Hydrogen in Aluminium-Coated Steels Exposed to Synthetic Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
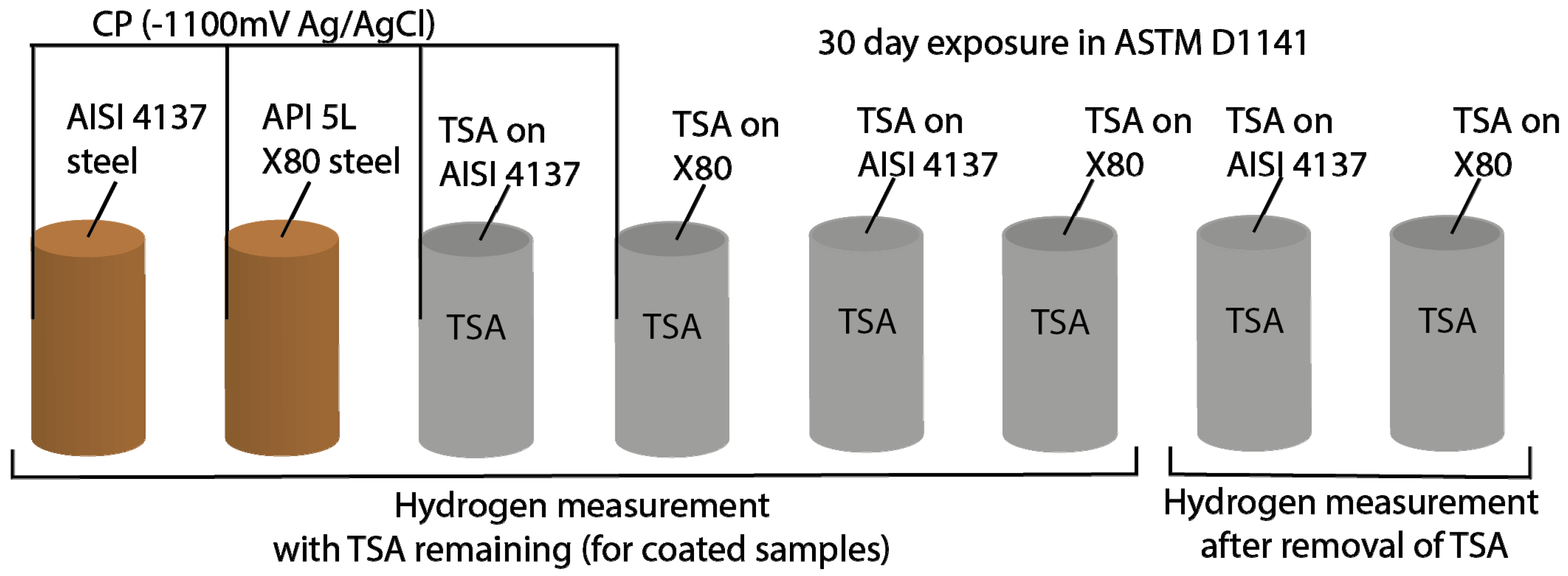
2.2. Exposure Tests
2.3. Visual and Microstructural Examination
2.4. Hydrogen Measurements
3. Results
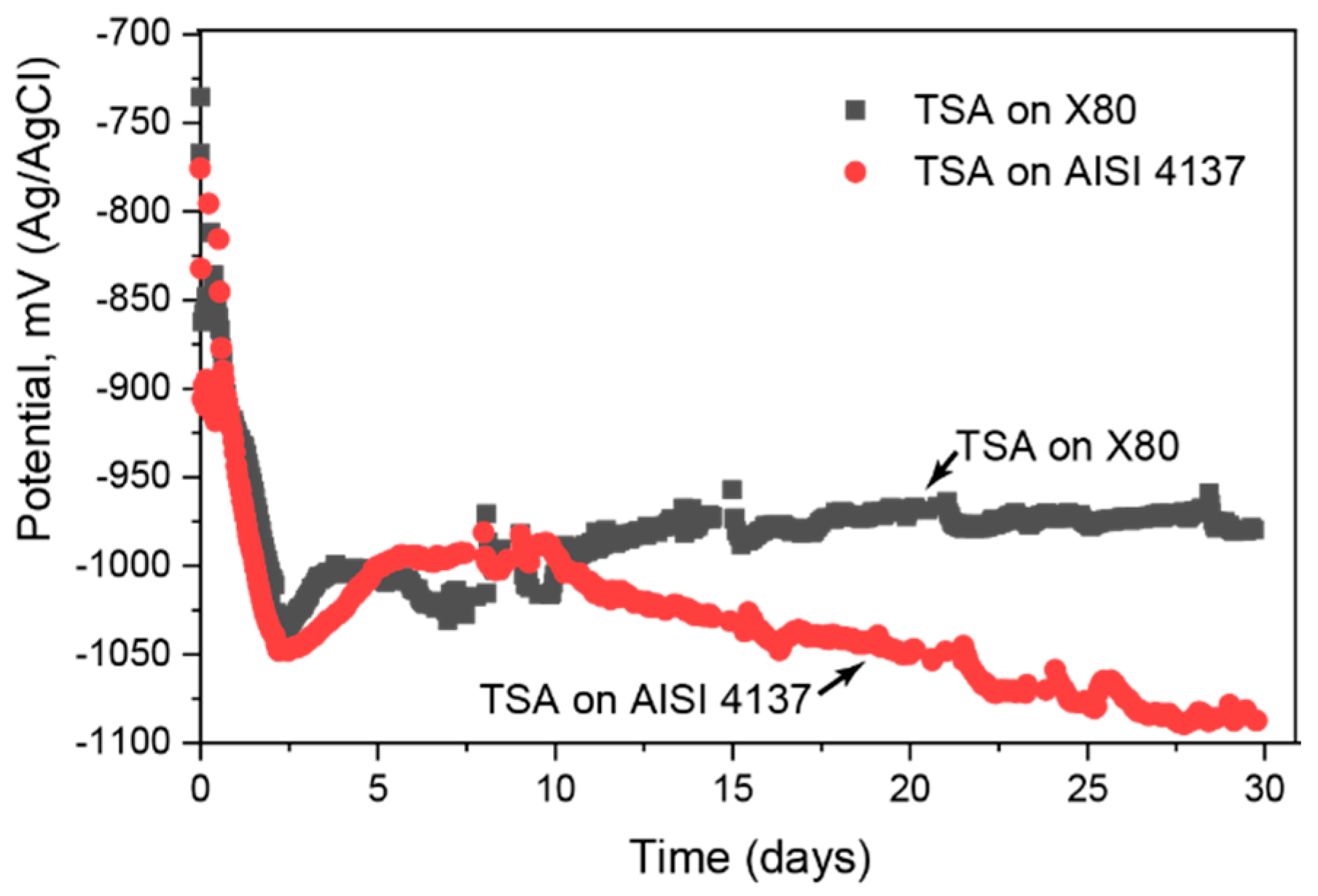
3.1. Electrochemcial Measurements
3.2. Visual and Microstructural Observations
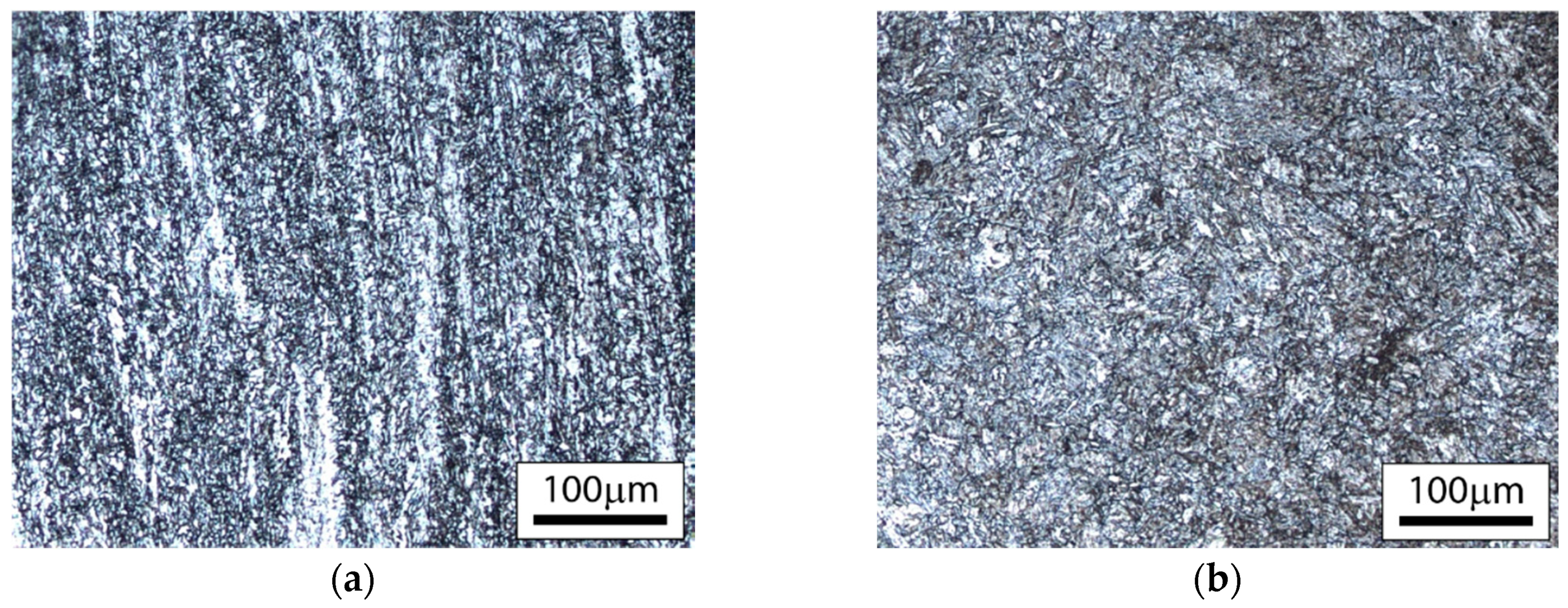
3.2.1. Substrate and Coating Microstructure
3.2.2. Visual Observations during Testing
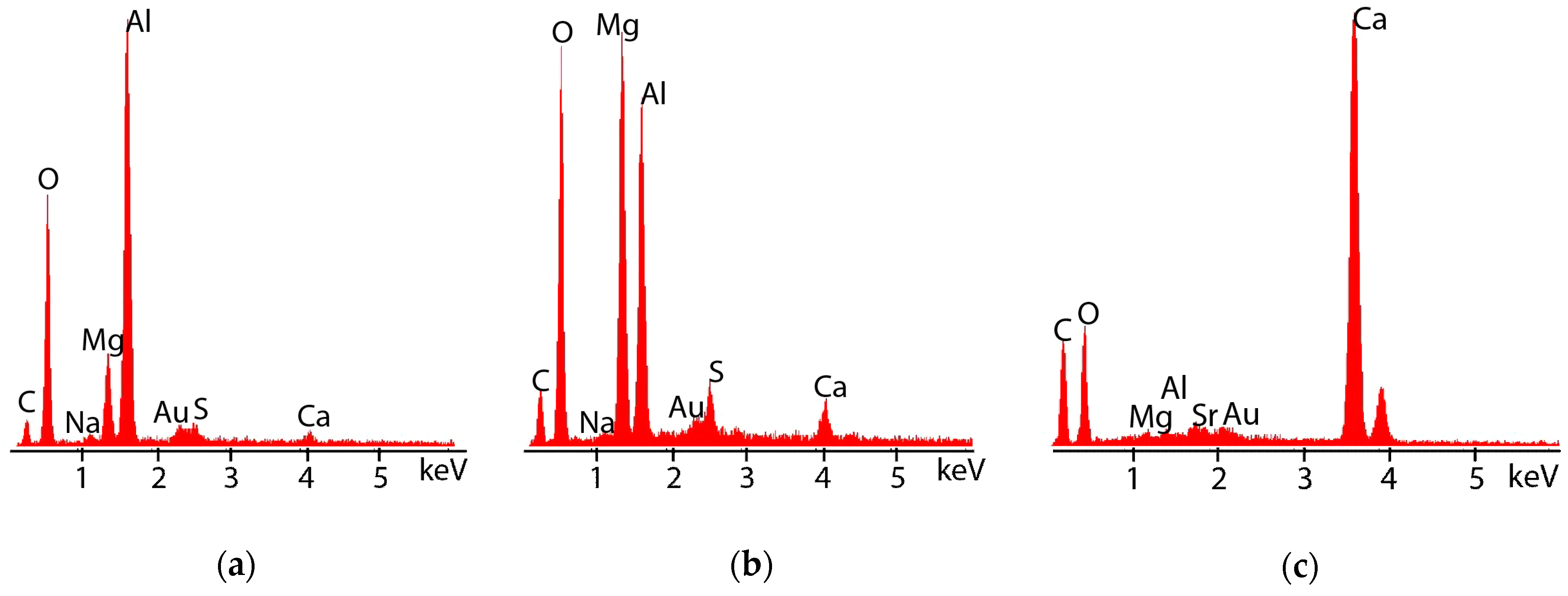
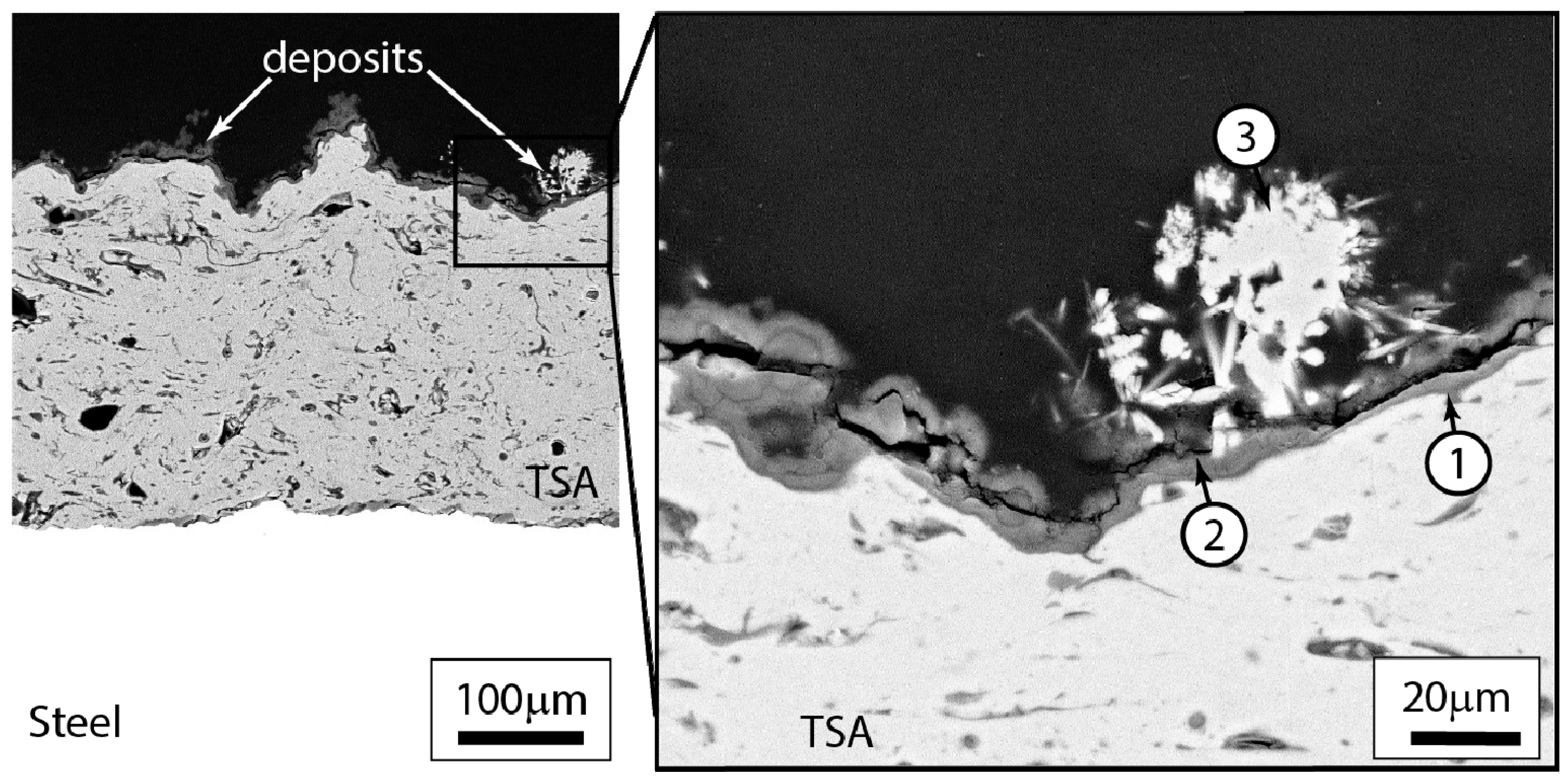
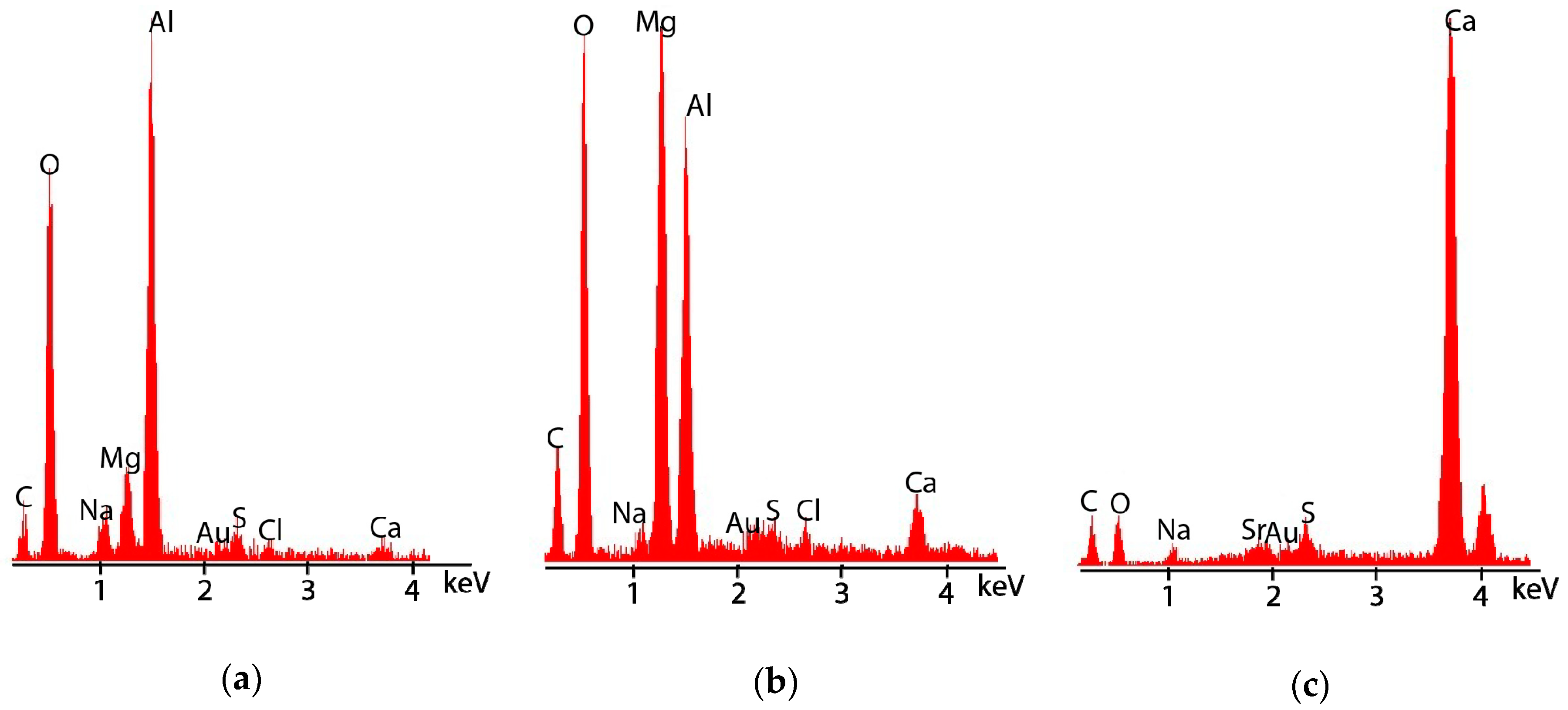
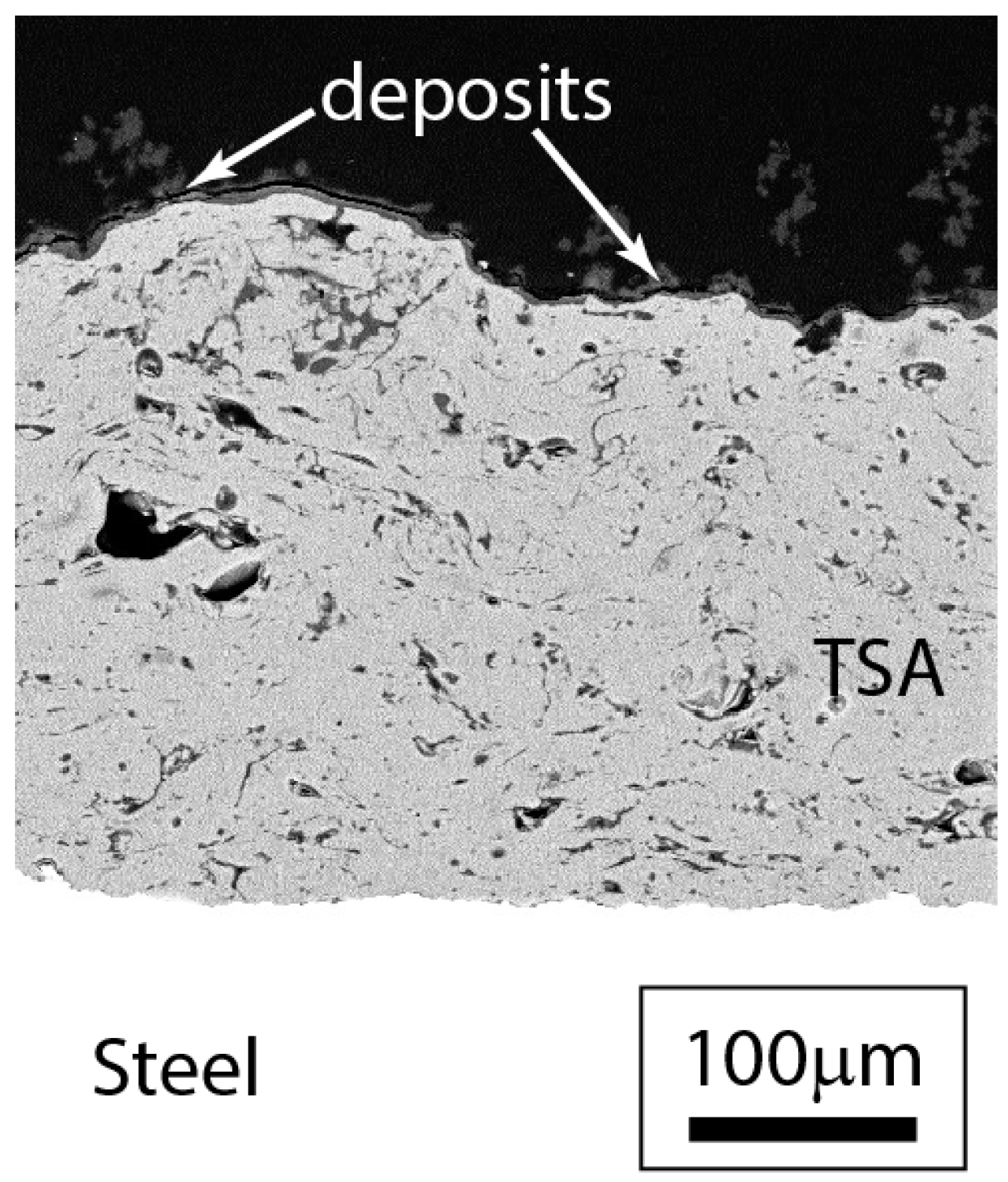
3.2.3. Post-Test Microstructural Observations
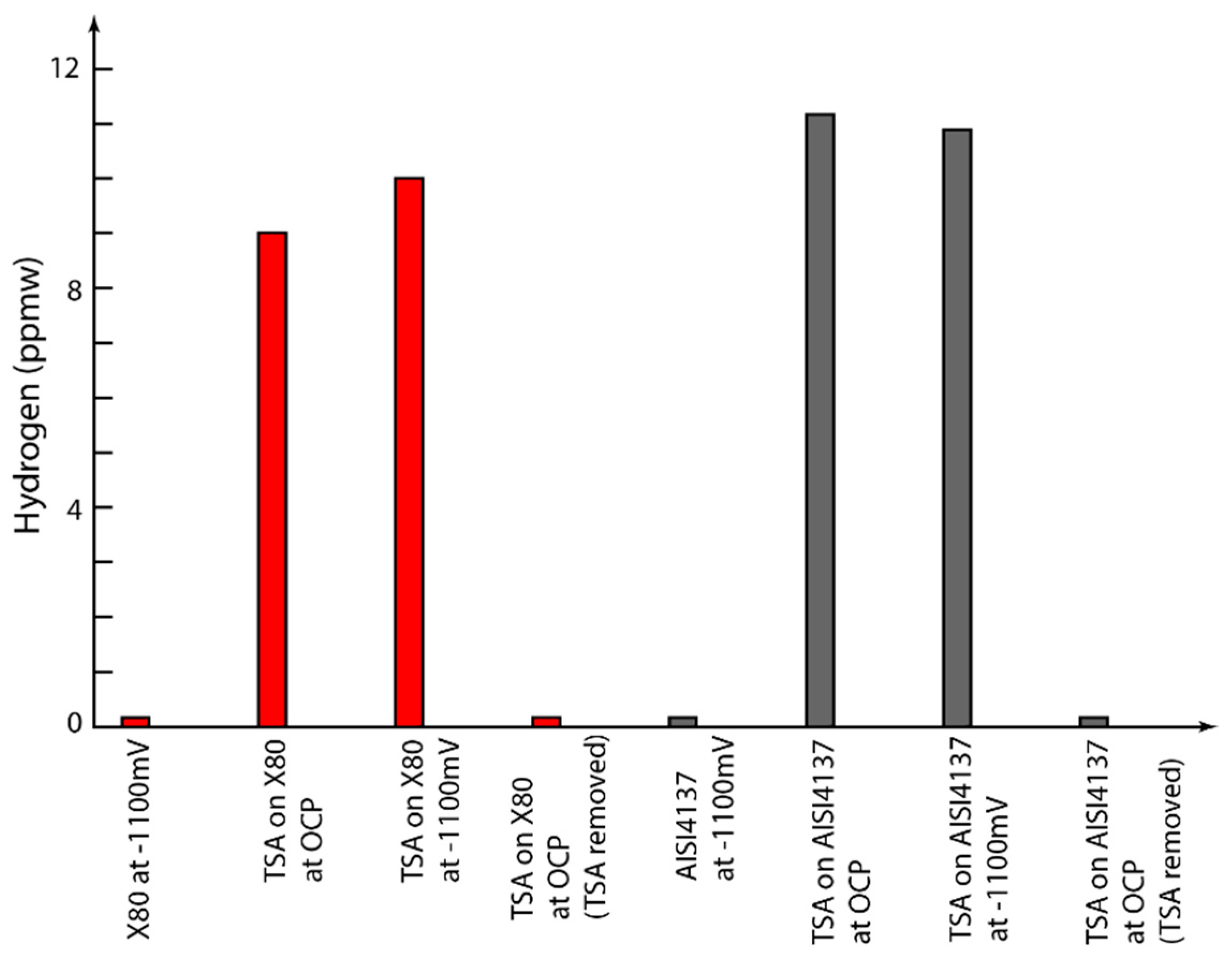
3.3. Hydrogen Measurements
4. Discussion
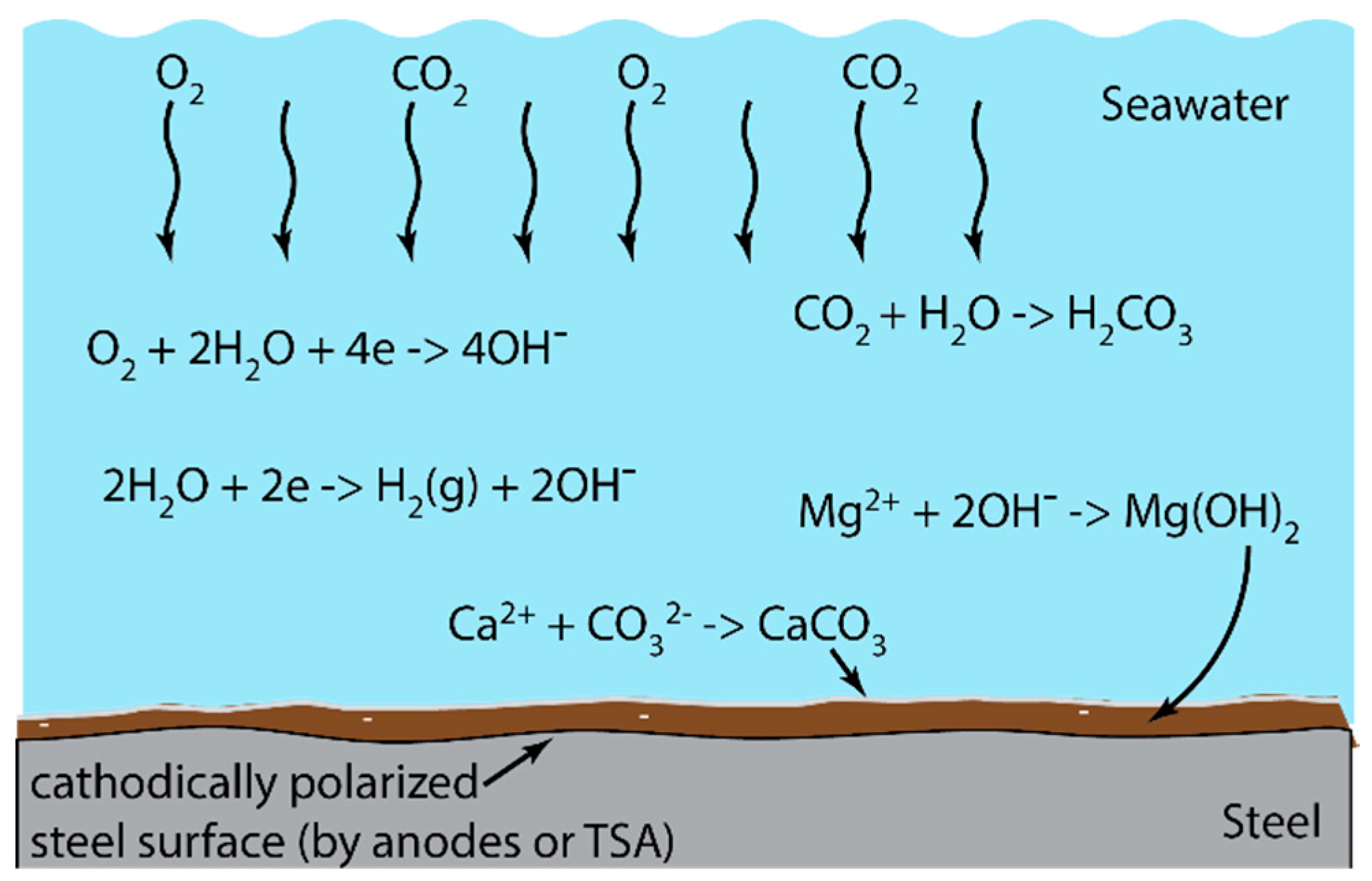
4.1. Mechanism of Hydrogen Generation
4.2. Location of Hydrogen in TSA-Coated Steel
4.3. Influence of Calcareous Deposits
5. Conclusions
- API 5L X80 and AISI 4137 steels show similar levels of hydrogen after 30 days of charging at −1.1 V (Ag/AgCl/saturated KCl)
- The level of hydrogen observed in TSA-coated steel specimens were ~100 times more than that observed in uncoated specimens
- The hydrogen in TSA-coated steel was localised in the TSA layer/steel-TSA interface
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Refait, P.; Paul, S. Preface. Mater. Corros. Werkst. Und Korros. 2019, 70, 949. [Google Scholar] [CrossRef]
- Wyatt, B.S. Cathodic protection of offshore structures. Anti Corros. Methods Mater. 1977, 24, 5–10. [Google Scholar] [CrossRef]
- Wyatt, B.S. Cathodic protection of offshore structures. 2. Anti Corros. Methods Mater. 1977, 24, 9–15. [Google Scholar] [CrossRef]
- Wang, W.; Hartt, W.H.; Chen, S. Sacrificial anode cathodic polarization of steel in seawater. 1. A novel experimental and analysis methodology. Corrosion 1996, 52, 419–427. [Google Scholar] [CrossRef]
- Smith, W.R.; Paul, S. Natural deposit coatings on steel during cathodic protection and hydrogen ingress. Coatings 2015, 5, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Thodla, R.; Sridhar, N.; Amaya, H.; Fahimi, B.; Taylor, C. Hydrogen embrittlement of low alloy steels under cathodic polarization. Corrosion 2020, 76, 299–311. [Google Scholar] [CrossRef]
- Beck, W.; Bockris, J.O.; Genshaw, M.A.; Subraman, P.K. Diffusivity and solubility of hydrogen as a function of composition in Fe-Ni alloys. Metall. Trans. 1971, 2, 883–888. [Google Scholar] [CrossRef]
- Bockris, J.O.; Subramanyan, P.K. Hydrogen embrittlement and hydrogen traps. J. Electrochem. Soc. 1971, 118, 1114–1118. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Bernstein, I.M. An example of the effect of hydrogen trapping on hydrogen embrittlement. Metall. Trans. A Phys. Metall. Mater. Sci. 1981, 12, 835–844. [Google Scholar] [CrossRef]
- Robinson, M.J.; Sharp, R.M. The effect of post-exposure heat-treatment on the hydrogen embrittlement of high-carbon steel. Corrosion 1985, 41, 582–586. [Google Scholar] [CrossRef]
- Figueroa, D.; Robinson, M.J. Hydrogen transport and embrittlement in 300 M and AerMet100 ultra high strength steels. Corros. Sci. 2010, 52, 1593–1602. [Google Scholar] [CrossRef] [Green Version]
- Asadipoor, M.; Anaraki, A.P.; Kadkhodapour, J.; Sharifi, S.M.H.; Barnoush, A. Macro- and microscale investigations of hydrogen embrittlement in X70 pipeline steel by in-situ and ex-situ hydrogen charging tensile tests and in-situ electrochemical micro-cantilever bending test. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2020, 772, 12. [Google Scholar] [CrossRef]
- Spengler, E.; Fragata, F.L.; Margarit, I.C.P.; Mattos, O.R. Corrosion protection of low toxicity paints. Prog. Org. Coat. 1997, 30, 51–57. [Google Scholar] [CrossRef]
- Kendig, M.; Mills, D.J. An historical perspective on the corrosion protection by paints. Prog. Org. Coat. 2017, 102, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Guenbour, A.; Benbachir, A.; Kacemi, A. Evaluation of the corrosion performance of zinc-phosphate-painted carbon steel. Surf. Coat. Technol. 1999, 113, 36–43. [Google Scholar] [CrossRef]
- Ferry, M.; Nik, W.B.W.; Noor, C.W.M. The Influence of seawater velocity to the corrosion rate and paint degradation at mild steel plate immersed in sea water. Appl. Mech. Mat. 2014, 554, 218–221. [Google Scholar] [CrossRef]
- Fischer, K.P.; Thomason, W.H.; Rosbrook, T.; Murali, J. Performance history of thermal-sprayed aluminum coatings in offshore service. Mater. Perform. 1995, 34, 27–35. [Google Scholar]
- Syrek-Gerstenkorn, B.; Paul, S.; Davenport, A.J. Sacrificial thermally sprayed aluminium coatings for marine environments: A review. Coatings 2020, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Shrestha, S.; Lee, C.M.; Harvey, M.D.F. Thermally sprayed aluminum (TSA) coatings for extended design life of 22%Cr duplex stainless steel in marine environments. J. Therm. Spray Technol. 2013, 22, 328–336. [Google Scholar] [CrossRef]
- Paul, S.; Lu, Q.; Harvey, M.D.F. Mitigating localized corrosion using thermally sprayed aluminum (TSA) coatings on welded 25% Cr superduplex stainless steel. J. Therm. Spray Technol. 2015, 24, 629–636. [Google Scholar] [CrossRef]
- Ce, N.; Paul, S. Thermally sprayed aluminum coatings for the protection of subsea risers and pipelines carrying hot fluids. Coatings 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Ce, N.A.; Paul, S. Protection of hot subsea risers by using thermally sprayed aluminium. Anti Corros. Methods Mater. 2017, 64, 299–305. [Google Scholar] [CrossRef]
- Syrek-Gerstenkorn, B.; Paul, S.; Davenport, A.J. Use of thermally sprayed aluminium (TSA) coatings to protect offshore structures in submerged and splash zones. Surf. Coat. Technol. 2019, 374, 124–133. [Google Scholar] [CrossRef]
- Echaniz, R.G.; Paul, S.; Thornton, R. Effect of seawater constituents on the performance of thermal spray aluminum in marine environments. Mater. Corros. Werkst. Und Korros. 2019, 70, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Hartt, W.H.; Culberson, C.H.; Smith, S.W. Calcareous deposits on metal-surfaces in seawater—A critical-review. Corrosion 1984, 40, 609–618. [Google Scholar] [CrossRef]
- Wilson, H.; Johnsen, R.; Rodriguez, C.T.; Hesjevik, S.M. Properties of TSA in natural seawater at ambient and elevated temperature. Mater. Corros. 2018. [Google Scholar] [CrossRef]
- Pollock, W.J. Statistical treatment of slow strain rate data for assessment of hydrogen embrittlement in high-strength 4340 steel. Corrosion Sci. 1992, 33, 1105–1119. [Google Scholar] [CrossRef]
- Carr, M.J.; Robinson, M.J. The effects of zinc alloy electroplating on the hydrogen embrittlement of high-strength steels. Trans. Inst. Met. Finish. 1995, 73, 58–64. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhang, X.Y.; Wu, Y.H.; Wang, L.; Zhao, P.W.; Yang, L. The character of hydrogen embrittlement in mooring chain steel. JOM 2020, 72, 2003–2010. [Google Scholar] [CrossRef]
- Chalaftris, G.; Robinson, M.J. Hydrogen re-embrittlement of high strength steel by corrosion of cadmium and aluminium based sacrificial coatings. Corros. Eng. Sci. Technol. 2005, 40, 28–32. [Google Scholar] [CrossRef]
- Figueroa, D.; Robinson, M.J. The effects of sacrificial coatings on hydrogen embrittlement and re-embrittlement of ultra high strength steels. Corros. Sci. 2008, 50, 1066–1079. [Google Scholar] [CrossRef]
- Cardilli, E.; Robinson, M.J. Aluminium based coatings for optimum sacrificial protection of high strength steel. Corros. Eng. Sci. Technol. 2011, 46, 782–789. [Google Scholar] [CrossRef]
- ASTM D1141. Standard Practice for the Preparation of Substitute Ocean Water; ASTM International: West Conshohocken, PA, USA, 2013; Volume 11.02. [Google Scholar]
- Ce, N.; Paul, S. The effect of temperature and local pH on calcareous deposit formation in damaged thermal spray aluminum (TSA) coatings and its implication on corrosion mitigation of offshore steel structures. Coatings 2017, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Kishor, K.; Saha, S.; Parashtekar, A.; Pala, R.G.S. Increasing Chlorine Selectivity through Weakening of Oxygen Adsorbates at Surface in Cu Doped RuO2 during Seawater Electrolysis. J. Electrochem. Soc. 2018, 165, J3276–J3280. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D.K. Recent progress in alkaline water electrolysis for hydrogen production and applications (vol 36, pg 307, 2010). Prog. Energy Combust. Sci. 2011, 37, 631. [Google Scholar] [CrossRef]
- Hsu, G.-S.W.; Hsia, C.-W.; Hsu, S.Y. Effects of electrode settings on chlorine generation efficiency of electrolyzing seawater. J. Food Drug Anal. 2015, 23, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.-H.; Miao, J.; Zhang, L.; Gao, J.; Wang, H.; Tao, H.; Hung, S.-F.; Vasileff, A.; Qiao, S.Z.; Liu, B. An earth-abundant catalyst-based seawater photoelectrolysis system with 17.9% solar-to-hydrogen efficiency. Adv. Mater. 2018, 30, 1707261. [Google Scholar] [CrossRef]
- Bockris, J.O. Hydrogen. Materials 2011, 4, 2073–2091. [Google Scholar] [CrossRef] [Green Version]
- Bockris, J.O.M.; Uosaki, K. Photoelectrochemical production of hydrogen. In Advances in Chemistry, Vol 163; American Chemical Society: Washington, DC, USA, 1977; pp. 33–70. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.M.; Tribollet, B.; Sutter, E.M.M. New insights into the cathodic dissolution of aluminium using electrochemical methods. Electrochim. Acta 2016, 216, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Serdechnova, M.; Volovitch, P.; Brisset, F.; Ogle, K. On the cathodic dissolution of Al and Al alloys. Electrochim. Acta 2014, 124, 9–16. [Google Scholar] [CrossRef]
- Ogle, K.; Serdechnova, M.; Mokaddem, M.; Volovitch, P. The cathodic dissolution of Al, Al2Cu, and Al alloys. Electrochim. Acta 2011, 56, 1711–1718. [Google Scholar] [CrossRef]
- Mokaddem, M.; Volovitch, P.; Rechou, F.; Oltra, R.; Ogle, K. The anodic and cathodic dissolution of Al and Al-Cu-Mg alloy. Electrochim. Acta 2010, 55, 3779–3786. [Google Scholar] [CrossRef]
- Moon, S.M.; Pyun, S.I. The corrosion of pure aluminium during cathodic polarization in aqueous solutions. Corros. Sci. 1997, 39, 399–408. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F. The mechanism of hydrogen evolution during anodic polarization of aluminium. Electrochim. Acta 2015, 180, 712–721. [Google Scholar] [CrossRef]
- Paul, S. Assessing coating reliability through pore architecture evaluation. J. Therm. Spray Technol. 2010, 19, 779–786. [Google Scholar] [CrossRef]
- Bhattacharya, I.N.; Das, S.C.; Mukherjee, P.S.; Paul, S.; Mitra, P.K. Thermal decomposition of precipitated fine aluminium trihydroxide. Scand. J. Metall. 2004, 33, 211–219. [Google Scholar] [CrossRef]
- Villalobos, J.C.; Del-Pozo, A.; Mayen, J.; Serna, S.; Campillo, B. Hydrogen embrittlement suscetibility on X-120 microalloyed steel as function of tempering temperature. Int. J. Hydrog. Energy 2020, 45, 9137–9148. [Google Scholar] [CrossRef]
- Folena, M.C.; Ponciano, J.A.D. Assessment of hydrogen embrittlement severity of an API 5LX80 steel in H2S environments by integrated methodologies. Eng. Fail. Anal. 2020, 111, 14. [Google Scholar] [CrossRef]
- Ishikawa, T.; McLellan, R.B. The diffusivity of hydrogen in aluminum. Acta Metall. 1986, 34, 1091–1095. [Google Scholar] [CrossRef]
- Talbot, D.E.J.; Anyalebechi, P.N. Solubility of hydrogen in liquid aluminum. Mater. Sci. Technol. 1988, 4, 1–4. [Google Scholar] [CrossRef]
- Ambat, R.; Dwarakadasa, E.S. Effect of hydrogen in aluminium and aluminium alloys: A review. Bull. Mater. Sci. 1996, 19, 103–114. [Google Scholar] [CrossRef]
- Fanicchia, F.; Maeder, X.; Ast, J.; Taylor, A.A.; Guo, Y.; Polyakov, M.N.; Michler, J.; Axinte, D.A. Residual stress and adhesion of thermal spray coatings: Microscopic view by solidification and crystallisation analysis in the epitaxial CoNiCrAlY single splat. Mater. Des. 2018, 153, 36–46. [Google Scholar] [CrossRef]
- Ohaeri, E.; Omale, J.; Rahman, K.M.M.; Szpunar, J. Effect of post-processing annealing treatments on microstructure development and hydrogen embrittlement in API 5L X70 pipeline steel. Mater. Charact. 2020, 161, 18. [Google Scholar] [CrossRef]
- Chan, W.K.; Kwok, C.T.; Lo, K.H. Mechanical properties and hydrogen embrittlement of laser-surface melted AISI 430 ferritic stainless steel. Coatings 2020, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Arafin, M.A.; Szpunar, J.A. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Chang, K.-D.; Gu, J.-L.; Fang, H.-S.; Yang, Z.-G.; Bai, B.-Z.; Zhang, W.-Z. Effects of heat-treatment process of a novel bainite/martensite dual-phase high strength steel on its susceptibility to hydrogen embrittlement. ISIJ Int. 2001, 41, 1397–1401. [Google Scholar] [CrossRef]
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corros. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Möller, H. The influence of Mg2+on the formation of calcareous deposits on a freely corroding low carbon steel in seawater. Corros. Sci. 2007, 49, 1992–2001. [Google Scholar] [CrossRef]
- Hartt, W.H.; Lemieux, E. A principal determinant in cathodic protection design of offshore structures—The mean current density. Corrosion 2000, 56, 988–997. [Google Scholar] [CrossRef]
- Deslouis, C.; Festy, D.; Gil, O.; Maillot, V.; Touzain, S.; Tribollet, B. Characterization of calcareous deposits in artificial sea water by impedances techniques: 2-deposit of Mg(OH)(2) without CaCO3. Electrochim. Acta 2000, 45, 1837–1845. [Google Scholar] [CrossRef]
- Deslouis, C.; Festy, D.; Gil, O.; Rius, G.; Touzain, S.; Tribollet, B. Characterization of calcareous deposits in artificial sea water by impedance techniques—I. Deposit of CaCO3 without Mg(OH)(2). Electrochim. Acta 1998, 43, 1891–1901. [Google Scholar] [CrossRef]
- Lin, S.H.; Dexter, S.C. Effects of temperature and magnesium-ions on calcareous deposition. Corrosion 1988, 44, 615–622. [Google Scholar] [CrossRef]
- Kunjapur, M.M.; Hartt, W.H.; Smith, S.W. Influence of temperature and exposure time upon calcareous deposits. Corrosion 1987, 43, 674–679. [Google Scholar] [CrossRef]
- Neville, A.; Morizot, A.P. Calcareous scales formed by cathodic protection—An assessment of characteristics and kinetics. J. Cryst. Growth 2002, 243, 490–502. [Google Scholar] [CrossRef]
- Hartt, W.H. Discussion: Does calcareous scale formation on cathodically protected steel affect hydrogen uptake? Corrosion 2006, 62, 947–949. [Google Scholar] [CrossRef]
- Hinds, G.; Turnbull, A. Technical note: Does calcareous scale formation on cathodically protected steel affect hydrogen uptake? Corrosion 2005, 61, 835–837. [Google Scholar] [CrossRef]
- Ou, K.C.; Wu, J.K. Effect of calcareous deposits formation on the hydrogen absorption of steel. Mater. Chem. Phys. 1997, 48, 52–55. [Google Scholar] [CrossRef]
- Simoni, L.; Caselani, J.Q.; Ramos, L.B.; Schroeder, R.M.; Malfatti, C.D. The influence of calcareous deposits on hydrogen uptake and embrittlement of API 5CT P110 steel. Corros. Sci. 2017, 118, 178–189. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Basha, B.I.Y.; Rivera-Díaz-Del-Castillo, P.E.J. Hydrogen transport in metals: Integration of permeation, thermal desorption and degassing. J. Mater. Sci. Technol. 2017, 33, 1433–1447. [Google Scholar] [CrossRef]
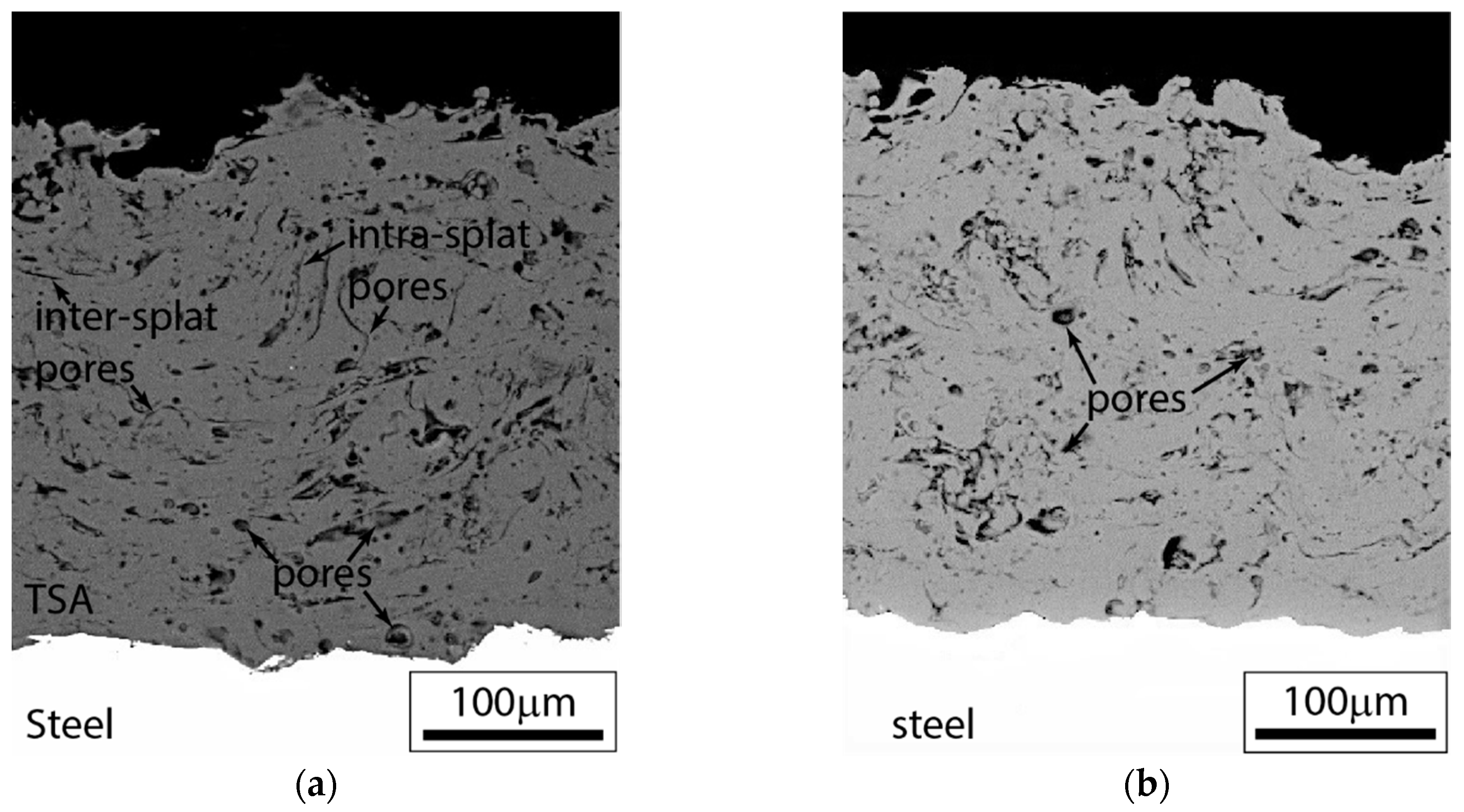
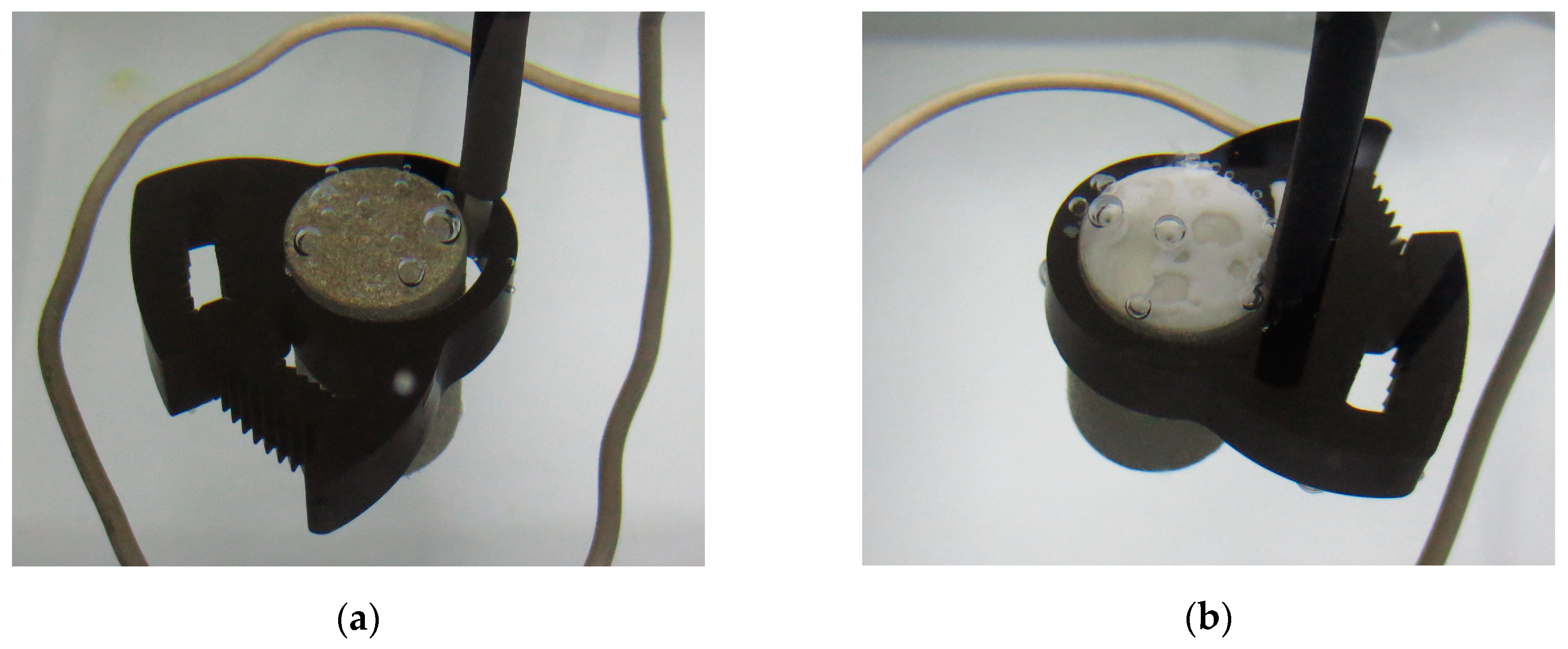
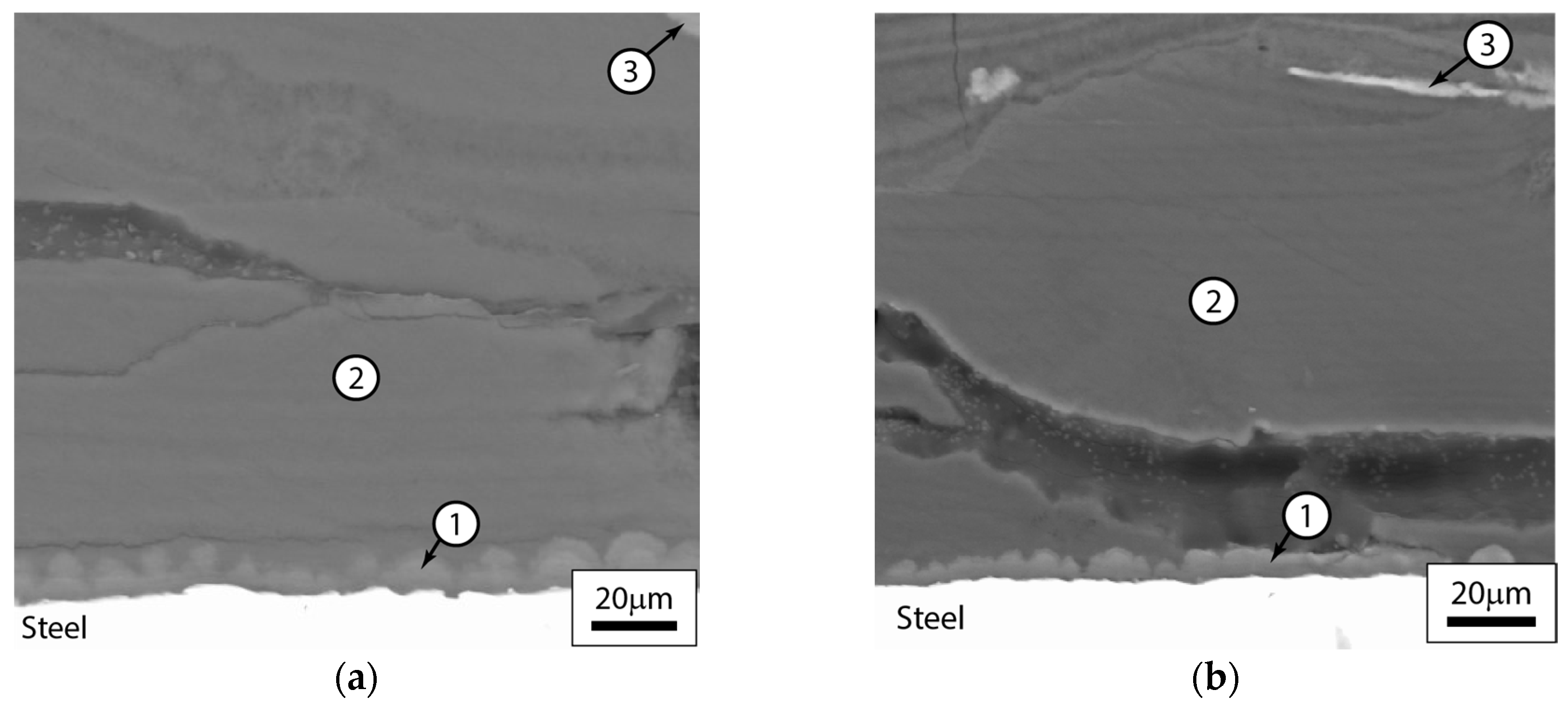

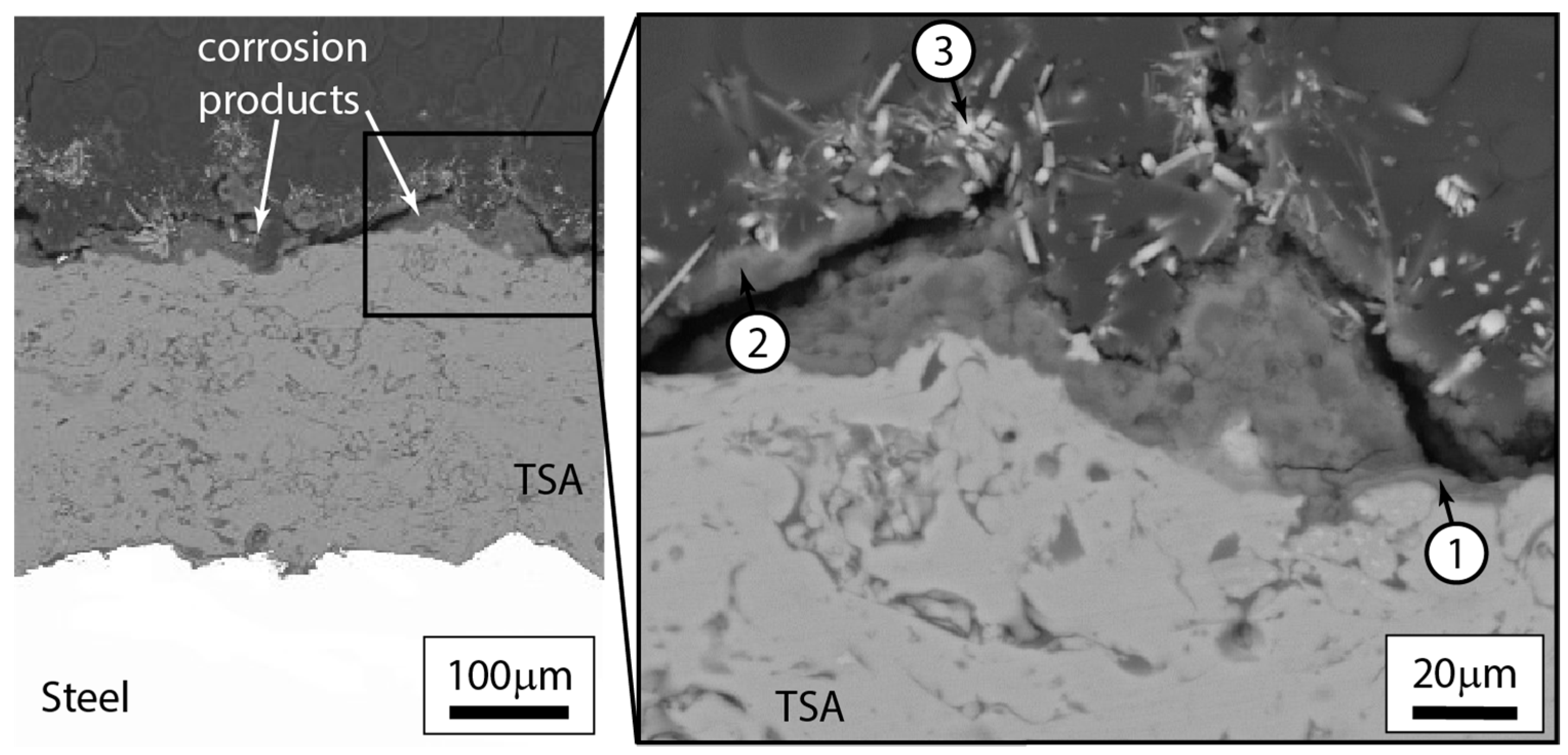
| Material | C | Mn | Si | S | P | Fe | N | V | Cu | Al | Cr | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| API 5L X80 | 0.03 | 1.76 | 0.21 | 0.004 | 0.016 | Balance | 0.006 | 0.025 | 0.01 | 0.029 | 0.16 | 0.18 |
| AISI 4137 | 0.34 | 0.80 | 0.22 | 0.010 | 0.018 | Balance | - | - | - | 0.026 | 1.0 | 0.20 |
| Al wire (coating consumable) | - | <0.01 | 0.07 | - | - | 0.21 | 0.01 | - | <0.01 | Balance | - | - |
| Wire Diameter (mm) | Wire Feed Rate (g/min) | Spray Distance (mm) | Increment Step (mm) | Traverse Speed (m/s) | Nominal Thickness (µm) |
|---|---|---|---|---|---|
| 2.3 | 98.7 | 95 | 15 | 0.5 | 200–300 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H3BO3 | SrCl2 | NaF |
|---|---|---|---|---|---|---|---|---|---|
| 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 | 0.101 | 0.025 | 0.025 | 0.003 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, S. Hydrogen in Aluminium-Coated Steels Exposed to Synthetic Seawater. Surfaces 2020, 3, 282-300. https://doi.org/10.3390/surfaces3030021
Paul S. Hydrogen in Aluminium-Coated Steels Exposed to Synthetic Seawater. Surfaces. 2020; 3(3):282-300. https://doi.org/10.3390/surfaces3030021
Chicago/Turabian StylePaul, Shiladitya. 2020. "Hydrogen in Aluminium-Coated Steels Exposed to Synthetic Seawater" Surfaces 3, no. 3: 282-300. https://doi.org/10.3390/surfaces3030021
APA StylePaul, S. (2020). Hydrogen in Aluminium-Coated Steels Exposed to Synthetic Seawater. Surfaces, 3(3), 282-300. https://doi.org/10.3390/surfaces3030021





