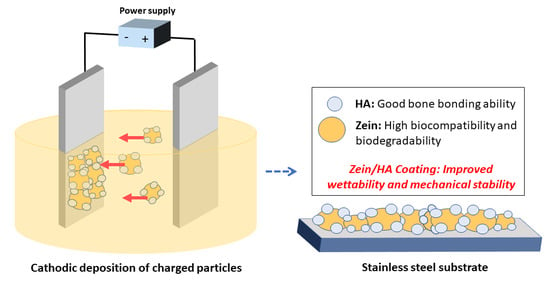
Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Suspension
2.3. EPD Coating
2.4. Optimization of EPD Parameters and Designing the Array
2.5. Characterization of the Coatings
3. Results and Discussion
3.1. Suspension Stability
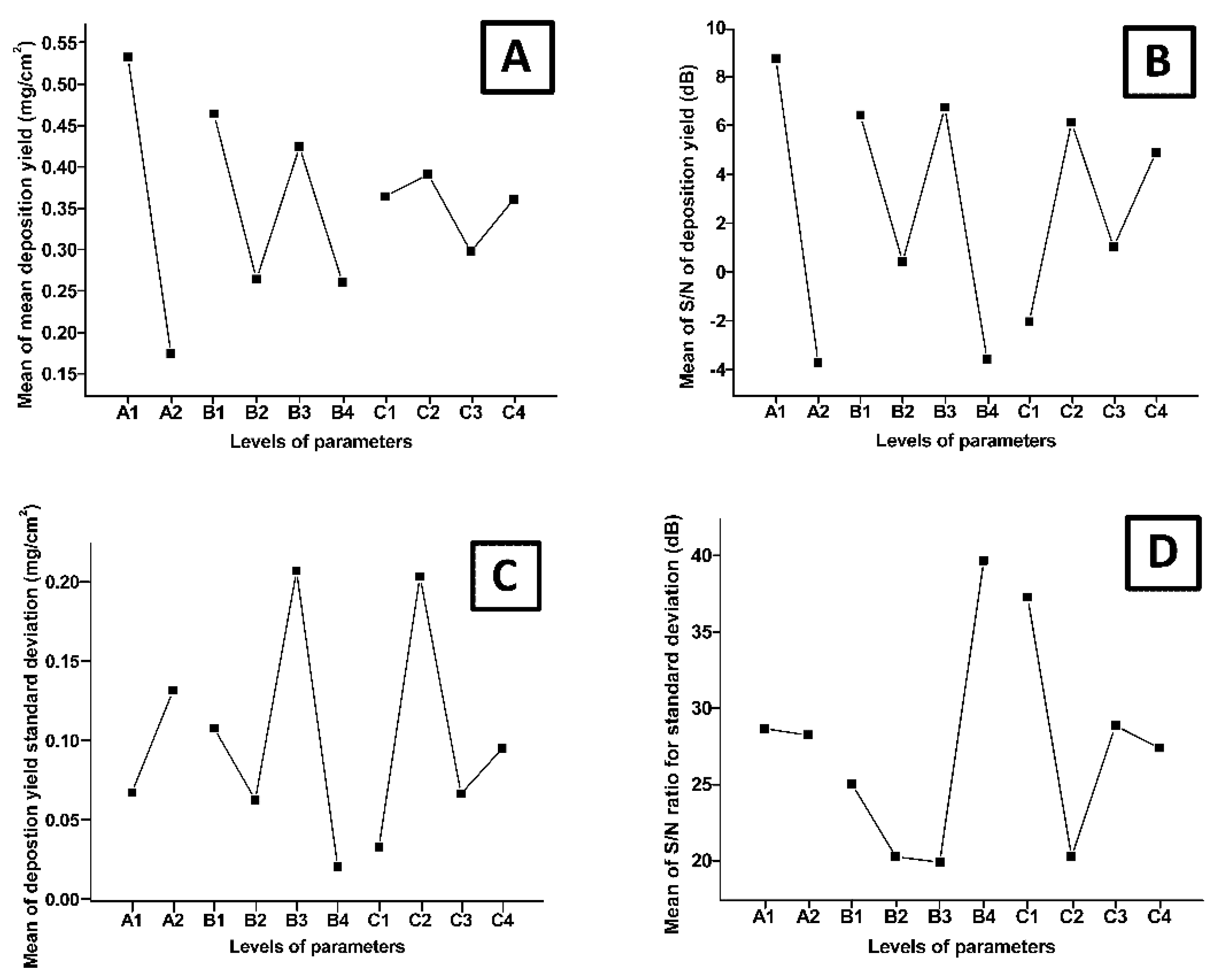
3.2. Design of Experiments Study
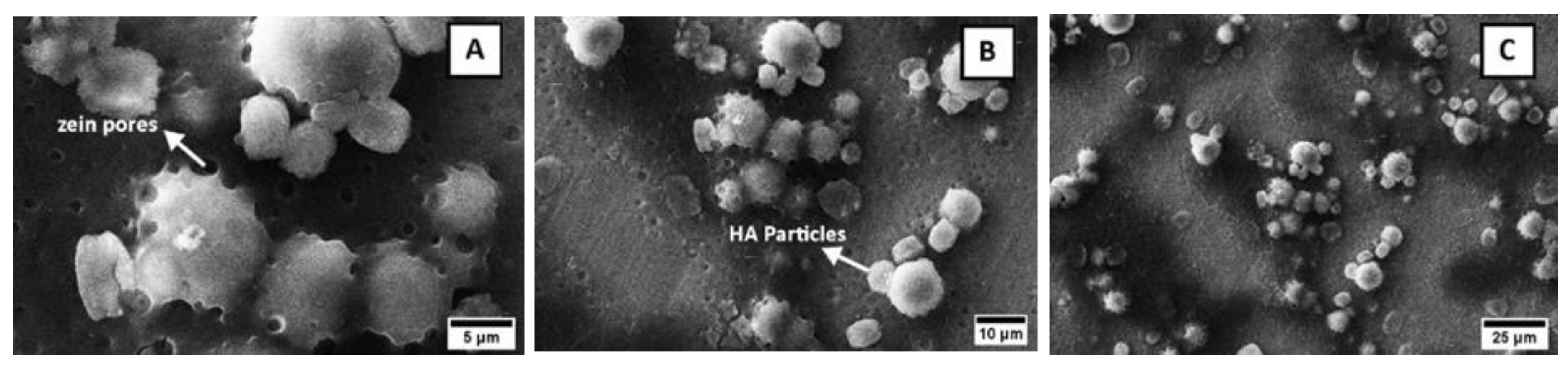
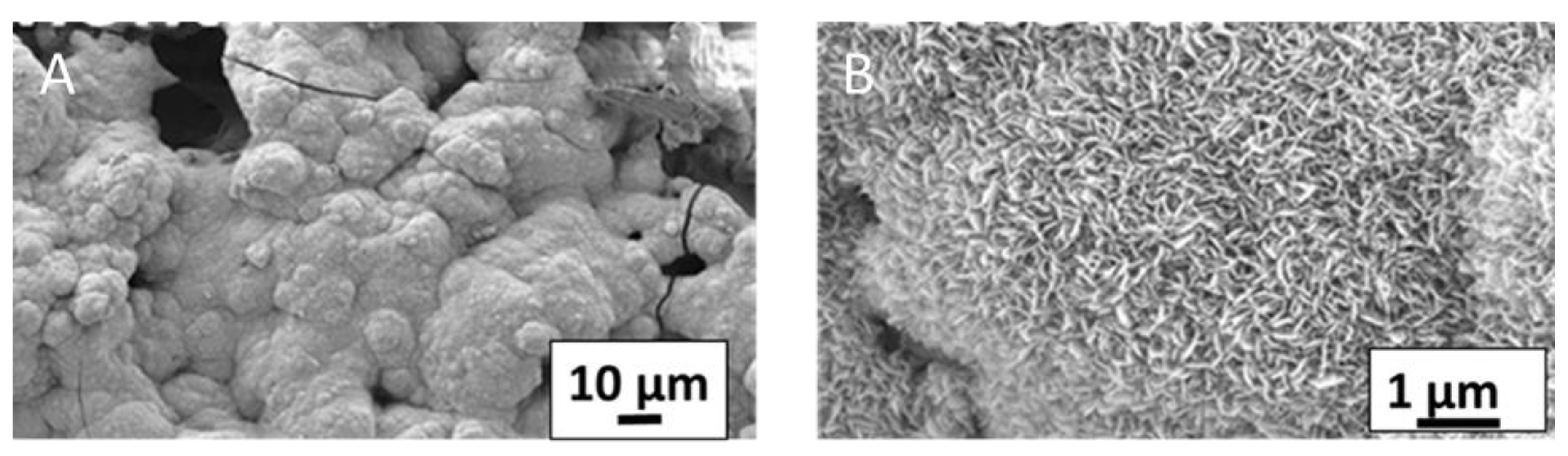
3.3. Morphological Analysis
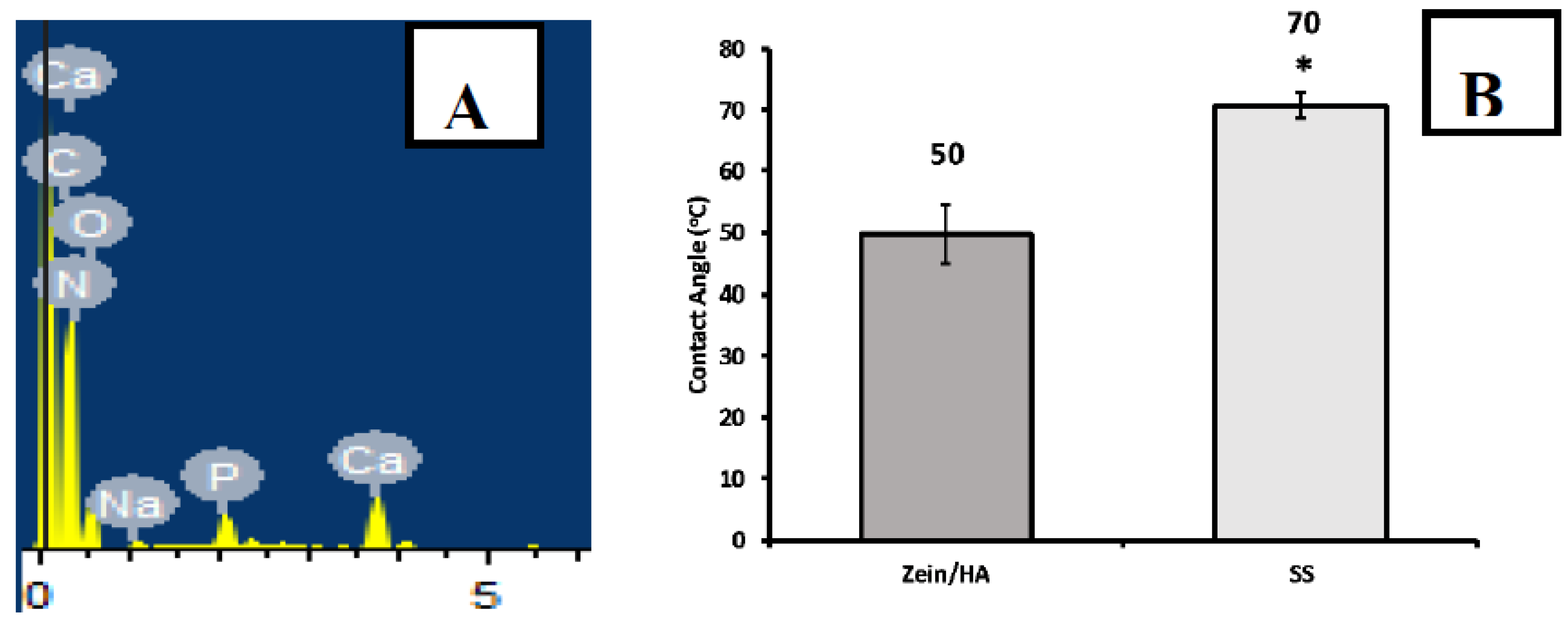
3.4. Composition Analysis (Qualitative)
3.5. Wettability Studies
3.6. Roughness Measurements
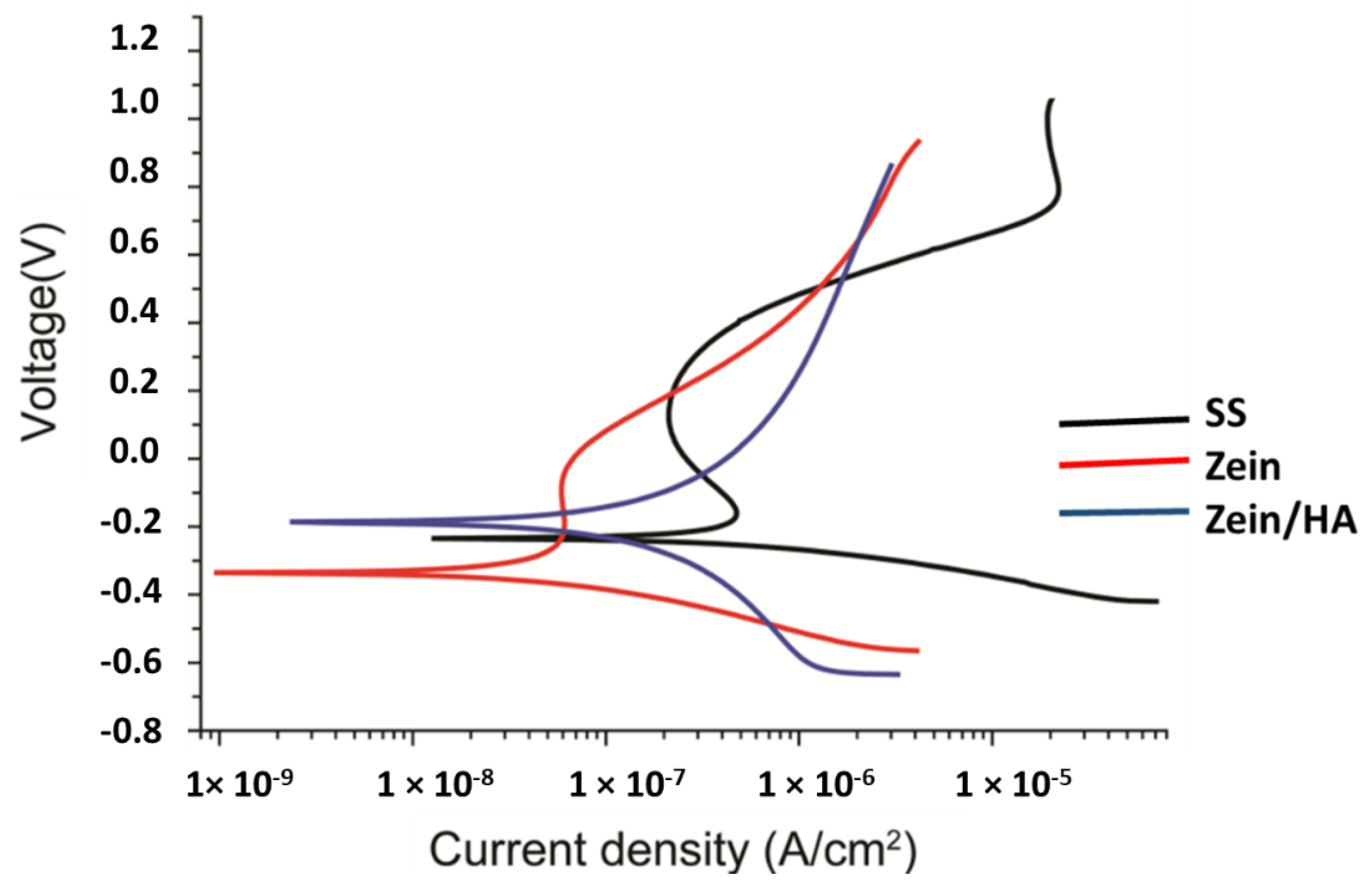
3.7. Corrosion Studies
3.8. Adhesion Strength (Scratch Test)
3.9. In-Vitro Bioactivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prateeksha Kaul, P.J. Bioimplants Market by Type (Cardiovascular Bioimplants, Dental Bioimplants, Orthopedic Bioimplants, Spinal Bioimplants, and Ophthalmology Bioimplants), Material (Metallic Biomaterials, Ceramic Biomaterials, Polymers Biomaterials, and Natural Biomaterials)—Global Opportunity Analysis and Industry Forecast, 2017–2023. Available online: https://www.alliedmarketresearch.com/bioimplants-market (accessed on 1 May 2020).
- Ralls, A.; Kumar, P.; Misra, M.; Menezes, P.L. Material Design and Surface Engineering for Bio-implants. JOM 2020, 72, 684–696. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating techniques for functional enhancement of metal implants for bone replacement: A review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Lee, J.-M.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Choi, J.-H.; Lee, B.-K.; Kim, J.-W.; Kim, H.-E.; et al. Enhanced bioactivity and biocompatibility of nanostructured hydroxyapatite coating by hydrothermal annealing. Thin Solid Films. 2011, 519, 8085–8090. [Google Scholar] [CrossRef]
- Meyer, N.; Rivera, L.R.; Ellis, T.; Qi, J.; Ryan, M.P.; Boccaccini, A.R. Bioactive and antibacterial coatings based on zein/bioactive glass composites by electrophoretic deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. I. Morphology and thermal properties. J. Appl. Polym. Sci. 2010, 118, 778–789. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Influence of deposition conditions on the composition, texture and microstructure of RF-magnetron sputter-deposited hydroxyapatite thin films. Thin Solid Films 2015, 591, 368–374. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Chaikina, M.V.; Zaikovskiy, V.I.; Pichugin, V.F.; Buck, V.; Prymak, O.; Epple, M.; Surmenev, R.A. The structure of an RF-magnetron sputter-deposited silicate-containing hydroxyapatite-based coating investigated by high-resolution techniques. Surf. Coat. Technol. 2013, 218, 39–46. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Mukhametkaliyev, T.M.; Khakbaz, H.; Surmenev, R.A.; Bobby Kannan, M. Ultrathin film coating of hydroxyapatite (HA) on a magnesium–calcium alloy using RF magnetron sputtering for bioimplant applications. Mater. Lett. 2015, 152, 280–282. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, G.-S.; Lee, Y.-T.; Huang, T.-C.; Chang, T.-W.; Chen, Y.-W.; Lee, B.-S.; Tung, K.-L. Microstructures and cell reaction of porous hydroxyapatite coatings on titanium discs using a novel vapour-induced pore-forming atmospheric plasma spraying. Surf. Coat. Technol. 2020, 393, 125837. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, biological, and antibacterial characteristics of plasma-sprayed (Sr, Zn) substituted Hydroxyapatite coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366. [Google Scholar] [CrossRef]
- Xu, L.; Shi, X.; Qian, Q.; Bai, X.; Xu, L.; Wang, Q. Hydrothermal sterilization in silver nitrate solution endows plasma sprayed hydroxyapatite coating with antibacterial property. Mater. Lett. 2020, 263, 127258. [Google Scholar] [CrossRef]
- Melero, H.C.; Sakai, R.T.; Vignatti, C.A.; Benedetti, A.V.; Fernández, J.; Guilemany, J.M.; Suegama, P.H. Corrosion resistance evaluation of HVOF produced hydroxyapatite and TiO2-hydroxyapatite coatings in Hanks’ solution. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Vilardell, A.; Cinca, N.; Garcia-Giralt, N.; Dosta, S.; Cano, I.; Nogués, X.; Guilemany, J. In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater. Sci. Eng. C 2020, 107, 110306. [Google Scholar] [CrossRef]
- Choi, G.; Choi, A.H.; Evans, L.A.; Akyol, S.; Ben-Nissan, B. A review: Recent advances in sol-gel-derived hydroxyapatite nanocoatings for clinical applications. J. Am. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Pawlik, A.; Rehman, M.A.U.; Nawaz, Q.; Bastan, F.E.; Sulka, G.D.; Boccaccini, A.R. Fabrication and characterization of electrophoretically deposited chitosan-hydroxyapatite composite coatings on anodic titanium dioxide layers. Electrochim. Acta 2019, 307, 465–473. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef]
- Petrovicova, E.; Schadler, L. Thermal spraying of polymers. Int. Mater. Rev. 2002, 47, 169–190. [Google Scholar] [CrossRef]
- Das, A.; Shukla, M. New generation hopeite coating on Ti6Al4V (TC4) by radio frequency magnetron sputtering for prosthetic-orthopaedic implant applications: Synthesis and characterisation. Trans. IMF 2020, 98, 88–96. [Google Scholar] [CrossRef]
- Liao, S.-C.; Chen, C.-Y.; Hsu, Y.-H.; Li, C.-T.; Hsieh, C.-C.; Tsai, M.-S.; Chan, M.-Y.; Lee, C.-H.; Wang, S.-H.; Ng, S.-K.; et al. In vitro and in vivo biocompatibility study of surface modified TiN deposited on Ti6Al4V using high-power impulse magnetron sputtering technique. Surf. Coat. Technol. 2020, 394, 125814. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Boccaccini, A.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Boccaccini, A.R. Electrophoretic deposition of chitosan/gelatin/bioactive glass composite coatings on 316L stainless steel: A design of experiment study. Surf. Coat. Technol. 2019, 358, 976–986. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic deposition of PEEK/bioactive glass composite coatings for orthopedic implants: A design of experiments (DoE) study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef]
- Heise, S.; Höhlinger, M.; Hernández, Y.T.; Palacio, J.J.P.; Rodriquez Ortiz, J.A.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition and characterization of chitosan/bioactive glass composite coatings on Mg alloy substrates. Electrochim. Acta 2017, 232, 456–464. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.; Boccaccini, A. Electrophoretic deposition of chitosan/45S5 Bioglass® composite coatings for orthopaedic applications. Surf. Coat. Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Murugan, V.; Mathews, P.K. Optimization of Heat Treatment Process Using Taguchi’s Parameter Design Approach. Int. J. Res. Mech. Eng. 2013, 1, 16–21. [Google Scholar]
- Ramos Rivera, L.; Dippel, J.; Boccaccini, A.R. Formation of Zein/Bioactive Glass Layers Using Electrophoretic Deposition Technique. ECS Trans. 2018, 82, 73–80. [Google Scholar] [CrossRef]
- Mariotti, C.E.; Ramos-Rivera, L.; Conti, B.; Boccaccini, A.R. Zein-Based Electrospun Fibers Containing Bioactive Glass with Antibacterial Capabilities. Macromol. Biosci. 2020, 2000059. [Google Scholar] [CrossRef]
- Qu, Z.-H.; Wang, H.-J.; Tang, T.-T.; Zhang, X.-L.; Wang, J.-Y.; Dai, K.-R. Evaluation of the zein/inorganics composite on biocompatibility and osteoblastic differentiation. Acta Biomater. 2008, 4, 1360–1368. [Google Scholar] [CrossRef]
- Lian, H.; Liu, X.; Meng, Z. Enhanced mechanical and osteogenic differentiation performance of hydroxyapatite/zein composite for bone tissue engineering. J. Mater. Sci. 2019, 54, 719–729. [Google Scholar] [CrossRef]
- Babaei, M.; Ghaee, A.; Nourmohammadi, J. Poly (sodium 4-styrene sulfonate)-modified hydroxyapatite nanoparticles in zein-based scaffold as a drug carrier for vancomycin. Mater. Sci. Eng. C 2019, 100, 874–885. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part A 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Kaya, S.; Boccaccini, A.R. Electrophoretic deposition of zein coatings. J. Coat. Technol. Res. 2017, 14, 683–689. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Heise, S.; Virtanen, S.; Boccaccini, A.R. Taguchi Design of Experiments approach to determine process parameter for the electrophoretic deposition of chitosan/bioactive glass on Mg alloy substrates. ECS Trans. 2018, 82, 81. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Q.; Wang, J.-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials 2004, 25, 4691–4697. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Jia, Y.; Han, H.; Sun, D. Preparation and Evaluation of Electrospun Zein/HA Fibers Based on Two Methods of Adding HA Nanoparticles. J. Bionic Eng. 2014, 11, 115–124. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yu, X.; He, J.; Xiao, Z. Molecular Mechanism of Nano-Hydroxyapatite Surface Changes from Hydrophilic to Hydrophobic. Asian J. Chem. 2014, 26, 5355. [Google Scholar] [CrossRef]
- Aldana, A.A.; Malatto, L.; Rehman, M.A.U.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano-and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, M.A.; Ferraris, S.; Goldmann, W.H.; Perero, S.; Bastan, F.E.; Nawaz, Q.; Confiengo, G.G.d.; Ferraris, M.; Boccaccini, A.R. Antibacterial and Bioactive Coatings Based on Radio Frequency Co-Sputtering of Silver Nanocluster-Silica Coatings on PEEK/Bioactive Glass Layers Obtained by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2017, 9, 32489–32497. [Google Scholar] [CrossRef]

| Symbol | Control Factor | Levels 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| A | Concentration of HA in the suspension (g/L) | 1.25 | 5 | ||
| B | Voltage (V) | 3 | 5 | 7 | 9 |
| C | Time (min) | 6 | 9 | 12 | 15 |
| Concentration | Time | Voltage |
|---|---|---|
| 1.25 | 3 | 6 |
| 1.25 | 5 | 9 |
| 1.25 | 7 | 12 |
| 1.25 | 9 | 15 |
| 5 | 3 | 15 |
| 5 | 5 | 12 |
| 5 | 7 | 9 |
| 5 | 9 | 6 |
| Control Factors | Deposition Yield | S/N Ratio | Standard Deviation | S/N Ratio | ||
|---|---|---|---|---|---|---|
| Concentration (g/L) | Time (min) | Voltage (V) | (mg/cm2) | (dB) | (dB) | |
| 1.25 | 3 | 6 | 0.23333 | 1.716821 | 0.158605 | 20.80852 |
| 1.25 | 5 | 9 | 0.093333 | −6.24198 | 0.041096 | 32.53885 |
| 1.25 | 7 | 12 | 0.346667 | 5.155527 | 0.322215 | 14.65196 |
| 1.25 | 9 | 15 | 0.033333 | −15.1851 | 0.009428 | 45.32639 |
| 5 | 3 | 15 | 0.7 | 11.25925 | 0.058878 | 29.41574 |
| 5 | 5 | 12 | 0.44 | 7.226339 | 0.08641 | 26.08359 |
| 5 | 7 | 9 | 0.50667 | 8.451732 | 0.094281 | 25.32639 |
| 5 | 9 | 6 | 0.49333 | 8.220095 | 0.033993 | 34.18695 |
| Factors | 1 | 2 | 3 | 4 | Δ (Maximum − Minimum) | |
|---|---|---|---|---|---|---|
| Conc. (g/L) | A | 0.535 | 0.176667 | 0.358333 | ||
| Time (min) | B | 0.466667 | 0.266667 | 0.426667 | 0.263333 | 0.203333 |
| Voltage (V) | C | 0.366667 | 0.393333 | 0.3 | 0.363333 | 0.093333 |
| Factors | 1 | 2 | 3 | 4 | Δ (Maximum − Minimum) | |
|---|---|---|---|---|---|---|
| Conc. (g/L) | A | 8.789353 | −3.63869 | 12.42805 | ||
| Time (min) | B | 6.488034 | 0.49218 | 6.80363 | −3.48252 | 6.31145 |
| Voltage (V) | C | −1.96295 | 6.190933 | 1.104877 | 4.968458 | 5.086057 |
| Factors | 1 | 2 | 3 | 4 | Δ (Maximum − Minimum) | |
|---|---|---|---|---|---|---|
| Conc. (g/L) | A | 0.068391 | 0.132836 | 0.064445 | ||
| Time (min) | B | 0.108742 | 0.063753 | 0.208248 | 0.021711 | 0.186537 |
| Voltage (V) | C | 0.034153 | 0.204312 | 0.067688 | 0.096299 | 0.170159 |
| Factors | 1 | 2 | 3 | 4 | Δ (Maximum − Minimum) | |
|---|---|---|---|---|---|---|
| Conc. (g/L) | A | 28.75317 | 28.33143 | 0.421739 | ||
| Time (min) | B | 25.11213 | 20.36778 | 19.98917 | 39.75667 | 19.7675 |
| Voltage (V) | C | 37.37106 | 20.36778 | 28.93262 | 27.49774 | 17.00329 |
| Control Factor | Concentration | Time | Voltage |
|---|---|---|---|
| p-value | 0.050 | 0.421 | 0.814 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Y.; Yasir, M.; Ur Rehman, M.A. Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces 2020, 3, 237-250. https://doi.org/10.3390/surfaces3020018
Ahmed Y, Yasir M, Ur Rehman MA. Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces. 2020; 3(2):237-250. https://doi.org/10.3390/surfaces3020018
Chicago/Turabian StyleAhmed, Yusra, Muhammad Yasir, and Muhammad Atiq Ur Rehman. 2020. "Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications" Surfaces 3, no. 2: 237-250. https://doi.org/10.3390/surfaces3020018
APA StyleAhmed, Y., Yasir, M., & Ur Rehman, M. A. (2020). Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces, 3(2), 237-250. https://doi.org/10.3390/surfaces3020018