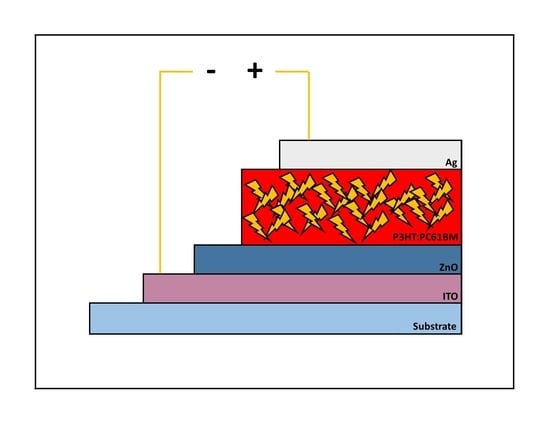
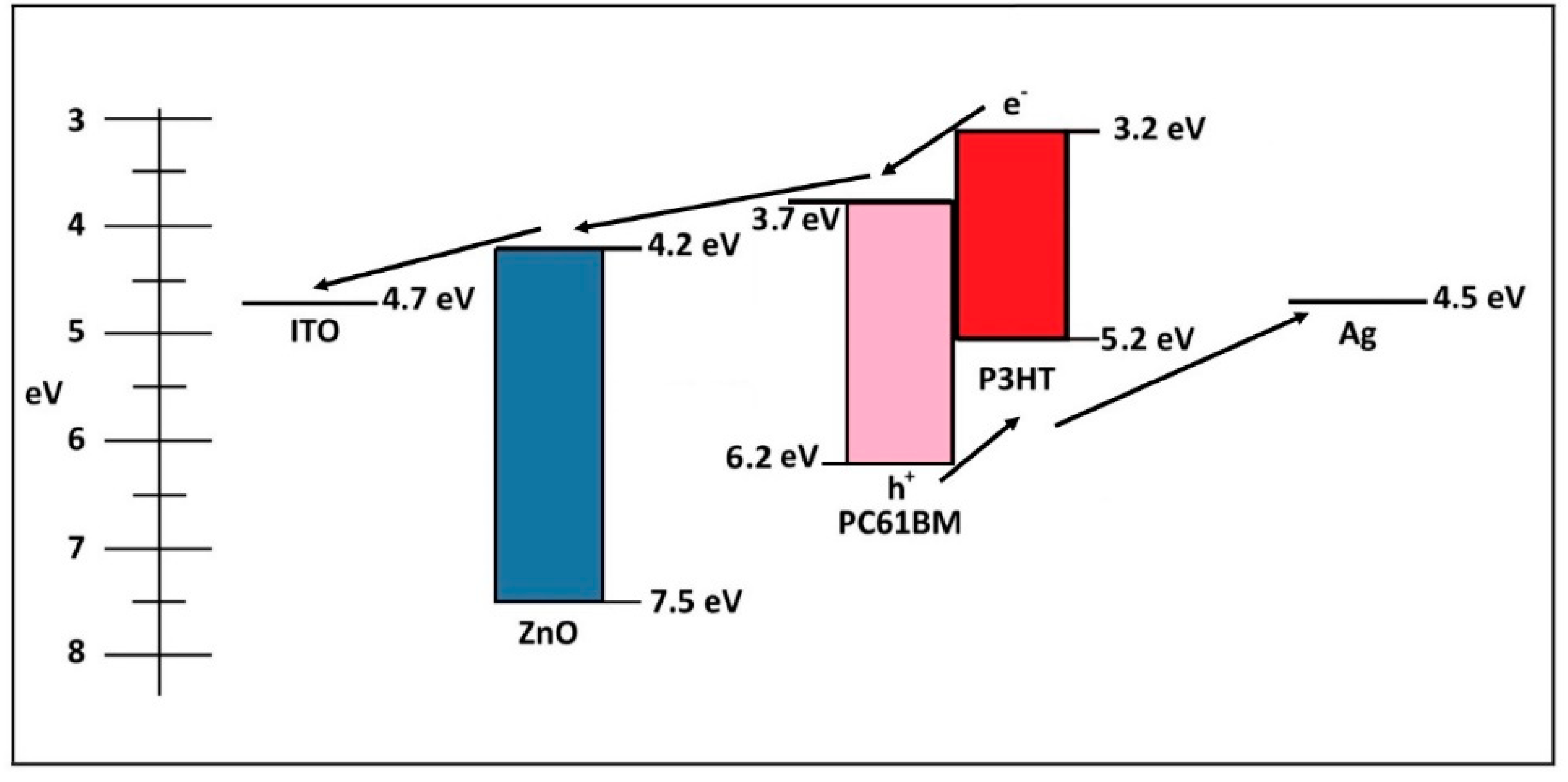
Stability of Non-Flexible vs. Flexible Inverted Bulk-Heterojunction Organic Solar Cells with ZnO as Electron Transport Layer Prepared by a Sol-Gel Spin Coating Method
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals & Materials
2.2. Synthesis
2.3. Analysis
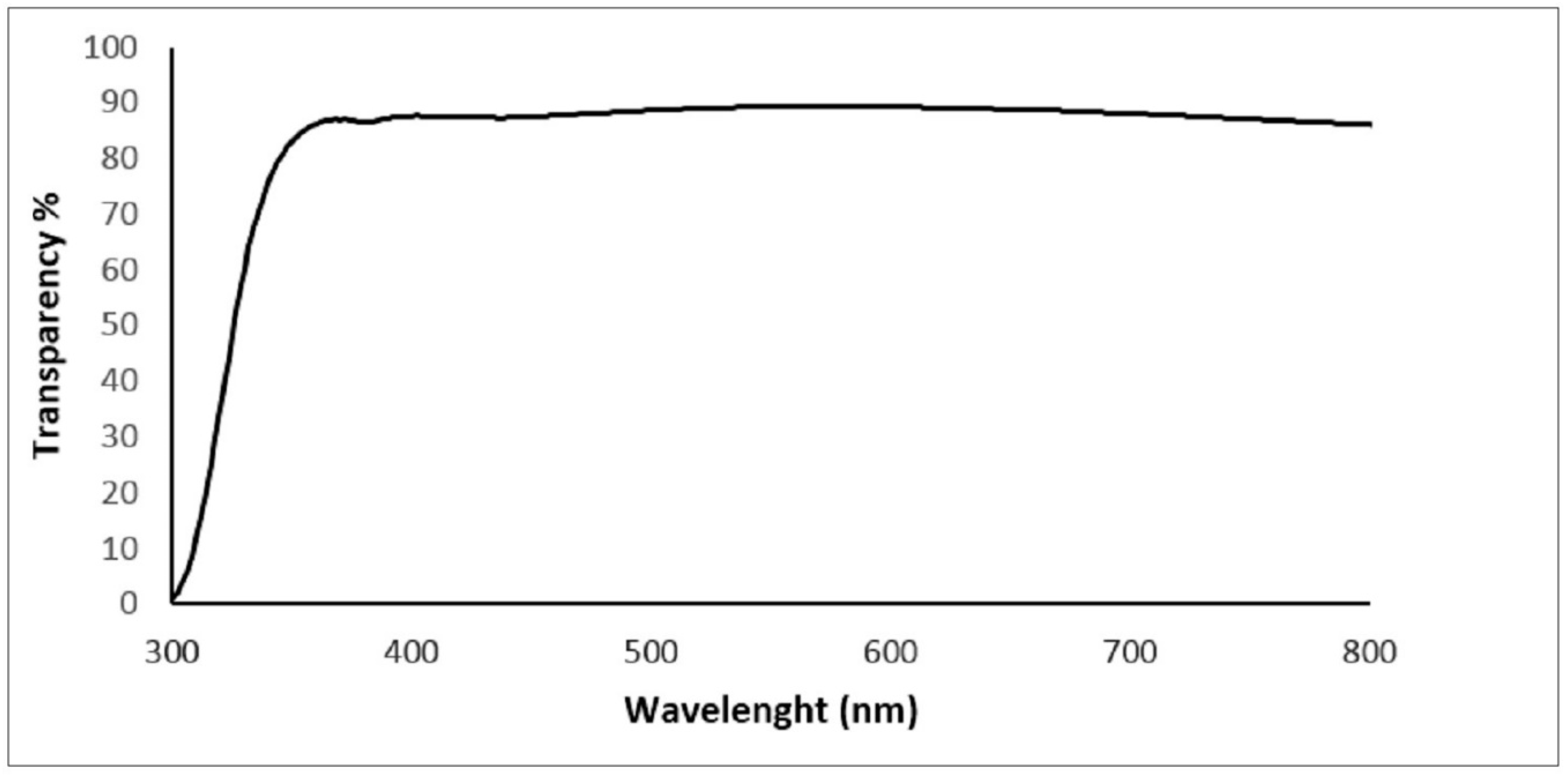
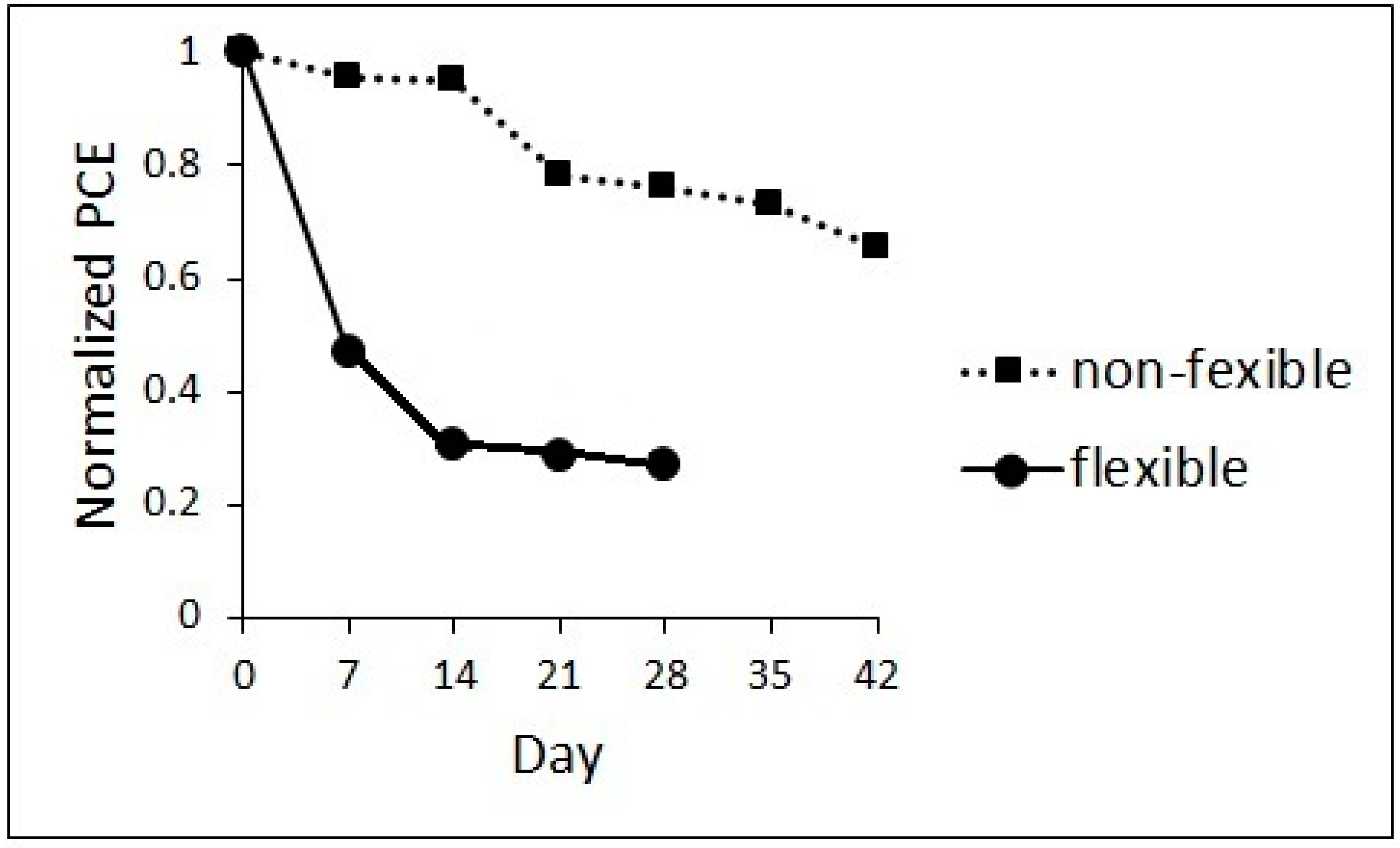
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, D.J.; Kim, H.K. Optimization of titanium-doped indium oxide anodes for heterojunction organic solar cells. Phys. Stat. Solidi A 2016, 214, 1600463. [Google Scholar] [CrossRef]
- Mutlu, A.; Can, M.; Tozlu, C. Performance improvement of organic solar cell via incorporation of donor type self- assembled interfacial monolayer. Thin Solid Films 2019, 685, 88–96. [Google Scholar] [CrossRef]
- Khanmohammadi, K.; Sohrabi, B.; Zamani, M.R. Effect of electron-donating and -withdrawing substitutions in naphthoquinone sensitizers: The structure engineering of dyes for DSSC. J. Mol. Struct. 2018, 1167, 274–279. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Vorontsov, A.S.; Forsh, P.A. Investigation of Photoelectron Properties of Polymer Films with Silicon Nanoparticles. Surfaces 2019, 2, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Fallah, M.; Maleki, I.; Zamani-Meymian, M.R.; Abdi, Y. Enhancing the efficiency of dye-sensitized solar cell by increasing the light trapping and decreasing the electron-hole recombination rate due to Ag@TiO2 core-shell photoanode structure. Mater. Res. Express 2019, 7, 016409. [Google Scholar] [CrossRef]
- Hussain, C.M. Handbook of Nanomaterials for Industrial Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ramki, K.; Venkatesh, N.; Sathiyan, G.; Thangamuthu, R.; Sakthivel, P. A comprehensive review on the reasons behind low power conversion efficiency of dibenzo derivatives based donors in bulk heterojunction organic solar cells. Org. Electron. 2019, 73, 182–204. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Ye, L.; Li, S.; Liu, X.; Zhang, S.; Ghasemi, M.; Xiong, Y.; Hou, J.; Ade, H. Quenching to the percolation Threshold in Organic Solar Cells. Joule 2019, 3, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.W.; Peng, K.K.; Lin, Y.W.; Su, Y.J.; Ma, K.J.; Hong, L.; Chang, C.C.; Hou, J.; Hsu, C.S. A chlorinated nonacyclic carbazole-based acceptor affords over 15% efficiency in organic solar cells. J. Mat. Chem. A 2020, 8, 1131–1137. [Google Scholar] [CrossRef]
- Jiang, K.; Wei, Q.; Lai, J.Y.L.; Peng, Z.; Kim, H.K.; Yuan, J.; Ye, L.; Ade, H.; Zou, Y.; Yan, H. Alkyl Chain Tuning of Small Molecule Acceptors for Efficient Organic Solar Cells. Joule 2019, 3, 3020–3033. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Souza, F.L.D.; Leite, E. Nanoenergy: Nanotechnology Applied for Energy Production, 2nd ed.; Springer International Publishing: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Rwenyagila, E.R. A Review of Organic Photovoltaic Energy Source and Its Technological Designs. Int. J. Photoenergy 2017, 1656512. [Google Scholar] [CrossRef]
- Ram, K.S.; Singh, J. Highly Efficient and Stable Solar Cells with Hybrid of Nanostructures and Bulk Heterojunction Organic Semiconductors. Adv. Theory Simul. 2019, 2, 1900030. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Shen, W.; Zhong, M.; Guo, S. Graphene Quantum Dots Band Structure Tuned by Size for Efficient Organic Solar Cells. Phys. Stat. Solidi A 2019, 216, 1900657. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Sulaiman, K.; Iwamoto, M. Fundamentals of bulk heterojunction organic solar cells: An overview of stability/degradation issues and strategies for improvement Renew. Sust. Energy Rev. 2018, 84, 43–53. [Google Scholar] [CrossRef]
- Shafket, R.; Doan, V.V.; Lee, H.K.; Lee, S.K.; Lee, J.C.; Moon, S.J.; So, W.W.; Song, C.E.; Shin, W.S. Enhanced photostability in polymer solar cells achieved with modified electron transport layer. Thin Solid Films 2019, 669, 42–48. [Google Scholar] [CrossRef]
- Gupta, S.K.; Banerjee, S.; Singh, A.; Pali, L.S.; Garg, A. Modeling of degradation in normal and inverted OSC devices. Sol. Energy Mat. Sol. Cells 2019, 191, 329–338. [Google Scholar] [CrossRef]
- Chander, N.; Singh, S.; Iyer, S.S.K. Stability and reliability of P3HT:PC61BM inverted organic solar cells. Sol. Energy Mat. Sol. Cells 2017, 161, 407–415. [Google Scholar] [CrossRef]
- Aslan, F.; Adam, G.; Stadler, P.; Goktas, A.; Mutlu, I.H.; Sariciftci, N.S. Sol–gel derived In2S3 buffer layers for inverted organic photovoltaic cells. Sol. Energy 2014, 108, 230–237. [Google Scholar] [CrossRef]
- Fallah, M.; Zamani-Meymian, M.R.; Rabbani, M. Influence of two gradual steps of vacuum annealing on structural and opto-electronic characteristics of Nb-doped TiO2 transparent conducting oxide. Superlattice Microst. 2018, 123, 242–250. [Google Scholar] [CrossRef]
- Chu, A.K.; Tien, W.C.; Lai, S.W.; Tsai, H.L.; Bai, R.Y.; Lin, X.Z.; Chen, L.Y. High-resistivity sol-gel ITO thin film as an interfacial buffer layer for bulk heterojunction organic solar cells. Org. Electron. 2017, 46, 99–104. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Schaffer, C.J.; Song, L.; Körstgens, V.; Pröller, S.; Indari, E.D.; Wang, T.; Abdelsamie, A.; Bernstorff, S.; Müller-Buschbaum, P. In-Operando Morphology Investigation of Inverted Bulk Heterojunction Organic Solar Cells with GISAXS. J. Mater. Chem. A 2015, 3, 8324–8331. [Google Scholar] [CrossRef]
- Dupuis, A.; Tournebize, A.; Bussiere, P.O.; Rivaton, A.; Gardette, J.L. Morphology and photochemical stability of P3HT:PCBM active layers of organic solar cells. Eur. Phys. J. Appl. Phys. 2011, 56, 34104. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Guo, S.; Herzig, E.M.; Sarkar, K.; Schindler, M.; Magerl, D.; Philipp, M.; Perlich, J.; Müller-Buschbaum, P. Investigation of Morphological Degradation of P3HT:PCBM Bulk Heterojunction Films Exposed to Long-Term Host Solvent Vapor. J. Mater. Chem. A 2016, 4, 3743–3753. [Google Scholar] [CrossRef] [Green Version]
- Sahdan, M.Z.; Malek, M.F.; Alias, M.S.; Kamaruddin, S.A.; Norhidayah, C.A.; Sarip, N.; Nafarizal, N.; Rusop, M. Fabrication of inverted bulk heterojunction organic solar cells basedon conjugated P3HT:PCBM using various thicknesses of ZnO bufferlayer. Optik 2015, 126, 645–648. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Braden, E.V.; Bonke, S.A.; Bach, U.; Mìller-Buschbaum, P. Screen-Printing of ZnO Nanostructures from Sol–Gel Solutions for Their Application in Dye-Sensitized Solar Cells. Chem. Sus. Chem. 2015, 8, 2696–2704. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia-Belmonte, G. Recent Advances to Understand Morphology Stability of Organic Photovoltaics. Nano-Micro Lett. 2017, 9, 10–25. [Google Scholar] [CrossRef] [Green Version]
- You, H.; Zhang, J.; Zhang, C.; Lin, Z.; Chen, D.; Chang, J.; Zhang, J. Efficient flexible inverted small-bandgap organic solar cells with low-temperature zinc oxide interlayer. Jpn. J. Appl. Phys. 2016, 55, 122302. [Google Scholar] [CrossRef]
- Liu, F.; Chen, D.; Wang, C.; Luo, K.; Gu, W.; Briseno, A.L.; Hsu, J.W.P.; Russell, T.P. Molecular Weight Dependence of the Morphology in P3HT: PCBM Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 19876–19887. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.P.; Ulum, S.; Sista, P.; Burke, K.B.; Wilson, M.G.; Stefan, M.C.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. The effect of polymer molecular weight on P3HT: PCBM nanoparticulate organic photovoltaic device performance. Sol. Energy Mater Sol. 2014, 128, 369–377. [Google Scholar] [CrossRef]
- Spoltore, D.; Vangerven, T.; Verstappen, P.; Piersimoni, F.; Bertho, S.; Vandewal, K.; Brande, N.V.D.; Mele, B.V.; Sio, A.D.; Parisi, J.; et al. Effect of molecular weight on morphology and photovoltaic 4 properties in P3HT:PCBM solar cells. Org. Electron. 2015, 21, 160–170. [Google Scholar] [CrossRef]
- Fallah, M.; Zamani-Meymian, M.-R.; Rahimi, R.; Rabbani, M. Effect of annealing treatment on electrical and optical properties of Nb doped TiO2thin films as a TCO prepared by sol–gel spin coating method. Appl. Surf. Sci. 2014, 316, 456–462. [Google Scholar] [CrossRef]
- Khorami, A.; Joodaki, M. Extracting voltage-dependent series resistance of single diode model for organic solar cells. SN Appl. Sci. 2019, 1, 619. [Google Scholar] [CrossRef] [Green Version]
- Zellmeier, M.; Kuhnapfel, S.; Rech, B.; Nickel, N.H.; Rappich, J. Enhanced stability of P3HT/poly-crystalline Si thin film hybrid solar cells. Phys. Stat. Solidi A 2016, 213, 1904–1908. [Google Scholar] [CrossRef]
- Yang, R.Y.; Chen, H.Y.; Lai, F.D. Performance Degradation of Dye-Sensitized Solar Cells Induced by Electrolytes. Adv. Mat. Sci. Eng. 2012, 902146. [Google Scholar] [CrossRef] [Green Version]
- Schrecongost, D.; Aziziha, M.; Zhang, H.T.; Tung, I.C.; Tessmer, J.; Dai, W.; Wang, Q.; Engel-Herbert, R.; Wen, H.; Picard, Y.N. On-Demand Nanoscale Manipulations of Correlated Oxide Phases. Adv. Funct. Mater. 2019, 26, 1905585. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.Q. Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Ríos-Ramirez, B.; Nair, P.K. On the Stability of Operation of Antimony Sulfide Selenide Thin Film Solar Cells Under Solar Radiation. Phys. Stat. Solidi A 2018, 215, 1800479. [Google Scholar] [CrossRef]
- Polo, A.; Lhermitte, C.R.; Dozzi, M.V.; Selli, E.; Sivula, K. Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces 2020, 3, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Koide, N.; Islam, A.; Chiba, Y.; Han, L. Improvement of efficiency of dye-sensitized solar cells based on analysis of equivalent circuit. J. Photochem. Photobiol. A Chem. 2006, 182, 296–305. [Google Scholar] [CrossRef]
- Girardi, L.; Bardini, L.; Michieli, N.; Kalinic, B.; Maurizio, C.; Rizzi, G.A.; Mattei, G. Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics. Surfaces 2019, 2, 41–53. [Google Scholar] [CrossRef] [Green Version]
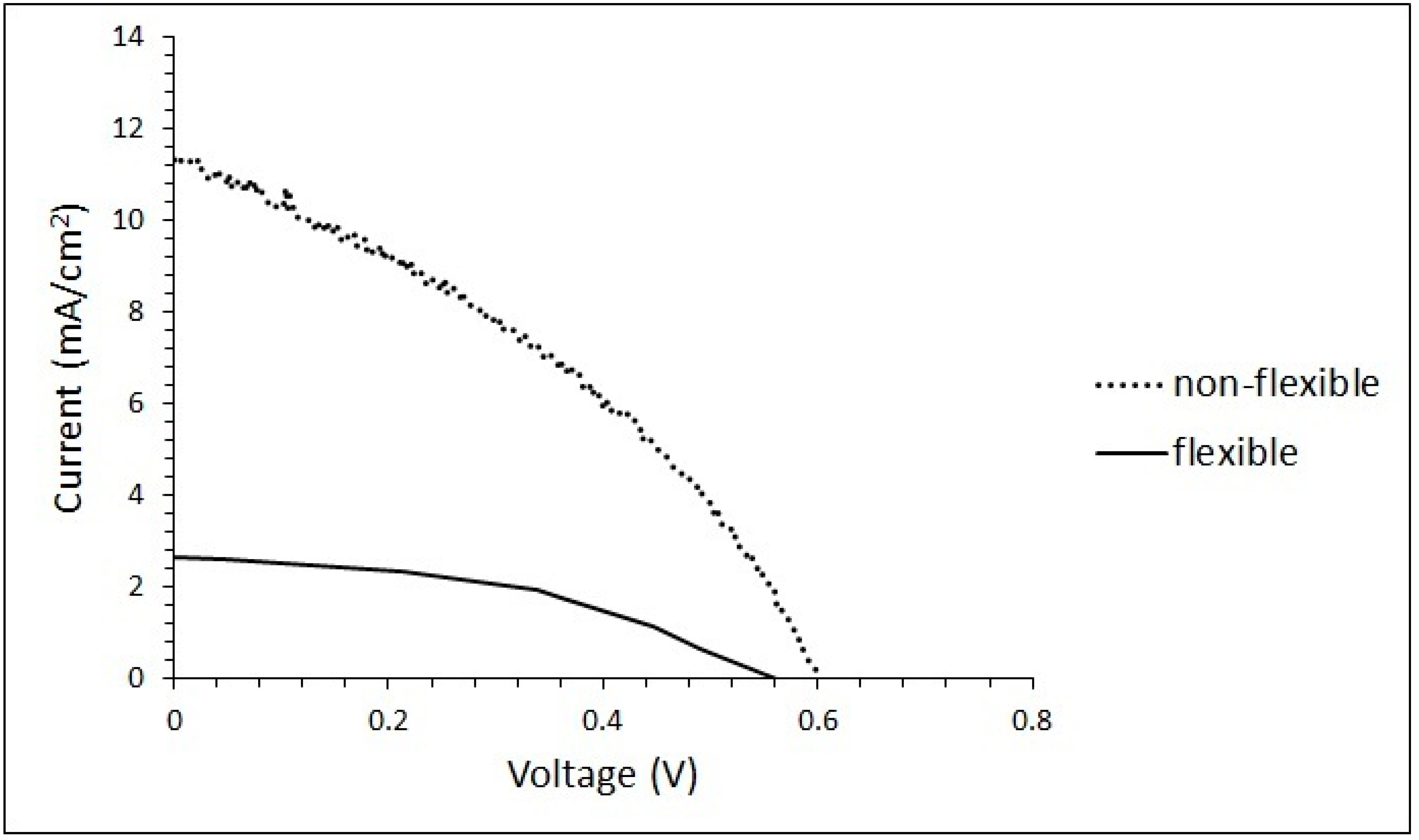
| Day | Jsc (mA.cm−2) | Voc (V) | FF (%) | PCE (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Glass | PET | Glass | PET | Glass | PET | Glass | PET | |
| 0 | 11.35 ± 0.56 | 2.65 ± 0.75 | 0.60 ± 0.01 | 0.56 ± 0.01 | 37 ± 0.016 | 45 ± 0.019 | 2.52 ± 0.17 | 0.67 ± 0.20 |
| 7 | 10.44 ± 0.53 | 1.68 ± 0.32 | 0.60 ± 0.01 | 0.53 ± 0.02 | 37 ± 0.023 | 33 ± 0.025 | 2.32 ± 0.16 | 0.30 ± 0.07 |
| 14 | 10.25 ± 0.48 | 1.37 ± 0.43 | 0.58 ± 0.02 | 0.53 ± 0.01 | 37 ± 0.013 | 33 ± 0.013 | 2.20 ± 0.18 | 0.24 ± 0.08 |
| 21 | 8.23 ± 0.43 | 0.96 ± 0.14 | 0.57 ± 0.01 | 0.53 ± 0.01 | 37 ± 0.019 | 33 ± 0.027 | 1.74 ± 0.13 | 0.17 ± 0.3 |
| 28 | 7.63 ± 0.40 | 0.75 ± 0.28 | 0.57 ± 0.02 | 0.52 ± 0.01 | 37 ± 0.020 | 33 ± 0.016 | 1.61 ± 0.25 | 0.13 ± 0.05 |
| 35 | 7.32 ± 0.43 | 0.56 ± 0.01 | 35 ± 0.026 | 1.43 ± 0.13 | ||||
| 42 | 6.95 ± 0.39 | 0.52 ± 0.01 | 36 ± 0.017 | 1.30 ± 0.1 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani-Meymian, M.-R.; Sheikholeslami, S.; Fallah, M. Stability of Non-Flexible vs. Flexible Inverted Bulk-Heterojunction Organic Solar Cells with ZnO as Electron Transport Layer Prepared by a Sol-Gel Spin Coating Method. Surfaces 2020, 3, 319-327. https://doi.org/10.3390/surfaces3030023
Zamani-Meymian M-R, Sheikholeslami S, Fallah M. Stability of Non-Flexible vs. Flexible Inverted Bulk-Heterojunction Organic Solar Cells with ZnO as Electron Transport Layer Prepared by a Sol-Gel Spin Coating Method. Surfaces. 2020; 3(3):319-327. https://doi.org/10.3390/surfaces3030023
Chicago/Turabian StyleZamani-Meymian, Mohammad-Reza, Saeb Sheikholeslami, and Milad Fallah. 2020. "Stability of Non-Flexible vs. Flexible Inverted Bulk-Heterojunction Organic Solar Cells with ZnO as Electron Transport Layer Prepared by a Sol-Gel Spin Coating Method" Surfaces 3, no. 3: 319-327. https://doi.org/10.3390/surfaces3030023
APA StyleZamani-Meymian, M.-R., Sheikholeslami, S., & Fallah, M. (2020). Stability of Non-Flexible vs. Flexible Inverted Bulk-Heterojunction Organic Solar Cells with ZnO as Electron Transport Layer Prepared by a Sol-Gel Spin Coating Method. Surfaces, 3(3), 319-327. https://doi.org/10.3390/surfaces3030023