Abstract
Polymer-based molecular tweezers have emerged as a prominent research area due to their enhanced ability to form host–guest complexes, driven by advancements in their design and synthesis. The impact of the spacer structure on the tweezers is predominant. They can be rigid, flexible, and stimuli-responsive. Herein, a new generation of molecular tweezers is introduced as polymer-based molecular tweezers. The integration of molecular tweezers onto biopolymers has significantly expanded their potential applications, making them promising candidates, especially in drug delivery, owing to their biocompatibility, adaptive structural features, and versatile interaction capabilities. The unique structure of polymer-based molecular tweezers, particularly when integrated with biopolymers, creates a unique nano-environment that enhances their interaction with guest molecules. This minireview focuses on the synthesis and applications of polymer-based molecular tweezers and examines how the incorporation of various spacers affects their binding affinity and specificity. These features highlight the advancement of these polymer-based systems, emphasizing their potential applications, particularly in drug delivery, water treatment technology, and future research opportunities.
1. Introduction
The concept of molecular tweezers and the ability of such systems to form host–guest complexes have received tremendous attention in recent years [1,2,3]. Various kinds of spacers have been used to make molecular tweezers [4]. It is worth noting that the type of spacer within the molecular structure of the tweezers is a determining factor in its binding affinity [5]. Furthermore, the introduction of rigid, flexible, and stimuli-responsive tweezers, a new generation of tweezers, is presented in this review in the form of molecular tweezers on the backbone of a biopolymer. There are a few examples of this category in the literature. Considering their unique applications, mainly in drug delivery, it is likely to see greater numbers of research studies in this area in the coming years [6]. The term “molecular tweezers” was first described for artificial receptors with two flat, generally aromatic pincers attached through a spacer unit [7]. The open cavity created between the molecular receptors offers a recognition site for different guests via noncovalent interactions such as hydrogen bonding, π-π stacking, ion–dipole and dipole–dipole interactions, including weaker interactions like van der Waals forces [8,9,10]. The morphological dynamics and properties of the polymer chains in polymer-based molecular tweezers, such as chain flexibility and conformation, directly influence their supramolecular behavior. These factors impact the binding affinity, selectivity, and overall stability of the host–guest complexes, thereby playing a crucial role in the tweezers’ functional performance, as described elsewhere [11].
History of Molecular Tweezers
In recent decades, these synthetic architectures have attracted increasing attention in the realm of supramolecular chemistry [12]. In their pioneering work, Chen and Whitlock synthesized tweezers that contained two caffeine pincers separated by a diene spacer (1) as represented in Figure 1 [7]. The non-rigidity of the spacer unit designed by Whitlock et al. led Zimmerman and coworkers to synthesize U-shaped molecular tweezers based on dibenzoacridine (2). The latter contained two parallel pincers attached to C-2 and C-12 positions, which was further modified by incorporation of extra-functional groups in the cleft. This modification led to an increase in the binding affinity of the molecular tweezers (3 versus 2), especially for adenine [13,14]. This research was the start of the recognition of amino acids using synthetic molecular tweezers, which further continued the treatment of Alzheimer’s disease through preventing amyloid-β protein folding using CLR01 and CLR03 tweezers [15,16]. The tweezers were fabricated based on the result obtained by Klärner and coworkers, who introduced a novel class of preorganized tweezers with a belt-type structure, denoted as 4 in Figure 1 [17,18]. In these new groups, which contain only non-conjugated benzene and/or naphthalene rings, the cavity size can be tuned by changing the number of methylene-bridged spacer units.
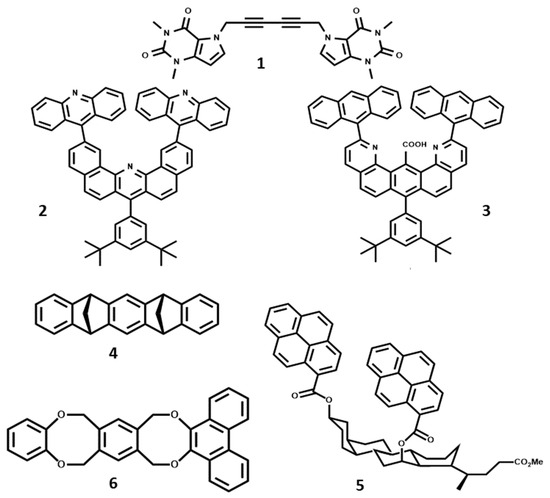
Figure 1.
The molecular structure of some well-known molecular tweezers synthesized by different research groups. Redrawn from references [7,13,14,17,19,20], respectively.
Maitra et al. [19] constructed tweezers based on the use of bile acids as one of the most available and well-organized molecular scaffolds. The arrangement of hydroxyl groups on the structure of bile acids makes them appropriate units to attach to large aromatic pincers and make tweezers (5).
Harmata et al. introduced one of the first examples of chiral molecular tweezers based on Kagan’s ether (6) [20]. These chiral hosts can be employed to recognize chiral guests in their deep host cavity.
Following the developments reported above, different classes of molecular tweezers with various kinds of spacers were synthesized by other research groups [21,22,23,24,25,26,27,28]. In short, it can be stated that for all of these synthetic hosts, spacers play a crucial role, not only in the structure of the molecular tweezers, but also in the molecular recognition process.
Molecular tweezers are a sub-division of the synthetic host that can be varied extensively based on their spacers and pincers. By choosing several structural building blocks or synthons (tectons), different types of noncovalent interactions can be involved in the guest recognition process. In previous reports, the spacers were divided into three main categories: rigid, flexible, and switchable or stimuli-responsive tweezers (Scheme 1) [4,29].
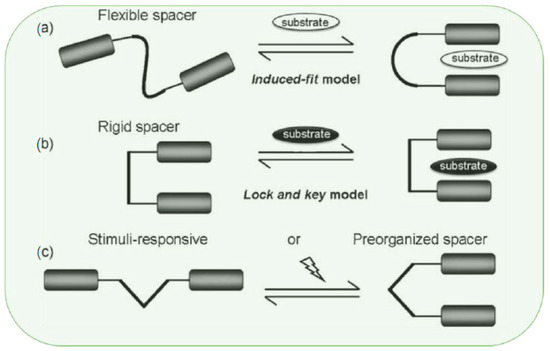
Scheme 1.
Schematic representation of three different molecular tweezers based on the type of the spacer, including (a) flexible, (b) rigid, and (c) stimuli-responsive tweezers activated by external triggers, such as light or other forms of stimulation denoted by the zig-zag line. Adapted with permission from reference [4].
2. Different Types of Polymer-Based Molecular Tweezers
Although many reviews were published related to molecular tweezers [30,31,32], none of them specifically discussed tweezers with a (bio)polymer backbone. Herein, the macromolecular based tweezers are reviewed with the aim of studying the chemical structure of the arms that are attached to a polymer scaffold, along with the types of guests that can interact with these polymer-based tweezers. In addition, examples of this class of tweezers were discussed and as was their application in the pharmaceutical area, like drug delivery.
For ease of understanding, tweezer-based polymers are categorized into two groups:
- (i)
- The architecture of molecular tweezers, containing both spacer and pincers, are bonded to a polymer chain;
- (ii)
- The pincers are directly attached to the polymer chain. It means a short part of the polymer chain (a monomer unit or an oligomer) is the spacer of the molecular tweezers.
The structural variations in the first group (i) are more numerous and more examples are reported in the literature since it is possible to attach all the known synthesized tweezers to a polymeric scaffold through some structural modification. In contrast, the pincers are connected to a polymer backbone in the second group (ii), where limited examples of this category are reported in the literature.
It is worth bearing in mind that these classes of tweezers are entirely different from supramolecular polymers based on molecular tweezers. The supramolecular polymers denote the polymeric arrays of tweezers or tweezer–guest complexes formed via self-assembly by special arrangement using noncovalent interactions (cf. Scheme 2) [33,34,35,36,37,38]. There are also many reports in the literature about grafting other types of hosts different from molecular tweezers on a linear polymer which are not mentioned here. The most well-known hosts are cyclodextrins onto a polymer [39], chains such as polyacrylamide [40], chitosan [41,42], polyethylene glycol [43], or attachment of cyclodextrins within the main polymer chain via cross-linking through epichlorohydrin [44], and diisocyanate linkers, among various other types of systems [45]. In addition, cavitands, calixarenes, and crown ethers have been immobilized onto a polymer support, which have been reported for their use in chromatographic separations and as catalysts [46].
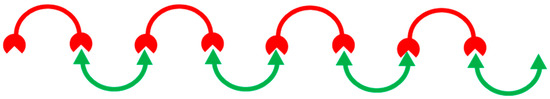
Scheme 2.
The schematic arrangement of tweezers (red) and guest (green) parts to show the supramolecular polymers connected by noncovalent interactions.
2.1. Molecular Tweezers Bound to a Polymer Chain
One of the first examples of polymer-bound tweezers was introduced by Shimazawa et al. in 1992 [47]. They coupled the water-insoluble Zimmerman’s tweezers of 2 and 3 (cf. Figure 1) to the high molecular weight dextran polymer (MW > 2,000,000) and synthesized a water-soluble molecular tweezer on a biopolymer chain with well-separated pincers separated by a distance of 7 Å. To attach the tweezers to the polymer scaffold, an amine handle was designed on the cleft, which was coupled to an oxidized dextran structure via periodate with a Schiff base reaction followed by reduction (cf. Figure 2). Products were purified using gel filtration column chromatography to obtain highly soluble polymers in aqueous media. The substituent content of Zimmerman’s tweezers bound to the biopolymer scaffold was determined by UV absorption spectroscopy which was estimated at 100 μmol/mg. These hosts had the same or higher affinity toward adenosine, as compared with Zimmerman’s tweezers.
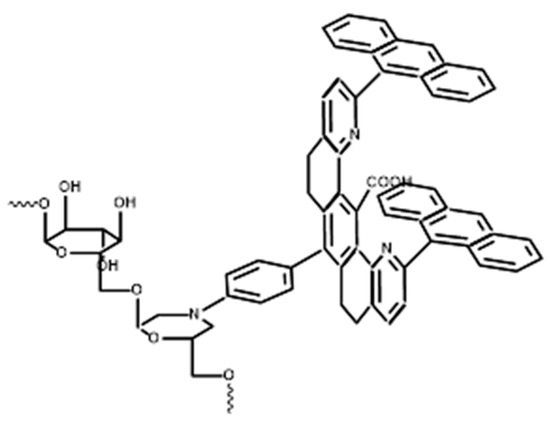
Figure 2.
Chemical structure of water-soluble Zimmerman molecular tweezers on the dextran scaffold. Redrawn from reference [47].
Attaching two flat π-surfaces like pyrene to the rigid bile acid spacer as molecular tweezers was reported by Maitra and D’Souza, with this method, which results in a high binding affinity with electron-deficient guests [19]. This type of host structure was prepared via the esterification of methyl deoxycholate with pyrene carbonyl chloride followed by the immobilization of this tweezer on chloromethylated polystyrene to install molecular tweezers onto a polymer backbone with less synthetic effort, as compared to Shimazawa tweezers (cf. Figure 3). There are some other modifications to this method to attach two aromatic pincers co-facially at a distance of 6–7 Å to the 3- and 12-positions of the bile acids [48]. Pyrene loading was determined through hydrolyzing the ester functionality and weighing the pyrene carboxylic acid residue, which was estimated at 0.54 mmol of steroid unit per gram of the polymer. Host–guest interactions with nitroaromatic guests were studied through fluorescence quenching experiments and NMR titrations for non-bonded molecular tweezers. It was shown that after the attachment of the host to the polymer backbone, binding constants decreased. The association constant (220 M−1) of the trinitrofluorenone host with the free tweezer was estimated in CDCl3 at 25 °C. The association constant was reduced to 97 M−1 for the bounded tweezer-based host. Although the exact reason for the reduction was not discussed, it could be due to microenvironment effects, steric hindrance, and reduced conformational mobility after attachment to the polymer backbone.
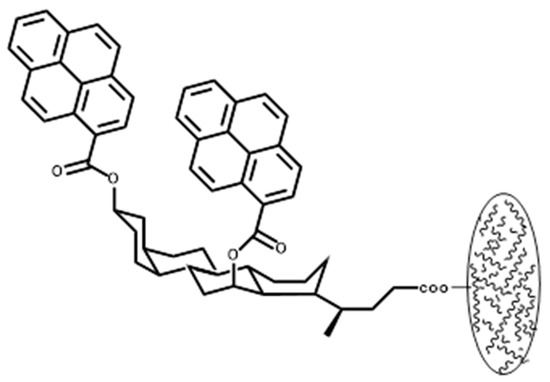
Figure 3.
Maitra’s molecular tweezers immobilized on the polystyrene. The image illustrates the attachment of the tweezers to a polystyrene bead. Redrawn from reference [19].
A chain-end based on the bis-pyrenyl tweezers on the polyamide backbone was prepared to study the effects of end-capped tweezers on the chain folding properties of the synthetic host with polyaromatic guests, where it is expected that stronger interactions occur over simple pyrenyl units (Figure 4a) [49]. These types of end-capped tweezers were synthesized using Jeffamine 400 (a polyether amine), isophthaloyl dichloride, and an aminomethyl derivative of dipyrenemethyl isophthalimide as the starting materials [50]. This structural attribute enables the formation of a charge transfer host–guest complex with a polymeric guest that contains naphthalene diimide rings, which can be observed as a profound red color change when mixing a solution of the two polymers. Evidence of charge transfer between the host and guest was provided using the UV-Vis spectra of the host–guest blend, which displayed a strong absorption band at 526 nm (Figure 4b). The individual solutions of each polymer in trichloroethanol are yellow. The π-π stacked complex between electron-rich groups of the tweezers and the electron-deficient guest is also retained in the solid-state when an elastomeric film is prepared, which was shown by the appearance of a dark red color of the film. Rheometry experiments demonstrate an enhanced tensile strength and considerable modulus of elongation and toughness compared to non-tweezer complexes.
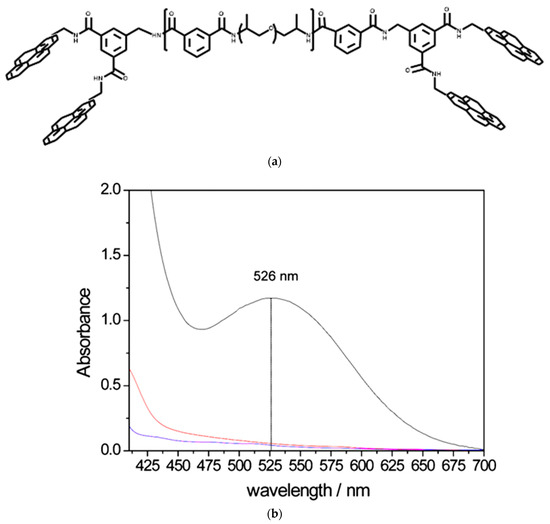
Figure 4.
(a) Bis-pyrenyl tweezers on a polyamide scaffold appropriate for aromatic guests. Redrawn from reference [49]. (b) The UV-visible spectra of host–guest blend reveals a prominent absorption band at 526 nm, indicating charge transfer between them. The blue line is a pure diimide guest, the red line is a polymer-based host, and the black line is a host–guest blend. Reprinted with permission from reference [49].
A solid-supported Zn(II) porphyrin tweezer system bound onto TentaGel beads was reported by Carofiglo et al. [51]. The immobilized tweezer system was used as a rapid and accurate sensing device for biogenic amines to indicate the occurrence of food spoilage. This sensor on a solid support can be used as a recyclable food freshness monitor. To synthesize these types of tweezers, an amino porphyrin was reacted with cyanuric chloride in a 2:1 ratio. The third chloride of cyanuric chloride was replaced by amino-functionalized TentaGel (cross-linked polystyrene network with grafted polyethylene glycol units), and finally, the macrocycle was metalated with zinc acetate, which afforded the corresponding zinc porphyrin (Figure 5). The free amine functionality of TentaGel is acetylated by acetic anhydride to prevent self-association of Zn(II) porphyrin with –NH2 groups. UV-Vis spectroscopy in the Q-band region (500–650 nm) was used for the study of a complex formation between the ditopic host tweezers with diamines, which are characterized by a red-shift in the spectral profile. The association constant of this host with different aliphatic diamines was determined spectrophotometrically and found to vary over an order of magnitude (4.2 × 106–3.4 × 107 M−1) in dichloromethane at 25 °C, which was dependent on the chain length of the diamines.
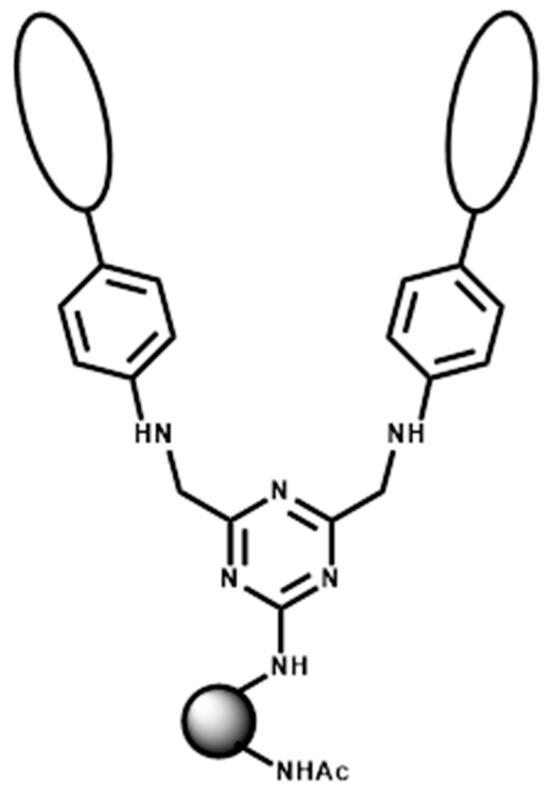
Figure 5.
Structure of zinc porphyrin tweezers immobilized on TentaGel polymer (cross-linked polystyrene network with grafted polyethylene glycol units). Redrawn from reference [51].
In a report by Leblond et al. [52], the macromolecular backbone of the tweezers provides water solubility for pH-responsive molecular tweezers. The corresponding macromolecular structure comprises three different parts:
- i.
- A polyethylene glycol (PEG) polymer chain as a water-soluble carrier;
- ii.
- Two naphthalene rings as the pincers of the tweezers;
- iii.
- A methoxyphenyl pyridine methoxyphenyl triad unit as the pH-responsive part of the tweezers.
Naphthalene arms are attached to the pyridine ring by two successive Suzuki couplings, which are further linked to the polyethylene glycol backbone through an amide linkage. The transformation of the switchable unit from a U-shape in neutral pH to a W-shape in acidic media can result in two free naphthalene units that do not feel the electronic effects of their neighbor (cf. Figure 6a). This conformational change was studied by 1H-NMR and 2D-NOESY experiments. The binding and release capacity of this host was evaluated against quinizarin and mitoxantrone as anticancer drugs since both contain π-systems that can intercalate with tweezers via π-π stacking and hydrophobic interactions. Fluorescence spectroscopy also demonstrated that this type of PEG-tweezers host formed complexes with the mentioned guests at near neutral pH (7.4), while there was no interaction in acidic media (pH = 4.5), as predicted from the U- and W-shaped conformers (cf. Figure 6b). The two different pH media were selected based on 1H-NMR studies that established the pKa value to be 6.3. These polymer-based tweezers may be a potent candidate due to their controlled release properties in anticancer medicinal formulations.
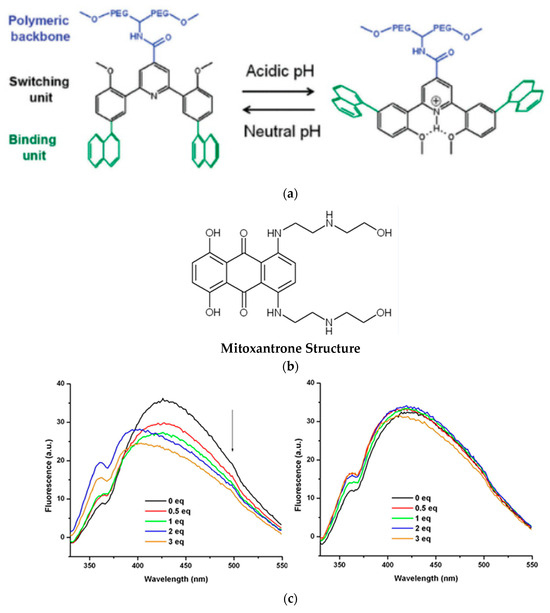
Figure 6.
(a) U- and W-shape of PEG-bound molecular tweezers in acidic and neutral pH. Adapted with permission from reference [52]. (b) Chemical structure of mitoxantrone which was used as the guest. The arrow shows decrease of fluorescence intensity. (c) Emission fluorescence of polymer based tweezers upon addition of mitoxantrone at neutral (left) and acidic pH (right). Reprinted with permission from reference [52].
2.2. Molecular Tweezers That Use the Polymer Chain as the Spacer
In some reports, the arms of the tweezers are connected to the polymer chain, so part of the chain is considered the spacer of the molecular tweezer unit. Copolymer formation of the porphyrin with methacrylic acid and N-vinylpyrrolidone monomers were investigated to make water-soluble artificial tweezers [53,54,55]. Scamporrino and coworkers have reported the synthesis of a charged and uncharged water-soluble tweezer containing bis-porphyrin attached onto peripheral positions of hydrophilic polyethylene glycol chains [56]. The ability of this host to bind a guest successfully depends on the length and flexibility of the bridges, the meso-porphyrin substituents, and the type and number of metals used in the structure of the porphyrin. The uncharged water-soluble tweezers were synthesized by the reaction of dibromomethane or its longer derivatives with PEGylated tetraphenylporphyrin, where each molecule has only one reactive hydroxyl group. For the charged species, the core hydrogen atom of the porphyrin was substituted with a cobalt or zinc ion to yield a metal-bis-porphyrin (cf. Figure 7). Two open and closed conformers of these soluble tweezers were investigated by the study of the Soret and Q-regions of the UV-Vis spectra at pH 7, which indicated the presence of a dynamic equilibrium between these tweezer conformations. These tweezers were used as the host for four different chiral amino acids, including l-alanine, l-phenyl alanine, l-lysine, and l-tryptophan in an aqueous solution at pH 7 and 9. Although there was no change in UV-Vis spectra, the appearance of an induced circular dichroism (ICD) band at the same wavelength of the Soret band, for a solution of achiral metal porphyrin with chiral amino acids, indicated the formation of a chiral host–guest complex using the amino acid guest as a bridge between two folded porphyrin arms. It is worth noting that the sign of the CD spectra was changed when D-amino acids were used as guests, which confirmed the formation of complexes with different chirality. These designed structures can be applied as potential candidates for amino acid sensing in aqueous media [57].
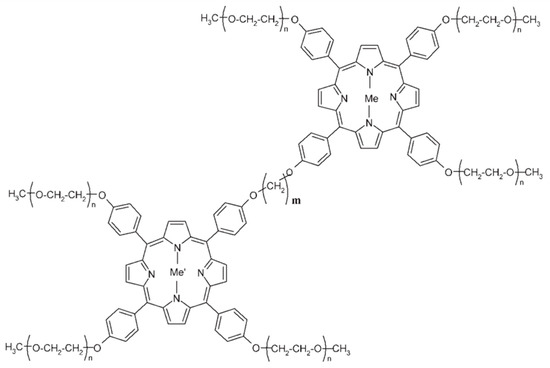
Figure 7.
Water-soluble metal-bis-porphyrin end-capped with PEG. Adapted with permission from reference [56].
Metalloporphyrin–peptoid conjugates (MPPCs) are another group of molecular tweezers that contain co-facial porphyrins onto helical peptoids [58]. Peptoids are defined as peptidomimetics which are made by employing a glycine backbone [59]. Secondary structures like helix, ribbon, and square helix can be formed by sequentially inserting a monomer. Co-facial porphyrins on MCCPs are fascinating pincers of preorganized molecular tweezers since they can be chiral through their chiral linkers. Lee and coworkers [58] have carried out elegant work on the preparation of three MCCPs onto different peptoid backbones. Porphyrins are attached to the peptoid via an amide linkage and finally metalized with Zn(OAc)2 or Cu(OAc)2 (cf. Figure 8a). The investigation of the host–guest complexes using UV-Vis and CD spectra identified several classes of complexes: achiral host–chiral guest, chiral host–achiral guest, and chiral host–chiral guest. Graphical representations of the formed complexes are shown in Figure 8b. When a chiral guest like l- or d-Lys-OMe was intercalated between two porphyrin rings attached to an achiral peptoid scaffold, induced chiral orientation generated two exciton-coupled circular dichroism (ECCD) signals. With two enantiomeric guests, the apparent Cotton effects in the CD spectra were mirror images that indicated a right- or left-handed orientation of the porphyrins. With a chiral peptoid scaffold, chiral porphyrin tweezers were made, which was detectable from its ECCD signal even in the pure host without any guest molecule. In this case, the intensity of the ECCD signal decreased upon complexation with an achiral guest due to the weakening of chiral interaction with porphyrins. Aromatic and aliphatic mono- and diamines, besides cysteamine as a biological guest, were selected for this special type of porphyrin tweezers, attributed to their relevance for the treatment of neurodegenerative diseases like Parkinson’s and Huntington’s [60]. Spectroscopic titrations demonstrated clear isosbestic points, indicating the formation of host–guest complexes. Calculated Ka values range from 1.1 × 105–1.5 × 105 M−1 for aliphatic and aromatic diamines. In the case of cysteamine, the host–guest complex was studied via UV and CD spectroscopy, which resulted in an estimated Ka value of 1.1 × 106 M−1 upon complex formation between the amino and thiol groups of the guest and porphyrin units.
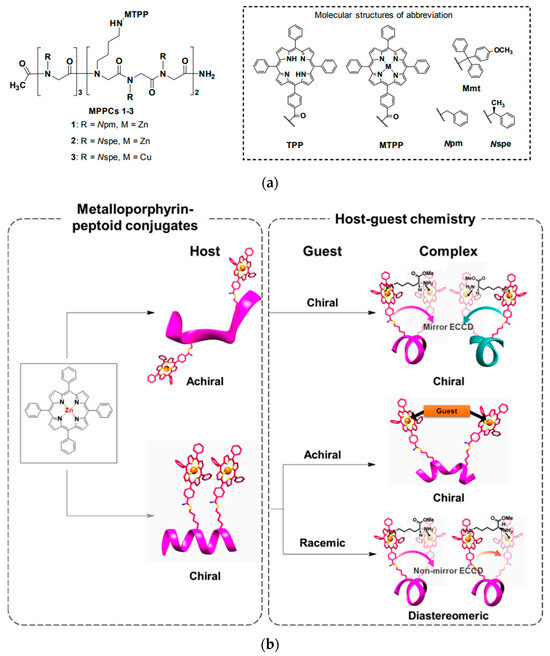
Figure 8.
(a) Structure of three different metalloporphyrin–peptoid conjugates (MPPCs) and (b) host–guest complex formation between MPPCs guests as follows: achiral host–chiral guest, chiral host–chiral guest, and chiral host–chiral guest. Adapted with permission from reference [58].
If a chiral MPPC was mixed with a racemic mixture of guests, two diastereomeric complexes were formed. The corresponding UV spectra are the same, whereas the ECCD signals revealed characteristic signatures. Future research should continue to explore the diverse molecular recognition properties of polymer-based molecular tweezers by investigating different spacer configurations and the role of various types of biopolymer platforms due to their chiral nature [61]. In turn, biopolymer-based tweezer systems may offer advantages over synthetic polymer platforms due to their multifunctional structure and versatile utility as hosts for a diverse range of guest systems [62].
3. Future Perspectives
The emergence of unique physicochemical properties biopolymer-based tweezer systems is ongoing, as evidenced by a number of recent studies that reveal hierarchical structural complexity and incremental advancements in the physicochemical and biological properties, especially for various macromolecular systems. For the case of chitosan biopolymers, Vafakish and Wilson reported the first such examples [63,64] of biopolymer tweezer systems. These biopolymer hosts build upon concepts of conventional molecular tweezers and host–guest chemistry that reveal unique structural attributes with emergent properties.
In a recent study, Dolatkhah et al. [65] reported the design of chitosan grafted with PAA containing an iron oxide core with immobilized Ag nanoparticles denoted as PAAgCHI/Fe3O4/Ag (L or H), where L and H refer to low and high loading of Ag nanoparticles (NPs) in the biopolymer-PAA shell, as outlined in Figure 9 below.
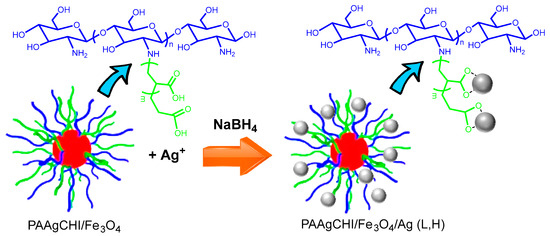
Figure 9.
Schematic proposed structure of the stimuli-responsive catalyst. Formation of hybrid metal nanomaterial, PAAgCHI/Fe3O4/Ag (L, H), where L and H refer to low and high levels of Ag nanoparticle loadings. The following color scheme defines the various components: red sphere (Fe3O4), gray sphere (Ag NPs), blue line (chitosan), and green line (grafted PAA; PAAg). Copied with permission from Ref. [65].
In the context of Type (ii) polymer-based molecular tweezers discussed in Section 2 above, the core–shell polymer brush system displays structural features that resemble molecular tweezer systems described in Section 2 of this mini-review. The polymer-based tweezer systems by Dolatkhah et al. [65] revealed excellent catalytic efficiency for reduction reactions of low molecular weight dye molecules (4-nitrophenol, 4-NP; and methyl orange, MO). In particular, rapid catalytic reduction occurs for 4-NP, as compared with pristine Ag nanoparticles and other known catalyst materials, as summarized in the following Table 1.

Table 1.
Comparison of the catalytic activities of PAAgCHI/Fe3O4/Ag (H, L) for the reduction of 4-nitrophenol with other known Ag catalyst materials. Copied with permission from ref. [65].
The results in Table 1 highlight the strategy of conjugating AgNPs onto stimuli-responsive magnetic polyelectrolyte brushes, which yield well-dispersed and magnetically retrievable nanocatalysts with excellent performance that exceed trends observed for pristine metal nanoparticles.
A related development of grafted chitosan with thiol carboxylic acid residue by incorporating S-acetyl mercaptosuccinic anhydride (SAMSA) to yield CHT-SAMSA was recently reported by Vafaskish & Wilson [69]. Ag NPs were immobilized onto the CHT-SAMSA (Ag@CS-SAMSA), as illustrated in Figure 10. The Ag@CS-SAMSA sensor displayed a high sensitivity for the detection of methylene blue (MB), with an enhancement factor ca. 108 with practical reusability. This modified chitosan sensor system for the detection of MB over three cycles displayed good reproducibility and stability upon storage. The large surface enhanced Raman scattering (SERS) enabled the detection of MB at relatively low levels from 1 nM to 100 µM. These impressive results highlight the utility and sustainable nature of this ‘tweezer-based’ hybrid chitosan sensor, and its relatively low-cost development for the detection of cationic dyes such as MB. The noteworthy properties of the modified chitosan for this facile “tweezer-like” sensor material are further highlighted in a comparison of other SERS sensors reported in Table 2 [69]. The enhancement factor (EF) and the limit of detection (LOD) rank favorably among the various state-of-the-art sensor materials reported in the literature.

Table 2.
Comparison of EF and LOD of substrates from the literature, along with the results reported in this study. Copied with permission from reference [69].
Mir and Wilson [77] recently reported a ‘quick dip’ method for the preparation of coated flax biomass fiber systems, where chitosan was immobilized noncovalently onto pristine flax fibers (cf. Figure 10, upper panel). The resulting flax fiber composites (FFCs) reveal distinctive physicochemical properties in terms of their stimuli-responsiveness to pH and solvent, which become switchable according to the extent of chitosan surface coverage onto the fiber surface. The incremental chitosan loading further contributes unique adsorption properties toward cationic and anionic dyes, according to the dual adsorption sites (cf. Figure 10, lower panel) for anion (RB) and cation (MB) dyes. The resulting FFC construct bears hallmark features akin to polymer-based tweezers, where the potential role of cooperative binding of the RB dye within the “brush-like” chitosan structures is inferred near the fiber-chitosan interface. The surface patterned fibers are inferred to have a “tweezer-like” function that vary according to the degree of surface coverage of chitosan. The FFCs have promising utility as sustainable sorbents or as filter media in “smart textiles”, along with wound dressing materials for biomedical devices.
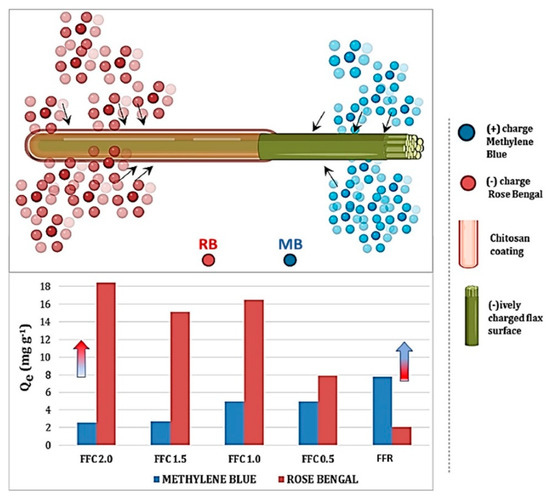
Figure 10.
Schematic illustration of the dual-function adsorption properties on surface-patterned flax with variable chitosan loading. The surface properties vary from a predominantly positive charge due to chitosan surface coating effects to a predominantly negative surface charge for pristine flax fiber (FFR; without the chitosan coating). The arrows in the lower panel highlight the incremental adsorption of RB (right to left), whereas MB increases from left to right. Copied with permission from ref. [77].
Nia et al. [78] recently reported the preparation of silica nanoparticles that are referred to as ‘sea urchin-like nanostructured silica’ (SNS). This because the structure of SNS consists of many silica fibrils protruding from the core, similar to the hairs of a sea urchin. In parallel, a mesoporous organic silica (MOS) material with an internally cross-linked organic network was prepared that contains organic domains, as shown in Scheme 3 (cf. expanded region in the red rectangle).
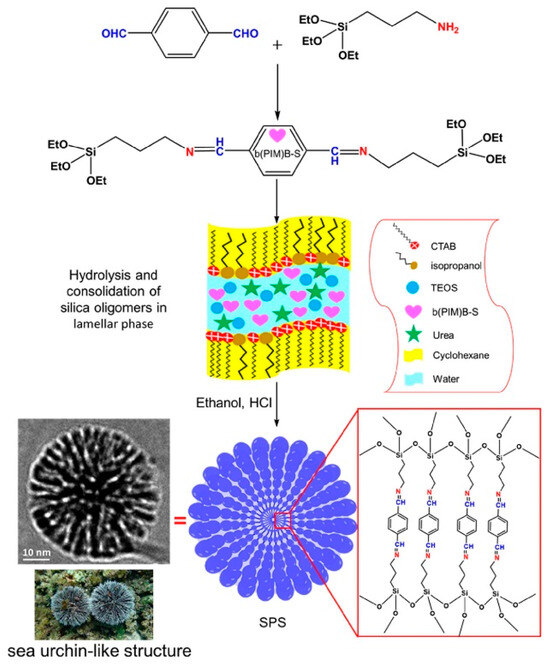
Scheme 3.
Schematic illustration of the stepwise synthesis process of SPS. Copied with permission from ref. [78].
The organosilane cross-linker in Scheme 3 bears structural features of a highly preorganized “tweezer-like” system. The role of this organosilane unit was evaluated for the controlled release of doxorubicin-loaded SPS and SNS systems. In brief, the SPS systems display controlled release features that are pH dependent, in contrast to SNS (without organic domains) that serve as a control system for drug delivery. The effect observed for SNS was attributed to the intercalation of doxorubicin between the organosilane linkers of SPS (cf. Scheme 3). This effect concurs with conventional tweezer-like host–guest complexes, as described by Chen and Whitlock [7] in their seminal work on tweezer-like systems nearly half a century ago.
The formation of polymer-based tweezer systems that extend to hierarchical chitosan and cellulose composites, described above, provide abundant inspiration for the development of advanced materials that are often limited by our collective imagination. The selected examples [63,64,65,69,77,78] outlined above highlight versatile and innovative systems with “tweezer-like” functionality, based on covalent modification of biopolymers. By contrast, the unique example reported by Mir and Wilson [77] highlight the utility of noncovalent surface immobilization of a natural fiber with chitosan that display tailored properties, according to the surface coverage of the biopolymer.
Future work on tweezer-based systems is recommended that explores aspects of supramolecular chemistry with various guest compounds. The future development of responsive polymer-based tweezer systems will serve to stimulate the development of hosts with ‘on-off’ binding capability and molecular selectivity by controlling the surface chemistry through external stimuli such as pH and salt gradients [78,79]. Inevitably, the use of large macromolecular hosts, as described above, will necessitate fundamental studies to gain insight on the role of various intermolecular interactions (e.g., electrostatic interactions and hydrogen bonding) and solvation processes, such as hydrophobic effects [80,81,82]. Thus, comprehensive and systematic thermodynamic, kinetic, and computational studies of host–guest systems are encouraged to establish structure–function relationships for these remarkable and unique macromolecular tweezer-like host systems. An improved understanding of structure–function relationships can be advanced in the context of supramolecular chemistry, which will contribute immensely to the field of biocomposites. In turn, the future development of tweezer-based systems and their technological applications in catalysis, sensors, advanced drug delivery, and environmental remediation are envisaged, among a growing list of examples [79,83,84,85,86].
4. Conclusions
In summary, this mini-review has outlined recent strides in the development of polymer-based molecular tweezers, with a specific focus on their integration with biopolymers. The unique structure-function relationships of these (bio)polymer-based systems, such as their enhanced interaction with guest molecules, reveal distinct features apart from traditional molecular tweezers that will catalyze new avenues of inquiry and applications. Polymer-based tweezers reveal how the choice of spacer affects the efficacy of these constructs in the formation of stable host–guest complexes, which may be further enhanced due to cooperativity effects. The incorporation of these spacers into a polymer matrix not only improves binding affinity, but also extends the range of potential applications such as drug delivery and other biomedical devices. Future research should continue to explore the diverse potential of (bio)polymer-based molecular tweezers by investigating different spacer configurations and polymer combinations [61]. This exploration will be critical in optimizing the performance and versatility of these systems [62]. As the field progresses, the development of polymer-based molecular tweezers will support their utility in diverse scientific and industrial applications, which will pave the way for innovative solutions and technological advancements.
Author Contributions
Conceptualization, B.V.; investigation, B.V.; resources, L.D.W.; writing—original draft preparation, B.V.; writing—review and editing, B.V. and L.D.W.; visualization, B.V.; supervision, L.D.W.; funding acquisition, L.D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Government of Canada through the Natural Sciences and Engineering Research Council (NSERC), Discovery Grant Number: RGPIN 04315-2021. The financial support provided by the University of Saskatchewan in the form of a Dean’s Scholarship for B.V. is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shahpasand-Kroner, H.; Siddique, I.; Malik, R.; Linares, G.R.; Ivanova, M.I.; Ichida, J.; Weil, T.; Müunch, J.; Sanchez-Garcia, E.; Klüarner, F.G.; et al. Molecular Tweezers: Supramolecular Hosts with Broad-Spectrum Biological Applications. Pharmacol. Rev. 2023, 75, 263–308. [Google Scholar] [CrossRef] [PubMed]
- El-Refaey, A.; Kozawa, D.; Kameda, T.; Kato, Y.K.; Ito, Y.; Kawamoto, M. Diameter-Selective Sorting of Single-Walled Carbon Nanotubes Using π-Molecular Tweezers for Energy Materials. ACS Appl. Nano Mater. 2023, 6, 1919–1926. [Google Scholar] [CrossRef]
- Qian, C.; Chen, J.; Wang, C.; Wang, Q.; Wang, X.; Wang, X. Light-Controlled Molecular Tweezers Capture Specific Amyloid Oligomers. Aggregate 2024, 5, e463. [Google Scholar] [CrossRef]
- Leblond, J.; Petitjean, A. Molecular Tweezers: Concepts and Applications. ChemPhysChem 2011, 12, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Norrehed, S.; Andersson, C.H.; Huang, H.; Light, M.E.; Bergquist, J.; Grennberg, H.; Gogoll, A. Synthesis and Properties of Bis-Porphyrin Molecular Tweezers: Effects of Spacer Flexibility on Binding and Supramolecular Chirogenesis. Molecules 2016, 21, 16. [Google Scholar] [CrossRef]
- Mbarek, A.; Moussa, G.; Chain, J.L. Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips. Molecules 2019, 24, 1803. [Google Scholar] [CrossRef]
- Chen, C.W.; Whitlock, H.W. Molecular Tweezers: A Simple Model of Bifunctional Intercalation. J. Am. Chem. Soc. 1978, 100, 4921–4922. [Google Scholar] [CrossRef]
- Zimmerman, S.C. Rigid Molecular Tweezers as Hosts for the Complexation of Neutral Guests. Top. Curr. Chem. 1993, 165, 71–102. [Google Scholar]
- Meiners, A.; Bäcker, S.; Hadrović, I.; Heid, C.; Beuck, C.; Ruiz-Blanco, Y.B.; Mieres-Perez, J.; Pörschke, M.; Grad, J.N.; Vallet, C.; et al. Specific Inhibition of the Survivin–CRM1 Interaction by Peptide-Modified Molecular Tweezers. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Msellem, P.; Dekthiarenko, M.; Seyd, N.H.; Vives, G. Switchable Molecular Tweezers: Design and Applications. Beilstein J. Org. Chem. 2024, 20, 504–539. [Google Scholar] [CrossRef]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkov, A.S. Buckybowl Molecular Tweezers for Recognition of Fullerenes. ChemPhysChem 2024, 25, 1–12. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; Zeng, Z.; Wu, W.; Reichert, D.E. Synthesis and Structure of Molecular Tweezers Containing Active Site Functionality. J. Am. Chem. Soc. 1991, 113, 183–196. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; VanZyl, C.M.; Hamilton, G.S. Rigid Molecular Tweezers: Preorganized Hosts for Electron Donor-Acceptor Complexation in Organic Solvents. J. Am. Chem. Soc. 1989, 111, 1373–1381. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, D.; Klärner, F.G.; Schrader, T.; Bitan, G.; Bowers, M.T. Amyloid β-Protein Assembly: The Effect of Molecular Tweezers CLR01 and CLR03. J. Phys. Chem. B 2015, 119, 4831–4841. [Google Scholar] [CrossRef]
- Bier, D.; Rose, R.; Bravo-Rodriguez, K.; Bartel, M.; Ramirez-Anguita, J.M.; Dutt, S.; Wilch, C.; Klärner, F.-G.; Sanchez-Garcia, E.; Schrader, T.; et al. Molecular Tweezers Modulate 14-3-3 Protein-Protein Interactions. Nat. Chem. 2013, 5, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Klärner, F.G.; Schrader, T. Aromatic Interactions by Molecular Tweezers and Clips in Chemical and Biological Systems. Acc. Chem. Res. 2013, 46, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Klärner, F.G.; Kahlert, B. Molecular Tweezers and Clips as Synthetic Receptors. Molecular Recognition and Dynamics in Receptor-Substrate Complexes. Acc. Chem. Res. 2003, 36, 919–932. [Google Scholar] [CrossRef] [PubMed]
- D’Souz, L.J.; Maitra, U. Design, Synthesis, and Evaluation of Bile Acid-Based Molecular Tweezers. J. Org. Chem. 1996, 61, 9494–9502. [Google Scholar] [CrossRef]
- Harmata, M. Chiral Molecular Tweezers. Acc. Chem. Res. 2004, 37, 862–873. [Google Scholar] [CrossRef]
- Legouin, B.; Gayral, M.; Uriac, P.; Cupif, J.F.; Levoin, N.; Toupet, L.; Van De Weghe, P. Molecular Tweezers: Synthesis and Formation of Host-Guest Complexes. Eur. J. Org. Chem. 2010, 5503–5508. [Google Scholar] [CrossRef]
- Dutt, S.; Wilch, C.; Gersthagen, T.; Talbiersky, P.; Bravo-Rodriguez, K.; Hanni, M.; Sánchez-García, E.; Ochsenfeld, C.; Klärner, F.G.; Schrader, T. Molecular Tweezers with Varying Anions: A Comparative Study. J. Org. Chem. 2013, 78, 6721–6734. [Google Scholar] [CrossRef][Green Version]
- Lindqvist, M.; Borre, K.; Axenov, K.; Kótai, B.; Nieger, M.; Leskelä, M.; Pápai, I.; Repo, T. Chiral Molecular Tweezers: Synthesis and Reactivity in Asymmetric Hydrogenation. J. Am. Chem. Soc. 2015, 137, 4038–4041. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.; Sowislok, A.; Schaller, T.; Niemeyer, F.; Klärner, F.G.; Schrader, T. Molecular Tweezers with Additional Recognition Sites. Chem. Eur. J. 2018, 24, 11332–11343. [Google Scholar] [CrossRef]
- Schrader, T.; Bitan, G.; Klärner, F.G. Molecular Tweezers for Lysine and Arginine-Powerful Inhibitors of Pathologic Protein Aggregation. Chem. Commun. 2016, 52, 11318–11334. [Google Scholar] [CrossRef]
- Attar, A.; Ripoli, C.; Riccardi, E.; Maiti, P.; Li Puma, D.D.; Liu, T.; Hayes, J.; Jones, M.R.; Lichti-Kaiser, K.; Yang, F.; et al. Protection of Primary Neurons and Mouse Brain from Alzheimer’s Pathology by Molecular Tweezers. Brain 2012, 135, 3735–3748. [Google Scholar] [CrossRef]
- Prabhudesai, S.; Sinha, S.; Attar, A.; Kotagiri, A.; Fitzmaurice, A.G.; Lakshmanan, R.; Ivanova, M.I.; Loo, J.I.; Klärner, F.; Schrader, T.; et al. A Novel “Molecular Tweezer” Inhibitor of α-Synuclein Neurotoxicity in Vitro and in Vivo. Neurotherapeutics 2012, 9, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.C. A Journey in Bioinspired Supramolecular Chemistry: From Molecular Tweezers to Small Molecules That Target Myotonic Dystrophy. Beilstein J. Org. Chem. 2016, 12, 125–138. [Google Scholar] [CrossRef]
- Hardouin-Lerouge, M.; Hudhomme, P.; Sallé, M. Molecular Clips and Tweezers Hosting Neutral Guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef]
- Weil, T.; Kirupakaran, A.; Le, M.; Rebmann, P.; Mieres-Perez, J.; Issmail, L.; Conzelmann, C.; Müller, J.A.; Rauch, L.; Gilg, A.; et al. Advanced Molecular Tweezers with Lipid Anchors against SARS-CoV-2 and Other Respiratory Viruses. JACS Au 2022, 2, 2187–2202. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, H.G.; Shon, M.J.; Yoon, T.Y. High-Resolution Single-Molecule Magnetic Tweezers. Annu. Rev. Biochem. 2022, 91, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Taghuo, E.S.; Schrader, T. Molecular tweezers—A new class of potent broad-spectrum antivirals against enveloped viruses. Chem. Commun. 2022, 58, 2954–2966. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wei, C.; Han, Y.; Yuan, M.; Yan, X.; Wang, F. Near-Infrared-Emissive Self-Assembled Polymers via the Implementation of Molecular Tweezer/Guest Complexation on a Supramolecular Coordination Complex Platform. Chin. J. Polym. Sci. 2018, 36, 399–405. [Google Scholar] [CrossRef]
- Tian, Y.K.; Shi, Y.G.; Yang, Z.S.; Wang, F. Responsive Supramolecular Polymers Based on the Bis[Alkynylplatinum(II)] Terpyridine Molecular Tweezer/Arene Recognition Motif. Angew. Chem.–Int. Ed. 2014, 53, 6090–6094. [Google Scholar] [CrossRef]
- Tian, Y.K.; Han, Y.F.; Yang, Z.S.; Wang, F. Donor-Acceptor-Type Supramolecular Polymers Derived from Robust Yet Responsive Heterodimeric Tweezers. Macromolecules 2016, 49, 6455–6461. [Google Scholar] [CrossRef]
- Dial, B.E.; Shimizu, K.D. Applications of Supramolecular Chemistry; CRC Press: Boca Raton, FL, USA, 2012; pp. 301–320. [Google Scholar] [CrossRef]
- Zhu, Z.; Cardin, C.J.; Gan, Y.; Colquhoun, H.M. Sequence-Selective Assembly of Tweezer Molecules on Linear Templates Enables Frameshift-Reading of Sequence Information. Nat. Chem. 2010, 2, 653–660. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Z.; Wang, F. Donor-Acceptor-Type Supramolecular Polymers on the Basis of Preorganized Molecular Tweezers/Guest Complexation. Chem. Soc. Rev. 2018, 47, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; van Nostrum, C.F.; Hennink, W.E.; van de Manakker, F. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Song, X.; Mensah, N.N.; Wen, Y.; Zhu, J.; Zhang, Z.; Tan, W.S.; Chen, X.; Li, J. β-Cyclodextrin-Polyacrylamide Hydrogel for Removal of Organic Micropollutants from Water. Molecules 2021, 26, 5031. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan Derivatives Obtained by Chemical Modifications for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(Ethylene Glycol) and Cyclodextrin-Grafted Chitosan: From Methodologies to Preparation and Potential Biotechnological Applications. Front. Chem. 2017, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sanada, Y.; Tamura, A.; Yui, N.; Sakurai, K. Chain Architecture and Flexibility of α-Cyclodextrin/PEG Polyrotaxanes in Dilute Solutions. Polym. J. 2015, 47, 464–467. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-Insoluble β-Cyclodextrin–Epichlorohydrin Polymers for Removal of Pollutants from Aqueous Solutions by Sorption Processes Using Batch Studies: A Review of Inclusion Mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Anne, J.M.; Boon, Y.H.; Saad, B.; Miskam, M.; Yusoff, M.M.; Shahriman, M.S.; Zain, N.N.M.; Lim, V.; Raoov, M. B-Cyclodextrin Conjugated Bifunctional Isocyanate Linker Polymer for Enhanced Removal of 2,4-Dinitrophenol from Environmental Waters. R. Soc. Open Sci. 2018, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rafai Far, A.; Lag Cho, Y.; Rang, A.; Rudkevich, D.M.; Rebek, J. Polymer-Bound Self-Folding Cavitands. Tetrahedron 2002, 58, 741–755. [Google Scholar] [CrossRef]
- Shimazawa, R.; Hashimoto, Y.; Iwasaki, S. Water Soluble Zimmerman Mlecular Tweezers Analogs: Dextran Coupling Method for Solubilization. Tetrahedron Lett. 1992, 33, 7197–7200. [Google Scholar] [CrossRef]
- Tamminen, J.; Kolehmainen, E. Bile Acids as Building Blocks of Supramolecular Hosts. Molecules 2001, 6, 21–46. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Hayes, W.; MacKay, M.E.; Rowan, S.J.; Colquhoun, H.M. A Supramolecular Polymer Based on Tweezer-Type π-π Stacking Interactions: Molecular Design for Healability and Enhanced Toughness. Chem. Mater. 2011, 23, 6–8. [Google Scholar] [CrossRef]
- Colquhoun, H.; Zhu, Z.; Cardin, C.J.; Drew, M.G.B.; Gan, Y. Recognition of Sequence-Information in Synthetic Copolymer Chains by a Conformationally-Constrained Tweezer Molecule. Faraday Discuss. 2009, 143, 205–220. [Google Scholar] [CrossRef]
- Elisa, L.; Baldini, F.; Giannetti, A.; Trono, C.; Carofiglio, T. Solid-Supported Zn(II) Porphyrin Tweezers as Optical Sensors for Diamines. Chem. Commun. 2010, 46, 3678–3680. [Google Scholar] [CrossRef]
- Leblond, J.; Gao, H.; Petitjean, A.; Leroux, J.C. PH-Responsive Molecular Tweezers. J. Am. Chem. Soc. 2010, 132, 8544–8545. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, A.; Mondal, P.; Saha, B.; Rath, S.P. Induction, Control, and Rationalization of Supramolecular Chirogenesis Using Metalloporphyrin: Tweezers: A Structure-Function Correlation. Dalton Trans. 2020, 49, 10679–10700. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.; Brückner, C. Modifications of Porphyrins and Hydroporphyrins for Their Solubilization in Aqueous Media. Molecules 2017, 22, 980. [Google Scholar] [CrossRef] [PubMed]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Scamporrino, E.; Mineo, P.; Dattilo, S.; Vitalini, D.; Spina, E. Uncharged Water-Soluble Metal-Bis- Porphyrins like Molecular Tweezers for Amino Acids. Macromol. Rapid Commun. 2007, 28, 1546–1552. [Google Scholar] [CrossRef]
- Li, Z.; Siddique, I.; Hadrović, I.; Kirupakaran, A.; Li, J.; Zhang, Y.; Klärner, F.G.; Schrader, T.; Bitan, G. Lysine-Selective Molecular Tweezers Are Cell Penetrant and Concentrate in Lysosomes. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, B.; Seo, J. Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition. Molecules 2018, 23, 2741. [Google Scholar] [CrossRef]
- Wolf, L.M.; Servoss, S.L.; Moss, M.A. Peptoids: Emerging Therapeutics for Neurodegeneration. J. Neurol. Neuromed. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Besouw, M.; Masereeuw, R.; Van Den Heuvel, L.; Levtchenko, E. Cysteamine: An Old Drug with New Potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef]
- Sayaheen, M.; Otero, N.; Peña-Gallego, A. A Computational Study of Two Promising Tweezers. Theor. Chem. Acc. 2023, 142, 1–12. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Bhashini Wijesinghe, W.C.; Min, D. Robust Membrane Protein Tweezers Reveal the Folding Speed Limit of Helical Membrane Proteins. eLife 2023, 12, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Vafakish, B.; Wilson, L.D. Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein. Surfaces 2019, 2, 468–484. [Google Scholar] [CrossRef]
- Vafakish, B.; Wilson, L.D. Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties. Molecules 2020, 25, 1052. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, A.; Dewani, C.; Kazem-Rostami, M.; Wilson, L.D. Magnetic Silver Nanoparticles Stabilized by Superhydrophilic Polymer Brushes with Exceptional Kinetics and Catalysis. Polymers 2024, 16, 2500. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Li, Y.; Tu, J.; Zhao, L.; Li, N.; Li, X. Synthesis of Fe3O4@SiO2-Ag Magnetic Nanocomposite Based on Small-Sized and Highly Dispersed Silver Nanoparticles for Catalytic Reduction of 4-Nitrophenol. J. Coll. Interface Sci. 2012, 383, 96–102. [Google Scholar] [CrossRef]
- Wang, M.; Tian, D.; Tian, P.; Yuan, L. Synthesis of Micron-SiO2@nano-Ag Particles and Their Catalytic Performance in 4-Nitrophenol Reduction. Appl. Surf. Sci. 2013, 283, 389–395. [Google Scholar] [CrossRef]
- Liang, M.; Su, R.; Qi, W.; Yu, Y.; Wang, L.; He, Z. Synthesis of Well-Dispersed Ag Nanoparticles on Eggshell Membrane for Catalytic Reduction of 4-Nitrophenol. J. Mater. Sci. 2014, 49, 1639–1647. [Google Scholar] [CrossRef]
- Vafakish, B.; Wilson, L.D. A Highly Sensitive Chitosan-Based SERS Sensor for the Trace Detection of a Model Cationic Dye. Int. J. Mol. Sci. 2024, 25, 9327. [Google Scholar] [CrossRef]
- Xiao, G.N.; Man, S.Q. Surface-Enhanced Raman Scattering of Methylene Blue Adsorbed on Cap-Shaped Silver Nanoparticles. Chem. Phys. Lett. 2007, 447, 305–309. [Google Scholar] [CrossRef]
- Srichan, C.; Ekpanyapong, M.; Horprathum, M.; Eiamchai, P.; Nuntawong, N.; Phokharatkul, D.; Danvirutai, P.; Bohez, E.; Wisitsoraat, A.; Tuantranont, A. Highly-Sensitive Surface-Enhanced Raman Spectroscopy (SERS)-Based Chemical Sensor Using 3D Graphene Foam Decorated with Silver Nanoparticles as SERS Substrate. Sci. Rep. 2016, 6, 23733. [Google Scholar] [CrossRef]
- Prikhozhdenko, E.S.; Lengert, E.V.; Parakhonskiy, B.V.; Gorin, D.A.; Sukhorukov, G.B.; Yashchenok, A.M. Biocompatible Chitosan Nanofibers Functionalized with Silver Nanoparticles for Sers Based Detection. Acta Phys. Pol. A 2016, 129, 247–249. [Google Scholar] [CrossRef]
- Wang, C.; Wong, K.W.; Wang, Q.; Zhou, Y.; Tang, C.; Fan, M.; Mei, J.; Lau, W.M. Silver-Nanoparticles-Loaded Chitosan Foam as a Flexible SERS Substrate for Active Collecting Analytes from Both Solid Surface and Solution. Talanta 2019, 191, 241–247. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Maniu, D.; Astilean, S. Gelatin-Nanogold Bioconjugates as Effective Plasmonic Platforms for SERS Detection and Tagging. Coll. Surf. B Biointerfaces 2013, 103, 475–481. [Google Scholar] [CrossRef]
- He, C.; Bai, H.; Yi, W.; Liu, J.; Li, X.; Li, X.; Xi, G. A Highly Sensitive and Stable SERS Substrate Using Hybrid Tungsten Dioxide/Carbon Ultrathin Nanowire Beams. J. Mater. Chem. C 2018, 6, 3200–3205. [Google Scholar] [CrossRef]
- Hariprasad, E.; Radhakrishnan, T.P. In Situ Fabricated Polymer-Silver Nanocomposite Thin Film as an Inexpensive and Efficient Substrate for Surface-Enhanced Raman Scattering. Langmuir 2013, 29, 13050–13057. [Google Scholar] [CrossRef]
- Mir, M.; Wilson, L.D. Flax fiber-chitosan biocomposites with tailored structure and switchable physicochemical properties. Carbohydr. Polym. Technol. Appl. 2023, 6, 100397. [Google Scholar] [CrossRef]
- Nia, M.H.; Wilson, L.D.; Kiasat, A.R.; Munguia-Lopez, J.G.; Kinsella, J.M.; van de Ven, T. Internally bridged nanosilica for loading and release of sparsely soluble compounds. J. Coll. Interface Sci. 2023, 649, 456–470. [Google Scholar]
- Venegas-García, D.J.; Mir, M.; Steiger, B.G.K.; Wilson, L.D. Furfuryl-pyridinium-functionalization of flaxseed gum for effective methylene blue removal from aqueous solution. Can. J. Chem. 2024, 1–13. [Google Scholar] [CrossRef]
- Jozeliūnaitė, A.; Javorskis, T.; Vaitkevičius, V.; Klimavičius, V.; Orentas, E. Fully Supramolecular Chiral Hydrogen-Bonded Molecular Tweezer. J. Amer. Chem. Soc. 2022, 144, 8231–8241. [Google Scholar] [CrossRef]
- Dehabadi, L.; Karoyo, A.H.; Wilson, L.D. Spectroscopic and Thermodynamic Study of Biopolymer Adsorption Phenomena in Heterogeneous Solid−Liquid Systems. ACS Omega 2018, 3, 15370–15379. [Google Scholar] [CrossRef]
- Rekharsky, M.; Inoue, Y. Solvation Effects in Supramolecular Recognition. In Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–17. [Google Scholar] [CrossRef]
- Yusof, W.R.W.; Awang, N.Y.F.; Laile, M.A.A.; Azizi, J.; Husaini, A.A.S.A.; Seeni, A.; Wilson, L.D.; Sabar, S. Chemically modified water-soluble chitosan derivatives: Modification strategies, biological activities, and applications. Polym. Technol. Mater. 2023, 62, 2182–2220. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Varma, R.S.; Thakur, V.K. Nano/Micro-Structural Supramolecular Biopolymers: Innovative Networks with the Boundless Potential in Sustainable Agriculture. Nano-Micro Lett. 2024, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kubota, R.; Aoyama, T.; Urayama, K.; Hamachi, I. Four distinct network patterns of supramolecular/polymer composite hydrogels controlled by formation kinetics and interfiber interactions. Nat. Commun. 2023, 14, 1696. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Jin, S.; Yu, Y.; Zeng, G.; Zhang, F.; Xiao, H.; Yang, R.; Li, K.; Li, J. Supramolecular and double network strategy toward strong and antibacterial protein films by introducing waterborne polyurethane and quaternized chitosan. Ind. Crop. Prod. 2023, 205, 117445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).