Solar Cells. J. Appl. Phys. 2015, 118, 014503. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Wu, Q.; Si, M.; Zhang, B.; Zhang, K.; Li, H.; Mi, L.; Jiang, Y.; Rong, Y.; Chen, J.; Fang, Y. Strong Damping of the Localized Surface Plasmon Resonance of Ag Nanoparticles by Ag2O. Nanotechnology 2018, 29, 295702. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Campos, A.; Troc, N.; Cottancin, E.; Pellarin, M.; Weissker, H.-C.; Lermé, J.; Kociak, M.; Hillenkamp, M. Plasmonic Quantum Size Effects in Silver Nanoparticles Are Dominated by Interfaces and Local Environments. Nat. Phys. 2019, 15, 275–280. [Google Scholar] [CrossRef]
- Mandal, S.; Ananthakrishnan, R. Double Effects of Interfacial Ag Nanoparticles in a ZnO Multipod@Ag@Bi2S3 Z-Scheme Photocatalytic Redox System: Concurrent Tuning and Improving Charge-Transfer Efficiency. Inorg. Chem. 2020, 59, 7681–7699. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Wang, C.; Qiu, G.; Ye, W.; Li, Y.; Wang, D. ZnO/Ag Nanorods as a Prominent SERS Substrate Contributed by Synergistic Charge Transfer Effect for Simultaneous Detection of Oral Antidiabetic Drugs Pioglitazone and Phenformin. Sens. Actuators B Chem. 2020, 307, 127634. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Q.; Bai, L.-H.; Su, X.; Wang, T.-T.; Peng, T.-W.; Zhai, X.-Q.; Huo, Y.; Wu, H.; Liu, C.; et al. Enhanced UV Emission from ZnO on Silver Nanoparticle Arrays by the Surface Plasmon Resonance Effect. Nanoscale Res. Lett. 2021, 16, 8. [Google Scholar] [CrossRef]
- Rauwel, P.; Galeckas, A.; Rauwel, E. Enhancing the UV Emission in ZnO–CNT Hybrid Nanostructures via the Surface Plasmon Resonance of Ag Nanoparticles. Nanomaterials 2021, 11, 452. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, I. Kelvin Probe Force Microscopy, Current Mapping, and Optical Properties of Hybrid ZnO Nanorods/Ag Nanoparticles. Surfaces 2024, 7, 770-785. https://doi.org/10.3390/surfaces7030050
Musa I. Kelvin Probe Force Microscopy, Current Mapping, and Optical Properties of Hybrid ZnO Nanorods/Ag Nanoparticles. Surfaces. 2024; 7(3):770-785. https://doi.org/10.3390/surfaces7030050
Chicago/Turabian StyleMusa, Ishaq. 2024. "Kelvin Probe Force Microscopy, Current Mapping, and Optical Properties of Hybrid ZnO Nanorods/Ag Nanoparticles" Surfaces 7, no. 3: 770-785. https://doi.org/10.3390/surfaces7030050
APA StyleMusa, I. (2024). Kelvin Probe Force Microscopy, Current Mapping, and Optical Properties of Hybrid ZnO Nanorods/Ag Nanoparticles. Surfaces, 7(3), 770-785. https://doi.org/10.3390/surfaces7030050






