Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
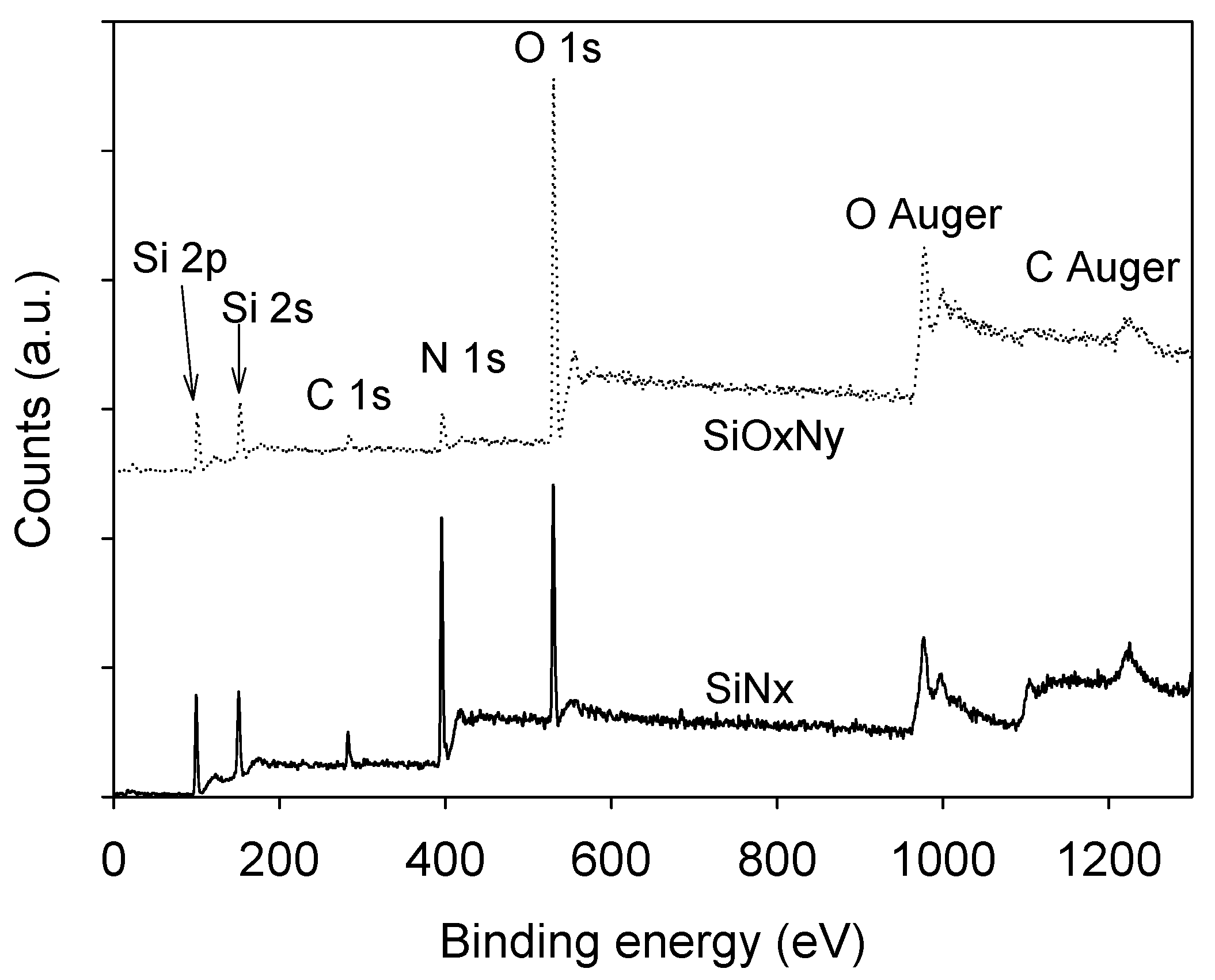
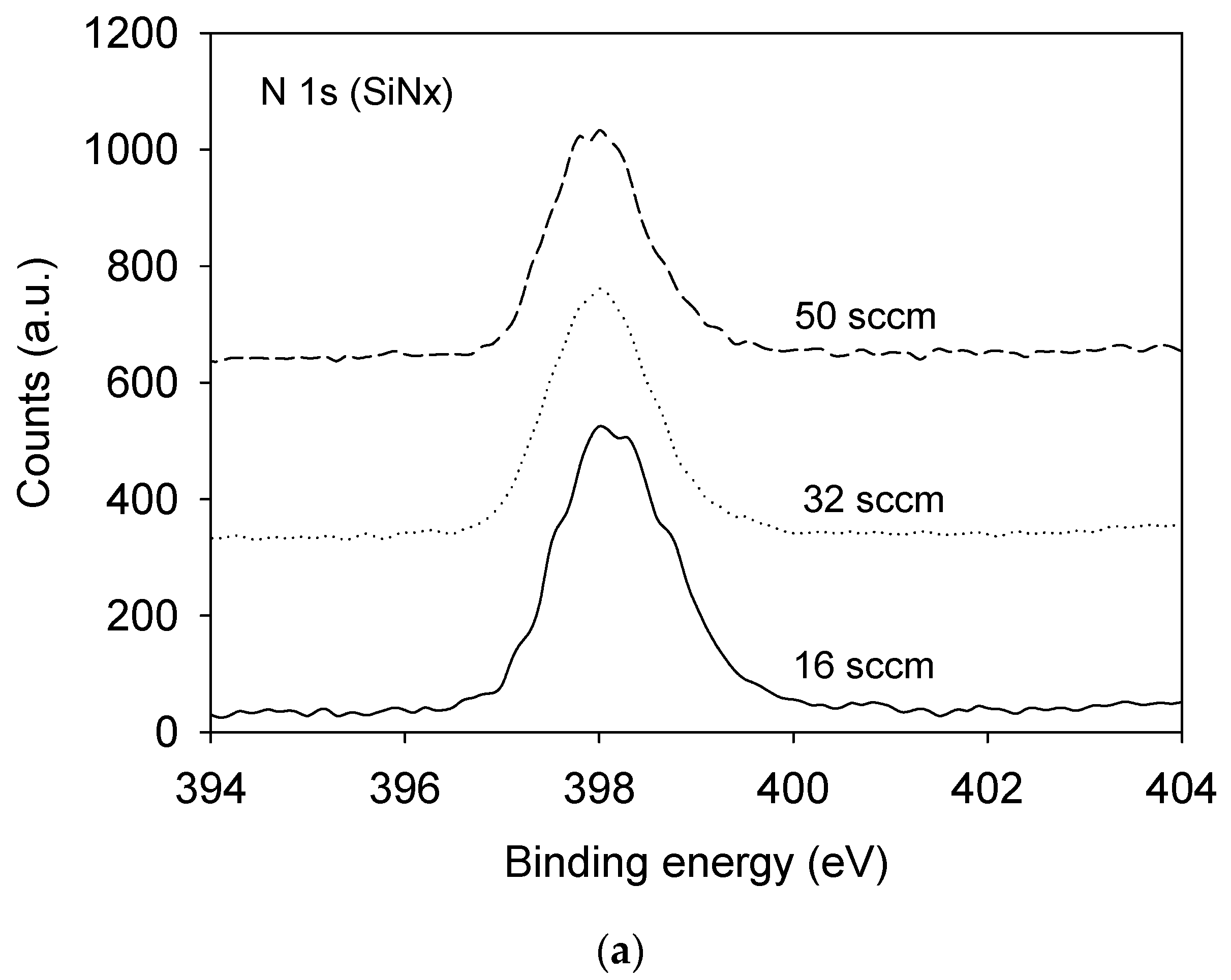
3.1. Film Composition and Bonding State Analysis
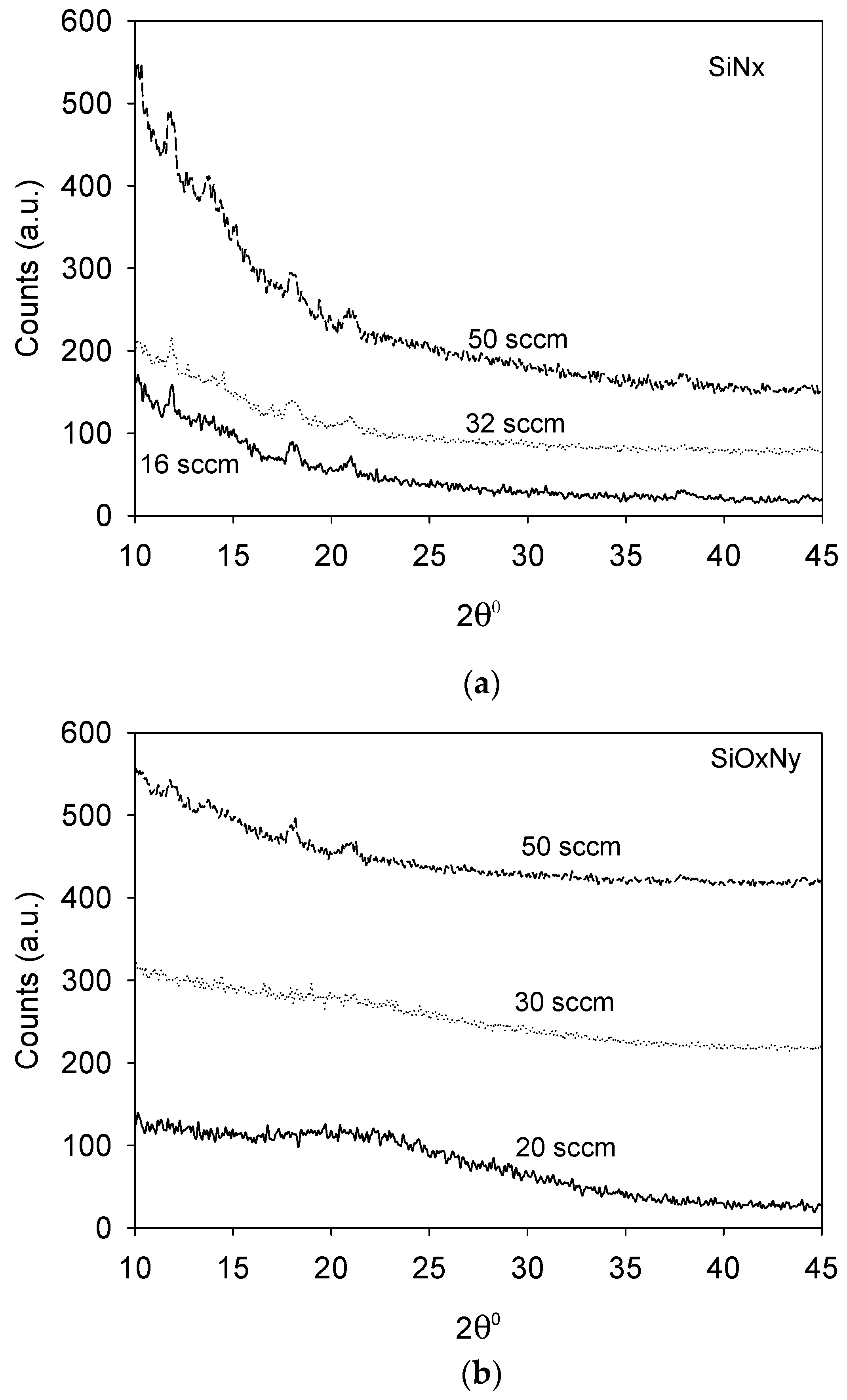
3.2. Crystallinity and Phase Identification
3.3. Young’s Modulus and Hardness
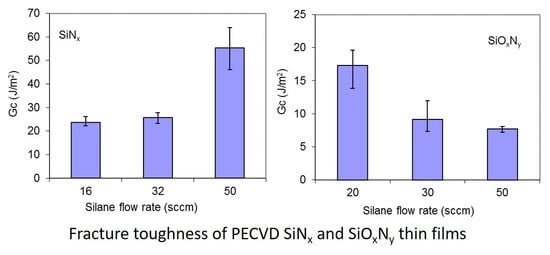
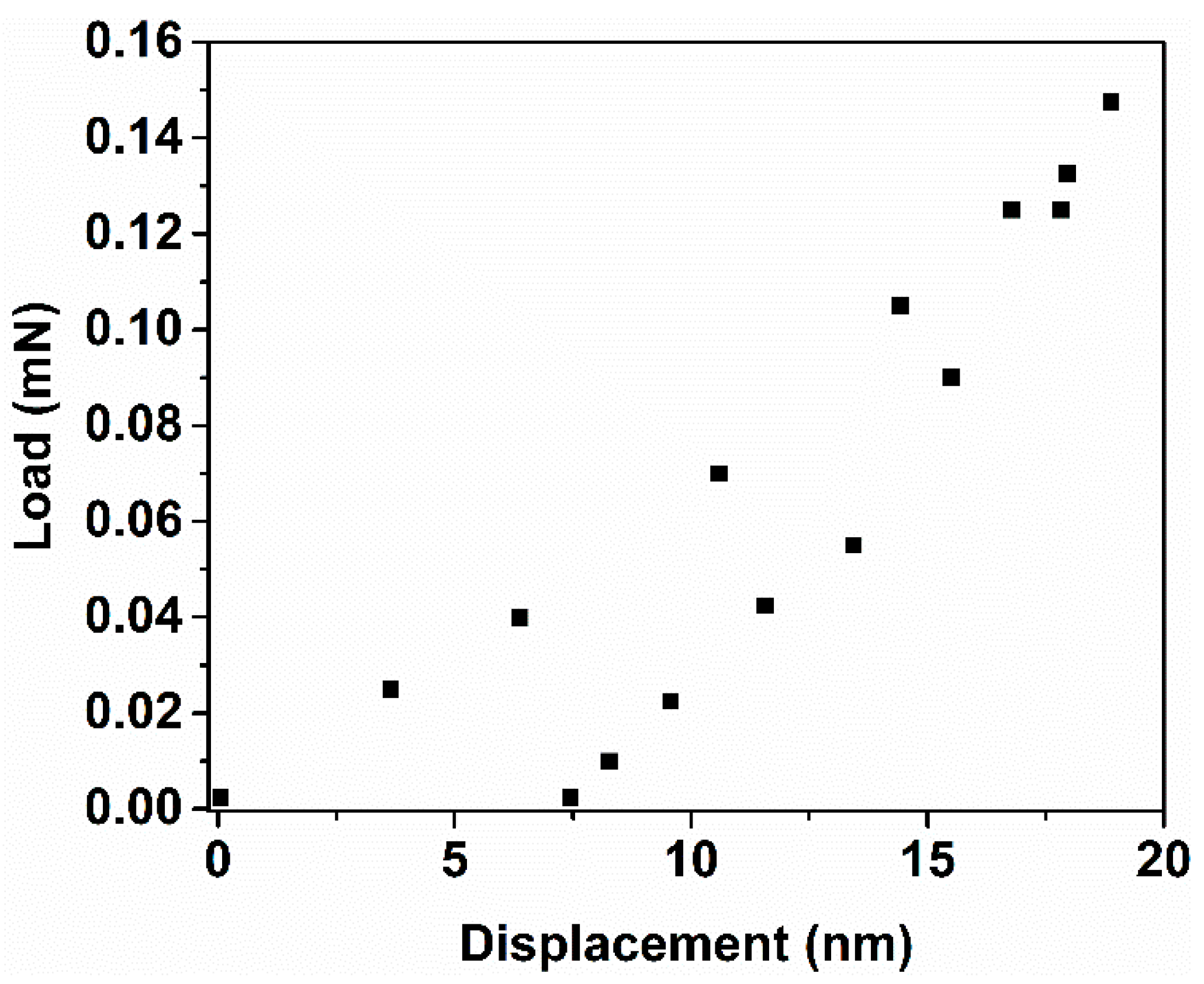
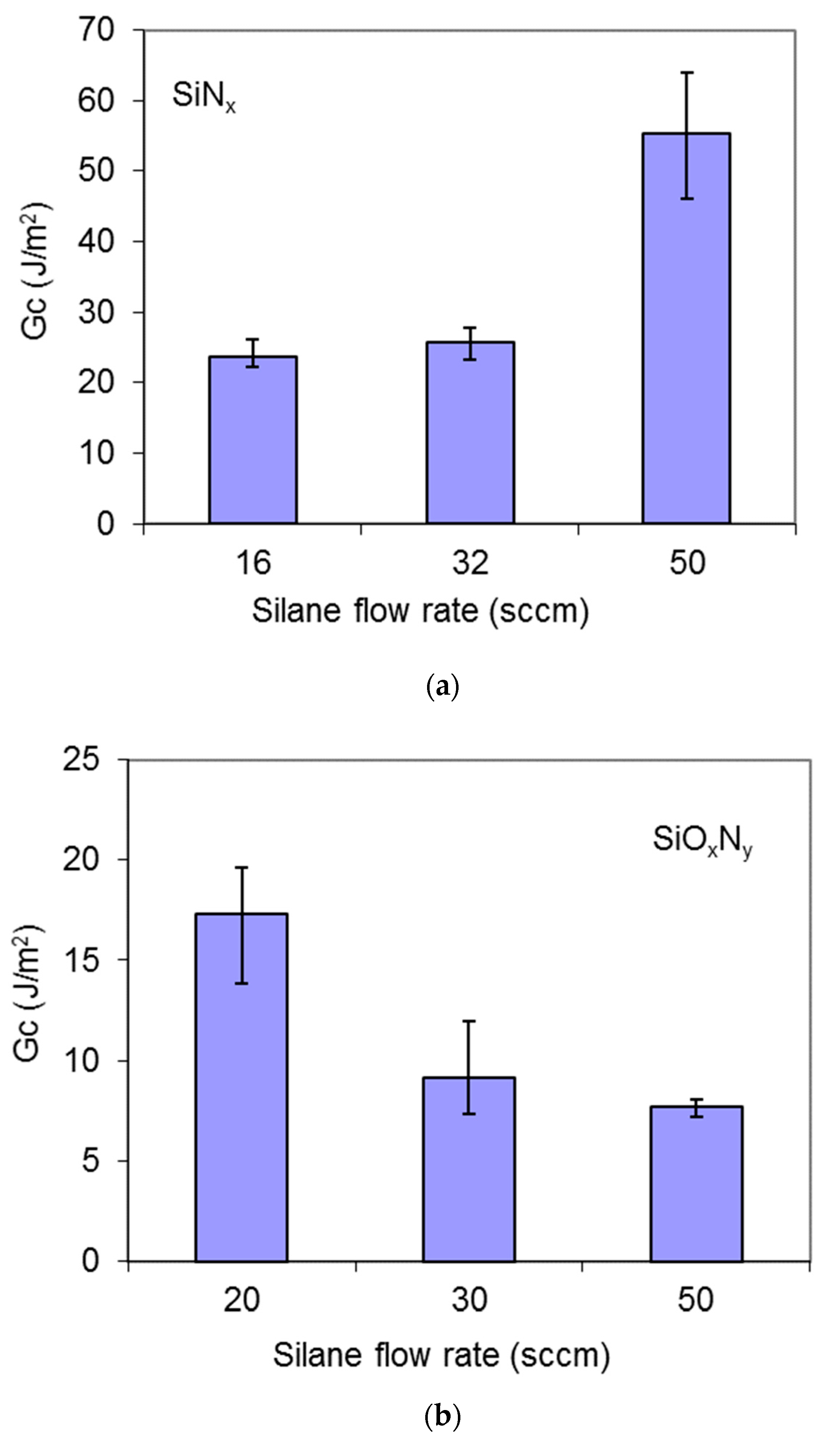
3.4. Fracture Toughness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jozwik, M.; Delobelle, P.; Gorecki, C.; Sabac, A.; Nieradko, L.; Meunier, C.; Munnik, F. Optomechanical characterization of compressively prestressed silicon oxynitride films deposited plasma-enhanced chemical vapour deposition on silicon membranes. Thin Solid Films 2004, 468, 84–92. [Google Scholar] [CrossRef]
- Prodanović, V.; Chan, H.W.; Graaf, H.V.D.; Sarro, P.M. Ultra-thin alumina and silicon nitride MEMS fabricated membranes for the electron multiplication. Nanotechnology 2018, 29, 155703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagolini, A.; Savoia, A.S.; Picciotto, A.; Boscardin, M.; Bellutti, P.; Lamberti, N.; Caliano, G. PECVD low stress silicon nitride analysis and optimization for the fabrication of CMUT devices. J. Micromech. Microeng. 2015, 25, 015012. [Google Scholar] [CrossRef]
- Li, D.-L.; Feng, X.-F.; Wen, Z.-Y.; Shang, Z.-G.; She, Y. Stress control of silicon nitride films deposited by plasma enhanced chemical vapor deposition. Optoelectron. Lett. 2016, 12, 285–289. [Google Scholar] [CrossRef]
- Dong, J.; Du, P.; Zhang, X. Characterization of the Young’s modulus and residual stresses for a sputtered silicon oxynitride film using micro-structures. Thin Solid Films 2013, 545, 414–418. [Google Scholar] [CrossRef]
- Danaie, K.; Bosseboeuf, A.; Clerc, C.; Gousset, C.; Julie, G. Fabrication of UV-transparent SixOyNz membranes with a low frequency PECVD reactor. Sens. Actuators A 2002, 99, 78–81. [Google Scholar] [CrossRef]
- Jo, M.C.; Park, S.K.; Park, S.J. A study on resistance of PECVD silicon nitride thin film to thermal stress-induced cracking. Appl. Surf. Sci. 1999, 140, 12–18. [Google Scholar] [CrossRef]
- Yau, B.S.; Huang, J.L. Effects of nitrogen flow on R.F. reactive magnetron sputtered silicon nitride films on high speed steel. Surf. Coat. Technol. 2004, 176, 290–295. [Google Scholar] [CrossRef]
- Lane, M.W.; Liniger, E.G.; Lloyd, J.R. Relationship between interfacial adhesion and electromigration in Cu metallization. J. Appl. Phys. 2003, 93, 1417–1421. [Google Scholar] [CrossRef]
- Dauskardt, R.H.; Lane, M.; Ma, Q.; Krishna, N. Adhesion and debonding of multi-layer thin film structures. Eng. Fract. Mech. 1998, 61, 141–162. [Google Scholar] [CrossRef]
- Skjöldebrand, C.; Schmidt, S.; Vuong, V.; Pettersson, M.; Grandfield, K.; Högberg, H.; Engqvist, H.; Persson, C. Influence of Substrate Heating and Nitrogen Flow on the Composition, Morphological and Mechanical Properties of SiNx Coatings Aimed for Joint Replacements. Materials 2017, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Obrosov, A.; Gulyaev, R.; Zak, A.; Ratzke, M.; Naveed, M.; Dudzinski, W.; Weiß, S. Chemical and Morphological Characterization of Magnetron Sputtered at Different Bias Voltages Cr-Al-C Coatings. Materials 2017, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Sajgalik, P.; Dusza, J.; Hoffmann, M.J. Relationshop between microstructure, toughening mechanisms, and fracture toughness of reinforced silicon nitride ceramics. J. Am. Ceram. Soc. 1995, 78, 2619–2624. [Google Scholar] [CrossRef]
- Becher, P.F.; Sun, E.Y.; Plucknett, K.P.; Alexander, K.B.; Hsueh, C.-H.; Lin, H.-T.; Waters, S.B.; Westmoreland, C.G.; Kang, E.; Hirao, K.; et al. Microstructural design of silicon nitride with imporved fracture toughness: I, effects of grain shape and size. J. Am. Ceram. Soc. 1998, 81, 2821–2830. [Google Scholar] [CrossRef]
- Doblinger, M.; Winkelman, G.G.; Dwyer, C.; Marsh, C.; Kirkland, A.I.; Cockayne, D.J.H.; Hoffmann, M.J. Structural and compositional comparison of Si3N4 ceramics with different fracture modes. Acta Mater. 2006, 54, 1949–1956. [Google Scholar] [CrossRef]
- Satet, R.L.; Hoffmann, M.J. Influence of the rare-earth element on the mechanical properties of RE-Mg-bearing silicon nitride. J. Am. Ceram. Soc. 2005, 88, 2485–2490. [Google Scholar] [CrossRef]
- Fan, L.S.; Howe, R.T.; Muller, R.S. Fracture toughness characterization of brittle thin films. Sens. Actuators 1990, A21–A23, 872–874. [Google Scholar] [CrossRef]
- Cardinale, G.F.; Tustison, R.W. Fracture strength and biaxial modulus measurement of plasma silicon nitride films. Thin Solid Films 1992, 207, 126–130. [Google Scholar] [CrossRef]
- Chen, Z.; Cotterell, B.; Wang, W.; Guenther, E.; Chua, S.J. A mechanical assessment of flexible optoelectronic devices. Thin Solid Films 2001, 394, 202–206. [Google Scholar] [CrossRef]
- Chen, Z.; Cotterell, B.; Wang, W. The Fracture of Brittle Thin Films on Compliant Substrate in Flexible Displays. Eng. Fract. Mech. 2002, 69, 597–603. [Google Scholar] [CrossRef]
- Chen, Z.; Gan, Z.H. Fracture Toughness Measurement of Thin Films on Compliant Substrate Using Controlled Buckling Test. Thin Solid Films 2007, 515, 3305–3309. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X.; Wong, C.C.; Mhaisalkar, S. Effect of Plating Parameters on the Intrinsic Stress in Electroless Nickel Plating. Surf. Coat. Technol. 2003, 167, 170–176. [Google Scholar] [CrossRef]
- Hirohata, Y.; Shimamoto, N.; Hino, T.; Yamashima, T.; Yabe, K. Properties of silicon nitride films prepared by magnetron sputtering. Thin Solid Films 1994, 253, 425–429. [Google Scholar] [CrossRef]
- Mao, A.Y.; Son, K.A.; Hess, D.A.; Brown, L.A.; White, J.M.; Kwong, D.L.; Roberts, D.A.; Vrtis, R.N. Annealing ultra thin Ta2O5 films deposited on bare and nitrogen passivated Si(100). Thin Solid Films 1999, 349, 230–237. [Google Scholar] [CrossRef]
- Tolstoy, V.P.; Chernyshova, I.V.; Skryshevsky, V.A. Infrared spectroscopy of thin layers in silicon microelectronics. In Handbook of Infrared Spectroscopy of Ultrathin Films; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 435–436. [Google Scholar]
- Gupta, M.; Rathi, V.K.; Thangaraj, R.; Agnihotri, O.P.; Chari, K.S. Preparation, properties and applications of silicon nitride thin films deposited by plasma-enhanced chemical vapor deposition. Thin Solid Films 1991, 204, 77–106. [Google Scholar] [CrossRef]
- Han, S.S.; Jun, B.H.; No, K.; Bae, B.S. Preparation of a-SiNx thin film with low hydrogen content by inductively coupled plasma enhanced chemical vapor deposition. J. Electrochem. Soc. 1998, 145, 652–658. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Lindelov, H.; Gerward, L.; Stahl, K.; Recio, J.M.; Mori-Sanchez, P.; Carlson, S.; Mezouar, M.; Dooryhee, E.; Fitch, A.; et al. Compressibility and thermal expansion of cubic silicon nitride. Phys. Rev. B 2002, 65, 161202(R). [Google Scholar] [CrossRef]
- Lynch, C.T. (Ed.) CRC Handbook of Materials Science; CRC Press: Boca Raton, FL, USA, 1975; Volume II. [Google Scholar]
- Carlotti, G.; Colpani, P.; Piccolo, D.; Santucci, S.; Senez, V.; Socino, G.; Verdini, L. Measurement of the elastic and viscoelastic properties of dielectric films used in microelectronics. Thin Solid Films 2002, 414, 99–104. [Google Scholar] [CrossRef]
- Hong, S.; Weihs, T.P.; Bravman, J.C.; Nix, W.D. Measuring stiffness and residual stresses of silicon nitride thin films. J. Electron. Mater. 1990, 19, 903–910. [Google Scholar] [CrossRef]
- Toivola, Y.; Thurn, J.; Cook, R.F.; Cibuzar, G.; Roberts, K. Influence of deposition conditions on mechanical properties of low-pressure chemical vapor deposited low-stress silicon nitride films. J. Appl. Phys. 2003, 94, 6915–6922. [Google Scholar] [CrossRef]
- Huang, H.; Winchester, K.J.; Suvorova, A.; Lawn, B.R.; Liu, Y.; Hu, X.Z.; Dell, J.M.; Faraone, L. Effect of deposition conditions on mechanical properties of low-temperature PECVD silicon nitride films. Mater. Sci. Eng. A 2006, 435–436, 453–459. [Google Scholar] [CrossRef]
- Kramer, T.; Paul, O. Postbuckled micromachined square membranes under differential pressure. J. Micromech. Microeng. 2003, 12, 475–478. [Google Scholar] [CrossRef]
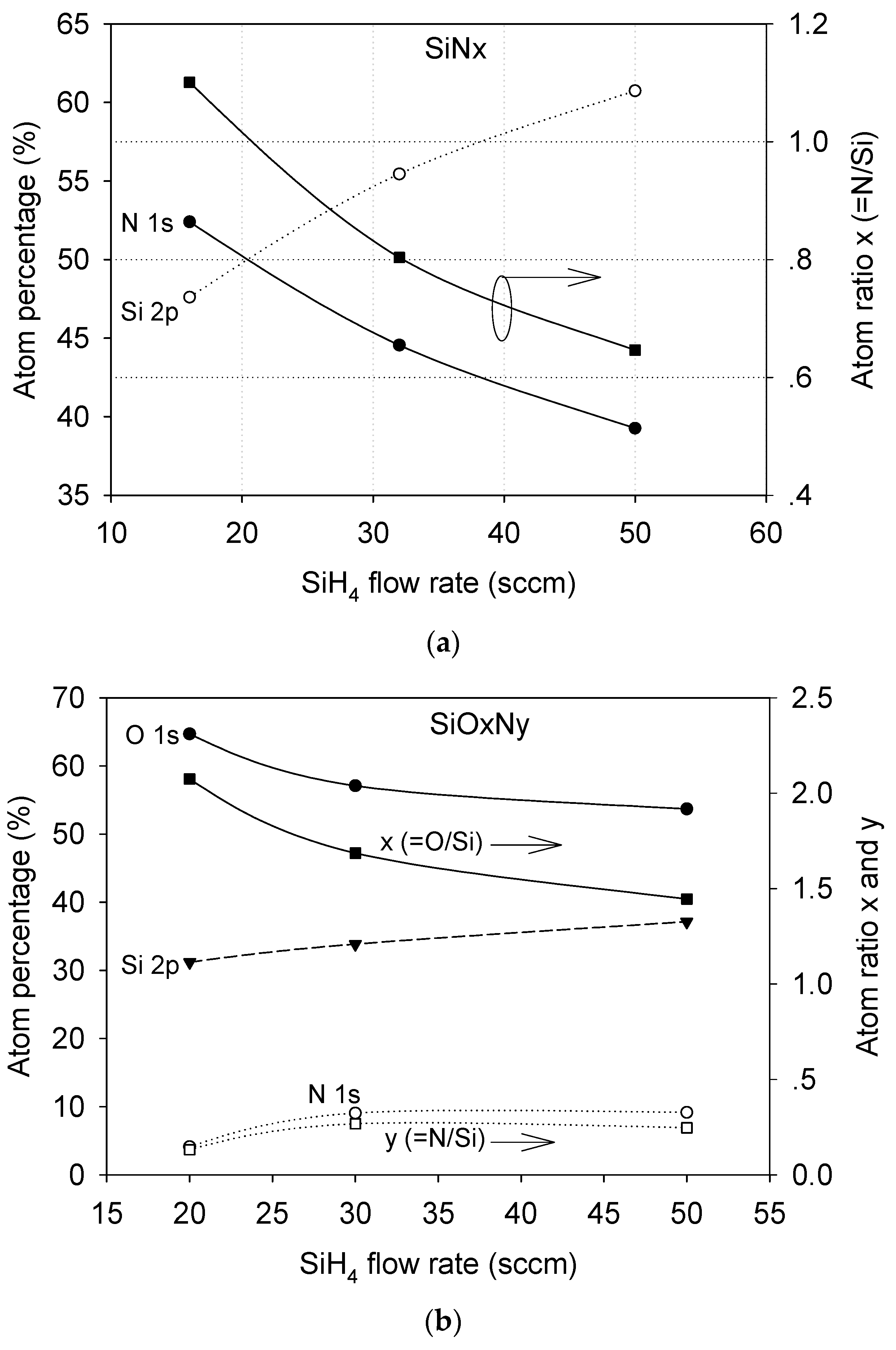
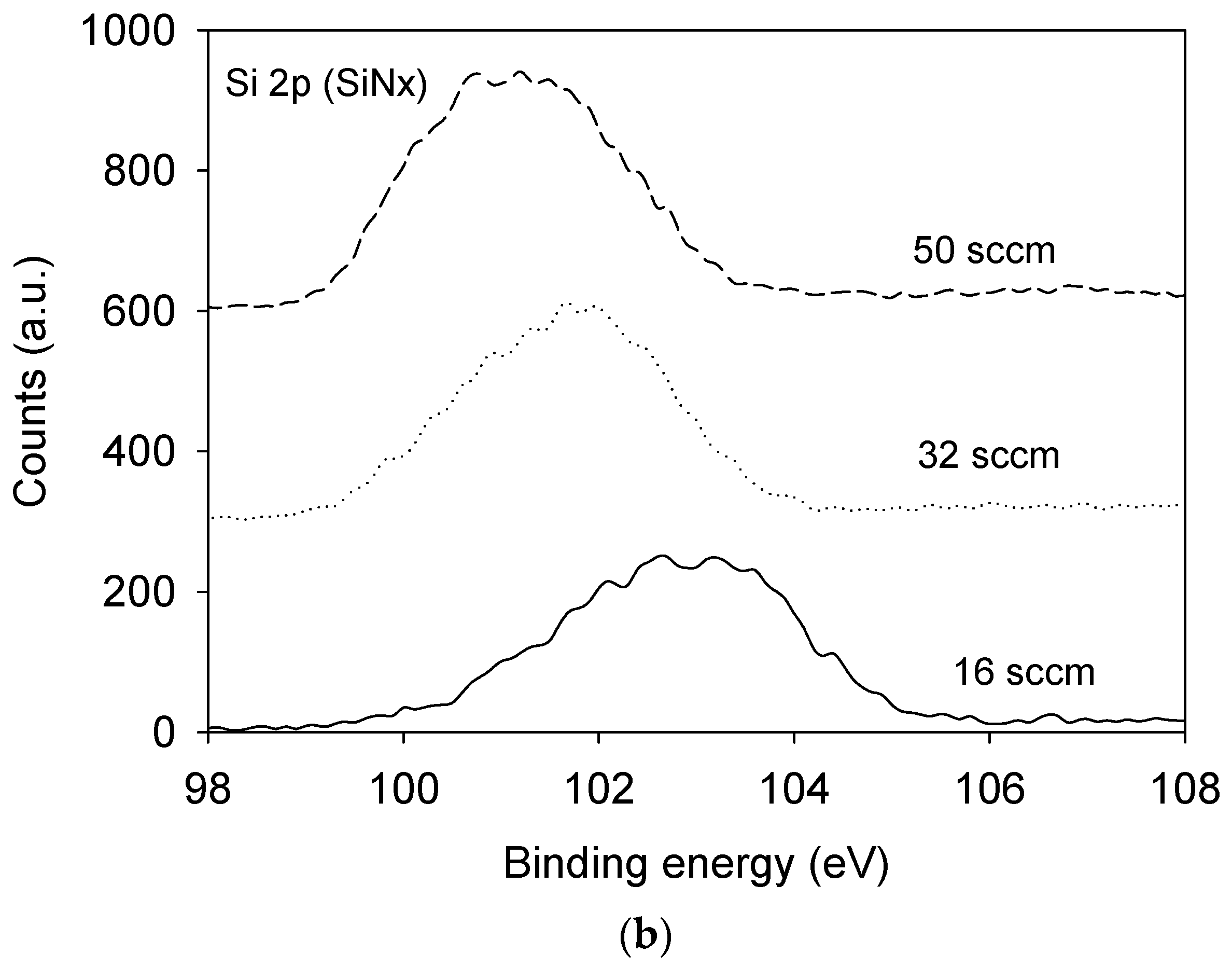
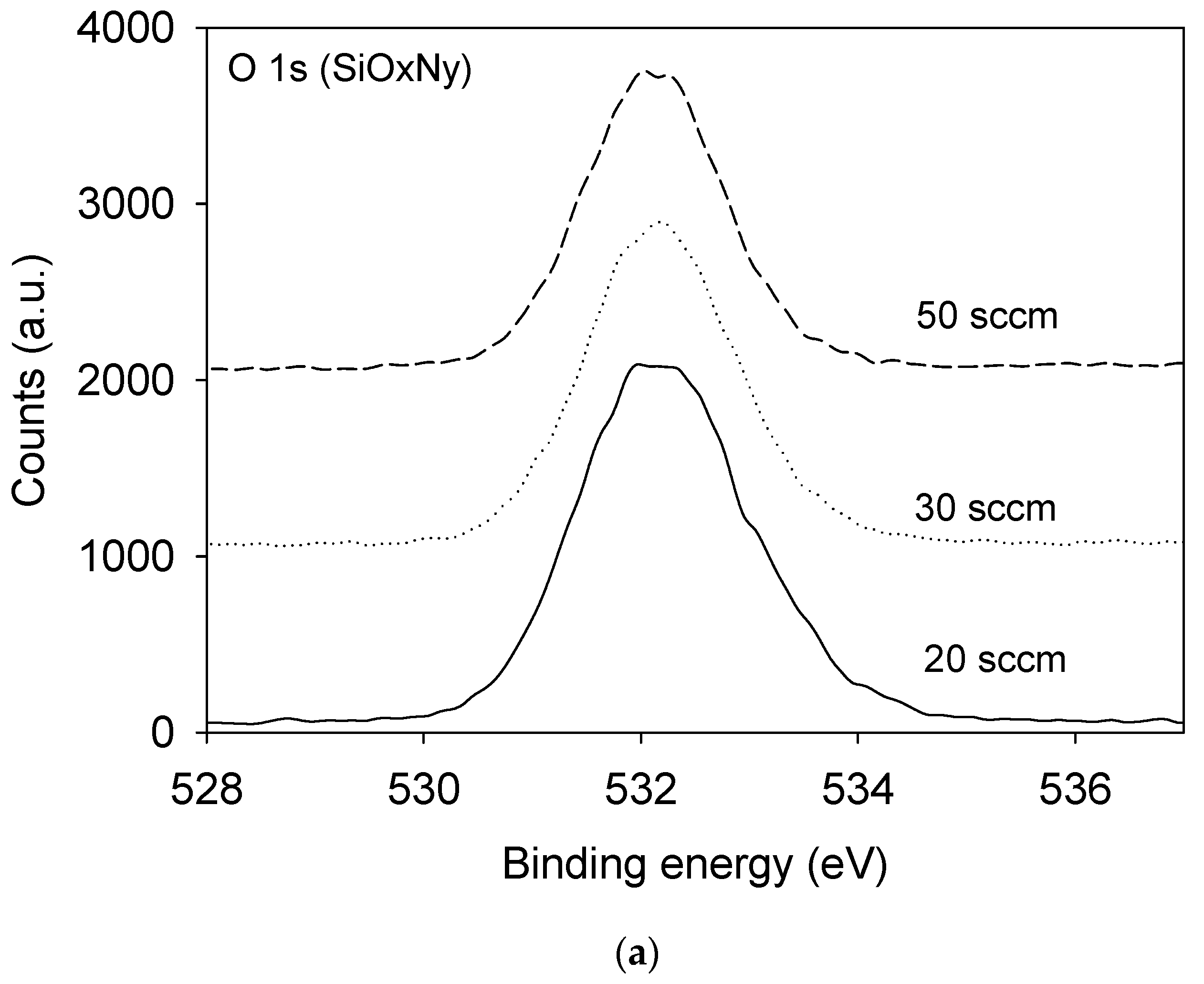
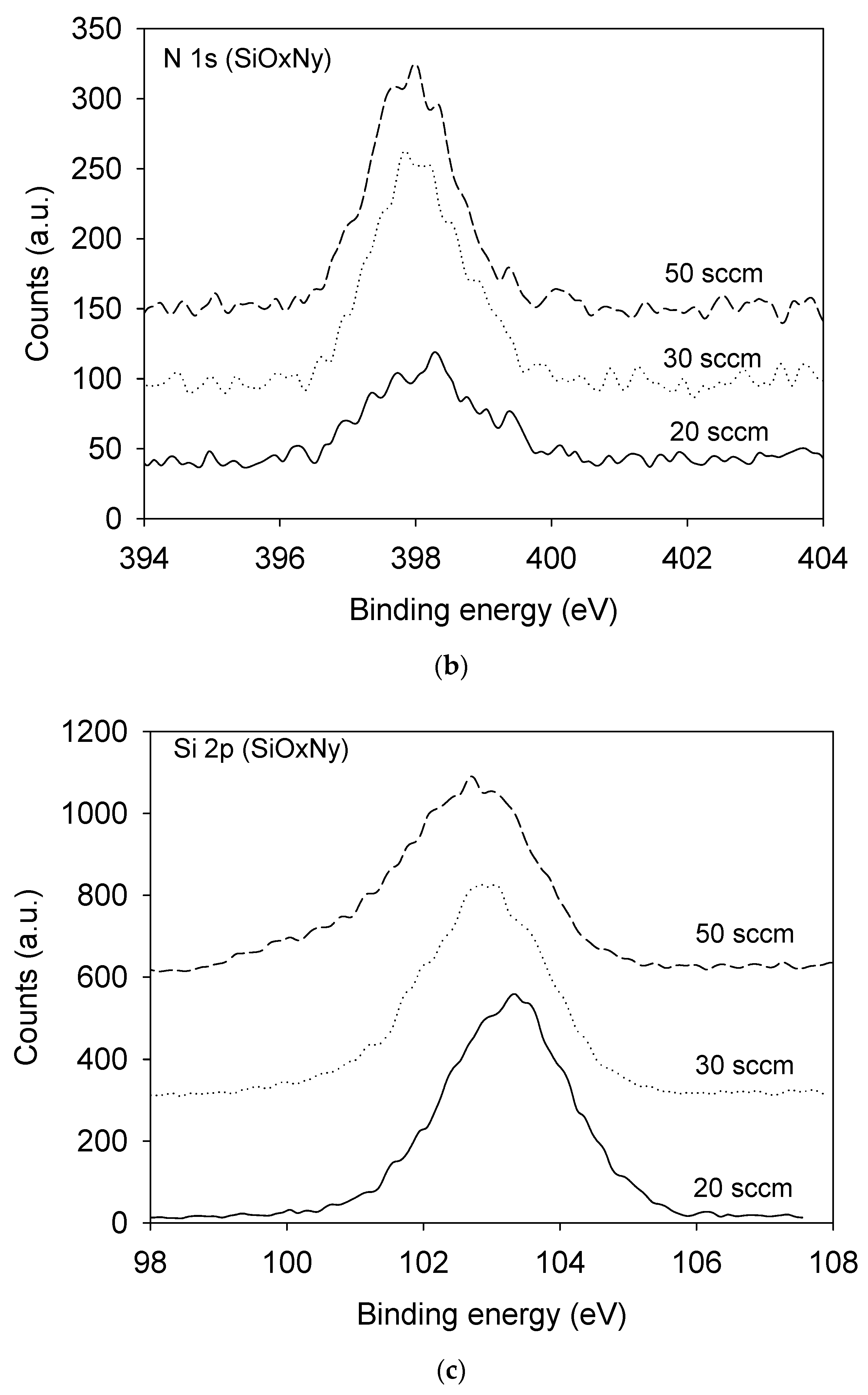
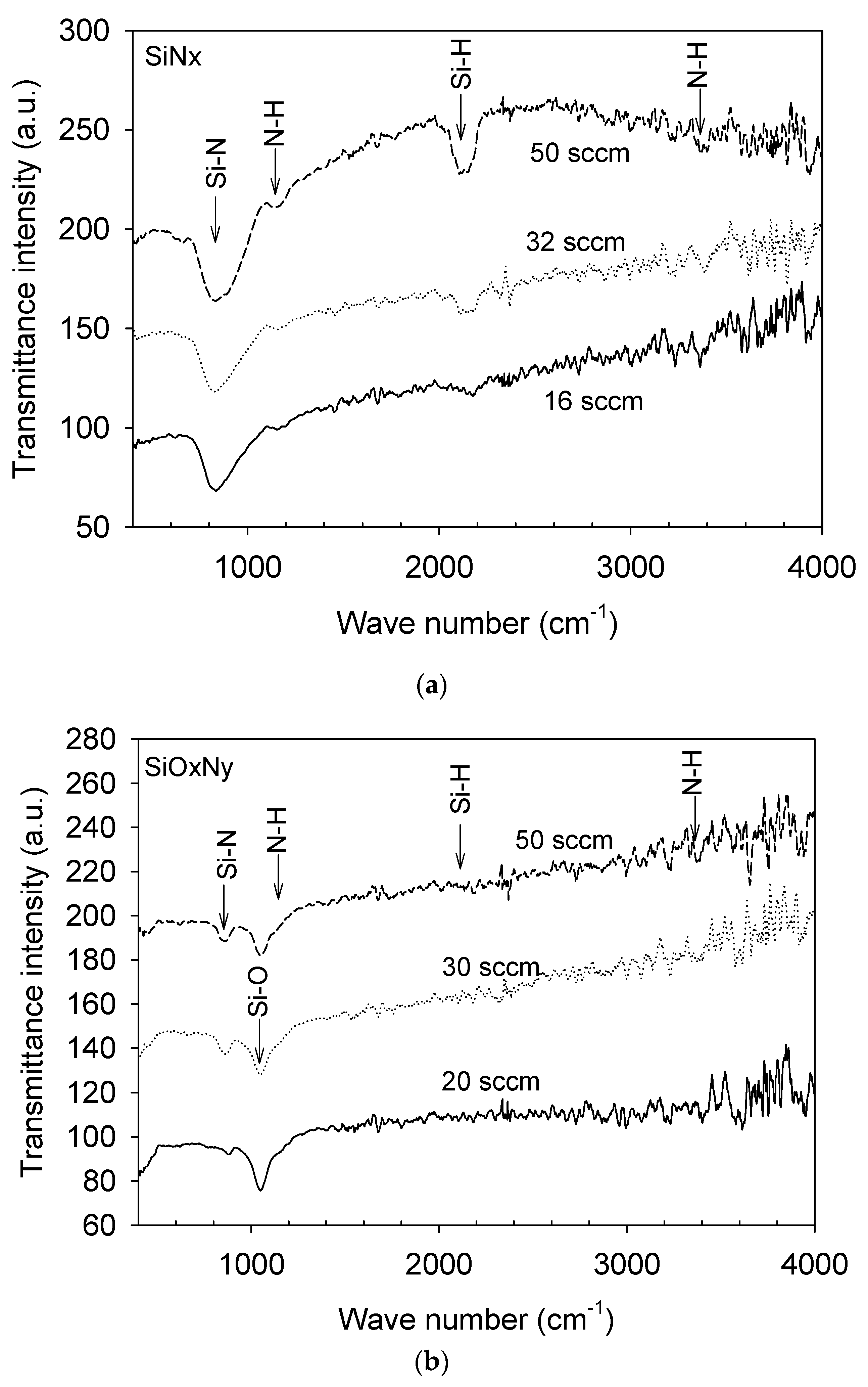
| Film | SiH4 Flow Rate (sccm) | N2O Flow Rate (sccm) | NH3 Flow Rate (sccm) | Pressure (mTorr) | Radio Frequency Power (W) |
|---|---|---|---|---|---|
| SiOxNy | 20, 30, 50 | 400 | -- | 730 | 100 |
| SiNx | 16, 32, 50 | -- | 160 | 620 | 250 |
| Film | Silane Flow Rate (sccm) | Young’s Modulus (GPa) | Hardness (GPa) | Poisson’s Ratio [27,28] |
|---|---|---|---|---|
| SiNx | 16 | 153.0 ± 14.9 | 13.6 ± 2.9 | 0.27 |
| 32 | 117.8 ± 9.5 | 11.8 ± 1.8 | ||
| 50 | 108.5 ± 10.6 | 10.2 ± 3.0 | ||
| SiOxNy | 20 | 89.2 ± 4.8 | 4.7 ± 0.7 | 0.23 |
| 30 | 78.9 ± 12.6 | 7.8 ± 0.9 | ||
| 50 | 76.8 ± 9.6 | 9.7 ± 4.0 |
| Film | Silane Flow Rate (sccm) | Film Thickness (nm) | Fracture Strain (%) | GIC (J/m2) | KIC (MPa⋅m1/2) |
|---|---|---|---|---|---|
| SiNx | 16 | 223.4 | 0.948 | 23.73 | 1.90 |
| 32 | 283.2 | 1.167 | 25.66 | 1.74 | |
| 50 | 358.5 | 1.367 | 55.41 | 2.44 | |
| SiOxNy | 20 | 198.9 | 1.497 | 17.29 | 1.24 |
| 30 | 245.6 | 1.072 | 9.16 | 0.85 | |
| 50 | 227.9 | 1.046 | 7.69 | 0.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Z.; Wang, C.; Chen, Z. Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition. Surfaces 2018, 1, 59-72. https://doi.org/10.3390/surfaces1010006
Gan Z, Wang C, Chen Z. Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition. Surfaces. 2018; 1(1):59-72. https://doi.org/10.3390/surfaces1010006
Chicago/Turabian StyleGan, Zhenghao, Changzheng Wang, and Zhong Chen. 2018. "Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition" Surfaces 1, no. 1: 59-72. https://doi.org/10.3390/surfaces1010006