ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of LTO/TiO2
2.3. Synthesis of ZnO/LTO/TiO2
2.4. Characterization
2.5. Photocatalytic Degradation of Methylene Blue (MB) under Visible Light Irradiation
3. Results
3.1. Characterization of LTO/TiO2 and ZnO/LTO/TiO2
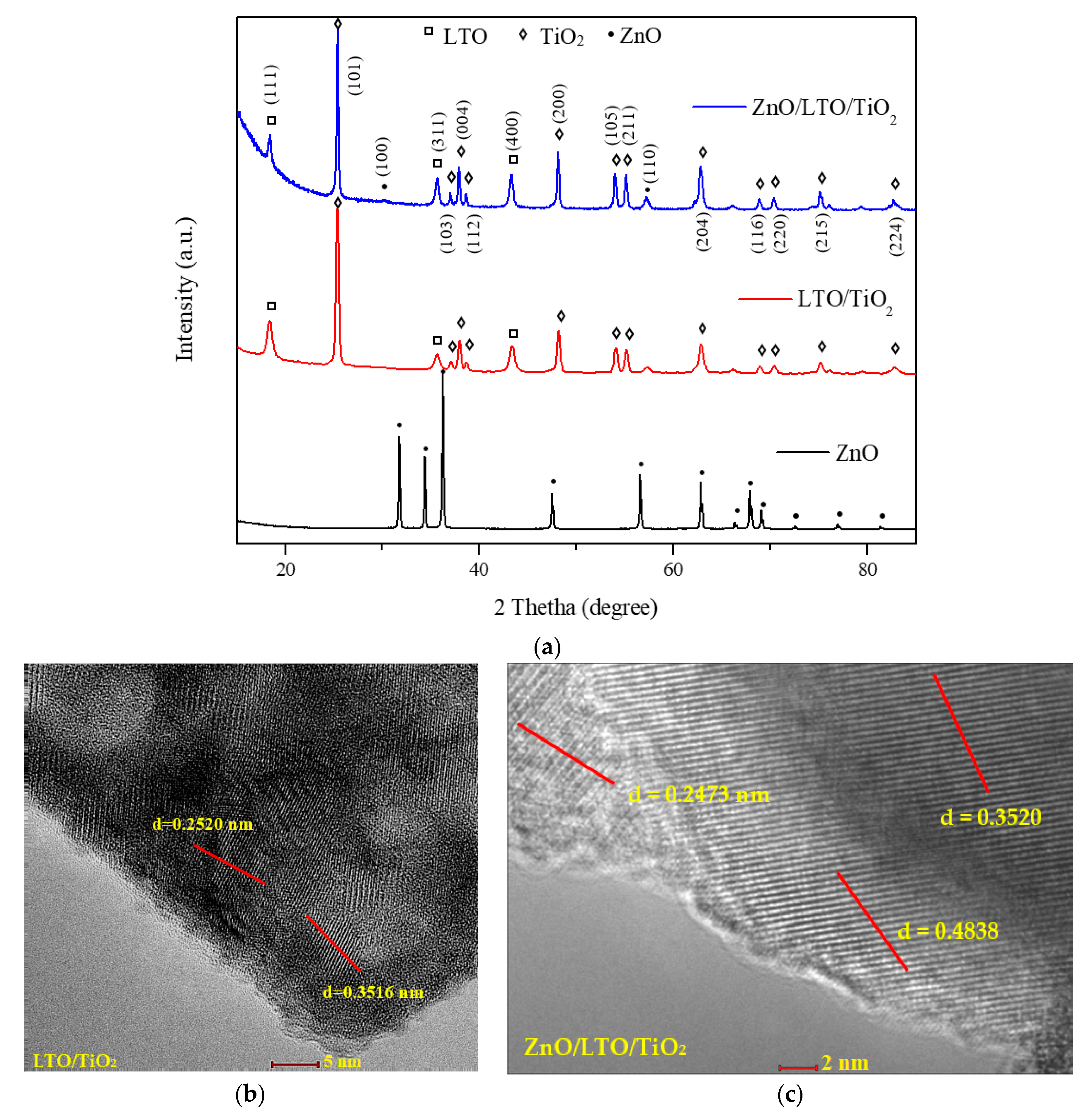
3.1.1. X-Ray Diffraction and HRTEM Analyses
3.1.2. FTIR and N2 Adsorption-Desorption Analyses
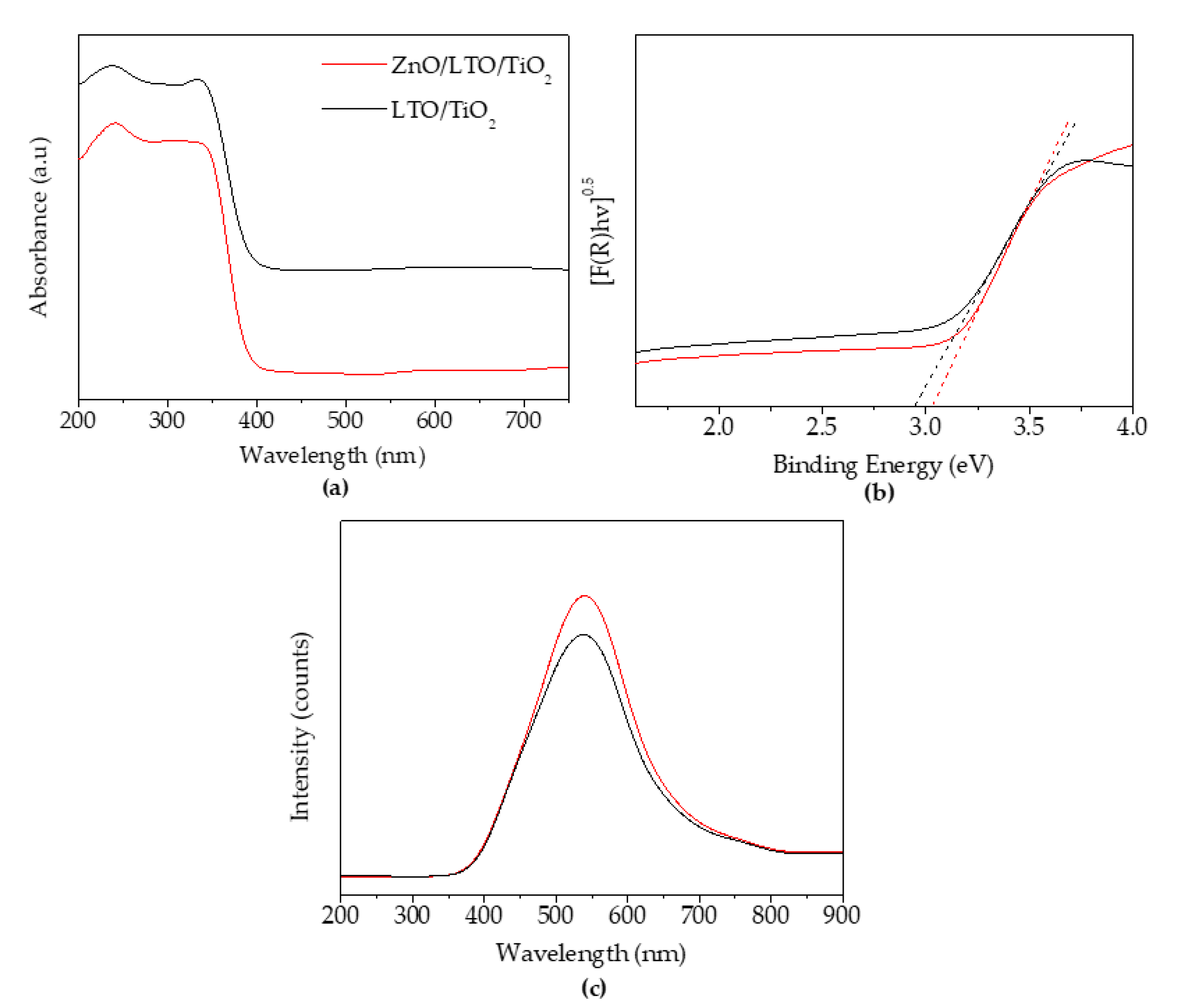
3.1.3. Diffuse Reflectance-UV/Vis and Photoluminescent Analyses
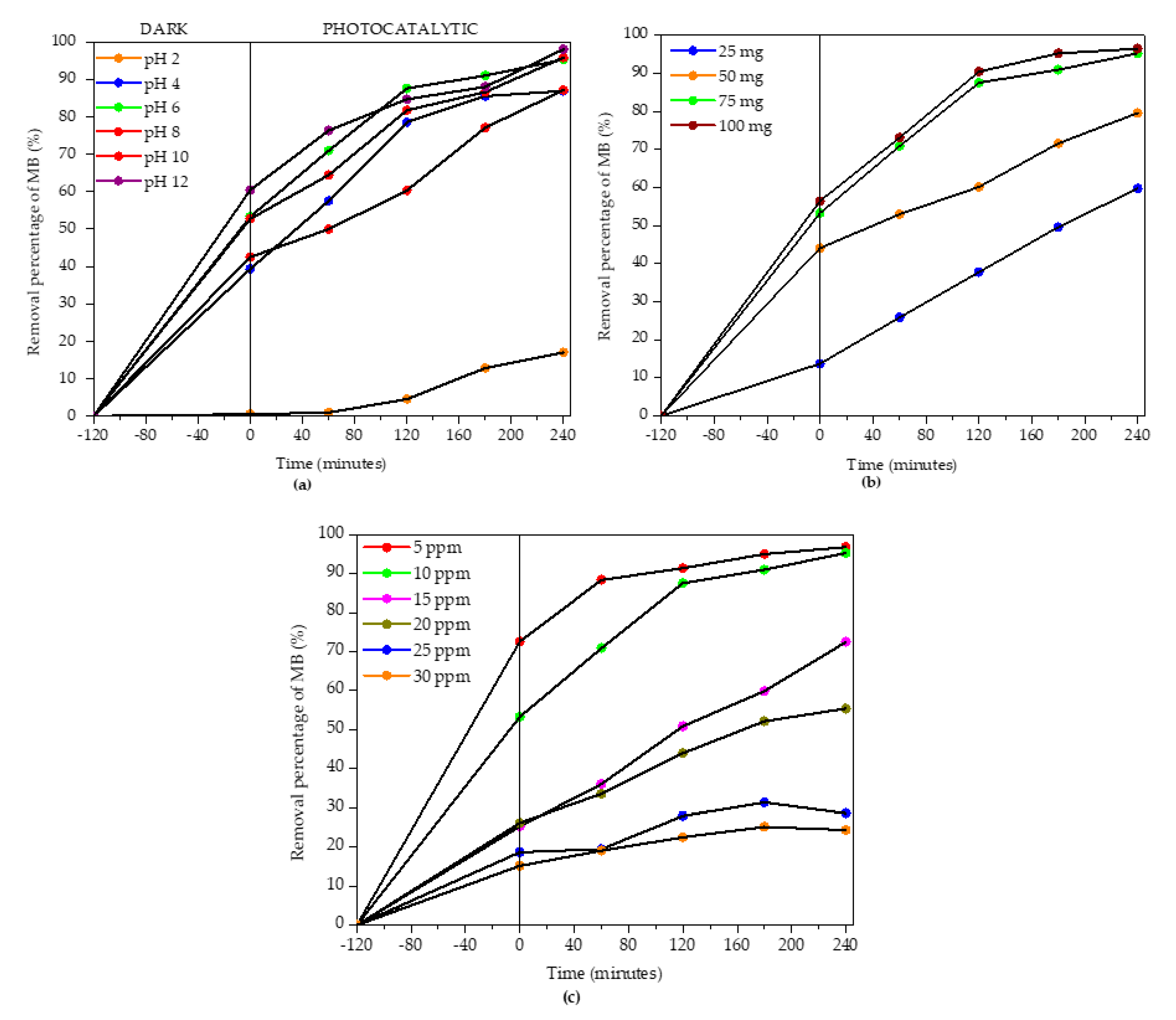
3.2. Photocatalytic Degradation of Methylene Blue (MB)
3.3. Free Radical Scavenging Study
3.4. Mineralization Study
3.5. Reusability Study
3.5.1. XPS Analysis of ZnO/LTO/TiO2
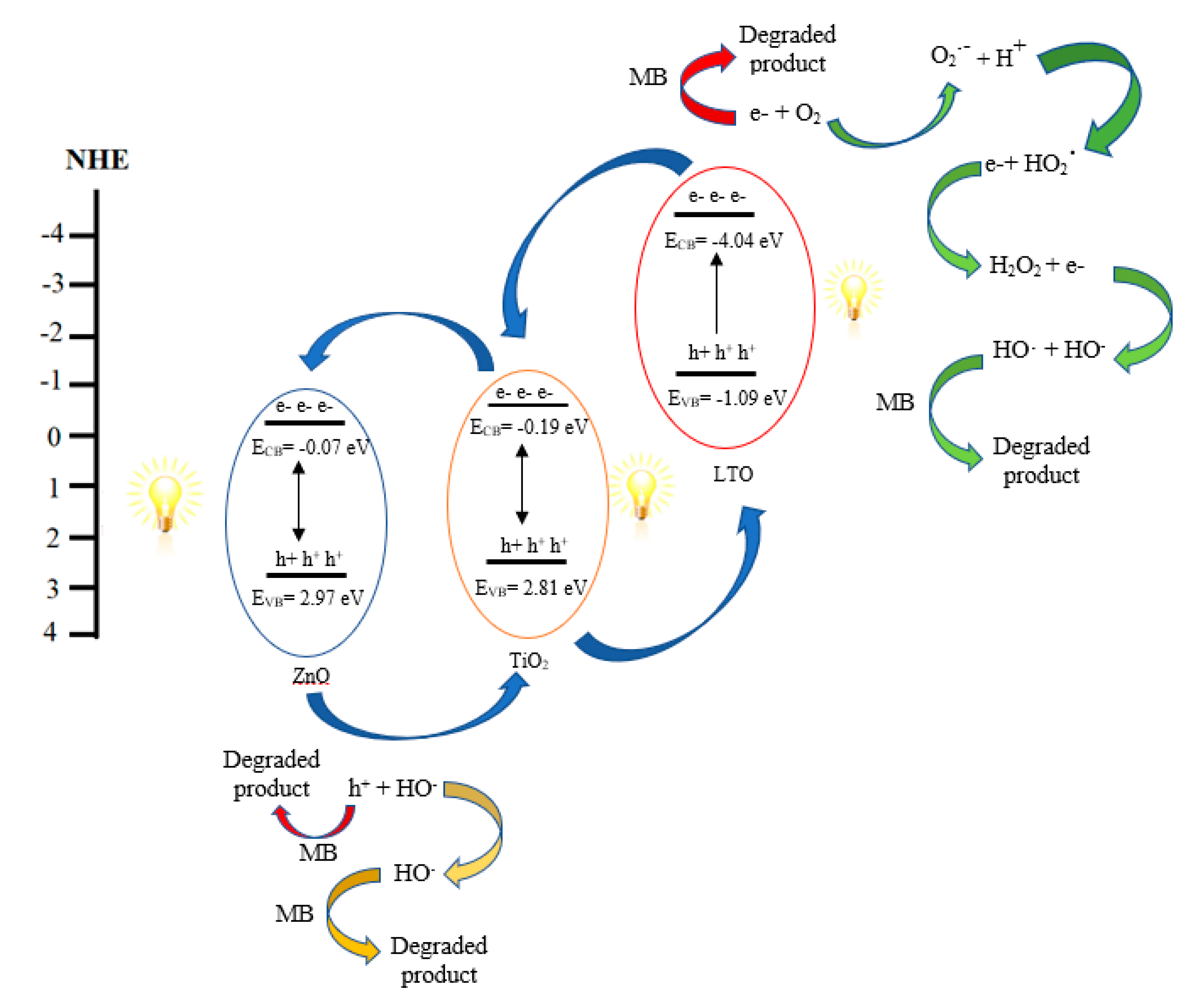
3.5.2. Proposed Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subki, N.S. Environmental contamination by batik wastewater and the potential application of activated carbon from pineapple waste for wastewater treatment. Ph.D. Thesis, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia, 2017. [Google Scholar]
- Adeleke, J.T.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl Surf. Sci. 2018, 455, 195–200. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kant, R.; Rai, A.; Gupta, A.; Bhattacharya, S. Facile synthesis of ZnO/GO nanoflowers over Si substrate for improved photocatalytic decolorization of MB dye and industrial wastewater under solar irradiation. Mater. Sci. Semicond. Process. 2019, 89, 6–17. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollution Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Bel Hadjltaief, H.; Ben Zina, M.; Galvez, M.E.; Da Costa, P. Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO-TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170, 153–161. [Google Scholar] [CrossRef]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayioǧlu, K.; Gedik, F.; Akin, A.N.; Aydinoǧlu, S.Ö. A comparative study for removal of different dyes over M/TiO2 (M = Cu, Ni, Co, Fe, Mn and Cr) photocatalysts under visible light irradiation. J. Photochem. Photobiol. A Chem. 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Chen, W.J.; Wu, C.T. Pt-TiO2/graphene photocatalysts for degradation of AO7 dye under visible light. Appl. Surf. Sci. 2015, 340, 9–17. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Dhatshanamurthi, P.; Shanthi, M. Preparation and characterization of SeO2/TiO2 composite photocatalyst with excellent performance for sunset yellow azo dye degradation under natural sunlight illumination. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 138, 489–498. [Google Scholar] [CrossRef]
- Thomas, M.; Naikoo, G.A.; Sheikh, M.U.D.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A Chem. 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Iqbal, M.; Nadeem, M.A.; Hussain, I.; Zeshan, S.Y.; Mustafa, G.; Zafar, M.I.; Nadeem, M.A. Photocatalytic degradation of textile dyes on Cu2O-CuO/TiO2 anatase powders. J. Environ. Chem. Eng. 2016, 4, 2138–2146. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 146–156. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Huang, J.; Fei, J.; Cao, L.; Li, C. In situ synthesis of mesoporous C-doped TiO2 single crystal with oxygen vacancy and its enhanced sunlight photocatalytic properties. Dye. Pigment. 2017, 144, 203–211. [Google Scholar] [CrossRef]
- Delsouz Khaki, M.R.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Swaminathan, M.; Houas, A. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. 2017, 60, 3–13. [Google Scholar] [CrossRef]
- Chauque, S.; Oliva, F.Y.; Visintin, A.; Barraco, D.; Leiva, E.P.M.; Cámara, O.R. Lithium titanate as anode material for lithium ion batteries: Synthesis, post-treatment and its electrochemical response. J. Electroanal. Chem. 2017, 799, 142–155. [Google Scholar] [CrossRef]
- Esteves, M.; Fernández-Werner, L.; Pignanelli, F.; Montenegro, B.; Belluzzi, M.; Pistón, M.; Chialanza, M.R.; Faccio, R.; Mombrú, Á.W. Synthesis, characterization and simulation of lithium titanate nanotubes for dye sensitized solar cells. Ceram. Int. 2019, 45, 708–717. [Google Scholar] [CrossRef]
- Ojha, M.; Le Houx, J.; Mukkabla, R.; Kramer, D.; Andrew Wills, R.G.; Deepa, M. Lithium titanate/pyrenecarboxylic acid decorated carbon nanotubes hybrid—Alginate gel supercapacitor. Electrochim. Acta. 2019, 309, 253–263. [Google Scholar] [CrossRef]
- Abdul Rahman, N.R.; Muniandy, L.; Adam, F.; Iqbal, A.; Ng, E.P.; Lee, H.L. Detailed photocatalytic study of alkaline titanates and its application for the degradation of methylene blue (MB) under solar irradiation. J. Photochem. Photobiol. A 2019, 375, 219–230. [Google Scholar] [CrossRef]
- Priya, A.; Arumugam, M.; Arunachalam, P.; Al-Mayouf, A.M.; Madhavan, J.; Theerthagiri, J.; Choi, M.Y. Fabrication of visible-light active BiFeWO6/ZnO nanocomposites with enhanced photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124294. [Google Scholar]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and characterization of ZnO/g-C3N4 nanocomposites photocatalyst for photocatalytic degradation of methylene blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Coelho, J.; Pokle, A.; Park, S.H.; McEvoy, N.; Berner, N.C.; Duesberg, G.S.; Nicolosi, V. Lithium titanates/carbon nanotubes composites processed by ultrasound irradiation as anodes for lithium ion batteries. Sci. Rep. 2017, 7, 7614. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.X.; Jiang, B.; Xie, S.K.; Zhang, L.C. Strong enhancement on dye photocatalytic degradation by ball-milled TiO2: A study of cationic and anionic dyes. J. Mater. Sci. Technol. 2017, 33, 856–863. [Google Scholar] [CrossRef]
- Al-Taweel, S.S.; Saud, H.R. New route for synthesis of pure anatase TiO2 nanoparticles via ultrasound - assisted sol—gel method. J. Chem. Pharm. Res. 2016, 8, 620–626. [Google Scholar]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol-Gel Synthesis of Zinc Oxide (ZnO) Nanoparticles: Study of Structural and Optical Properties. J. Sci. Islam. Repub. Iran. 2015, 26, 281–285. [Google Scholar]
- Bhatia, S.; Verma, N. Photocatalytic activity of ZnO nanoparticles with optimization of defects. Mater. Res. Bull. 2017, 95, 468–476. [Google Scholar] [CrossRef]
- Lan, C.K.; Chang, C.C.; Wu, C.Y.; Chen, B.H.; Duh, J.G. Improvement of the Ar/N2 binary plasma-treated carbon passivation layer deposited on Li4Ti5O12 electrodes for stable high-rate lithium ion batteries. RSC Adv. 2015, 5, 92554–92563. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and Analysis of Porosity and Pore Structures. MSA 2015, 80, 61–164. [Google Scholar] [CrossRef] [Green Version]
- Dewajani, H.; Rochmadi; Purwono, S.; Budiman, A. Effect of modification ZSM-5 catalyst in upgrading quality of organic liquid product derived from catalytic cracking of Indonesian nyamplung oil (Calpphyllum inophyllum). AIP Conf. Proc. 2016, 1755, 050002. [Google Scholar]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: Anatase versus rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef] [PubMed]
- Lavand, A.B.; Malghe, Y.S. Visible light photocatalytic degradation of 4-chlorophenol using C / ZnO / CdS nanocomposite. J. Saudi Chem. Soc. 2015, 19, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, A.; Dhanapandian, S.; Manoharan, C.; Sivakumar, G. Physical properties of Zn doped TiO2 thin films with spray pyrolysis technique and its effects in antibacterial activity. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 138, 105–112. [Google Scholar] [CrossRef]
- Nishanthi, S.T.; Iyyapushpam, S.; Sundarakannan, B.; Subramanian, E.; Padiyan, D.P. Inter-relationship between extent of anatase crystalline phase and photocatalytic activity of TiO2 nanotubes prepared by anodization and annealing method. Sep. Purif. Technol. 2014, 131, 102–107. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst, Chapter 2. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; Cao, W., Ed.; IntechOpen: Rijeka, Cratia, 2016; pp. 1–51. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.H.; Kim, J.S.; Kim, E.H.; Lee, S.K.; Kim, J.W.; Lim, H.J.; Koo, S.M. Enhanced photocatalytic activity of TiO2@mercapto-functionalized silica toward colored organic dyes. J. Mater. Sci. 2015, 50, 2577–2586. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Chemosphere Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sohrabnezhad, S.; Pourahmad, A.; Salavatiyan, T. CuO–MMT nanocomposite: Effective photocatalyst for the discoloration of methylene blue in the absence of H2O2. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–12. [Google Scholar] [CrossRef]
- Tabaei, H.S.M.; Kazemeini, M.; Fattahi, M. Preparation and characterization of visible light sensitive nano titanium dioxide photocatalyst. Sci. Iran. Trans. C 2012, 19, 1626–1631. [Google Scholar] [CrossRef] [Green Version]
- Sapawe, N.; Jalil, A.A.; Triwahyono, S.; Sah, R.N.R.A.; Jusoh, N.W.C.; Hairom, N.H.H.; Efendi, J. Electrochemical strategy for grown ZnO nanoparticles deposited onto HY zeolite with enhanced photodecolorization of methylene blue: Effect of the formation of Si-O-Zn bond. Appl. Catal. A Gen. 2013, 456, 144–158. [Google Scholar] [CrossRef]
- Taufik, A.; Albert, A.; Saleh, R. Sol-gel synthesis of ternary CuO/TiO2/ZnO nanocomposites for enhanced photocatalytic performance under UV and visible light irradiation. J. Photochem. Photobiol. A Chem. 2017, 344, 149–162. [Google Scholar] [CrossRef]
- Malik, R.; Chaudhary, V.; Tomer, V.K.; Rana, P.S.; Nehra, S.P. Visible light-driven mesoporous Au–TiO2/SiO2 photocatalysts for advanced oxidation process. Ceram. Int. 2016, 42, 10892–10901. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Perciani, N.; Moraes, D.; Nascimento, F.; Lucia, M.; Pinto, C.; Moreira, T.; Campos, B.; Thim, G.P.; Rodrigues, L.A. Methylene blue photodegradation employing hexagonal prism- shaped niobium oxide as heterogeneous catalyst: Effect of catalyst dosage, dye concentration, and radiation source. Mater. Chem. Phys. 2018, 214, 95–106. [Google Scholar]
- Jawad, A.H.; Shazwani, N.; Mubarak, A.; Azlan, M.; Ishak, M.; Ismail, K.; Nawawi, W.I. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. Integr. Med. Res. 2016, 10, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Paramarta, V.; Taufik, A.; Saleh, R. Comparison of photocatalytic performance of different types of grapheme in Fe3O4/SnO2 composites. J. Phys. Conf. Ser. 2017, 820, 012027. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, C.; Li, T. Rapid Photocatalytic Degradation of Methylene Blue under High Photon Flux UV Irradiation: Characteristics and Comparison with Routine Low Photon Flux. Int. J. Photoenergy. 2012, 398787. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Zhang, C.; Fu, L.; Wang, Y.; Yang, D. Preparation, characterization and photocatalytic application of TiO2–graphene photocatalyst under visible light irradiation. Ceram. Int. 2015, 41, 2502–2511. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Z.; Zhang, J.; Wu, H.; Xi, L.; Ruan, C. Synthesis of Ag/TiO2/graphene and its photocatalytic properties under visible light. Mater. Lett. 2016, 171, 182–186. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Wang, X.; Wang, Z.; Zhang, M.; He, G.; Sun, Z. A unique Cu2O/TiO2 nanocomposite with enhanced photocatalytic performance under visible light irradiation. Ceram. Int. 2017, 43, 4866–4872. [Google Scholar] [CrossRef]
- Harish, S.; Sabarinathan, M.; Archana, J.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Hayakawa, Y. Synthesis of ZnO/SrO nanocomposites for enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2017, 418, 147–155. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Dong, Y.; Li, H.; Xia, Y.; Wang, H. A facile solvothermal approach for the synthesis of novel W-doped TiO2 nanoparticles/reduced graphene oxide composites with enhanced photodegradation performance under visible light irradiation. New J. Chem. 2017, 41, 13382–13390. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.D.; Ponnusamy, S.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Cabir, B.; Yurderi, M.; Caner, N.; Agirtas, M.S.; Zahmakiran, M.; Kaya, M. Methylene blue photocatalytic degradation under visible light irradiation on copper phthalocyanine-sensitized TiO2 nanopowders. Mat. Sci. Eng. B Adv. 2017, 224, 9–17. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram. Int. 2018, 44, 7047–7056. [Google Scholar] [CrossRef]
- Neena, D.; Kondamareddy, K.K.; Bin, H.; Lu, D.; Kumar, P.; Dwivedi, R.K.; Fu, D. Enhanced visible light photodegradation activity of RhB/MB from aqueous solution using nanosized novel Fe-Cd co-modified ZnO. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2018, 160, 154–163. [Google Scholar] [CrossRef]
- Ilyas, U.; Lee, P.; Tan, T.L.; Ramanujan, R.V.; Zhang, S.; Chen, R.; Sun, H.D.; Rawat, R.S. High temperature ferromagnetic ordering in c-axis oriented ZnO:Mn nanoparticle thin films by tailoring substrate temperature. Int. J. Mod. Phys. Conf. Ser. 2014, 32, 1460341. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, X.; Zhang, W.; Wang, L.; Chen, X.; Gao, Y. Microwave-assisted synthesis of nanocomposite Ag/ZnO-TiO2 and photocatalytic degradation Rhodamine B with different modes. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 134–141. [Google Scholar] [CrossRef]
- Wu, L.; Wu, P.; Zhu, Y.; Zhu, N.; Dang, Z. Preparation and characterization of ZnTiO3–TiO2/pillared montmorillonite composite catalyst for enhanced photocatalytic activity. Res. Chem. Intermed. 2016, 42, 5253–5268. [Google Scholar] [CrossRef]
- Pereira-Nabais, C.; Swiatowska, J.; Chagnes, A.; Ozanam, F.; Gohier, A.; Tran-Vane, P.; Cojocaru, C.S.; Cassir, M.; Marcus, P. Interphase chemistry of Si electrodes used as anodes in Li-ion batteries. Appl. Surf. Sci. 2013, 266, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Kayaci, F.; Vempati, S.; Ozgit-Akgun, C.; Biyikli, N.; Uyar, T. Enhanced photocatalytic activity of homoassembled ZnO nanostructures on electrospun polymeric nanofibers: A combination of atomic layer deposition and hydrothermal growth. Appl. Catal. B: Environ. 2014, 156, 173–183. [Google Scholar] [CrossRef]
- Strauß, F.; Hüger, E.; Heitjans, P.; Trouillet, V.; Bruns, M.; Schmidt, H. Li-Si thin films for battery applications produced by ion-beam co-sputtering. RSC Adv. 2015, 5, 7192–7195. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.H.; Song, J.; Jung, Y.; Kweon, O.Y.; Song, H.; Jang, J. Electrospun ZnO/TiO2 composite nanofibers as a bactericidal agent. Chem. Commun. 2011, 47, 9164–9166. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Shen, J.; Cui, X. Facile synthesis of a heterogeneous Li2TiO3/TiO2 nanocomposite with enhanced photoelectrochemical water splitting. New J. Chem. 2017, 41, 6305–6314. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, C.; Park, D.; Kim, H.; Lee, J. Al-incorporation into Li7La3Zr2O12 solid electrolyte keeping stabilized cubic phase for all-solid-state Li batteries. J. Energy Chem. 2018, 27, 1501–1508. [Google Scholar] [CrossRef] [Green Version]
- Cai, A.; Sun, Y.; Du, L.; Wang, X. Hierarchical Ag2O—ZnO—Fe3O4 composites with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 644, 334–340. [Google Scholar] [CrossRef]
- Lee, M.S.; Park, M.; Kim, H.Y.; Park, S.J. Effects of Microporosity and Surface Chemistry on Separation Performances of N-Containing Pitch-Based Activated Carbons for CO2/N2 Binary Mixture. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
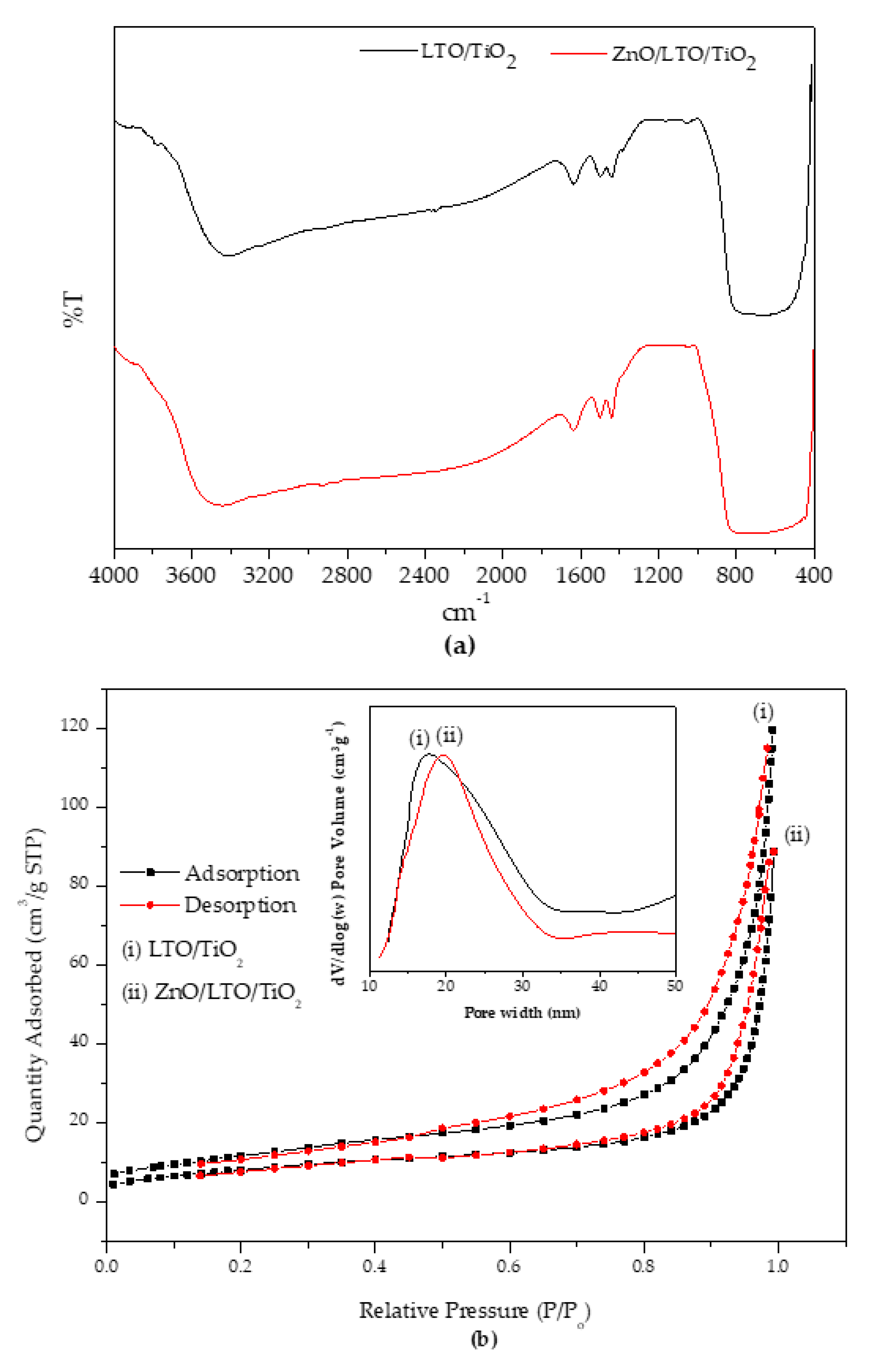


| Catalyst | BET Surface Area (m2g−1) | Average Pore Size (nm) | Average Pore Volume (cm3g−1) |
|---|---|---|---|
| LTO/TiO2 | 38.8 | 17.2 | 0.1854 |
| ZnO/LTO/TiO2 | 26.7 | 19.6 | 0.1363 |
| Initial Concentration, (ppm) | Rate Constant, k (h−1) | Correlation Coefficient (R2) | MB Decolorization (%) |
|---|---|---|---|
| 5 | 0.585 | 0.9395 | 96.8 |
| 10 | 0.586 | 0.9412 | 95.3 |
| 15 | 0.246 | 0.9788 | 72.5 |
| 20 | 0.0931 | 0.7407 | 45.4 |
| 25 | 0.0607 | 0.7911 | 28.6 |
| 30 | 0.006 | 0.0739 | 24.4 |
| Catalyst | Dye Removal (%) | Dye (ppm) | Time (min) | Reusability (Cycles) | Irradiation Source | Reference |
|---|---|---|---|---|---|---|
| TiO2-20% graphene | 98.8 | 10 | 100 | Three | Halogen-tungsten lamp (500 W) | [49] |
| Ag/TiO2/rGO | 79 | 10 | 240 | NA | Fluorescence xenon lamp (200 W) | [50] |
| Cu2O/TiO2 | 100 | 5.0 × 10−5 M | 90 | Three | Xenon light (300 W) | [51] |
| ZnO/3% SrO | 100 | 10 | 6 | Four | Halogen lamp (500 W) | [52] |
| W-TiO2/RGO | 99.8 | 10 | 90 | Four | Xenon lamp (400 W) | [53] |
| ZnO/1% CuO | 95.52 | 10 | 5 | Four | Halogen lamp (500 W) | [54] |
| CuPc/TiO2 | 100 | 20 mM | 150 | Five | Xenon lamp (150 W) | [55] |
| 0.5%NiO/m-TiO2 | 90 | 20 | 150 | Five | Visible light GE lamp (400 W) | [56] |
| Fe-Cd (2%):ZnO | 82 | 20 | 140 | Five | Xenon lamp (300 W) | [57] |
| 0.4ZnO:0.6TiO2 | 90 | 10 | 75 | NA | Tungsten lamp (100 W) | [58] |
| ZnO/LTO/TiO2 | 95 | 10 | 240 | Four | Fluorescent lamps (48 W) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Ibrahim, N.H.; Rahman, N.R.A.; Saharudin, K.A.; Adam, F.; Sreekantan, S.; Yusop, R.M.; Jaafar, N.F.; Wilson, L.D. ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation. Surfaces 2020, 3, 301-318. https://doi.org/10.3390/surfaces3030022
Iqbal A, Ibrahim NH, Rahman NRA, Saharudin KA, Adam F, Sreekantan S, Yusop RM, Jaafar NF, Wilson LD. ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation. Surfaces. 2020; 3(3):301-318. https://doi.org/10.3390/surfaces3030022
Chicago/Turabian StyleIqbal, Anwar, N. H. Ibrahim, Nur Ruzaina Abdul Rahman, K. A. Saharudin, Farook Adam, Srimala Sreekantan, Rahimi M. Yusop, N. F. Jaafar, and Lee D. Wilson. 2020. "ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation" Surfaces 3, no. 3: 301-318. https://doi.org/10.3390/surfaces3030022
APA StyleIqbal, A., Ibrahim, N. H., Rahman, N. R. A., Saharudin, K. A., Adam, F., Sreekantan, S., Yusop, R. M., Jaafar, N. F., & Wilson, L. D. (2020). ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation. Surfaces, 3(3), 301-318. https://doi.org/10.3390/surfaces3030022








