Effect of Corrosion Products and Deposits on the Damage Tolerance of TSA-Coated Steel in Artificial Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Governing Equations
2.2. Time-Dependent Model
2.3. Geometry and Boundary Conditions
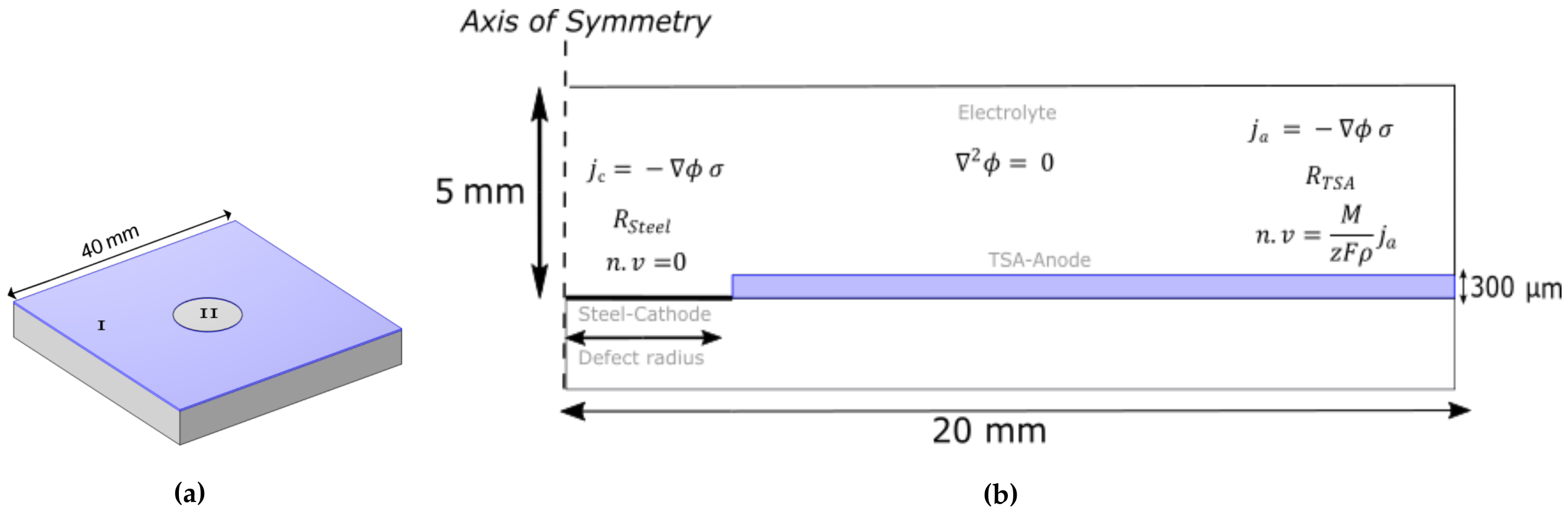
2.4. Parameters
- E0: No corrosion products or deposits;
- E1: Corrosion products and deposits formed in 10 d;
- E2: Corrosion products and deposits formed in 20 d.
3. Results
3.1. Potential
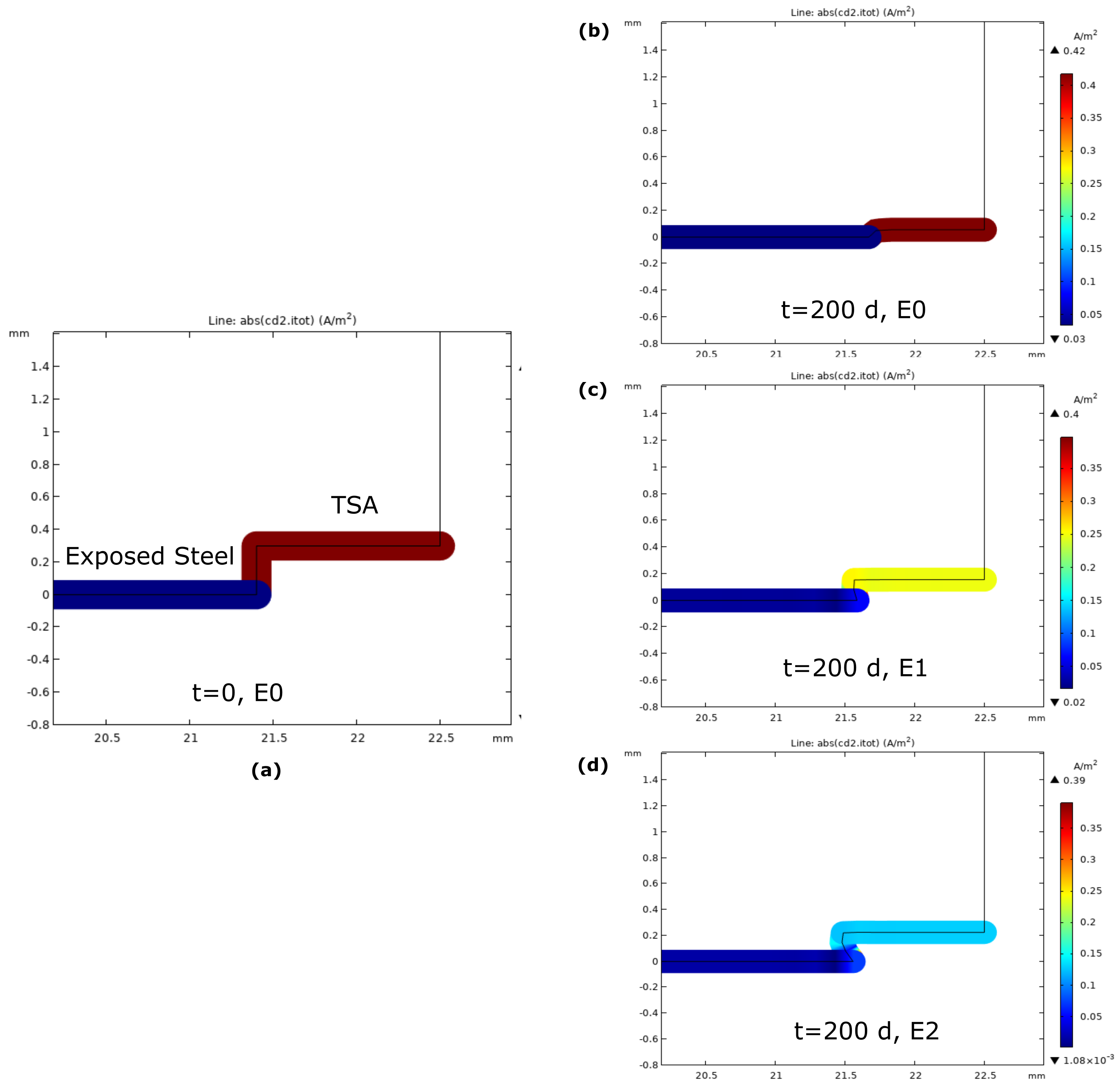
3.2. Current Density
3.3. Corrosion Rate
4. Discussion
4.1. Considerations of Modelling
- The ability of TSA coating to polarise exposed steel by varying surfaces of damage;
- The formation of calcareous deposits block the diffusion of dissolved O to steel;
- The precipitation of corrosion products fill the porous of the coating, reducing the corrosion of aluminium;
- The model replicates the behaviour of corrosion products and calcareous deposits assuming a film resistance on each electrode surface. The values were taken from a depth examination of cathodic reactions by Electrochemical Impedance Spectroscopy (EIS) conducted by R. Grinon et al. [14]. This investigation introduced a new methodology to separate anodic and cathodic processes. The samples were designed so bi-electrode and electrochemical measurements can be performed separately. From this study, the charge transfer resistance, and diffusion elements trough the corrosion products to TSA and deposits to the steel were selected as parameters in the simulation. Deposits build-up on steel surfaces and aluminium corrosion products on TSA can be compared to an electrically resistant layer [12] therefore, EIS is often used to determine quantitative data about dielectric and electrical elements at the interface electrode/solution [29,30]. The record of OCP in long-term exposure tests shows that the potential reaches a plateau after approximately 20–30 days [6,14] thus, it is considered that the deposited layers are stable enough to inhibit cathodic reactions and reduce the production of OH ions. The model assumed values of electrical resistance of deposits and corrosion products measured at two specific moments (10 d and 20 d);
- Physical measurements of thickness loss in the coating are difficult to obtain since the deposits and corrosion products fill the pores. In addition, the material losses are small and localised. For this reason, electrochemical measurements are generally adopted to estimate the corrosion rate [31]. This model simulates the CR by ALE and compares the results with values obtained by LPR [10].
4.2. Steel Protection by Damaged TSA Coating
4.3. Effect of Calcareous Deposits
4.4. Effect of Aluminium Corrosion Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, S.J.; Figueira, R.B. Corrosion protection systems and fatigue corrosion in offshore wind structures: Current status and future perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- Pakenham, B.; Ermakova, A.; Mehmanparast, A. A Review of Life Extension Strategies for Offshore Wind Farms Using Techno-Economic Assessments. Energies 2021, 14, 1936. [Google Scholar] [CrossRef]
- Masi, G.; Matteucci, F.; Tacq, J.; Balbo, A. State of the Art Study on Materials and Solutions Against Corrosion in Offshore Structures. North Sea Solutions for Innovation in Corrosion for Energy. 2018. Available online: http://nessieproject.com/library/reports-and-researches/NeSSIE%20Report%20Study%20on%20Materials%20and%20Solutions%20in%20Corrosion (accessed on 21 August 2021).
- Funke, S. German Renewables Award for Offshore Wind Project Arkona. 2017. Available online: https://www.offshorewindindustry.com/news/german-renewables-award-offshore-wind-project (accessed on 10 October 2021).
- Tiong, D.K.K.; Pit, H. Experiences on Thermal Spray Aluminum (TSA) Coating on Offshore Structures. In Proceedings of the CORROSION 2004, New Orleans, LA, USA, 28 March 2004. [Google Scholar]
- Grinon-Echaniz, R.; Paul, S.; Thornton, R.; Refait, P.; Jeannin, M.; Rodriguez, A. Prediction of Thermal Spray Coatings Performance in Marine Environments by Combination of Laboratory and Field Tests. Coatings 2021, 11, 320. [Google Scholar] [CrossRef]
- Fischer, K.P.; Thomason, W.H.; Rosbrook, T.; Murali, J. Performance history of thermal-sprayed aluminum coatings in offshore service. Mater. Perform. 1995, 34, 27–35. [Google Scholar]
- Syrek-Gerstenkorn, B.; Paul, S.; Davenport, A.J. Sacrificial thermally sprayed aluminium coatings for marine environments: A review. Coatings 2020, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Syrek-Gerstenkorn, B.; Paul, S.; Davenport, A.J. Use of thermally sprayed aluminium (TSA) coatings to protect offshore structures in submerged and splash zones. Surf. Coatings Technol. 2019, 374, 124–133. [Google Scholar] [CrossRef]
- Echaniz, R.G.; Paul, S.; Thornton, R. Effect of seawater constituents on the performance of thermal spray aluminum in marine environments. Mater. Corros. 2019, 70, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Paul, S. Behavior of Damaged Thermally Sprayed Aluminum (TSA) in Aerated and Deaerated Seawater. In Proceedings of the CORROSION 2019, Nashville, TN, USA, 26 March 2019. [Google Scholar]
- Yang, Y.; Scantlebury, J.D.; Koroleva, E.V. A study of calcareous deposits on cathodically protected mild steel in artificial seawater. Metals 2015, 5, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Ce, N.; Paul, S. The Effect of Temperature and Local pH on Calcareous Deposit Formation in Damaged Thermal Spray Aluminum (TSA) Coatings and its Implication on Corrosion Mitigation of Offshore Steel Structures. Coatings 2017, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Grinon-Echaniz, R.; Refait, P.; Jeannin, M.; Sabot, R.; Paul, S.; Thornton, R. Study of cathodic reactions in defects of thermal spray aluminium coatings on steel in artificial seawater. Corros. Sci. 2021, 187, 109514. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Wijesinghe, S.; Blackwood, D.J. Moving boundary simulation and mechanistic studies of the electrochemical corrosion protection by a damaged zinc coating. Corros. Sci. 2020, 163, 108296. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Van den Steen, N.; Wijesinghe, S.; Vafakhah, S.; Terryn, H.; Blackwood, D.J. Moving Boundary Simulation of Iron-Zinc Sacrificial Corrosion under Dynamic Electrolyte Thickness Based on Real-Time Monitoring Data. J. Electrochem. Soc. 2020, 167, 041503. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Allely, C.; Ogle, K. Modeling bimetallic corrosion under thin electrolyte films. Corros. Sci. 2011, 53, 201–207. [Google Scholar] [CrossRef]
- Cross, S.R.; Gollapudi, S.; Schuh, C.A. Validated numerical modeling of galvanic corrosion of zinc and aluminum coatings. Corros. Sci. 2014, 88, 226–233. [Google Scholar] [CrossRef]
- Sun, W.; Liu, G.; Wang, L.; Li, Y. A mathematical model for modeling the formation of calcareous deposits on cathodically protected steel in seawater. Electrochim. Acta 2012, 78, 597–608. [Google Scholar] [CrossRef]
- Sun, W.; Liu, G.; Wang, L.; Wu, T.; Liu, Y. An arbitrary Lagrangian–Eulerian model for studying the influences of corrosion product deposition on bimetallic corrosion. J. Solid State Electrochem. 2013, 17, 829–840. [Google Scholar] [CrossRef]
- Yin, L.; Li, W.; Wang, Y.; Jin, Y.; Pan, J.; Leygraf, C. Numerical simulation of micro-galvanic corrosion of Al alloys: Effect of density of Al (OH)3 precipitate. Electrochim. Acta 2019, 324, 134847. [Google Scholar] [CrossRef]
- Yin, L.; Jin, Y.; Leygraf, C.; Pan, J. A FEM model for investigation of micro-galvanic corrosion of Al alloys and effects of deposition of corrosion products. Electrochim. Acta 2016, 192, 310–318. [Google Scholar] [CrossRef]
- ASTM D1141-98; Standard Practice for the Preparation of Substitute Ocean Water. 2013.
- Deshpande, K.B. Validated numerical modelling of galvanic corrosion for couples: Magnesium alloy (AE44)–mild steel and AE44–aluminium alloy (AA6063) in brine solution. Corros. Sci. 2010, 52, 3514–3522. [Google Scholar] [CrossRef]
- Zheng, Z.; Fu, Y.; Liu, K.; Xiao, R.; Wang, X.; Shi, H. Three-stage vertical distribution of seawater conductivity. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cathodic Protection Design, Recommended Practice DNV-RP-B401. 2010. Available online: https://www.engr.mun.ca/~sbruneau/teaching/8751ocean/DNV%20cathode%20design.pdf (accessed on 14 August 2021).
- ISO 12944. Paints and Varnishes — Corrosion Protection of Steel Structures by Protective Paint Systems. 2019. Available online: https://www.iso.org/standard/77795.html (accessed on 14 August 2021).
- Paul, S. Cathodic Protection of Offshore Structures by Extreme Damage Tolerant Sacrificial Coatings. In Proceedings of the NACE International Corrosion Conference Proceedings. NACE International, Phoenix, AZ, USA, 15–19 April 2018; pp. 1–12. [Google Scholar]
- Esfahani, E.A.; Salimijazi, H.; Golozar, M.A.; Mostaghimi, J.; Pershin, L. Study of corrosion behavior of arc sprayed aluminum coating on mild steel. J. Therm. Spray Technol. 2012, 21, 1195–1202. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Park, J.H. Pore blocking characteristics of corrosion products formed on Aluminum coating produced by arc thermal metal spray process in 3.5 wt.% NaCl solution. Constr. Build. Mater. 2016, 113, 905–916. [Google Scholar] [CrossRef]
- Paul, S.; Harvey, D. Determination of the Corrosion Rate of Thermally Spayed Aluminum (TSA) in Simulated Marine Service. In Proceedings of the CORROSION 2020, Manchester, UK, 7 September 2020. [Google Scholar]
- Thomason, W. Offshore Corrosion Protection with Thermal-Sprayed Aluminum. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 17 May 1985. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rad, M.R.; McDonald, A.; Hussain, T. Beyond traditional coatings: A review on thermal-sprayed functional and smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef] [Green Version]
- C2.18-93R, A.A. Guide for the Protection of Steel with Thermal Sprayed Coatings of Aluminum and Zinc and their Alloys and Composites. Available online: https://webstore.ansi.org/preview-pages/AWS/preview_AWS+C2.18-93R.pdf (accessed on 20 June 2021).



| Parameter | Value | Description |
|---|---|---|
| Steel | −0.71 V | Corrosion potential of steel [14] |
| Steel | 9 × 10A/m | Exchange current density-cathode [14] |
| 0.114 V/dec | Anodic Tafel coefficient-steel [14] | |
| 0.538 V/dec | Cathodic Tafel coefficient-steel [14] | |
| TSA | −0.98 V | Corrosion potential of TSA [6,10] |
| TSA | 5.5 × 10A/m | Exchange current density-anode [6] |
| 0.57 V/dec | Anodic Tafel coefficient-TSA [6] | |
| 0.18 V/dec | Cathodic Tafel coefficient- TSA [6] | |
| 5 S/m | Electrolyte conductivity (Seawater) [25] | |
| M | 26.98 g/mol | Al molar mass |
| 2.7 g/cm | Al density | |
| z | 3 | Number of electrons for dissolving specie |
| F | 96,485.34 C/mol | Faraday constant |
| Parameter | Description | Exposure Time | |
|---|---|---|---|
| 10 d | 20 d | ||
| Film resistance on steel [14] | 15.5 k·cm | 46 k·cm | |
| Film resistance on TSA [14] | 2.7 k·cm | 8.7 k·cm | |
| Exposed Steel | Corrosion Rate of TSA (mm·y) | ||
|---|---|---|---|
| E0 | E1 | E2 | |
| 5% | 0.033 | 0.008 | 0.0035 |
| 50% | 0.279 | 0.102 | 0.045 |
| 90% | 0.438 | 0.256 | 0.135 |
| Compound | Salt Concentration (g/L) | Compound | Salt Concentration (g/L) |
|---|---|---|---|
| NaCl | 24.53 | NaHCO | 0.201 |
| MgCl | 5.2 | KBr | 0.101 |
| NaSO | 4.09 | HBO | 0.027 |
| CaCl | 1.16 | SrCl | 0.025 |
| KCl | 0.695 | NaF | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Vargas, A.; Gill, S.; Paul, S. Effect of Corrosion Products and Deposits on the Damage Tolerance of TSA-Coated Steel in Artificial Seawater. Surfaces 2022, 5, 113-126. https://doi.org/10.3390/surfaces5010005
Castro-Vargas A, Gill S, Paul S. Effect of Corrosion Products and Deposits on the Damage Tolerance of TSA-Coated Steel in Artificial Seawater. Surfaces. 2022; 5(1):113-126. https://doi.org/10.3390/surfaces5010005
Chicago/Turabian StyleCastro-Vargas, Adriana, Simon Gill, and Shiladitya Paul. 2022. "Effect of Corrosion Products and Deposits on the Damage Tolerance of TSA-Coated Steel in Artificial Seawater" Surfaces 5, no. 1: 113-126. https://doi.org/10.3390/surfaces5010005
APA StyleCastro-Vargas, A., Gill, S., & Paul, S. (2022). Effect of Corrosion Products and Deposits on the Damage Tolerance of TSA-Coated Steel in Artificial Seawater. Surfaces, 5(1), 113-126. https://doi.org/10.3390/surfaces5010005






