A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges
Abstract
1. Introduction
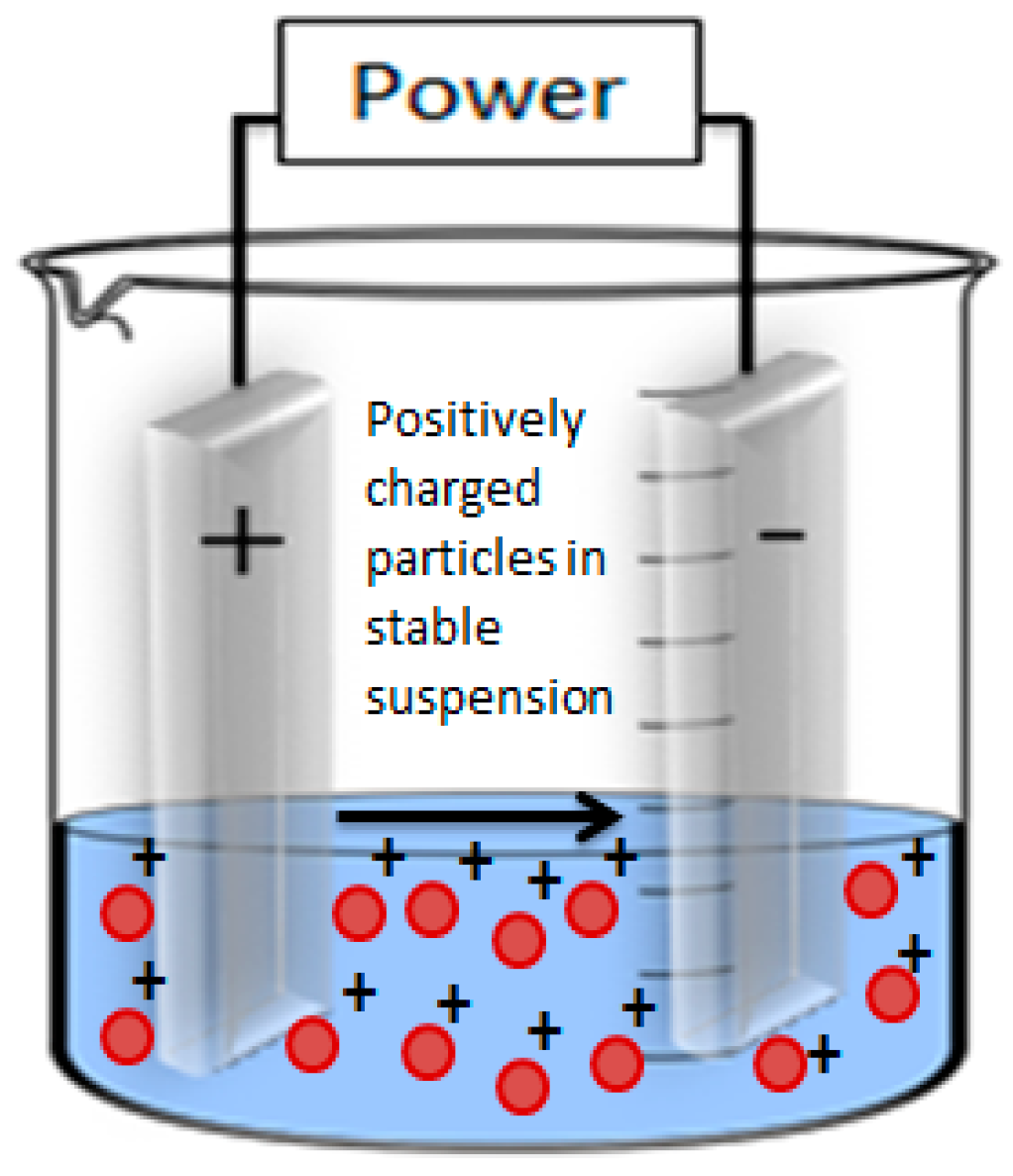
2. Electrophoretic Deposition
2.1. Classification of EPD
2.2. Key Parameters Affecting EPD
- µ, EM;
- ε, dielectric constant;
- ζ, zeta-potential;
- η, viscosity;
- f(ka), Henry’s function.
2.2.1. Parameters Related to Suspension Stability
2.2.2. Parameters Related to Processing
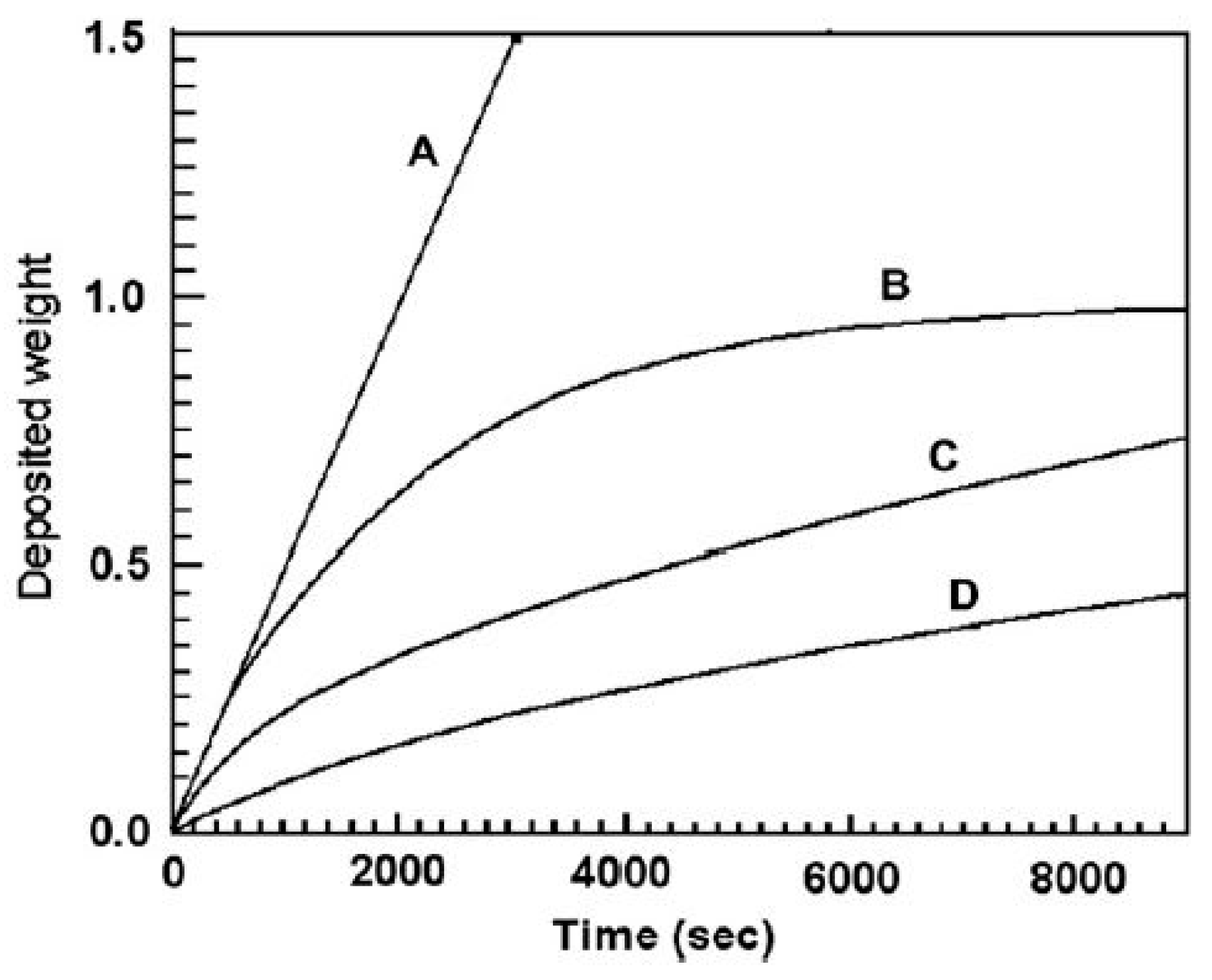
2.3. EPD Kinetics
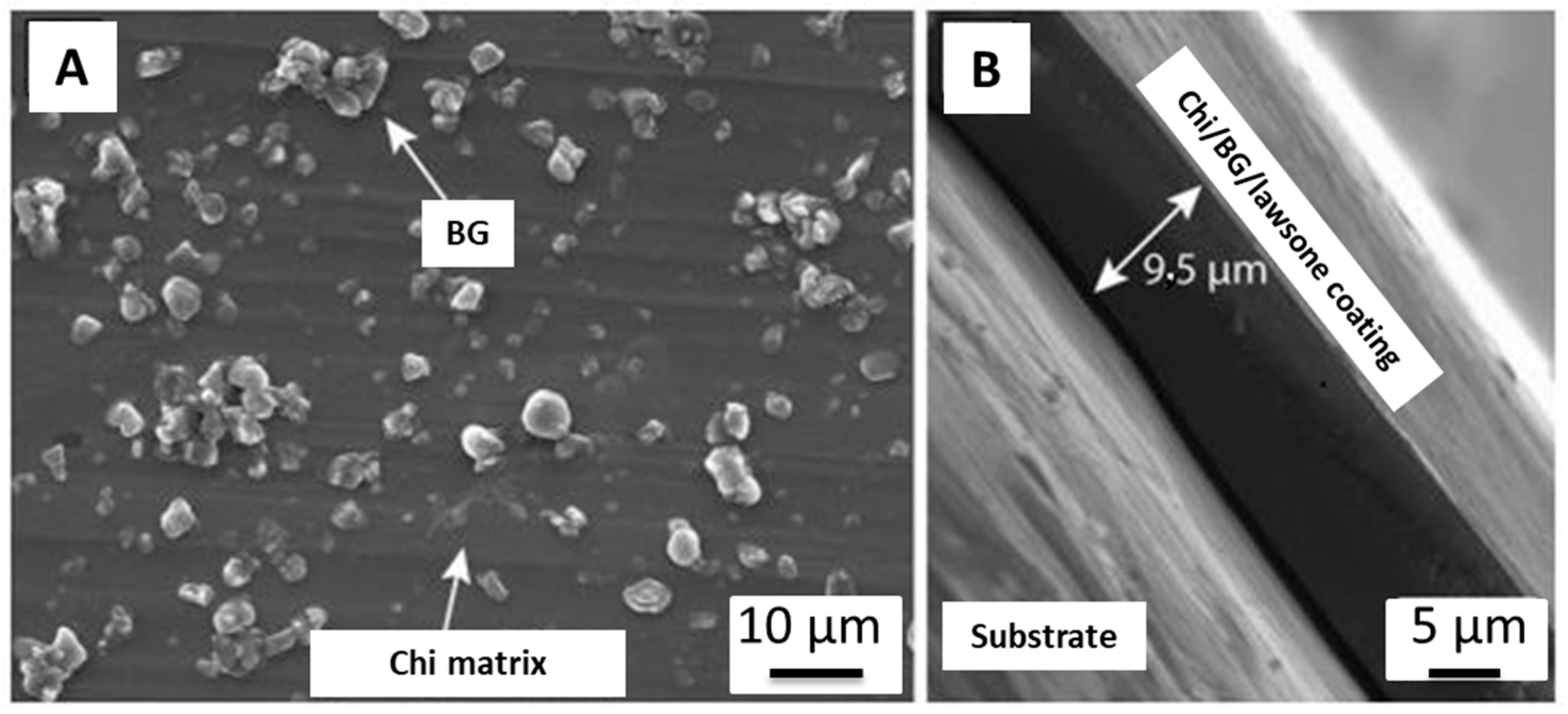
2.4. EPD of Chitosan-Based Coatings
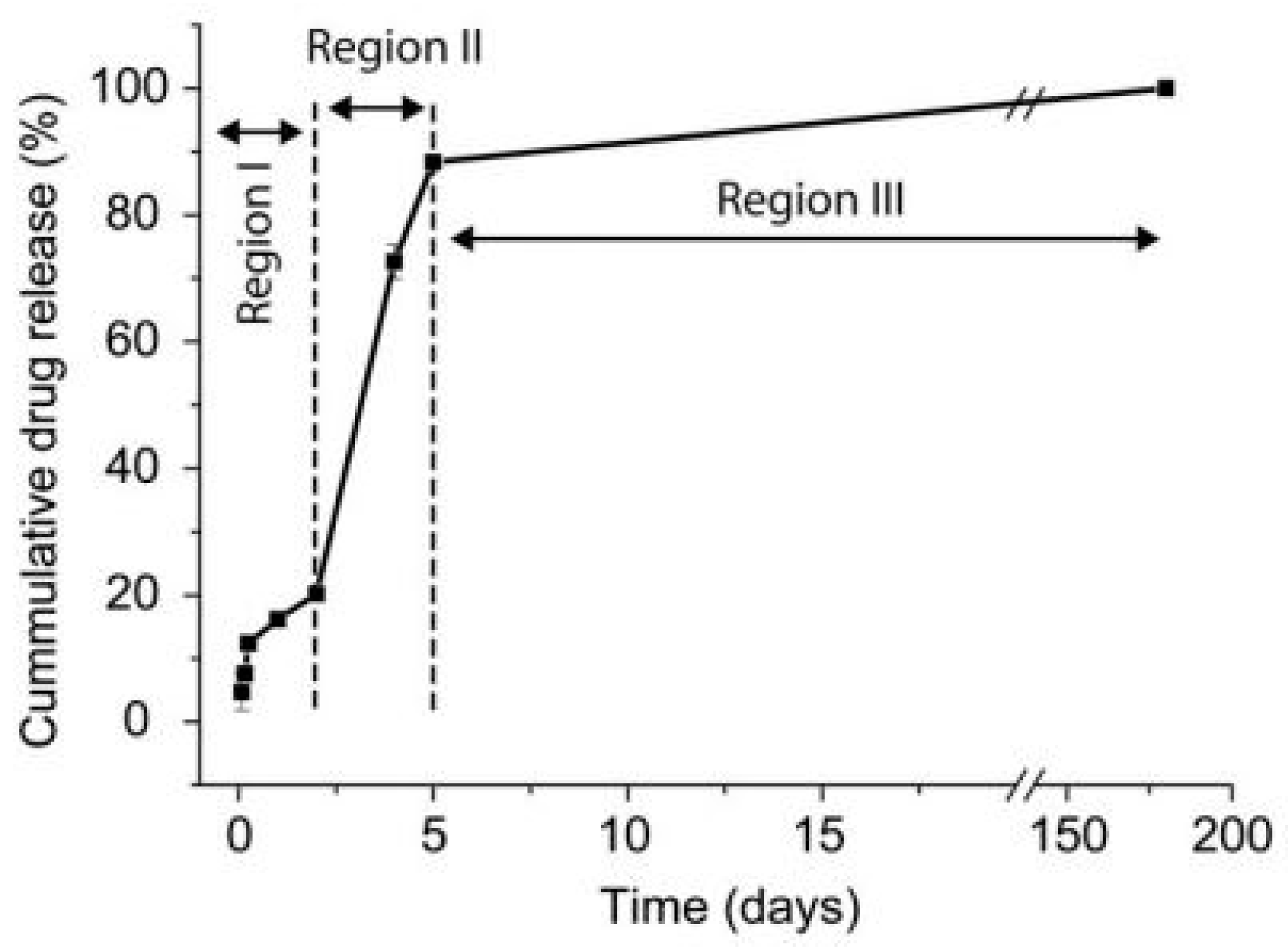
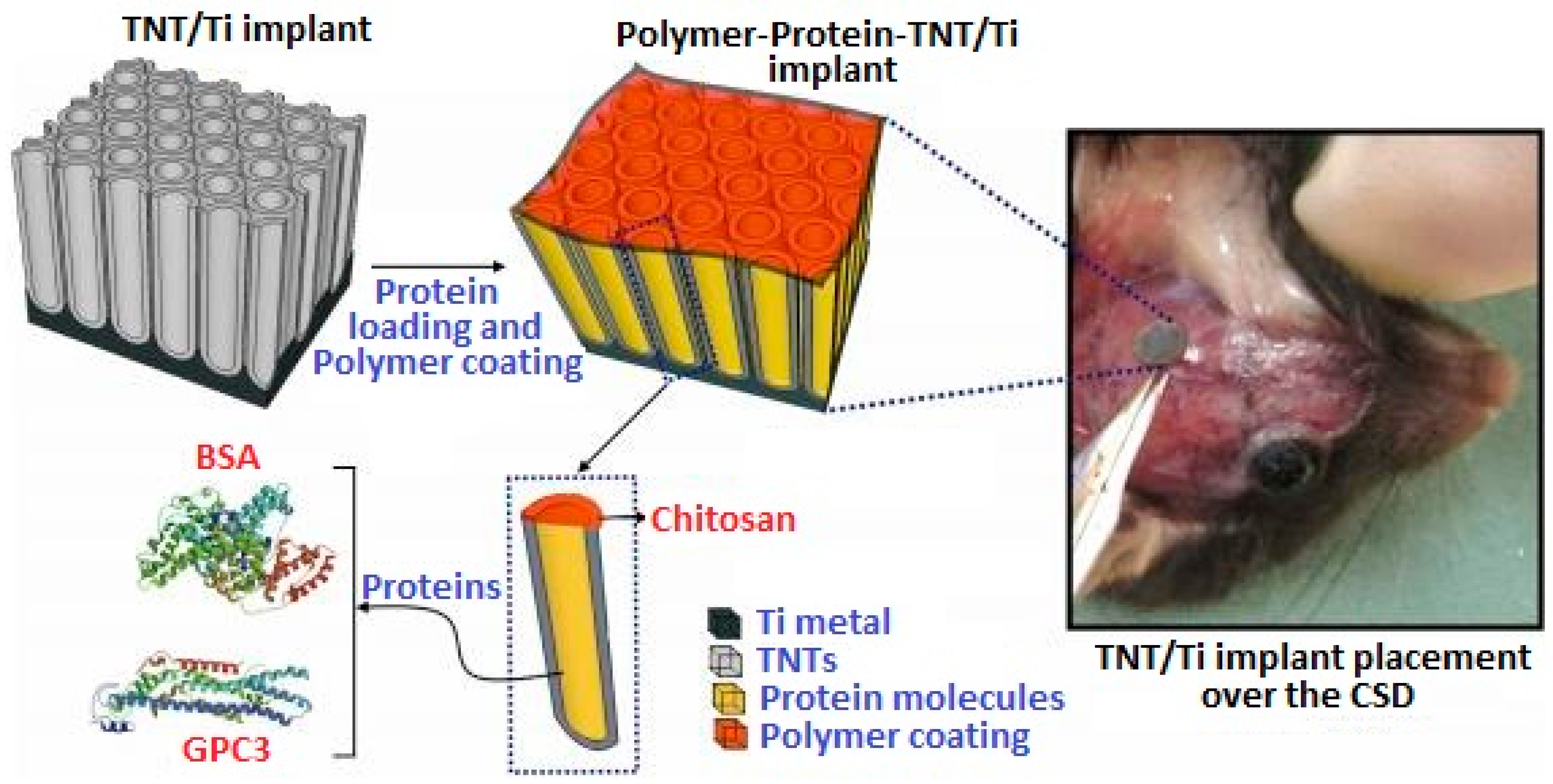
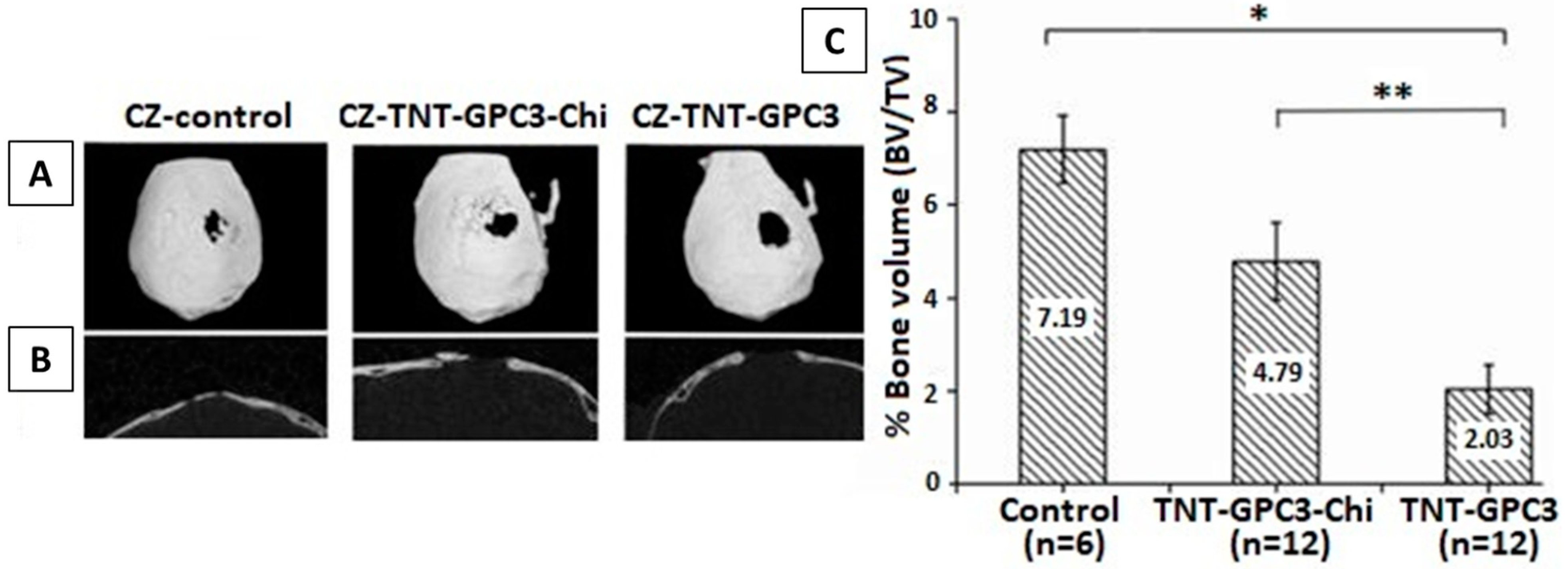
In Vivo Testing of Chi-Based Coatings
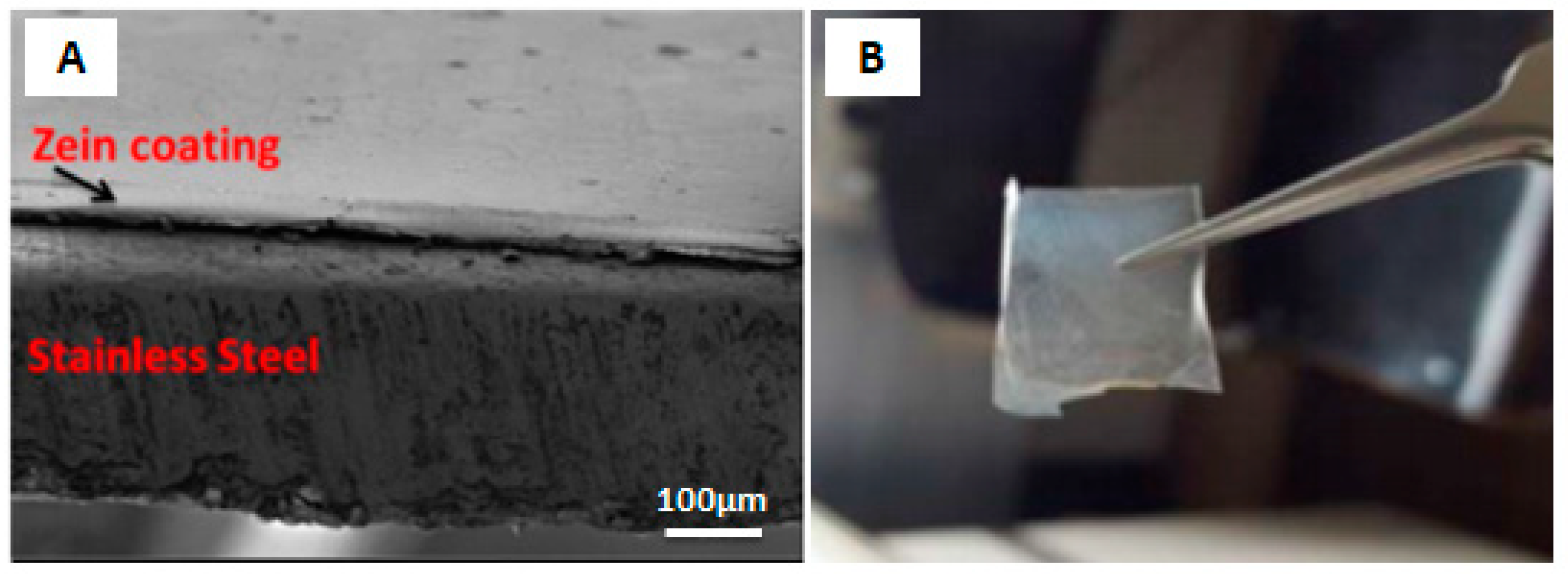
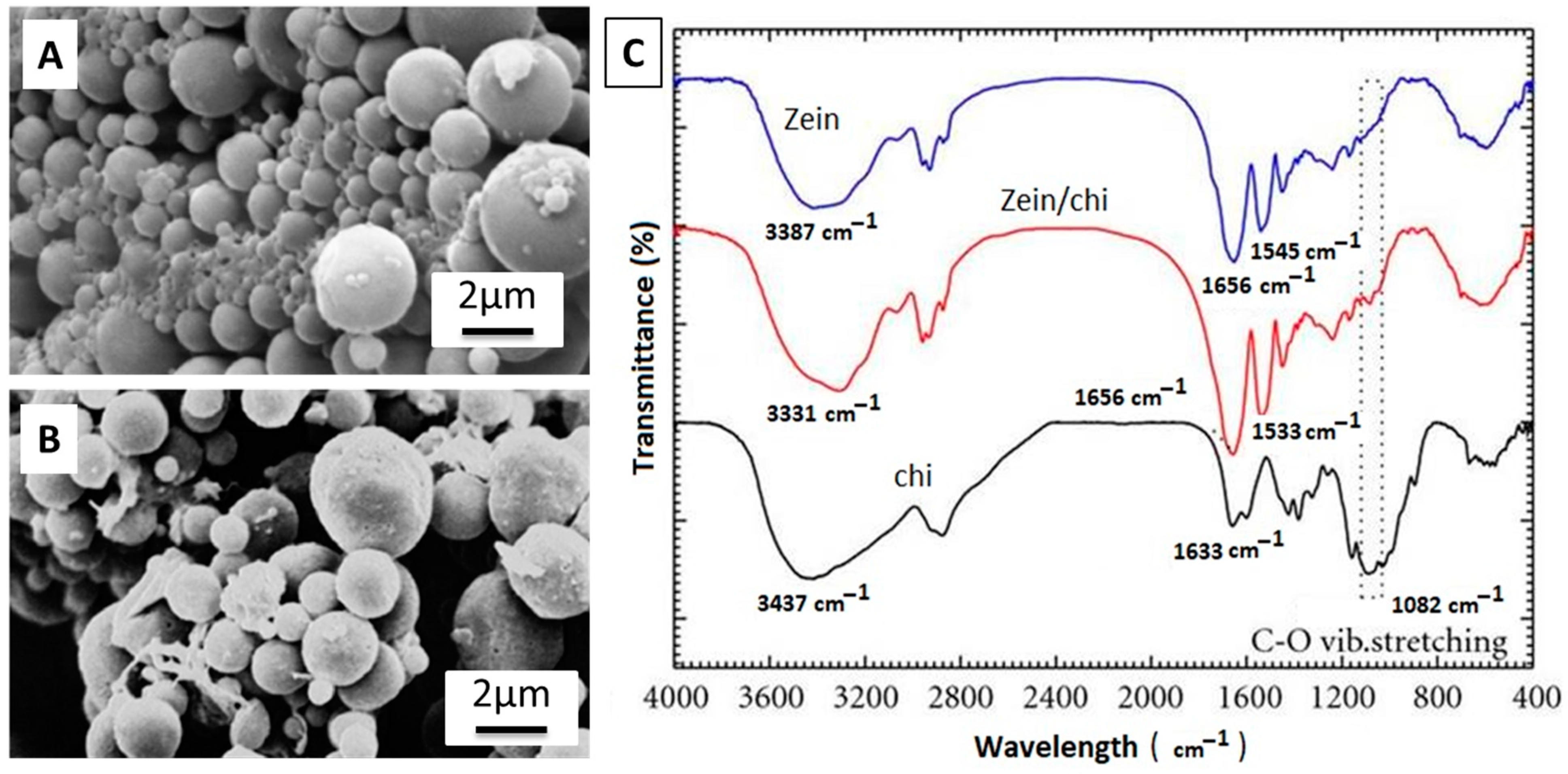
2.5. EPD of Zein Based Coatings
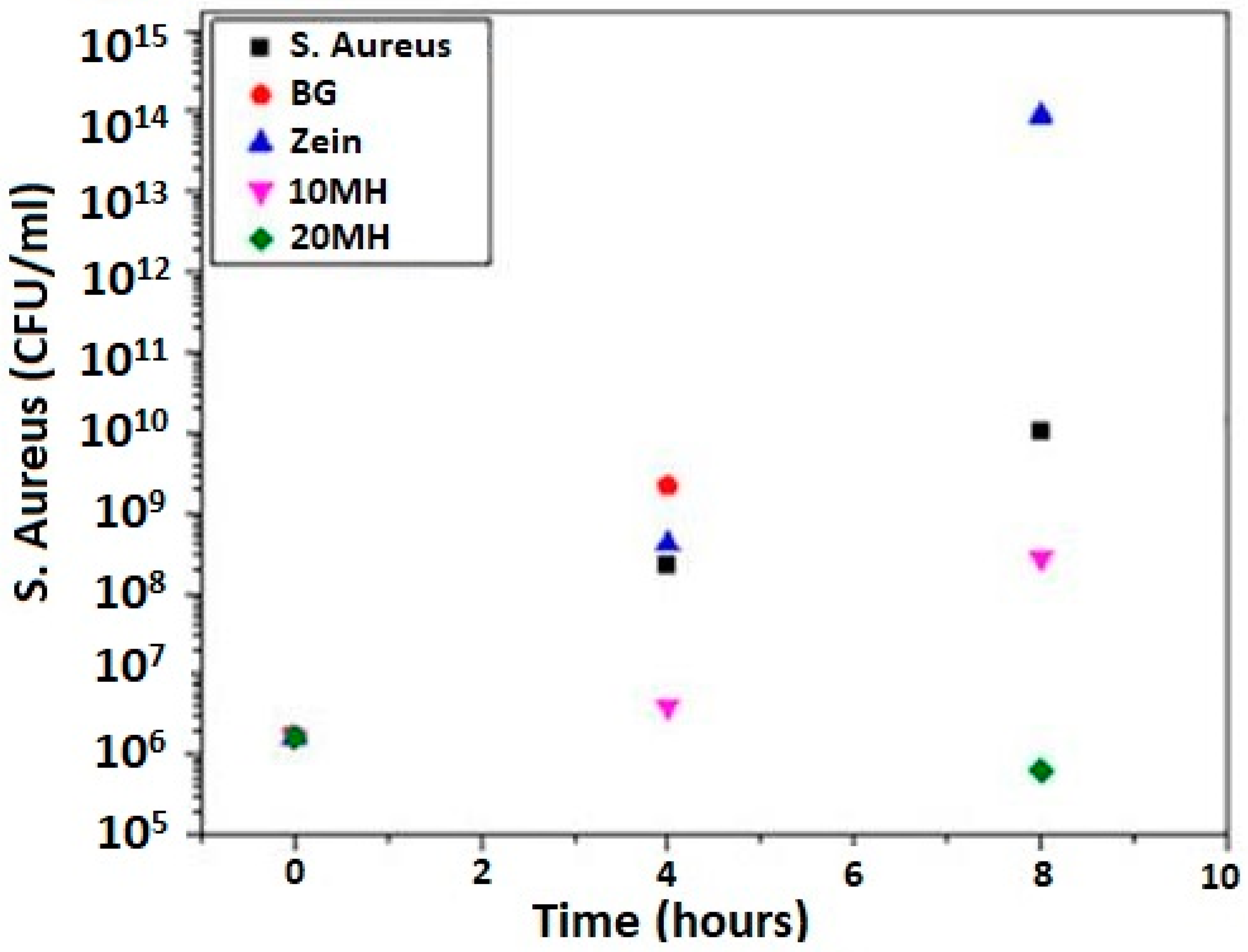
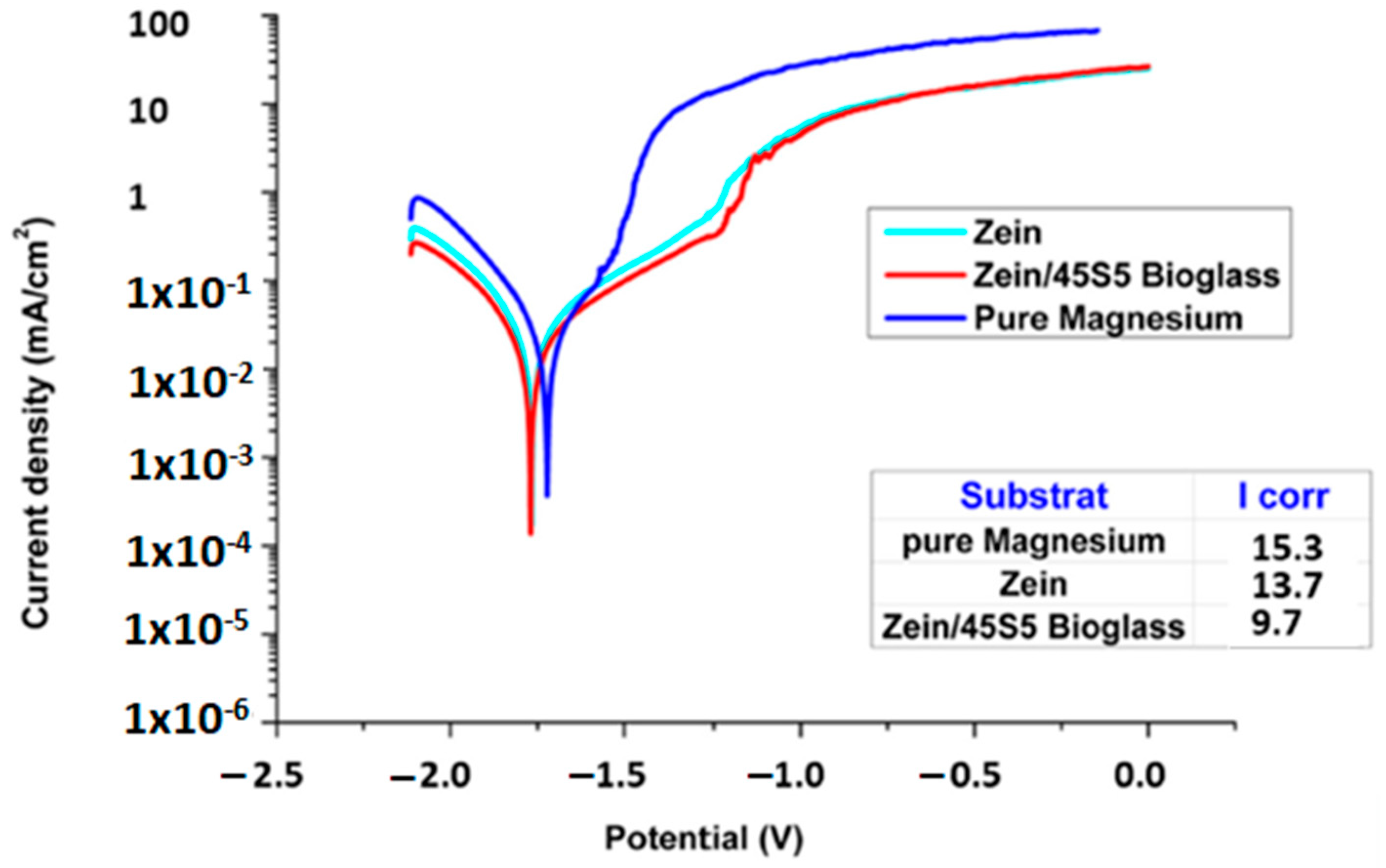
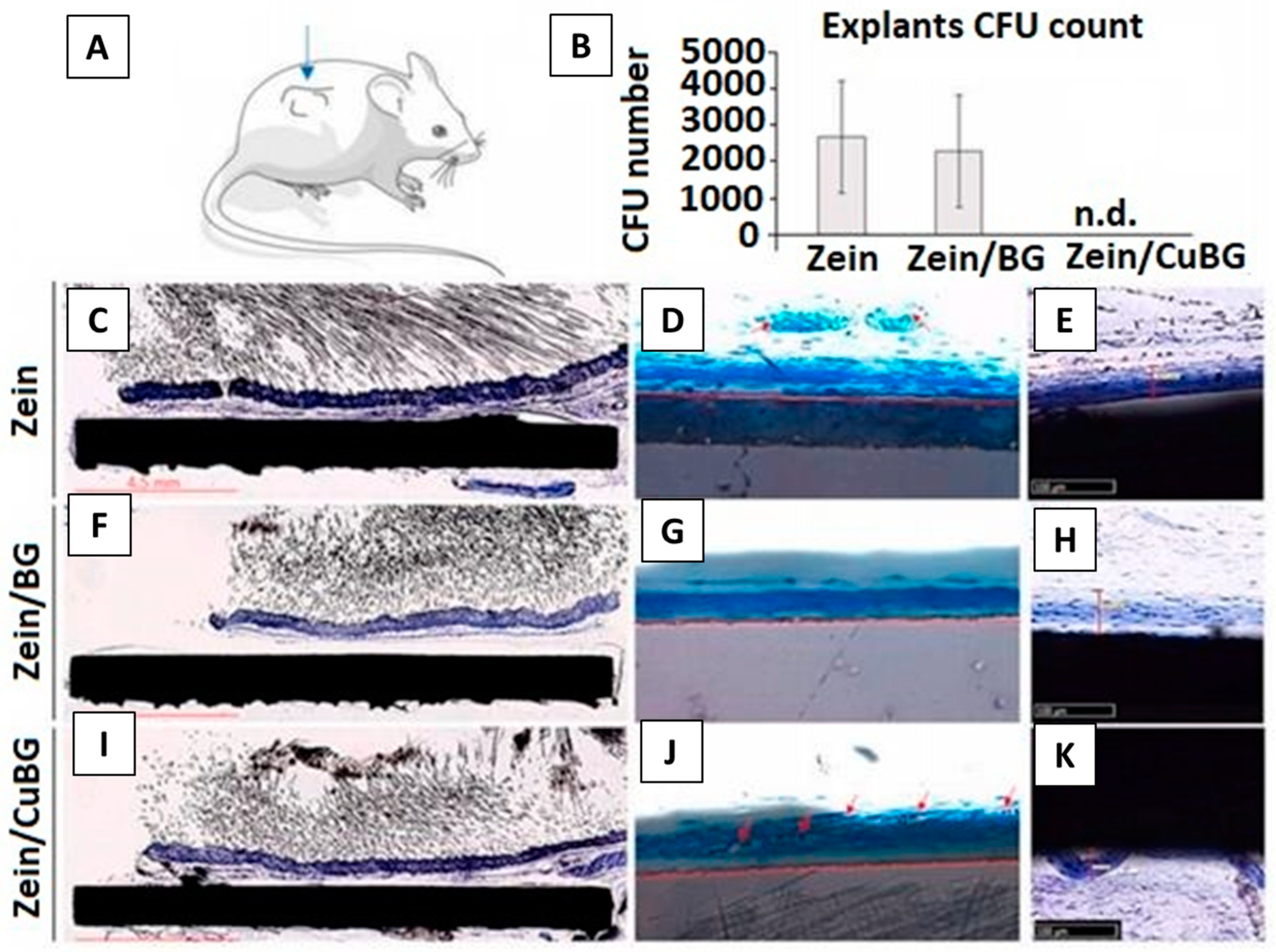
In Vivo Testing of Zein-Based Coating
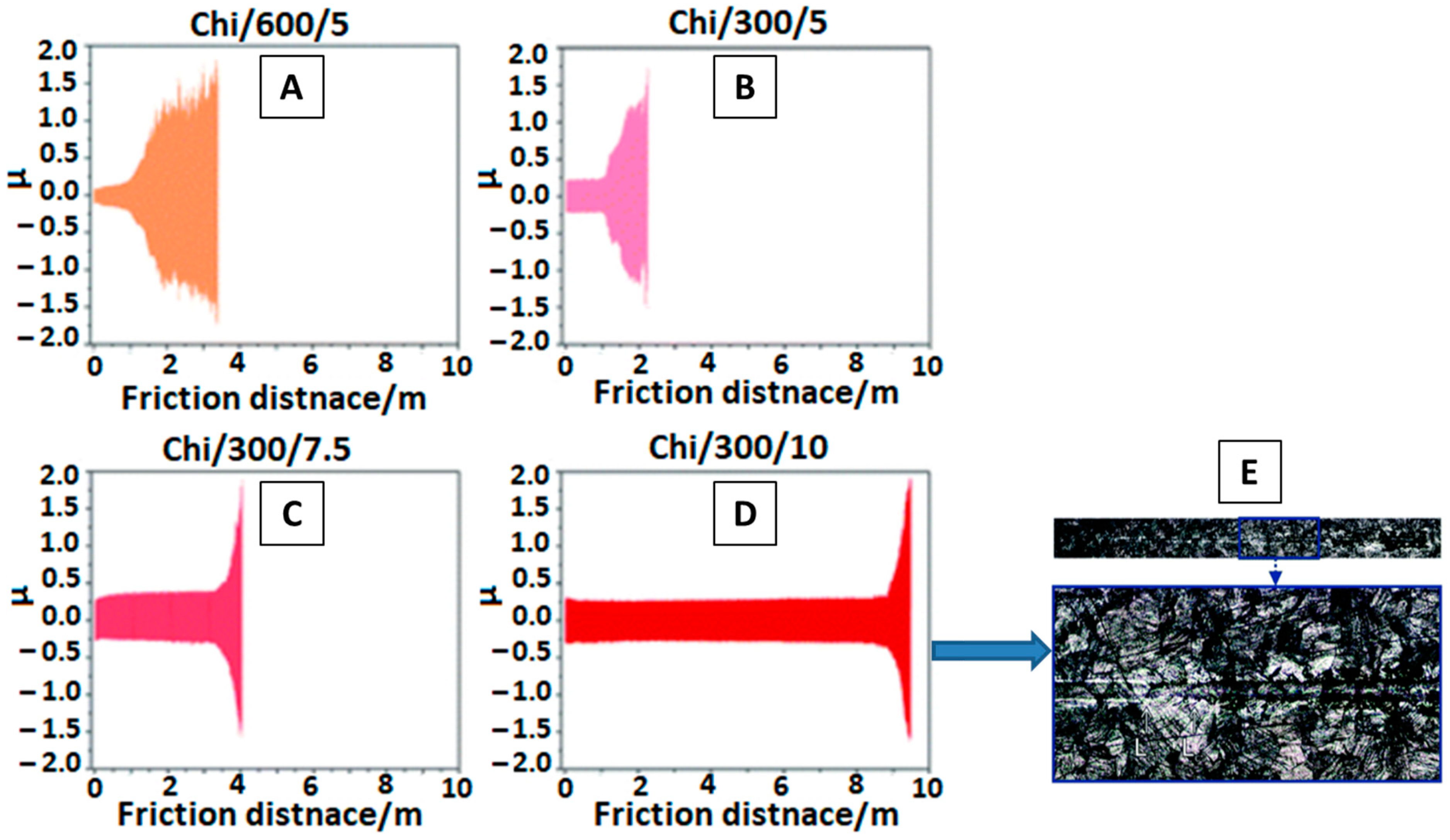
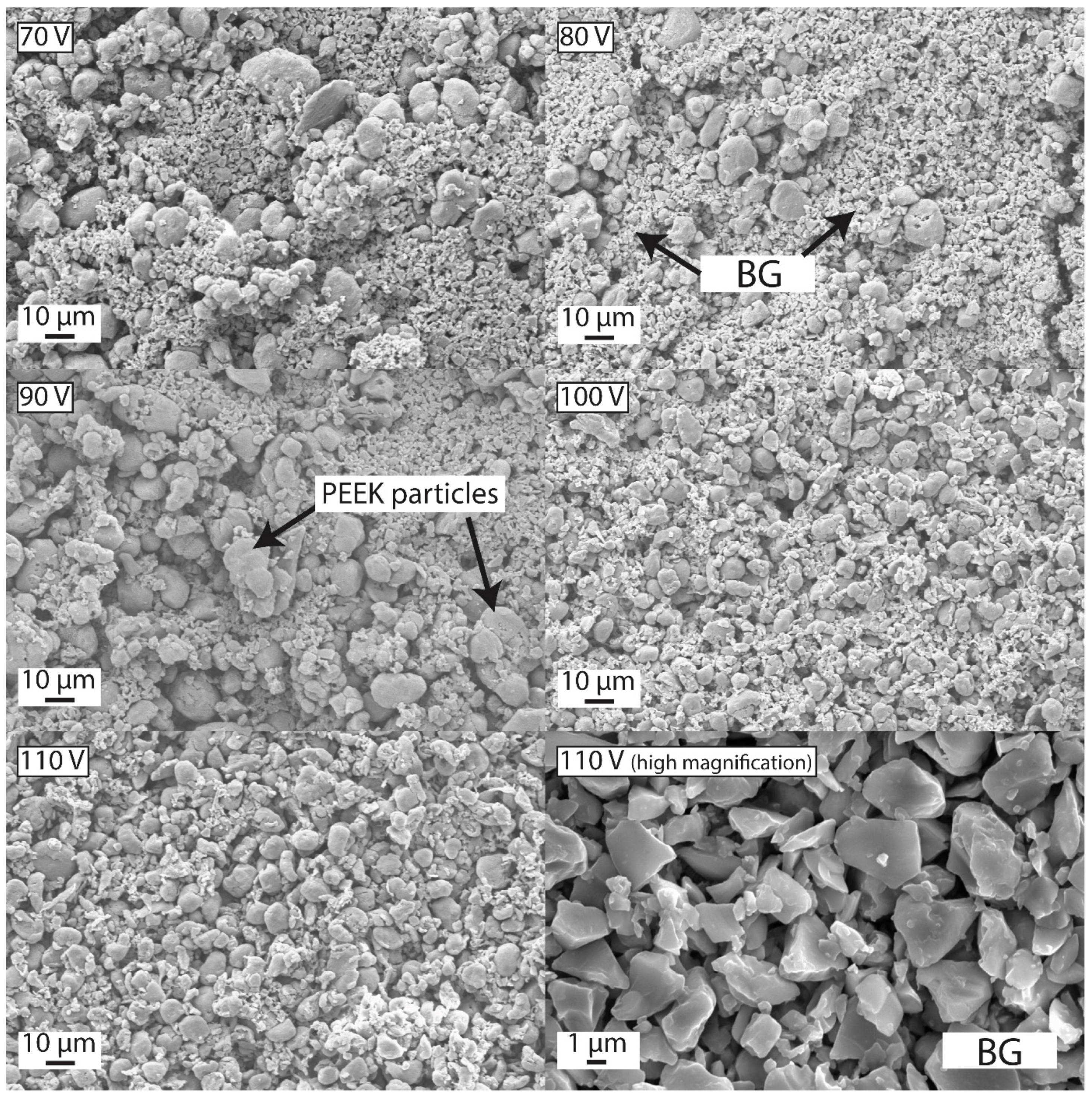
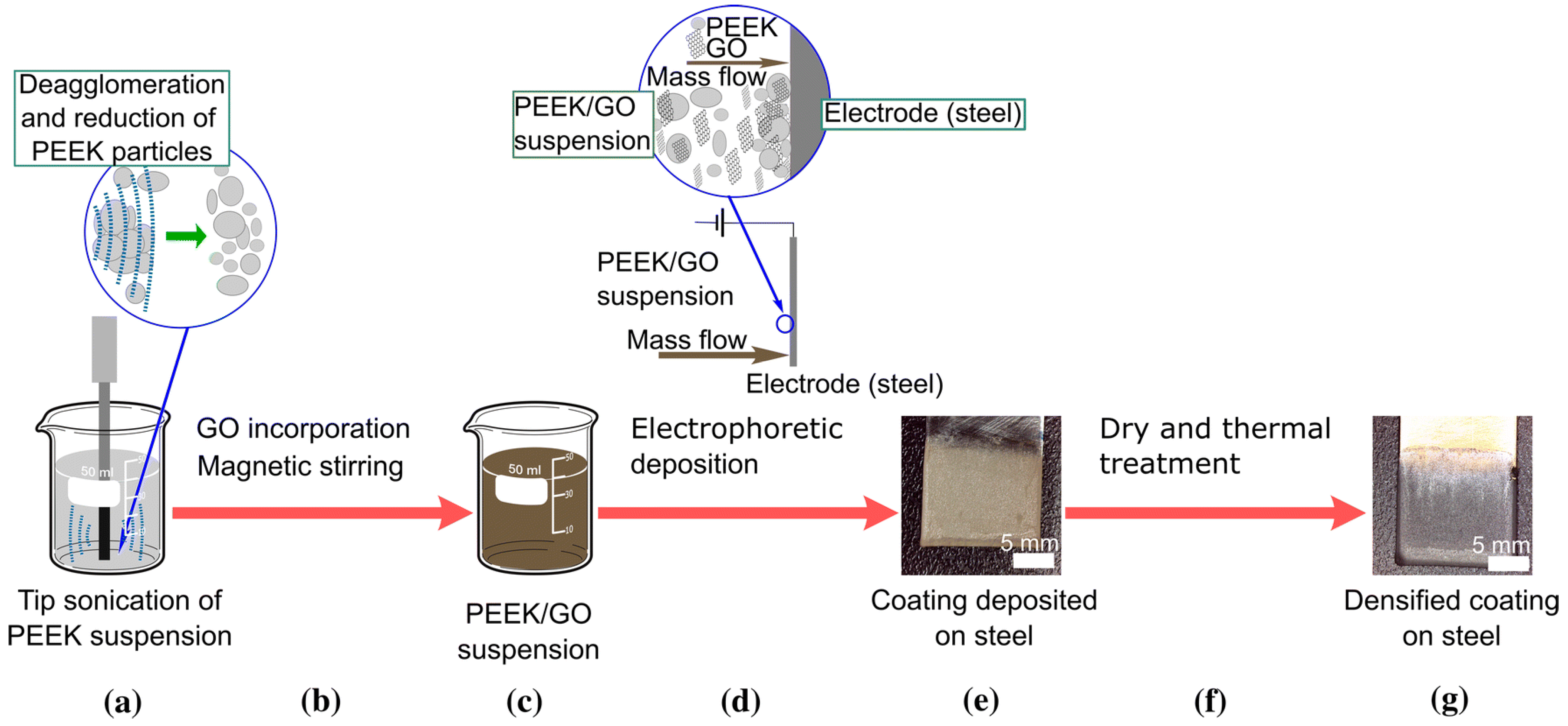
2.6. EPD of PEEK Based Coatings
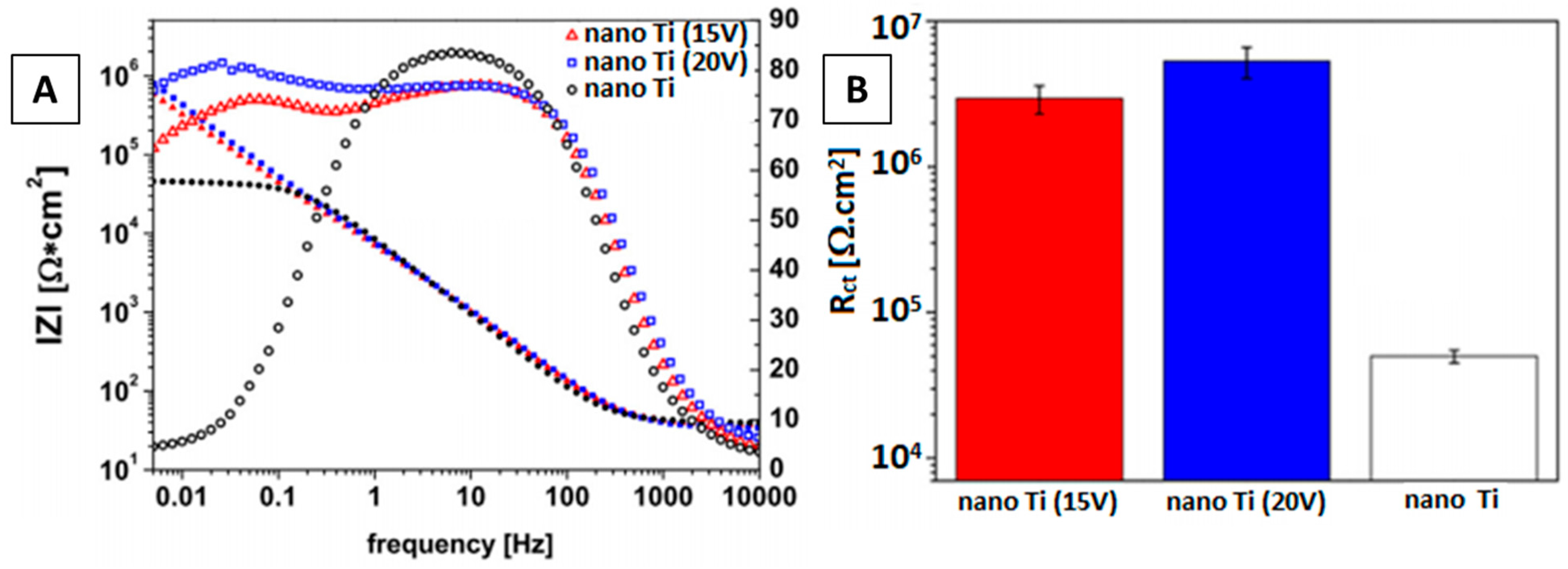
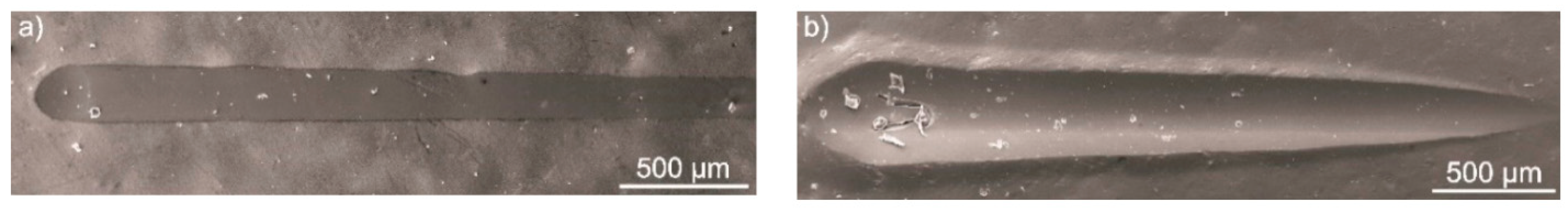
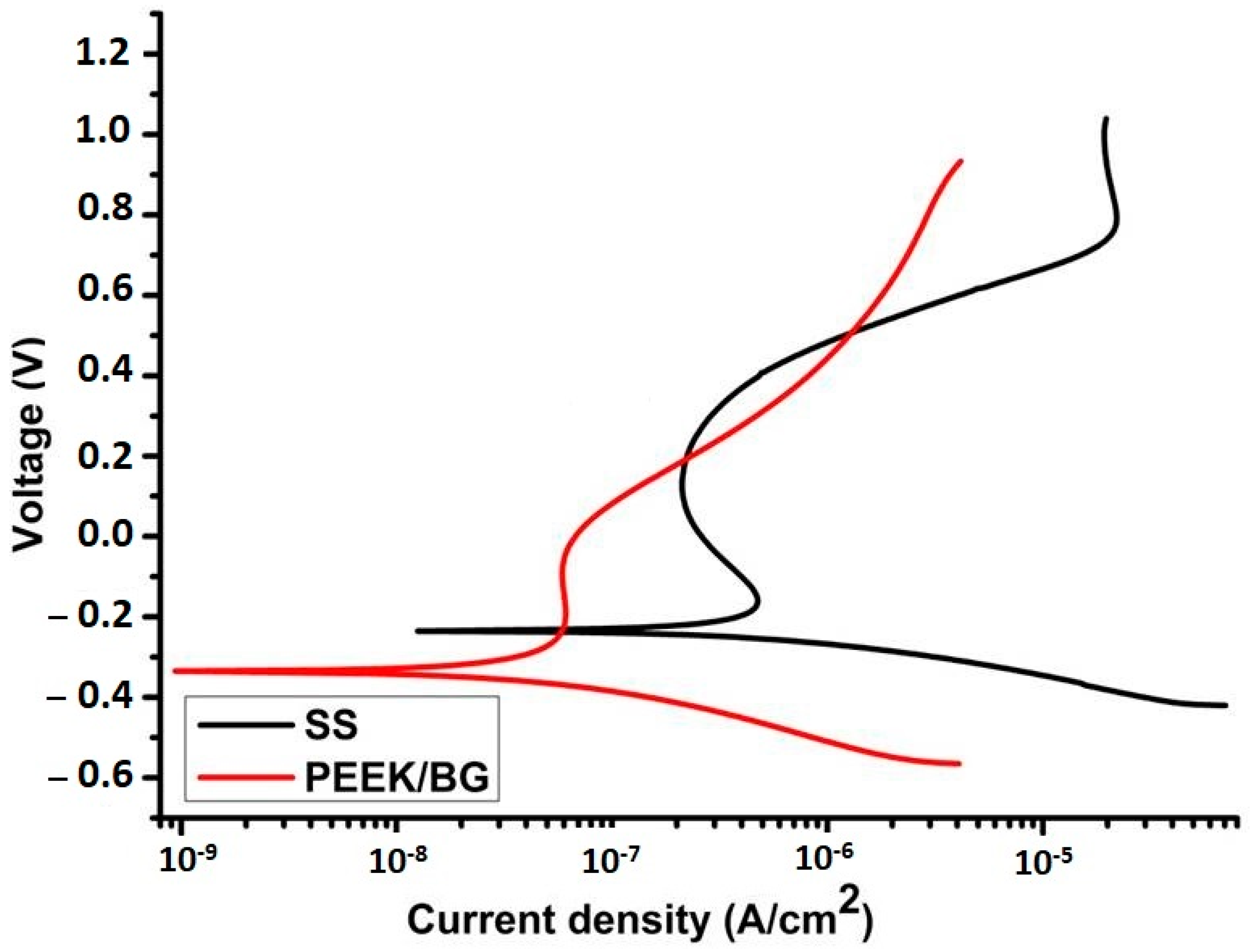
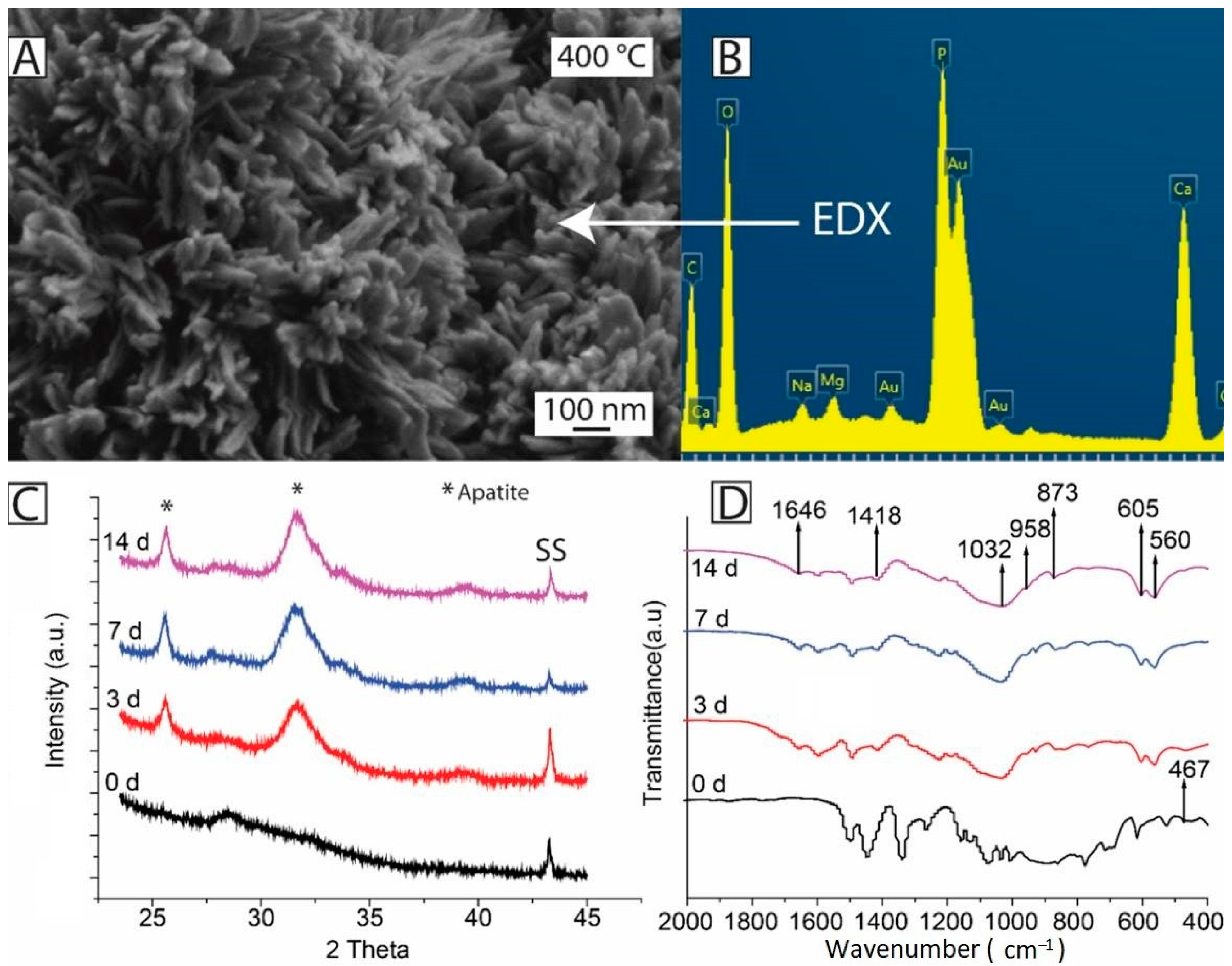
In Vivo Testing of PEEK-Based Coatings
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations and Acronyms
| 2D | two-dimensional |
| 3D | three-dimensional |
| AC | alternating current |
| BG | bioactive glass |
| CaP | calcium phosphates |
| CDC | constant direct current |
| CFs | carbon fibers |
| CFU | colony forming unit |
| Chi | chitosan |
| CNTs | carbon nanotubes |
| CSDs | critical sized defects |
| CTAB | cetyltrimethyl ammonium bromide |
| CZ | Crouzon |
| DC | direct current |
| DoE | design of experiment |
| DI | deionized |
| DLVO | Derjaguin–Landau–Verwy–Overbeek |
| DMEM | Dulbecco’s modified eagles medium |
| EDL | electric double layer |
| EDX | energy-dispersive X-ray spectroscopy |
| EIS | electrochemical impedance spectroscopy |
| EM | electrophoretic mobility |
| EPD | electrophoretic deposition |
| FDA | food and drug administration |
| FTIR | Fourier transform infrared spectroscopy |
| G | graphene |
| GO | graphene oxide |
| HA | hydroxyapatite |
| h-BN | hexagonal boron nitride |
| LTT | lymphocyte transformation testing |
| MBGNs | mesoporous bioactive glass nanoparticles |
| MH | manuka honey |
| NPs | nanoparticles |
| OPTIMA | commercialized PEEK polymer |
| OVAT | one variable at time |
| PBS | phosphate buffer saline |
| PDC | pulsed direct current |
| PEEK | polyetheretherketone |
| SBF | simulated body fluid |
| SEM | scanning electron microscopy |
| SS | stainless steel |
| STAC | stearyl-trimethyl-ammonium chloride |
| TCP | tissue culture plate |
| Tg | glass transition temperature |
| TNTs | titania nanotubes |
| XRD | X-ray diffractometry |
| TKA | total knee anthroplasty |
References
- Chiu, W.-H.; Lee, K.-M.; Hsieh, W.-F. High Efficiency Flexible Dye-Sensitized Solar Cells by Multiple Electrophoretic Depositions. J. Power Sources 2011, 196, 3683–3687. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R.; Hernán, L.; Melero, M.; Morales, J.; Caballero, A. EPD of Thick Films for Their Application in Lithium Batteries. J. Eur. Ceram. Soc. 2007, 27, 3823–3827. [Google Scholar] [CrossRef]
- Ishihara, T.; Sato, K.; Takita, Y. Electrophoretic Deposition of Y2O3-Stabilized ZrO2 Electrolyte Films in Solid Oxide Fuel Cells. J. Am. Ceram. Soc. 1996, 79, 913–919. [Google Scholar] [CrossRef]
- Castro, Y.; Ferrari, B.; Moreno, R.; Durán, A. Corrosion Behaviour of Silica Hybrid Coatings Produced from Basic Catalysed Particulate Sols by Dipping and EPD. Surf. Coat. Technol. 2005, 191, 228–235. [Google Scholar] [CrossRef]
- Du, C.; Pan, N. Supercapacitors Using Carbon Nanotubes Films by Electrophoretic Deposition. J. Power Sources 2006, 160, 1487–1494. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Ghorbani, M. Electrophoretic Deposition of Titania Nanoparticles in Different Alcohols: Kinetics of Deposition. J. Am. Ceram. Soc. 2011, 94, 2354–2361. [Google Scholar] [CrossRef]
- Rahighi, R.; Panahi, M.; Akhavan, O.; Mansoorianfar, M. Pressure-Engineered Electrophoretic Deposition for Gentamicin Loading within Osteoblast-Specific Cellulose Nanofiber Scaffolds. Mater. Chem. Phys. 2021, 272, 125018. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Amin Yavari, S. Electrophoretic Deposition: A Versatile Tool against Biomaterial Associated Infections. J. Mater. Chem. B 2018, 6, 1128–1148. [Google Scholar] [CrossRef]
- Taale, M.; Krüger, D.; Ossei-Wusu, E.; Schütt, F.; Rehman, M.A.U.; Mishra, Y.K.; Marx, J.; Stock, N.; Fiedler, B.; Boccaccini, A.R. Systematically Designed Periodic Electrophoretic Deposition for Decorating 3D Carbon-Based Scaffolds with Bioactive Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 4393–4404. [Google Scholar] [CrossRef]
- Arora, D.; Pant, P.; Sharma, P.K. Trends in Functional Biomaterials in Tissue Engineering and Regenerative Medicine BT-Biomaterials in Tissue Engineering and Regenerative Medicine: From Basic Concepts to State of the Art Approaches; Bhaskar, B., Sreenivasa Rao, P., Kasoju, N., Nagarjuna, V., Baadhe, R.R., Eds.; Springer: Singapore, 2021; pp. 215–269. ISBN 978-981-16-0002-9. [Google Scholar]
- Li, P.H.; Chu, P.K. 1-Thin Film Deposition Technologies and Processing of Biomaterials; Woodhead Publishing: Sawston, UK, 2016; pp. 3–28. ISBN 978-1-78242-453-6. [Google Scholar]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic Deposition of Bioactive Glass Composite Coating on Biomaterials and Electrochemical Behavior Study: A Review. Mater. Today Proc. 2018, 5, 20160–20169. [Google Scholar] [CrossRef]
- Hayashi, T.; Takasu, A. Design of Electrophoretic and Biocompatible Poly(2-Oxazoline)s Initiated by Perfluoroalkanesulfoneimides and Electrophoretic Deposition with Bioactive Glass. Biomacromolecules 2015, 16, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Anné, G.; Vanmeensel, K.; Vleugels, J.; Van der Biest, O. Electrophoretic Deposition as a Novel Near Net Shaping Technique for Functionally Graded Biomaterials. Mater. Sci. Forum 2005, 492–493, 213–218. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Chen, Q.; Braem, A.; Shaffer, M.S.P.; Boccaccini, A.R. Electrophoretic Deposition of Carbon Nanotubes: Recent Progress and Remaining Challenges. Int. Mater. Rev. 2020, 1–30. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Zięba, M.; Koter, S.; Korczeniewski, E.; Zięba, W.; Kowalczyk, P.; Kujawa, J. Recent Developments in the Electrophoretic Deposition of Carbon Nanomaterials BT-Porous Materials: Theory and Its Application for Environmental Remediation; Moreno-Piraján, J.C., Giraldo-Gutierrez, L., Gómez-Granados, F., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 113–137. ISBN 978-3-030-65991-2. [Google Scholar]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass® Composite Coatings on Stainless Steel Deposited by Direct Current and Alternating Current Electrophoretic Deposition. Surf. Coat. Technol. 2013, 233, 49–56. [Google Scholar] [CrossRef]
- Neirinck, B.; Fransaer, J.; Van der Biest, O.; Vleugels, J. Aqueous Electrophoretic Deposition in Asymmetric AC Electric Fields (AC–EPD). Electrochem. Commun. 2009, 11, 57–60. [Google Scholar] [CrossRef]
- Nawrotek, K.; Grams, J. Understanding Electrodeposition of Chitosan–Hydroxyapatite Structures for Regeneration of Tubular-Shaped Tissues and Organs. Materials 2021, 14, 1288. [Google Scholar] [CrossRef]
- Neirinck, B.; Mellaert, L.; Fransaer, J.; Van der Biest, O.; Anné, J.; Vleugels, J. Electrophoretic Deposition of Bacterial Cells. Electrochem. Commun. 2009, 11, 1842–1845. [Google Scholar] [CrossRef]
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Farè, S. Biopolymer-Based Strategies in the Design of Smart Medical Devices and Artificial Organs. Int. J. Artif. Organs 2018, 41, 337–359. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E.; Ciraldo, F.E.; Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic Deposition of Chitosan/45S5 Bioactive Glass Composite Coatings Doped with Zn and Sr. Front. Bioeng. Biotechnol. 2015, 3, 159. [Google Scholar] [CrossRef]
- Seuss, S.; Boccaccini, A.R. Electrophoretic Deposition of Biological Macromolecules, Drugs, and Cells. Biomacromolecules 2013, 14, 3355–3369. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M. Silica-Based Mesoporous Nanobiomaterials as Promoter of Bone Regeneration Process. J. Biomed. Mater. Res. Part A 2015, 103, 3703–3716. [Google Scholar] [CrossRef] [PubMed]
- Deraine, A.; Rebelo Calejo, M.T.; Agniel, R.; Kellomäki, M.; Pauthe, E.; Boissière, M.; Massera, J. Polymer-Based Honeycomb Films on Bioactive Glass: Toward a Biphasic Material for Bone Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2021, 13, 29984–29995. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic Deposition of Polymers and Proteins for Biomedical Applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef] [PubMed]
- Moskalewicz, T.; Warcaba, M.; Cieniek, Ł.; Sitarz, M.; Gajewska, M.; Boccaccini, A.R. Hydroxyapatite/Sodium Alginate Coatings Electrophoretically Deposited on Titanium Substrates: Microstructure and Properties. Appl. Surf. Sci. 2021, 540, 148353. [Google Scholar] [CrossRef]
- Ducheyne, P.; Van Raemdonck, W.; Heughebaert, J.C.; Heughebaert, M. Structural Analysis of Hydroxyapatite Coatings on Titanium. Biomaterials 1986, 7, 97–103. [Google Scholar] [CrossRef]
- Naghdi, S.; Jaleh, B.; Ehsani, A. Electrophoretic Deposition of Graphene Oxide on Aluminum: Characterization, Low Thermal Annealing, Surface and Anticorrosive Properties. Bull. Chem. Soc. Jpn. 2015, 88, 722–728. [Google Scholar] [CrossRef]
- Naghdi, S.; Jaleh, B.; Shahbazi, N. Reversible Wettability Conversion of Electrodeposited Graphene Oxide/Titania Nanocomposite Coating: Investigation of Surface Structures. Appl. Surf. Sci. 2016, 368, 409–416. [Google Scholar] [CrossRef]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. Characterization of biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Woodhead Publishing: Sawston, UK, 2017; pp. 97–115. ISBN 9780081009611. [Google Scholar]
- Sampath Kumar, T.S. Chapter 2-Physical and Chemical Characterization of Biomaterials; Bandyopadhyay, A., Bose, S., Eds.; Academic Press: Oxford, UK, 2013; pp. 11–47. ISBN 978-0-12-415800-9. [Google Scholar]
- Roeder, R.K. Chapter 3-Mechanical Characterization of Biomaterials; Bandyopadhyay, A., Bose, S., Eds.; Academic Press: Oxford, UK, 2013; pp. 49–104. ISBN 978-0-12-415800-9. [Google Scholar]
- Thasneem, Y.M.; Sharma, C.P. Chapter 5.1-In Vitro Characterization of Cell–Biomaterials Interactions; Academic Press: Oxford, UK, 2013; pp. 175–205. ISBN 978-0-12-415800-9. [Google Scholar]
- Jahanmard, F.; Dijkmans, F.M.; Majed, A.; Vogely, H.C.; van der Wal, B.C.H.; Stapels, D.A.C.; Ahmadi, S.M.; Vermonden, T.; Amin Yavari, S. Toward Antibacterial Coatings for Personalized Implants. ACS Biomater. Sci. Eng. 2020, 6, 5486–5492. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Zhang, Y.; Diba, M.; Kolwijck, E.; Shao, J.; Jansen, J.A.; Yang, F.; Boccaccini, A.R.; Leeuwenburgh, S.C.G. Electrophoretic Deposition of Chitosan Coatings Modified with Gelatin Nanospheres to Tune the Release of Antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 13785–13792. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.J.; Linley, M.D.; Best, S.M.; Cameron, R.E. Fabrication of Free Standing Collagen Membranes by Pulsed-Electrophoretic Deposition. Biofabrication 2019, 11, 45017. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.; Luo, D.; Zhitomirsky, I. Colloidal Strategies for Electrophoretic Deposition of Organic-Inorganic Composites for Biomedical Applications. Colloids Surf. Physicochem. Eng. Asp. 2017, 516, 219–225. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic Deposition of Biomaterials. J. R. Soc. Interface 2010, 7 (Suppl. 5), S581–S613. [Google Scholar] [CrossRef]
- Ammam, M. Electrophoretic Deposition under Modulated Electric Fields: A Review. RSC Adv. 2012, 2, 7633–7646. [Google Scholar] [CrossRef]
- Chávez-Valdez, A.; Boccaccini, A.R. Innovations in Electrophoretic Deposition: Alternating Current and Pulsed Direct Current Methods. Electrochim. Acta 2012, 65, 70–89. [Google Scholar] [CrossRef]
- Hu, X.; Bessette, P.H.; Qian, J.; Meinhart, C.D.; Daugherty, P.S.; Soh, H.T. Marker-Specific Sorting of Rare Cells Using Dielectrophoresis. Proc. Natl. Acad. Sci. USA 2005, 102, 15757–15761. [Google Scholar] [CrossRef]
- Novak, S.; Maver, U.; Peternel, Š.; Venturini, P.; Bele, M.; Gaberšček, M. Electrophoretic Deposition as a Tool for Separation of Protein Inclusion Bodies from Host Bacteria in Suspension. Colloids Surf. Physicochem. Eng. Asp. 2009, 340, 155–160. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Makhlouf, A.S.H. Handbook of Nanoelectrochemistry: Electrochemical Synthesis Methods, Properties, and Characterization Techniques; Springer: New York, NY, USA, 2016; ISBN 9783319152660. [Google Scholar]
- Ohshima, H. (Ed.) Chapter 8-Electrokinetic phenomena in a suspension of liquid drops. In Theory of Colloid and Interfacial Electric Phenomena; Elsevier: Amsterdam, The Netherlands, 2006; Volume 12, pp. 182–202. ISBN 1573-4285. [Google Scholar]
- Talebi, T.; Ghomashchi, R.; Talemi, P.; Aminorroaya, S. Suspension Characteristics and Electrophoretic Deposition Ofp-Type Bi2Te3Films for Thermoelectric Applications. J. Electrochem. Soc. 2018, 165, D364–D369. [Google Scholar] [CrossRef]
- LASKA, A. Parameters of the Electrophoretic Deposition Process and Its Influence on the Morphology of Hydroxyapatite Coatings. Review. Inżynieria Mater. 2020, 1, 20–25. [Google Scholar] [CrossRef]
- Kang, H.; Park, Y.; Hong, Y.-K.; Yoon, S.; Lee, M.-H.; Ha, D.-H. Solvent-Induced Charge Formation and Electrophoretic Deposition of Colloidal Iron Oxide Nanoparticles. Surf. Interfaces 2021, 22, 100815. [Google Scholar] [CrossRef]
- Malvern Instruments. Zeta Potential: An Introduction in 30 Minutes. Zetasizer Nano Serles Tech. Note MRK654-01 2011, 2, 1–6. [Google Scholar]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Jane Minay, E.; Shaffer, M.S.P. Electrophoretic Deposition of Carbon Nanotubes. Carbon N. Y. 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of Light and Temperature on Zeta Potential and Physical Stability in Solid Lipid Nanoparticle (SLNTM) Dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Medina-Rodríguez, S.; Ergeneman, O.; Pané, S.; Fernández-Sánchez, J.F.; Nelson, B.J.; Fernández-Gutiérrez, A. Electrophoretic Deposition as a New Approach to Produce Optical Sensing Films Adaptable to Microdevices. Nanoscale 2014, 6, 263–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadeghi, A.A.; Ebadzadeh, T.; Raissi, B.; Ghashghaie, S. Electrophoretic Deposition of TiO2 Nanoparticles in Viscous Alcoholic Media. Ceram. Int. 2013, 39, 7433–7438. [Google Scholar] [CrossRef]
- Vineetha, P. Fabrication of Dielectric Thick Films by Electrophoretic Deposition and Their Characterization; IntechOpen: Rijeka, Yugoslavia, 2020; ISBN 978-1-78984-687-4. [Google Scholar]
- Bintou, O.; Savadogo, O. Electrophoretic Deposition of Alumina and Nickel Oxide Particles. Ournal Sci. Res. Rep. 2013, 2, 190–205. [Google Scholar] [CrossRef]
- Kwok, C.T.; Wong, P.K.; Cheng, F.T.; Man, H.C. Characterization and Corrosion Behavior of Hydroxyapatite Coatings on Ti6Al4V Fabricated by Electrophoretic Deposition. Appl. Surf. Sci. 2009, 255, 6736–6744. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Schindler, U.; Krüger, H.-G. Ceramic Coatings on Carbon and Metallic Fibres by Electrophoretic Deposition. Mater. Lett. 2001, 51, 225–230. [Google Scholar] [CrossRef]
- Abdeltawab, A.A.; Shoeib, M.A.; Mohamed, S.G. Electrophoretic Deposition of Hydroxyapatite Coatings on Titanium from Dimethylformamide Suspensions. Surf. Coat. Technol. 2011, 206, 43–50. [Google Scholar] [CrossRef]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite Coating on Titanium Substrate by Electrophoretic Deposition Method: Effects of Titanium Dioxide Inner Layer on Adhesion Strength and Hydroxyapatite Decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Zhao, P.; Lesergent, L.J.; Farnese, J.; Wen, J.Z.; Ren, C.L. Electrochemistry Communications Electrophoretic Deposition of Carbon Nanotubes on Semi-Conducting and Non-Conducting Substrates. Electrochem. Commun. 2019, 108, 106558. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition of Chitosan/45S5 Bioglass® Composite Coatings for Orthopaedic Applications. Surf. Coat. Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Mosconi, D.; Giovannini, G.; Maccaferri, N.; Serri, M.; Agnoli, S.; Garoli, D. Electrophoretic Deposition of WS(2) Flakes on Nanoholes Arrays-Role of Used Suspension Medium. Materials 2019, 12, 3286. [Google Scholar] [CrossRef]
- Hamaker, H.C. Formation of a Deposit by Electrophoresis. Trans. Faraday Soc. 1940, 35, 279–287. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. EPD Kinetics: A Review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Sarkar, P.; Nicholson, P.P.S. Electrophoretic Deposition (EPD): Mechanisms, Kinetics and Application to Ceramics; Wiley-Blackwell: New York, NY, USA, 1996; Volume 79, pp. 1987–2002. [Google Scholar]
- Biesheuvel, P.M.; Verweij, H. Theory of Cast Formation in Electrophoretic Deposition. J. Am. Ceram. Soc. 1999, 82, 1451–1455. [Google Scholar] [CrossRef]
- Anné, G.; Vanmeensel, K.; Vleugels, J.; Van der Biest, O. A Mathematical Description of the Kinetics of the Electrophoretic Deposition Process for Al2O3-Based Suspensions. J. Am. Ceram. Soc. 2005, 88, 2036–2039. [Google Scholar] [CrossRef]
- Fukada, Y.; Nagarajan, N.; Mekky, W.; Bao, Y.; Kim, H.; Nicholson, P. Electrophoretic Deposition—Mechanisms, Myths and Materials. J. Mater. Sci. 2004, 39, 787–801. [Google Scholar] [CrossRef]
- Heise, S.; Virtanen, S.; Boccaccini, A.R. Taguchi Design of Experiments Approach to Determine Process Parameter for the Electrophoretic Deposition of Chitosan / Bioactive Glass on Mg Alloy Substrates. Electrochem. Soc. Tras 2018, 82, 81–87. [Google Scholar] [CrossRef]
- Pishbin, F.; Mouriño, V.; Gilchrist, J.B.; McComb, D.W.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Single-Step Electrochemical Deposition of Antimicrobial Orthopaedic Coatings Based on a Bioactive Glass/Chitosan/Nano-Silver Composite System. Acta Biomater. 2013, 9, 7469–7479. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.U.; Bastan, F.E.; Nawaz, Q.; Boccaccini, A.R.; Atiq, M.; Rehman, U.; Bastan, F.E.; Nawaz, Q.; Boccaccini, A.R.; Rehman, M.A.U.; et al. Electrophoretic Deposition of Lawsone Loaded Nanoscale Silicate Glass/Chitosan Composite on PEEK/BG Layers. Electrochem. Soc. Tras 2018, 82, 45–50. [Google Scholar] [CrossRef]
- Aqib, R. Ag–Sr Doped Mesoporous Bioactive Glass Nanoparticles Loaded Chitosan/Gelatin Coating for Orthopedic Implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Aydemir, T.; Liverani, L.; Pastore, J.I.; Ceré, S.M.; Goldmann, W.H.; Boccaccini, A.R.; Ballarre, J. Functional Behavior of Chitosan/Gelatin/Silica-Gentamicin Coatings by Electrophoretic Deposition on Surgical Grade Stainless Steel. Mater. Sci. Eng. C 2020, 115, 111062. [Google Scholar] [CrossRef]
- Saleem, O.; Wahaj, M.; Akhtar, M.A.; Ur Rehman, M.A. Fabrication and Characterization of Ag–Sr-Substituted Hydroxyapatite/Chitosan Coatings Deposited via Electrophoretic Deposition: A Design of Experiment Study. ACS Omega 2020, 5, 22984–22992. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Bano, S.S.S.; Yasir, M.; Wadood, A.; Ur Rehman, M.A.; Rehman, M.A.U. Ag and Mn-Doped Mesoporous Bioactive Glass Nanoparticles Incorporated into the Chitosan/Gelatin Coatings Deposited on PEEK/Bioactive Glass Layers for Favorable Osteogenic Differentiation and Antibacterial Activity. Mater. Adv. 2020, 1, 1273–1284. [Google Scholar] [CrossRef]
- Pishbin, F.; Mouriño, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition of Gentamicin-Loaded Bioactive Glass/Chitosan Composite Coatings for Orthopaedic Implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef]
- Gebhardt, F.; Seuss, S.; Turhan, M.C.; Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Characterization of Electrophoretic Chitosan Coatings on Stainless Steel. Mater. Lett. 2012, 66, 302–304. [Google Scholar] [CrossRef]
- Agata, S.; Heise, S.; Topolski, K.; Garbacz, H.; Boccaccini, A.R. Chitosan/Bioactive Glass Coatings as a Protective Layer against Corrosion of Nanocrystalline Titanium under Simulated Inflammation. Mater. Lett. 2020, 264, 127284. [Google Scholar] [CrossRef]
- Szklarska, M.; Łosiewicz, B.; Dercz, G.; Maszybrocka, J.; Rams-Baron, M.; Stach, S. Electrophoretic Deposition of Chitosan Coatings on the Ti15Mo Biomedical Alloy from a Citric Acid Solution. RSC Adv. 2020, 10, 13386–13393. [Google Scholar] [CrossRef]
- Ramos Rivera, L.; Dippel, J.; Boccaccini, A.R. Formation of Zein/Bioactive Glass Layers Using Electrophoretic Deposition Technique. ECS Trans. 2018, 82, 73–80. [Google Scholar] [CrossRef]
- Kaya, S.; Boccaccini, A.R. Electrophoretic Deposition of Zein Coatings. J. Coat. Technol. Res. 2017, 14, 683–689. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yasir, M.; Ur Rehman, M.A. Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces 2020, 3, 237–250. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rehman, M.A.U. Improvement in the Surface Properties of Stainless Steel via Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surf. Interfaces 2020, 20, 100589. [Google Scholar] [CrossRef]
- Rehman, U.; Atiq, M. Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants. Prosthesis 2020, 2, 211–224. [Google Scholar] [CrossRef]
- Ramos, L.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Bioactive Materials Copper Based Coatings for Implantable Stainless Steel Aimed at Bone Healing. Bioact. Mater. 2021, 6, 1479–1490. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zimowski, S.; Zych, A.; Łukaszczyk, A.; Reczyńska, K.; Pamuła, E. Electrophoretic Deposition, Microstructure and Selected Properties of Composite Alumina/Polyetheretherketone Coatings on the Ti-13Nb-13Zr Alloy. J. Electrochem. Soc. 2018, 165, D116–D128. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Peters, C.; Roether, J.A.; Eifler, D.; Misra, S.K.; Minay, E.J. Electrophoretic Deposition of Polyetheretherketone (PEEK) and PEEK/Bioglass® Coatings on NiTi Shape Memory Alloy Wires. J. Mater. Sci. 2006, 41, 8152–8159. [Google Scholar] [CrossRef]
- Iveković, A.; Novak, S.; Lukek, M.; Kalin, M. Aqueous Electrophoretic Deposition of Bulk Polyether Ether Ketone (PEEK). J. Mater. Process. Technol. 2015, 223, 58–64. [Google Scholar] [CrossRef]
- He, M.; Zhu, C.; Xu, H.; Sun, D.; Chen, C.; Feng, G.; Liu, L.; Li, Y.; Zhang, L. Conducting Polyetheretherketone Nanocomposites with an Electrophoretically Deposited Bioactive Coating for Bone Tissue Regeneration and Multimodal Therapeutic Applications. ACS Appl. Mater. Interfaces 2020, 12, 56924–56934. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A.; Rehman, M.A.U.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic Deposition of PEEK/Bioactive Glass Composite Coatings on Stainless Steel for Orthopedic Applications: An Optimization for in Vitro Bioactivity and Adhesion Strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Bastan, F.E.; Haider, B.; Boccaccini, A.R.; Rehman, M.A.U.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic Deposition of PEEK/Bioactive Glass Composite Coatings for Orthopedic Implants: A Design of Experiments (DoE) Study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Ferraris, S.; Goldmann, W.H.; Perero, S.; Bastan, F.E.; Nawaz, Q.; Di Confiengo, G.G.; Ferraris, M.; Boccaccini, A.R. Antibacterial and Bioactive Coatings Based on Radio Frequency Co-Sputtering of Silver Nanocluster-Silica Coatings on PEEK/Bioactive Glass Layers Obtained by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2017, 9, 32489–32497. [Google Scholar] [CrossRef] [PubMed]
- Moskalewicz, T.; Zimowski, S.; Fiołek, A.; Łukaszczyk, A.; Dubiel, B.; Cieniek, Ł. The Effect of the Polymer Structure in Composite Alumina / Polyetheretherketone Coatings on Corrosion Resistance, Micro-Mechanical and Tribological Properties of the Ti-6Al-4V Alloy. J. Mater. Eng. Perform. 2020, 29, 1426–1438. [Google Scholar] [CrossRef]
- Sak, A.; Moskalewicz, T.; Zimowski, S.; Cieniek, Ł.; Dubiel, B.; Radziszewska, A.; Kot, M.; Łukaszczyk, A. Influence of Polyetheretherketone Coatings on the Ti-13Nb-13Zr Titanium Alloy’s Bio-Tribological Properties and Corrosion Resistance. Mater. Sci. Eng. C 2016, 63, 52–61. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zych, A.; Kruk, A.; Kopia, A.; Zimowski, S.; Sitarz, M.; Cieniek, Ł. Electrophoretic Deposition and Microstructure Development of Si3N4/Polyetheretherketone Coatings on Titanium Alloy. Surf. Coat. Technol. 2018, 350, 633–647. [Google Scholar] [CrossRef]
- Seuss, S.; Heinloth, M.; Boccaccini, A.R. Development of Bioactive Composite Coatings Based on Combination of PEEK, Bioactive Glass and Ag Nanoparticles with Antibacterial Properties. Surf. Coat. Technol. 2015, 301, 100–105. [Google Scholar] [CrossRef]
- Virk, R.S.; Rehman, M.A.U.; Boccaccini, A.R. PEEK Based Biocompatible Coatings Incorporating H-BN and Bioactive Glass by Electrophoretic Deposition. ECS Trans. 2018, 82, 89–95. [Google Scholar] [CrossRef]
- Seuss, S.; Subhani, T.; Kang, M.Y.; Okudaira, K.; Aguilar Ventura, I.E.; Boccaccini, A.R. Electrophoretic Deposition of PEEK-TiO2 Composite Coatings on Stainless Steel. Key Eng. Mater. 2012, 507, 127–133. [Google Scholar] [CrossRef]
- Wang, C.; Ma, J.; Cheng, W. Formation of Polyetheretherketone Polymer Coating by Electrophoretic Deposition Method. Surf. Coat. Technol. 2003, 173, 271–275. [Google Scholar] [CrossRef]
- González-Castillo, E.I.; Costantini, T.; Shaffer, M.S.P.; Boccaccini, A.R. Nanocomposite Coatings Obtained by Electrophoretic Co-Deposition of Poly (Etheretherketone)/Graphene Oxide Suspensions. J. Mater. Sci. 2020, 55, 8881–8899. [Google Scholar] [CrossRef]
- Nawaz, Q.; Fastner, S.; Rehman, M.A.U.; Ferraris, S.; Perero, S.; Di Confiengo, G.G.; Yavuz, E.; Ferraris, M.; Boccaccini, A.R. Multifunctional Stratified Composite Coatings by Electrophoretic Deposition and RF Co-Sputtering for Orthopaedic Implants. J. Mater. Sci. 2021, 56, 7920–7935. [Google Scholar] [CrossRef]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Łukaszczyk, A.; Moskalewicz, T. Electrophoretic Co-Deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2020, 13, 3251. [Google Scholar] [CrossRef] [PubMed]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Moskalewicz, T. The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy. Coatings 2019, 9, 530. [Google Scholar] [CrossRef]
- Virk, R.S.; Atiq, M.; Rehman, U.; Munawar, M.A.; Schubert, D.W.; Goldmann, W.H.; Dusza, J.; Boccaccini, A.R. Curcumin-Containing Orthopedic Implant Coatings Deposited on Poly-Ether-Ether-Ketone/Bioactive Glass/Hexagonal Boron Nitride Layers by Electrophoretic Deposition. Coatings 2019, 9, 572. [Google Scholar] [CrossRef]
- Nawaz, A.; Ur Rehman, M.A. Chitosan/Gelatin-based Bioactive and Antibacterial Coatings Deposited via Electrophoretic Deposition. J. Appl. Polym. Sci. 2021, 138, 50220. [Google Scholar] [CrossRef]
- Atiq, M.; Rehman, U.; Bastan, F.E.; Nawaz, Q.; Goldmann, W.H.; Maqbool, M.; Virtanen, S.; Boccaccini, A.R. Electrophoretic Deposition of Lawsone Loaded Bioactive Glass (BG)/Chitosan Composite on Polyetheretherketone (PEEK)/ BG Layers as Antibacterial and Bioactive Coating. J. Biomed. Mater. Res. Part A 2018, 160, 3111–3112. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling Cell Adhesion and Degradation of Chitosan Films by N-Acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Aimin, C.; Chunlin, H.; Juliang, B.; Tinyin, Z.; Zhichao, D. Antibiotic Loaded Chitosan Bar. An in Vitro, in Vivo Study of a Possible Treatment for Osteomyelitis. Clin. Orthop. Relat. Res. 1999, 366, 239–247. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the Degree of Acetylation on Some Biological Properties of Chitosan Films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Yanovska, A.A.; Stanislavov, A.S.; Sukhodub, L.B.; Kuznetsov, V.N.; Illiashenko, V.Y.; Danilchenko, S.N.; Sukhodub, L.F. Silver-Doped Hydroxyapatite Coatings Formed on Ti-6Al-4V Substrates and Their Characterization. Mater. Sci. Eng. C 2014, 36, 215–220. [Google Scholar] [CrossRef]
- Bennett, B.L.; Littlejohn, L.F.; Kheirabadi, B.S.; Butler, F.K.; Kotwal, R.S.; Dubick, M.A.; Bailey, J.A. Management of External Hemorrhage in Tactical Combat Casualty Care: Chitosan-Based Hemostatic Gauze Dressings--TCCC Guidelines-Change 13-05. J. Spec. Oper. Med. Peer Rev. J. SOF Med. Prof. 2014, 14, 40–57. [Google Scholar]
- Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. Generation of the Pores on Graphene Surface and Their Reinforcement Effects on the Thermal and Mechanical Properties of Chitosan-Based Composites. Compos. Part B Eng. 2017, 114, 348–355. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic Deposition of Graphene Oxide Reinforced Chitosan–Hydroxyapatite Nanocomposite Coatings on Ti Substrate. J. Mater. Sci. Mater. Med. 2016, 27, 48. [Google Scholar] [CrossRef]
- Molaei, A.; Yousefpour, M. Electrophoretic Deposition of Chitosan–Bioglass®–Hydroxyapatite–Halloysite Nanotube Composite Coating. Rare Met. 2018, 1–8. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Witecka, A.; Valet, S.; Basista, M.; Boccaccini, A.R. Electrophoretically Deposited High Molecular Weight Chitosan/Bioactive Glass Composite Coatings on WE43 Magnesium Alloy. Surf. Coat. Technol. 2021, 418, 127232. [Google Scholar] [CrossRef]
- Ribeiro, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography Based on Chitin- and Chitosan-Derived Marine Polysaccharides. Symmetry 2017, 9, 190. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M. Preparation and Characterization of Fully Deacetylated Chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Ur Rehman, M.A.; Yıldıran Avcu, Y.; Boccaccini, A.R.; Ba, F.E.; Abdullah, H.Z. Electrophoretic Deposition of Chitosan-Based Composite Coatings for Biomedical Applications: A Review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Chew, K.-K.; Zein, S.H.S.; Ahmad, A.L. The Corrosion Scenario in Human Body: Stainless Steel 316L Orthopaedic Implants. Nat. Sci. 2012, 4, 184–188. [Google Scholar] [CrossRef]
- Li, Y.; Ho, J.; Ooi, C.P. Antibacterial Efficacy and Cytotoxicity Studies of Copper (II) and Titanium (IV) Substituted Hydroxyapatite Nanoparticles. Mater. Sci. Eng. C 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Song, Y.-H.; Kim, M.-K.; Park, E.-J.; Song, H.-J.; Anusavice, K.J.; Park, Y.-J. Cytotoxicity of Alloying Elements and Experimental Titanium Alloys by WST-1 and Agar Overlay Tests. Dent. Mater. 2014, 30, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Kennon, J.C.; Lee, J.; Songy, C.; Shukla, D.; Cofield, R.H.; Sanchez-Sotelo, J.; Sperling, J.W. The Effect of Patient-Reported Metal Allergies on the Outcomes of Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.G.; Barrack, R.L.; Schmalzried, T.P. Suspected Metal Allergy and Femoral Loosening After Total Knee Arthroplasty: A Diagnostic Dilemma. Arthroplast. Today 2021, 7, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Batmanghelich, F.; Ghorbani, M. Effect of PH and Carbon Nanotube Content on the Corrosion Behavior of Electrophoretically Deposited Chitosan–Hydroxyapatite–Carbon Nanotube Composite Coatings. Ceram. Int. 2013, 39, 5393–5402. [Google Scholar] [CrossRef]
- Molaei, A.; Amadeh, A.; Yari, M.; Reza Afshar, M. Structure, Apatite Inducing Ability, and Corrosion Behavior of Chitosan/Halloysite Nanotube Coatings Prepared by Electrophoretic Deposition on Titanium Substrate. Mater. Sci. Eng. C 2016, 59, 740–747. [Google Scholar] [CrossRef]
- Chew, K.-K.; Zein, S.H.S.; Ahmad, A.L.; McPhail, D.S.; Abdullah, M.F. The Electrochemical Studies of the Corrosion Resistance Behaviour of Hydroxyapatite Coatings on Stainless Steel Fabricated by Electrophoretic Deposition. J. Ind. Eng. Chem. 2013, 19, 1123–1129. [Google Scholar] [CrossRef]
- Goldmann, W.H. Biosensitive and Antibacterial Coatings on Metallic Material for Medical Applications. Cell Biol. Int. 2021, 45, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent Models in Neuroscience Research: Is It a Rat Race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Bariana, M.; Kaidonis, J.; Losic, D.; Ranjitkar, S.; Anderson, P. Titania Nanotube-Based Protein Delivery System to Inhibit Cranial Bone Regeneration in Crouzon Model of Craniosynostosis. Int. J. Nanomed. 2019, 14, 6313–6324. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Kaur, A.; Singh Bakshi, M. CHAPTER 10 Zein: Structure, Production, Film Properties and Applications. In Natural Polymers: Volume 1: Composites; The Royal Society of Chemistry: London, UK, 2012; Volume 1, pp. 204–218. ISBN 978-1-84973-402-8. [Google Scholar]
- Fereydouni, N.; Movaffagh, J.; Amiri, N.; Darroudi, S.; Gholoobi, A.; Goodarzi, A.; Hashemzadeh, A.; Darroudi, M. Synthesis of Nano-Fibers Containing Nano-Curcumin in Zein Corn Protein and Its Physicochemical and Biological Characteristics. Sci. Rep. 2021, 11, 1902. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant Activity of Zein Hydrolysates in a Liposome System and the Possible Mode of Action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Cheryan, M. Zein: The Industrial Protein from Corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. Zein in Controlled Drug Delivery and Tissue Engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Xu, H. Physicochemical Properties of Zein-Based Films by Electrophoretic Deposition Using Indium Tin Oxide Electrodes: Vertical and Horizontal Electric Fields. Int. J. Food Prop. 2016, 19, 945–957. [Google Scholar] [CrossRef]
- Meyer, N.; Rivera, L.R.; Ellis, T.; Qi, J.; Boccaccini, A.R. Bioactive and Antibacterial Coatings Based on Zein/Bioactive Glass Composites by Electrophoretic Deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef]
- Maci, F.; Moskalewicz, T.; Kowalski, K.; Łukaszczyk, A.; Hadzhieva, Z.; Boccaccini, A.R. The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates. Materials 2021, 14, 312. [Google Scholar]
- Ahmed, Y.; Nawaz, A.; Singh Virk, R.; Wadood, A.; Ur Rehman, M.A. Fabrication and Characterization of Zein/Bioactive Glass Deposited on Pretreated Magnesium via Electrophoretic Deposition. Int. J. Ceram. Eng. Sci. 2020, 2, 254–263. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, H.; Wang, J.; Wu, J.; Li, X.; Liu, G. Physicochemical Properties of Zein Films Cross-Linked with Glutaraldehyde. Polym. Bull. 2021, 1–19. [Google Scholar] [CrossRef]
- Gorji, M.R.; Sanjabi, S.; Edtmaier, C.; Katsich, C. Wear-Resistant Electrophoretic Deposition (EPD) Layer of Titanium Carbide. J. Alloy. Compd. 2019, 806, 1323–1338. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Fathi, M.; Bagri, A.; Boccaccini, A.R. Preparation and Characterization of Aligned Porous PCL/Zein Scaffolds as Drug Delivery Systems via Improved Unidirectional Freeze-Drying Method. Mater. Sci. Eng. C 2016, 68, 613–622. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Müller, V.; Piai, J.F.; Fajardo, A.R.; Fávaro, S.L.; Rubira, A.F.; Muniz, E.C. Preparation and Characterization of Zein and Zein-Chitosan Microspheres with Great Prospective of Application in Controlled Drug Release. J. Nanomater. 2011, 2011, 928728. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of Different Types of Cells on Polymer Surfaces with Wettability Gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Influence of Deposition Conditions on the Composition, Texture and Microstructure of RF-Magnetron Sputter-Deposited Hydroxyapatite Thin Films. Thin Solid Film. 2015, 591, 368–374. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Lasch, K.; Weidinger, J.; Boccaccini, A.R. Manuka Honey and Zein Coatings Impart Bioactive Glass Bone Tissue Scaffolds Antibacterial Properties and Superior Mechanical Properties. Front. Mater. 2021, 7, 449. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable Magnesium-Based Alloys for Biomedical Applications: The Role of Critical Alloying Elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable Magnesium Alloys as Temporary Orthopaedic Implants: A Review. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic Deposition of Nano-Zirconia Coating on AZ91D Magnesium Alloy for Bio-Corrosion Control Purposes. Surf. Coat. Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Seuss, S.; Boccaccini, A.R. Microstructure and Properties of Composite Polyetheretherketone/Bioglass® Coatings Deposited on Ti–6Al–7Nb Alloy for Medical Applications. Appl. Surf. Sci. 2013, 273, 62–67. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S.-U. The Electron Beam Deposition of Titanium on Polyetheretherketone (PEEK) and the Resulting Enhanced Biological Properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhao, Y.; Jin, G.; Lu, T.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of Sulfur Content on Bone Formation and Antibacterial Ability of Sulfonated PEEK. Biomaterials 2016, 83, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.-R.; Jin, K.; Hong, K.S.; Noh, J.H.; Lee, H.-K. In Vitro and in Vivo Evaluation of the Bioactivity of Hydroxyapatite-Coated Polyetheretherketone Biocomposites Created by Cold Spray Technology. Acta Biomater. 2013, 9, 6177–6187. [Google Scholar] [CrossRef]
- Zhitomirsky, D.; Roether, J.A.A.; Boccaccini, A.R.R.; Zhitomirsky, I. Electrophoretic Deposition of Bioactive Glass/Polymer Composite Coatings with and without HA Nanoparticle Inclusions for Biomedical Applications. J. Mater. Process. Technol. 2009, 209, 1853–1860. [Google Scholar] [CrossRef]
- Wenz, L.M.; Memitt, K.; Brown, S.A.; Moet, A. In Vitro Biocompatibility of Polyetheretherketone\nand Polysulfone Composites. J. Biomed. Mater. Res. 1990, 24, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and Proliferation of Osteoblasts and Fibroblasts on Biomaterials for Orthopaedic Use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Gu, X.; Sun, X.; Sun, Y.; Wang, J.; Liu, Y.; Yu, K.; Wang, Y.; Zhou, Y. Bioinspired Modifications of PEEK Implants for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 631616. [Google Scholar] [CrossRef]
- Corni, I.; Neumann, N.; Novak, S.; König, K.; Veronesi, P.; Chen, Q.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition of PEEK-Nano Alumina Composite Coatings on Stainless Steel. Surf. Coat. Technol. 2009, 203, 1349–1359. [Google Scholar] [CrossRef]
- Kruk, A.; Zimowski, S.; Łukaszczyk, A.; Cieniek, Ł.; Moskalewicz, T. The Influence of Heat Treatment on the Microstructure, Surface Topography and Selected Properties of PEEK Coatings Electrophoretically Deposited on the Ti-6Al-4V Alloy. Prog. Org. Coat. 2019, 133, 180–190. [Google Scholar] [CrossRef]
- Kuśmierczyk, F.; Zimowski, S.; Łukaszczyk, A.; Kopia, A.; Cieniek, Ł.; Moskalewicz, T. Development of Microstructure and Properties of Multicomponent MoS2/HA/PEEK Coatings on a Titanium Alloy Via Electrophoretic Deposition and Heat Treatment. Metall. Mater. Trans. A 2021, 1–16. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, W.; Yang, L.; Pan, Y.; Wang, X.; Wang, T.; Tang, S.; Yao, Y.; Hong, H.; Wei, J. Stimulation of Cell Responses and Bone Ingrowth into Macro-Microporous Implants of Nano-Bioglass/Polyetheretherketone Composite and Enhanced Antibacterial Activity by Release of Hinokitiol. Colloids Surf. B Biointerfaces 2018, 164, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F.; McNamara, A.; Turner, R.M. Potential of Polyetheretherketone (PEEK) and Carbon-Fibre-Reinforced PEEK in Medical Applications. J. Mater. Sci. Lett. 1987, 6, 188–190. [Google Scholar] [CrossRef]
- Katzer, A.; Marquardt, H.; Westendorf, J.; Wening, J.V.; Von Foerster, G. Polyetheretherketone-Cytotoxicity and Mutagenicity in Vitro. Biomaterials 2002, 23, 1749–1759. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Garle, M.J.; Batchelor, J.; Bradley, J.; Grant, D.M. Use of a Novel Carbon Fibre Composite Material for the Femoral Stem Component of a THR System: In Vitro Biological Assessment. Biomaterials 2003, 24, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guo, F.; Chen, J.; Wang, X.; Zhao, X.; Xiao, P. Bioactive Glass–Chitosan Composite Coatings on PEEK: Effects of Surface Wettability and Roughness on the Interfacial Fracture Resistance and in Vitro Cell Response. Appl. Surf. Sci. 2018, 440, 514–523. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, L.; Wang, T.; Tang, S.; Li, Q.; Tang, T.; Wei, S.; Qian, J.; Wei, J.; Su, J. Lithium Doped Silica Nanospheres/Poly(Dopamine) Composite Coating on Polyetheretherketone to Stimulate Cell Responses, Improve Bone Formation and Osseointegration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.S.; Cheng, M.H.W.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile Properties, Tension-Tension Fatigue and Biological Response of Polyetheretherketone-Hydroxyapatite Composites for Load-Bearing Orthopedic Implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Cheang, P.; Khor, K.A. Tensile Properties and Microstructural Analysis of Spheroidized Hydroxyapatite-Poly (Etheretherketone) Biocomposites. Mater. Sci. Eng. A 2003, 345, 55–63. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Chenag, P.; Khor, K.A. Mechanical Properties of Injection Molded Hydroxyapatite-Polyetheretherketone Biocomposites. Compos. Sci. Technol. 2003, 63, 421–425. [Google Scholar] [CrossRef]
- Ha, S.-W.; Mayer, J.; Koch, B.; Wintermantel, E. Plasma-Sprayed Hydroxylapatite Coating on Carbon Fibre Reinforced Thermoplastic Composite Materials. J. Mater. Sci. Mater. Med. 1994, 5, 481–484. [Google Scholar] [CrossRef]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Naing, M.W.; Cheah, C.M. Fabrication and Characterization of Three-Dimensional Poly(Ether-Ether-Ketone)/-Hydroxyapatite Biocomposite Scaffolds Using Laser Sintering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2005, 219, 183–194. [Google Scholar] [CrossRef]


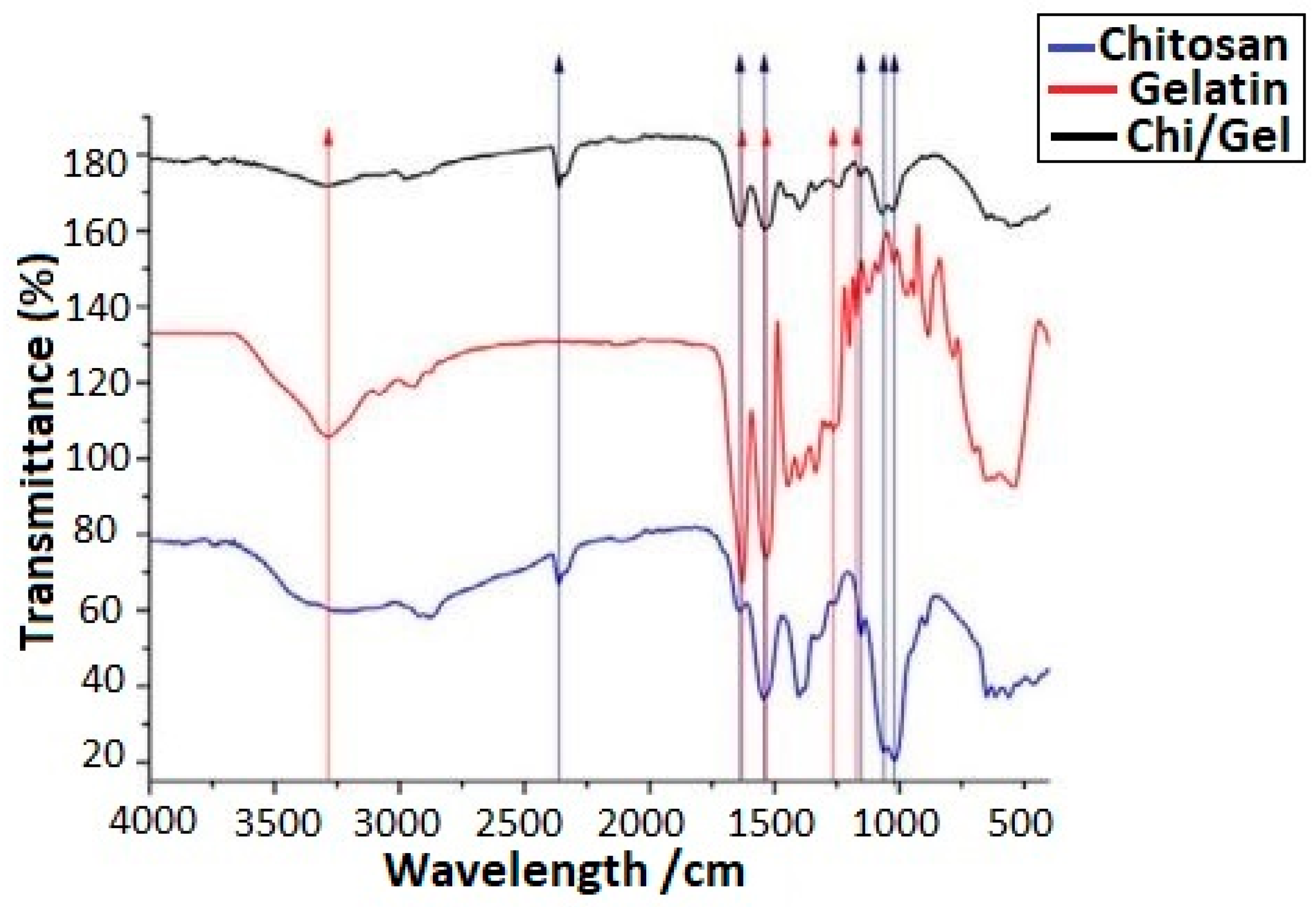
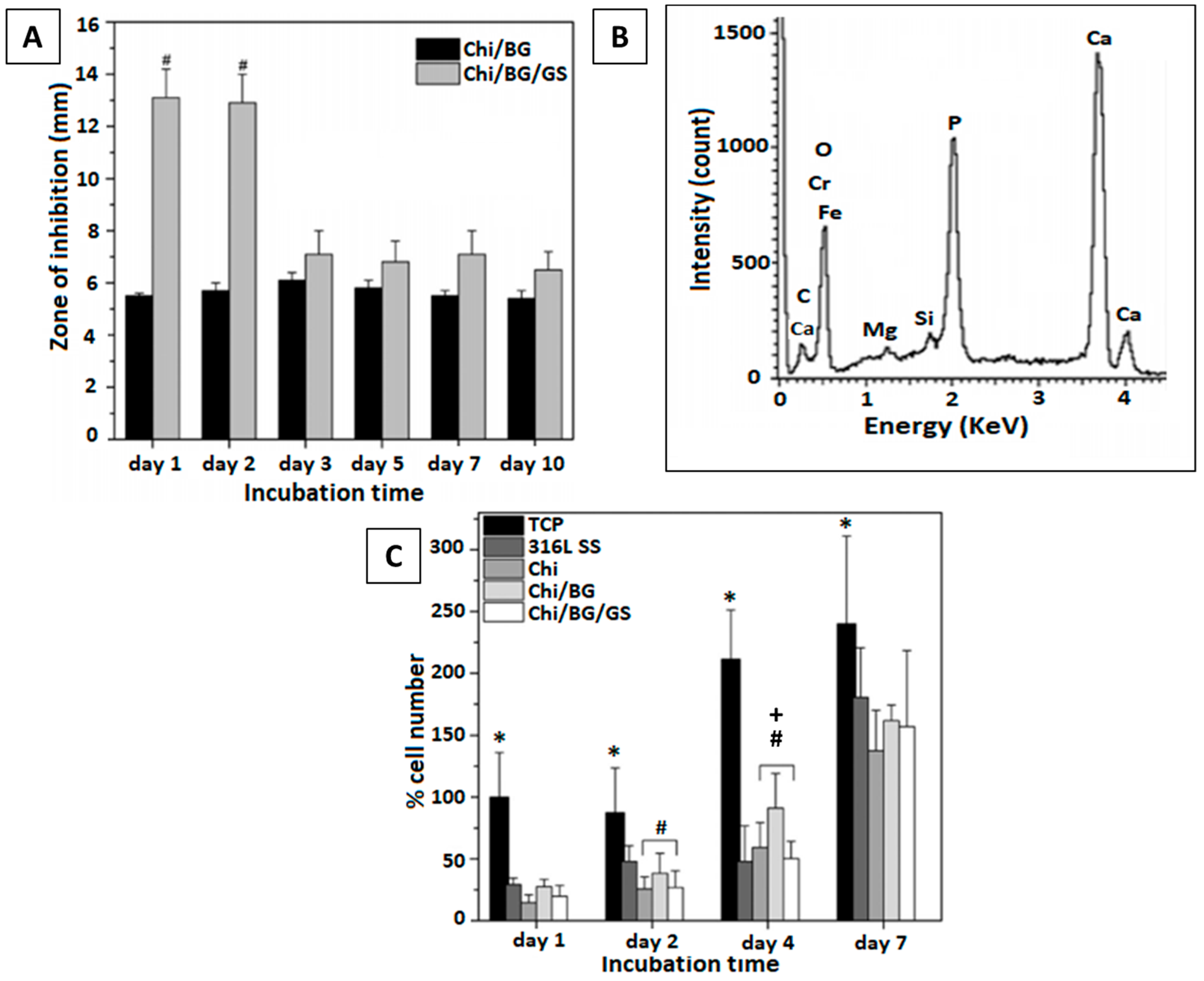
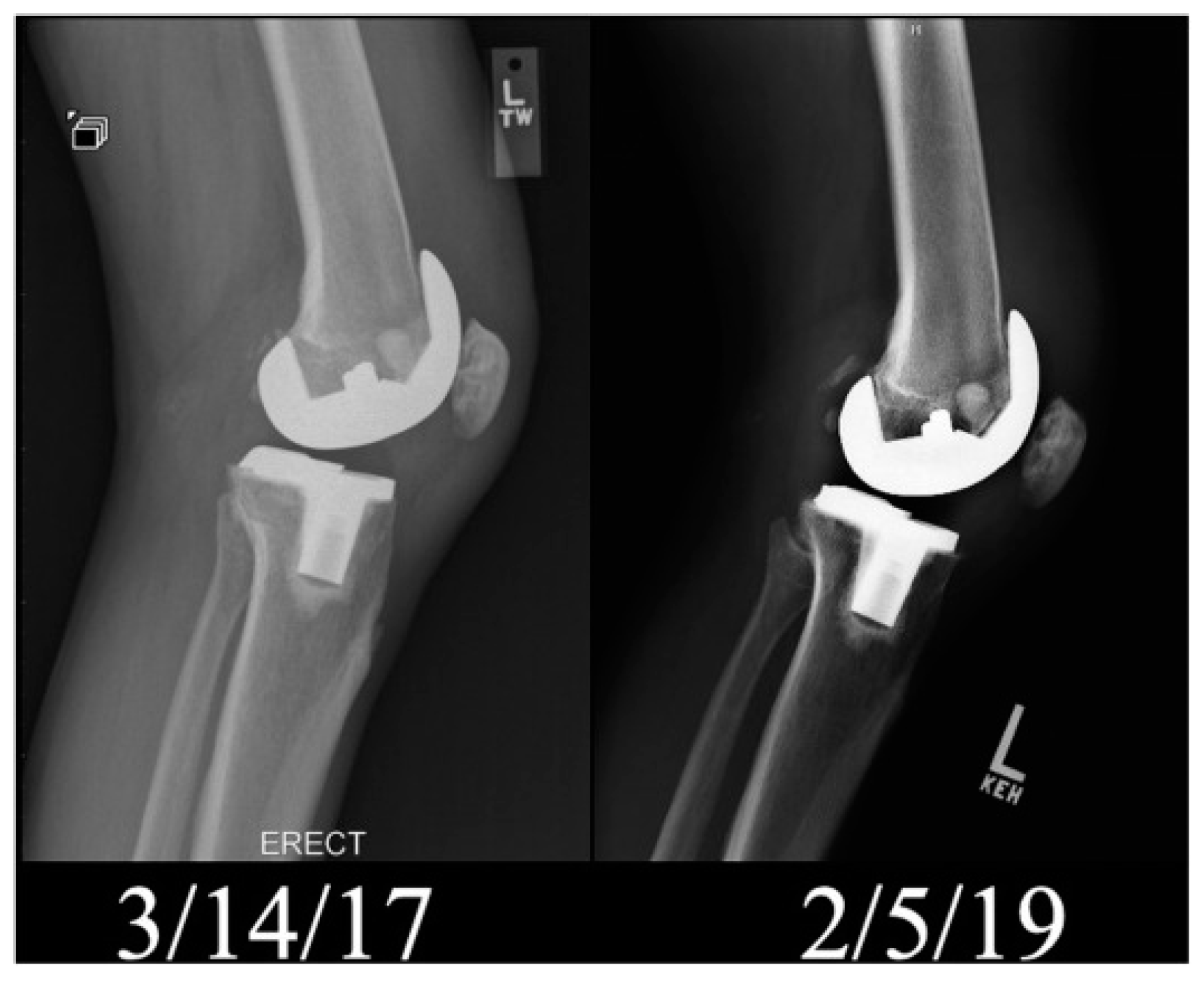
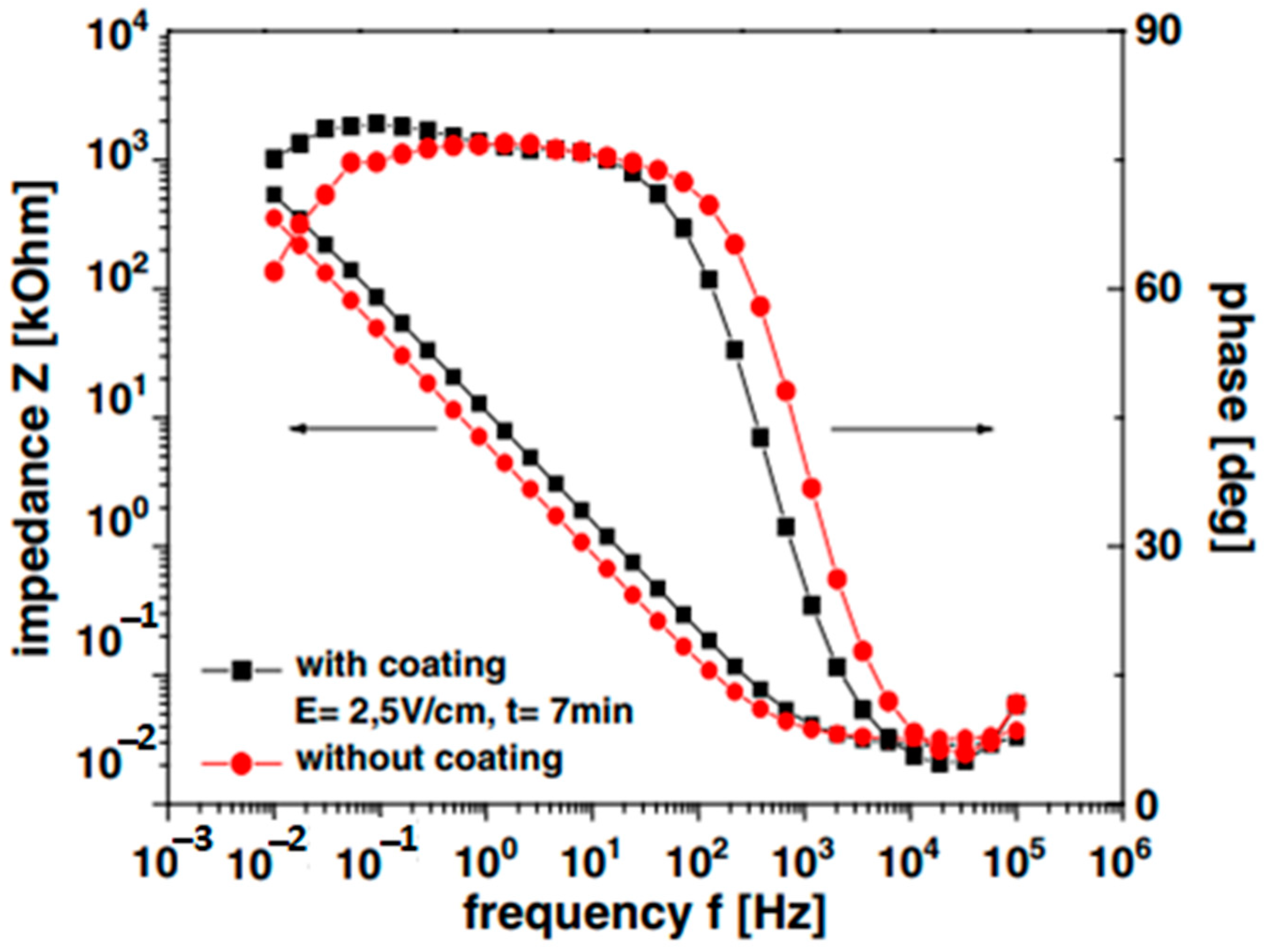

| No. | Suspension Composition | EPD Parameters | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Anode Material | Cathode Material | Voltage (V) | Time (min) | Interelectrode Spacing (cm) | |||
| 1. | 0.5 g/L chi + 20 vol % distilled water + 1 vol % acetic acid + 0.5 g/L BG powder + 0.5 g/L lawsone | 316L-SS | 316L-SS | 110 | 2 | 0.5 | [74] |
| 2. | (0.5 g/L chi + 1 vol % acetic acid+ 20 vol % distilled water + 79 vol % ethanol) + (1 g/L gelatin powder + 20 vol % distilled water + 1 vol % acetic acid + 79 vol % ethanol) | 316L-SS | 316L-SS | 15, 30, 45 | 3, 5, 7 | 1 | [75] |
| 3. | (0.5 g chi + 0.2 L DI water + 10 m L acetic acid + 0.79 L ethanol) + (1 g of gelatin type B + 0.2 L DI water + 10 m L acetic acid + 0.79 L ethanol)final composition: 33 wt % chi + 67 wt % gelatin + 2 g/L SiGe NPs | 316L-SS | 316L-SS | 18 | 5 | 1 | [76] |
| 4. | 0.5 g/L chi + 20 vol % distilled water + 1 vol % acetic acid + 79 vol % ethanol + AgSr-HA powder | 316L-SS | 316L-SS | 10, 20, 30, 40 | 3, 5, 7, 9 | 1 | [77] |
| 5. | (0.5 g chi + 0.2 L DI water + 10 m L acetic acid + 0.79 L ethanol) + (1 g of gelatin type B + 0.2 L DI water + 10 m L acetic acid + 0.79 L ethanol) + 2 g/L Ag-Mn MBGNs | 316L-SS | 316L-SS | 30 | 5 | 0.5 | [78] |
| 6. | 0.5 mg/mL chi + 1 vol % acetic acid in water + 5 mg/mL BG + 1 mL of gentamicin sulfate | Au | 316L-SS | 10 | 7, 13 | 1.5 | [79] |
| 7. | 0.33 wt % chi solution in DI water and acetic acid | - | 316L-SS | 2.5 | 7 | varied | [80] |
| 8. | chi + 1 g/L BG in ethanol and distilled water | 316L-SS | Ti | 10 to 50 | 1 to 3 | 1 | [81] |
| 9. | 1 g dm−3 chi + 4% aqueous citric acid | Pt | Ti15Mo | 2.5, 5, 7.5, 10 | 18 s, 1, 3, 5, 10 | 1.5 | [82] |
| 10. | 0.05 wt % Zein + 0.05 wt % BG + 74.9 wt % ethanol + 25 wt % DI water | 316L-SS | 316L-SS | 5 to 40 | 5 to 25 | 1 | [83] |
| 11. | 0.15 g/mL zein + 50 mL of 90 vol % aqueous ethanol-glycerol | 316L-SS | 316L-SS | 3, 5, 10 | 30 s, 1, 2, 5, 10 | 1 | [84] |
| 12. | 6 wt % zein + 74 wt % ethanol + 20 wt % distilled water + acetic acid + (1.25 g/L and 5 g/L) HA powder | 316L-SS | 316L-SS | 3, 5, 7, 9 | 6, 9, 12, 15 | 1 | [85,86] |
| 13. | 6 wt % zein + 20 wt % distilled water + 74 wt % ethanol + 5 g/L BG | 316L-SS | Mg | 14.5 V/cm (electric field) | 5 | - | [87] |
| 14. | 2.5 wt % CuO + 75 wt % zein solution in ethanol, glycerol, DI water + 10 g/L BG | - | 316L-SS | 10 | 5 | 1 | [88] |
| 15. | (0.1, 0.2, 0.3) g Al2O3 + 1.5 g of PEEK powder + 50 mL ethanol | 316L-SS | Ti-13Nb-13Zr | 30, 40, 50, 60, 70 | (10, 20, 30, 40, 50) s, 1 | 1 | [89] |
| 16. | 1–6 wt % PEEK + 0.5 wt % HCl + NaOH | SS | NiTi | 3–50 | 2–20 | 2 | [90] |
| 17. | 5–50 wt % PEEK + CTAB + anionic surfactants + distilled water | C/Cu | C/Cu | 2–60 | 10 | 2–5 | [91] |
| 18. | STAC + HA | C | PEEK/G10 | 55 | 90 | - | [92] |
| 19. | 2 wt % PEEK and 6.67 wt % BG + 13.34 wt % citric acid + ethanol | 316L-SS | 316L-SS | 110 | 2 | 0.5 | [93,94,95] |
| 20. | 0.2 g Al2O3 +1.5 g PEEK +50 mL ethanol | Ti-6Al-4V | 316L-SS | 20, 40, 60, 70, 80, 100 | 40 s | 1 | [96] |
| 21. | 1.5 g PEEK + 50 mL ethanol | 316L-SS | Ti-13Nb-13Zr | 70–115 | 1 | 1 | [97] |
| 22. | (0.02, 0.05, 0.1)Si3N4 + 1.5 g PEEK + 50 mL ethanol, and(0.02, 0.05, 0.1)Si3N4 + 1.5 g PEEK + 50 mL colloidal solution of chi | 316L-SS | Ti-13Nb-13Zr | 30, 40, 50, 60, 70, 80 | (20, 30, 40, 50, 60, 70, 80) s | 1 | [98] |
| 23. | 2 wt % PEEK + 3.3 wt % BG + Ag NPs | 316L-SS | 316L-SS | 200 V/cm (electric field) | 2 | - | [99] |
| 24. | 3.3 wt % BG + 2 wt % PEEK + 0.5 wt % h-BN + 6.6 wt % citric acid | 316L-SS | 316L-SS | 90 | 2 | 0.5 | [100] |
| 25. | Varying conc. Of PEEK/TiO2/PEEK + TiO2 in ethanol | 316L-SS | 316L-SS | 8–56 | 1–10 | 1.5 | [101] |
| 26. | 10–30 g/L PEEK + ethanol | C | 316L-SS | 0.167 mA/cm2 | - | 2 | [102] |
| 27. | 3 wt % PEEK + 95 vol % ethanol + 5 vol % isopropanol + (0.5, 1, 3) wt % GO | 316L-SS | 316L-SS | 10, 30 | 1–5 | 1 | [103] |
| 28. | 5 g/L (MBGNs/Ag-MBGNs + BG) + 20 g/L PEEK in ethanol | 316L-SS | 316L-SS | 100 V/cm (electric field) | 0.5 | - | [104] |
| 29. | (1, 2, 4)g/L G + 30 g/L of PEEK in ethanol | Ti-6Al-4V | SS | 10–100 | 40 s | 1 | [105] |
| 30. | 0.5 g/L Chi + 0.02 g Si3N4 +1.5 g PEEK in ethanol | 316L-SS | Ti-6Al-4V | 10–110 | 1.5 | 1.5 | [106] |
| 31. | 3.3 wt % BG + 6.6 wt % citric acid in ethanol + 2 wt % PEEK + 0.5 wt % h-BN | 316L-SS | 316L-SS | 90 | 1.5 | 1 | [107] |
| 32. | 1st layer (6.67 wt % BG + 13.34 wt % citric acid in ethanol + 2 wt % PEEK) + 2nd layer (chi/gelatin/2 g/L Ag-Mn MBGNs) | 316L-SS | 316L-SS | 80 (1st) 30 (2nd) | 1.5 (1st) 5 (2nd) | 0.5 | [78,108] |
| 33. | 1st layer (6.67 wt % BG + 13.34 wt % citric acid in ethanol + 2 wt % PEEK) + 2nd layer 0.5 g/L (chi/BG/lawsone) | 316L-SS | 316L-SS | 110 (1st) 50 (2nd) | 2 (1st) 5 (2nd) | 0.5 (1st) 1 (2nd) | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, S.A.; Wadood, A.; Hussain, S.W.; Yasir, M.; Ur Rehman, M.A. A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges. Surfaces 2021, 4, 205-239. https://doi.org/10.3390/surfaces4030018
Batool SA, Wadood A, Hussain SW, Yasir M, Ur Rehman MA. A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges. Surfaces. 2021; 4(3):205-239. https://doi.org/10.3390/surfaces4030018
Chicago/Turabian StyleBatool, Syeda Ammara, Abdul Wadood, Syed Wilayat Hussain, Muhammad Yasir, and Muhammad Atiq Ur Rehman. 2021. "A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges" Surfaces 4, no. 3: 205-239. https://doi.org/10.3390/surfaces4030018
APA StyleBatool, S. A., Wadood, A., Hussain, S. W., Yasir, M., & Ur Rehman, M. A. (2021). A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges. Surfaces, 4(3), 205-239. https://doi.org/10.3390/surfaces4030018






