1. Introduction
As reported by the International Center for the Study of Conservation and Restoration of Cultural Property (ICCROM), more than 60% of cultural property stored in uncontrolled environments exhibits signs of deterioration, in particular biodeterioration [
1].
The term “biodeterioration” refers to the physical, chemical, and/or aesthetic alterations of a material induced directly or indirectly by the metabolic activity of living organisms.
Microbial biofilms have been identified as a primary factor contributing to the biodeterioration of stone monuments, exerting a negative impact on both the aesthetic appearance and the structural integrity of the materials [
2]. Biofilms are composed of complex communities of microorganisms, including autotrophic and heterotrophic
Bacteria,
Fungi,
Algae,
Cyanobacteria, and
Lichens [
3,
4]. These microorganisms adhere to surfaces and are immersed in an extracellular polymeric matrix (EPS) composed mainly of polysaccharides.
The process of biofilm formation occurs through multiple stages. Initially, pioneer microorganisms, including cyanobacteria and microalgae, attach to the stone surface [
5]. Consequently, microcolonies emerge, producing EPS, thereby establishing a protected environment favoring the colonization of microorganisms, including
Fungi and heterotrophic
Bacteria [
6]. This process affects both indoor and outdoor artifacts.
The role of EPS in the protective response of microorganisms to adverse environmental conditions (including dehydration and UV exposure) is well documented [
7].
To preserve cultural heritage artifacts effectively, it is essential to develop strategies that prevent and remove microbial communities while minimizing the impact on original surfaces and maintaining the integrity of historic materials [
8].
The management of biodeterioration can be approached by using different indirect and direct methodologies, whose selection depends on the biodeteriogens present, their degree of diffusion, the prevailing environmental conditions, and the artifact’s state of preservation [
9]. As the term suggests, indirect methods do not act directly on the artwork but rather aim to modify or limit the effects of environmental factors that promote deterioration. These factors include, but are not limited to, temperature, pH, and light. These methods are particularly employed for works stored indoors and are regarded as environmentally sustainable, as they do not entail direct manipulation of the artifact [
10]. Nevertheless, the efficacy of these measures is frequently constrained, as they can address only a portion of the underlying problem. Direct methods can be categorized into three distinct classifications: physical, biological, and chemical.
A variety of physical methods are employed to address this issue, including the application of UV-C light [
11], the use of gamma radiation [
12], and the implementation of laser or mechanical cleaning procedures for surfaces [
13]; these methods have the disadvantage of failing to guarantee results over the long term. Biological methods primarily entail the utilization of biocides of natural provenance [
14,
15]. Conversely, chemical methods entail the utilization of synthetic biocides [
16,
17,
18]. Frequently, these techniques are used in combination with each other. For example, the mechanical removal of biofilm is frequently performed after preliminary treatment with biocides to complement its effectiveness [
19,
20].
In this context, any contribution to the development of innovative methods for eliminating microbial communities is of critical importance—especially when the selected molecules are not harmful to the environment or to the restorers themselves.
In recent years, scientific interest has increasingly focused on selenium-based compounds, owing to selenium’s distinctive chemical properties that set it apart from other elements [
21].
In biological systems, selenium displays a dual behavior, acting either as an antioxidant or a prooxidant depending on its chemical form, concentration, and the physiological environment. It can disrupt cellular redox balance by directly oxidizing thiol groups or indirectly generating reactive oxygen species (ROS), ultimately leading to oxidative stress and cellular damage [
21,
22].
The biocidal potential of selenium compounds has previously been documented, including in U.S. Patent No. 9,370,187 B2 [
23]. These biocidal formulations typically include at least one selenium atom capable of forming Se
− species, which can generate damaging free radicals against a wide range of target organisms.
Against this backdrop, the present study aimed to investigate a class of selenium-based biocides for the prevention of biodeterioration in cultural heritage materials, namely seleno-sugars that represent a class of compounds characterized by a distinctive structural modification: the replacement of an oxygen atom within the sugar backbone with a selenium atom. This substitution produces sugar isosteres that exhibit unique chemical and biological properties [
21].
The research began with the synthesis of organo-selenium compounds, specifically seleno-sugars derived from
d-mannose and
d-ribose. These molecules were selected based on previous studies demonstrating their favorable cell permeability, a key property considered critical for biocidal efficacy due to the requirement for penetration into biological systems, while concurrently exhibiting a complete lack of cytotoxicity toward human immortalized keratinocytes [
24].
To achieve comprehensive structural characterization, the synthesized compounds were analyzed using nuclear magnetic resonance (NMR) spectroscopy and gas chromatography–mass spectrometry (GC-MS).
The biocidal efficacy of selected compounds was assessed through a growth inhibition assay (OECD 201 [
25]), employing the unicellular alga
Raphidocelis subcapitata [
26], once known as
Pseudokirchneriella subcapitata and very well known for its fast growth in laboratory conditions and sensitivity to environmental stresses, as a model organism from the ones suggested by the protocol.
The efficacy demonstrated by selenium in the development of biocides paves the way for the design of new antimicrobial compounds capable of acting on a broad spectrum of microorganisms without compromising operators’ safety during use.
2. Materials and Methods
All the reagents were acquired (Aldrich, Fluka, and Sigma) at the highest purity available and used without further purification. Thin-layer chromatography was performed with silicagel plates, Merck 60 F254, and the display of the products on TLC was accomplished with UV lamp lighting, molecular iodine, and in H2SO4–EtOH (95:5) with further heating until the development of color. The column chromatographies were carried out using 70–230 mesh silica gel (Merck). The NMR spectra were recorded on Bruker DRX (400MHz) by Bruker Corporation, Karlsruhe, Germany and Varian Inova Marker (500MHz) by Varian, Inc., Palo Alto, California, spectrometers in CDCl3 solution unless otherwise specified. The chemical shifts are reported in ppm (δ). The TopSpin 4.0.6 software package was used for the analysis.
2.1. Synthesis
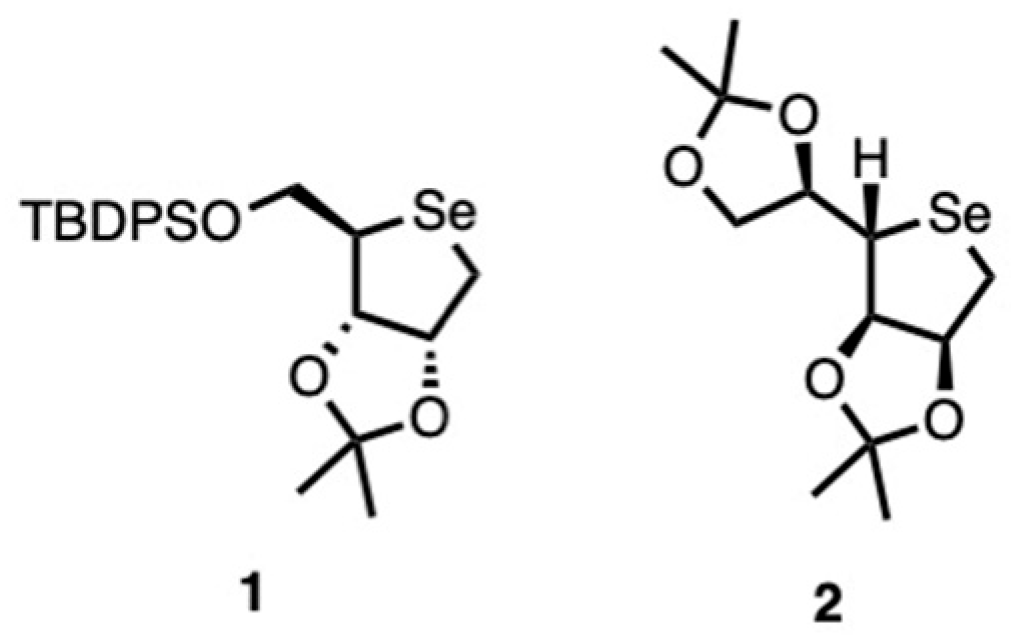
To obtain novel selenium-based biocides, two classes of seleno-sugars were synthesized (
Figure 1).
Specifically, the key intermediate
1 was prepared from a commercially available
d-ribonolactone derivative, following a strategy (
Scheme 1) previously reported in the literature [
27].
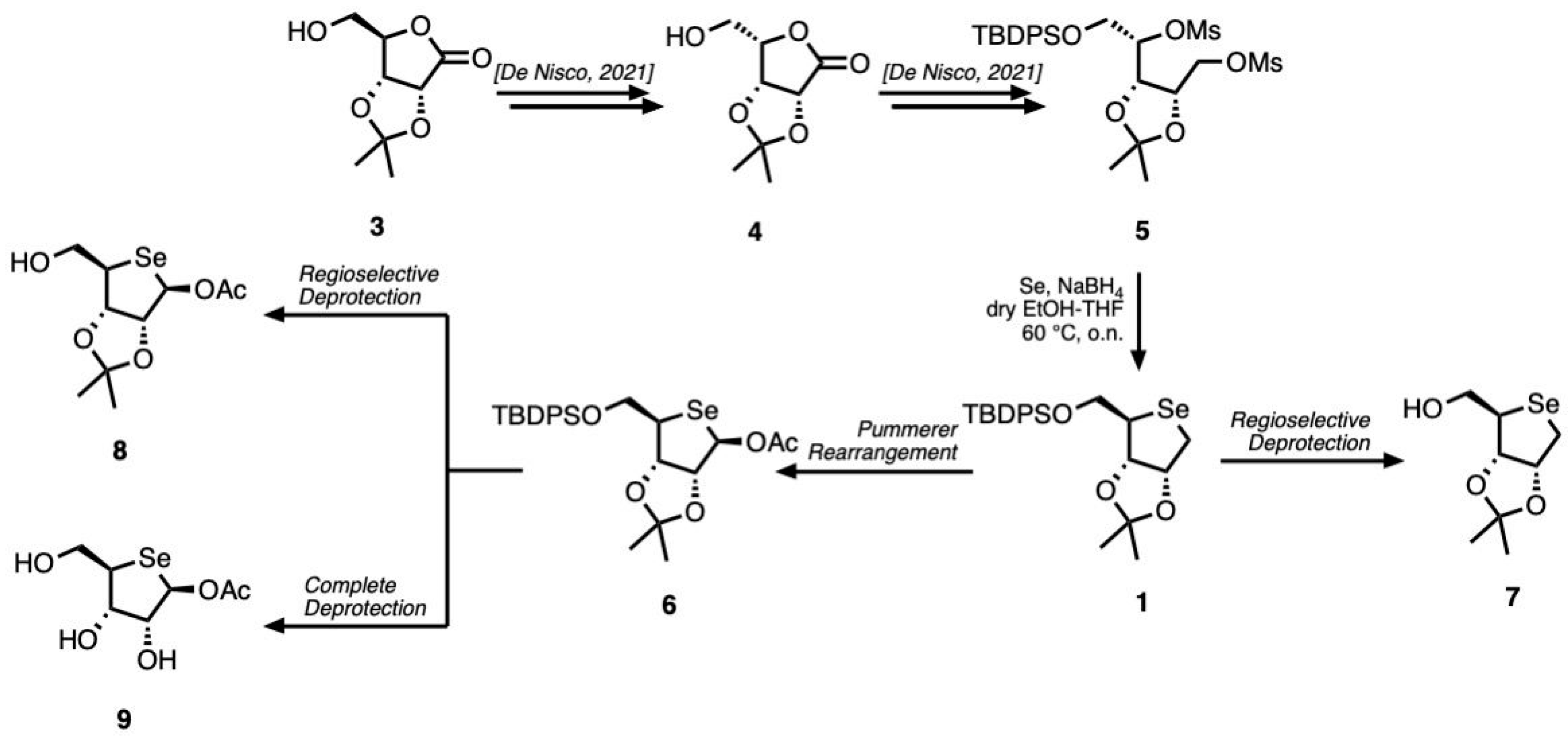
The synthesis strategy began with the preparation of compound 4. To achieve this, d-ribonolactone 3 was treated with mesyl chloride (MsCl) under basic conditions to afford an intermediate bearing a mesyl group at the C-5 hydroxyl position. This functionalization is crucial for facilitating the subsequent stereochemical inversion at the C-4 chiral center in compound 4. The latter was selectively protected at the primary hydroxyl at C-5 by introduction of the tert-butyldiphenylsilyl (TBDPS) group. The resulting protected lactone was then reduced with sodium borohydride (NaBH4) to afford the corresponding derivative in quantitative yields. This intermediate was subsequently subjected to a double mesylation reaction with mesyl chloride, introducing mesylate leaving groups at the C-1 and C-4 positions. The presence of these two activated leaving groups enabled a key modification in which the ring oxygen was replaced by a selenium atom, affording scaffold 1.
To introduce an acetyl group at the anomeric position, a Pummerer rearrangement was performed in the presence of acetic anhydride at 100 °C, following the oxidation of compound 1 with meta-chloroperbenzoic acid (m-CPBA), yielding compound 6.
Both compounds 1 and 6 underwent partial deprotection at C-5 by treatment with tetrabutylammonium fluoride (TBAF), affording compounds 7 and 8, respectively. On the other hand, the complete deprotection of compound 6 was accomplished to afford the fully deprotected product 9.
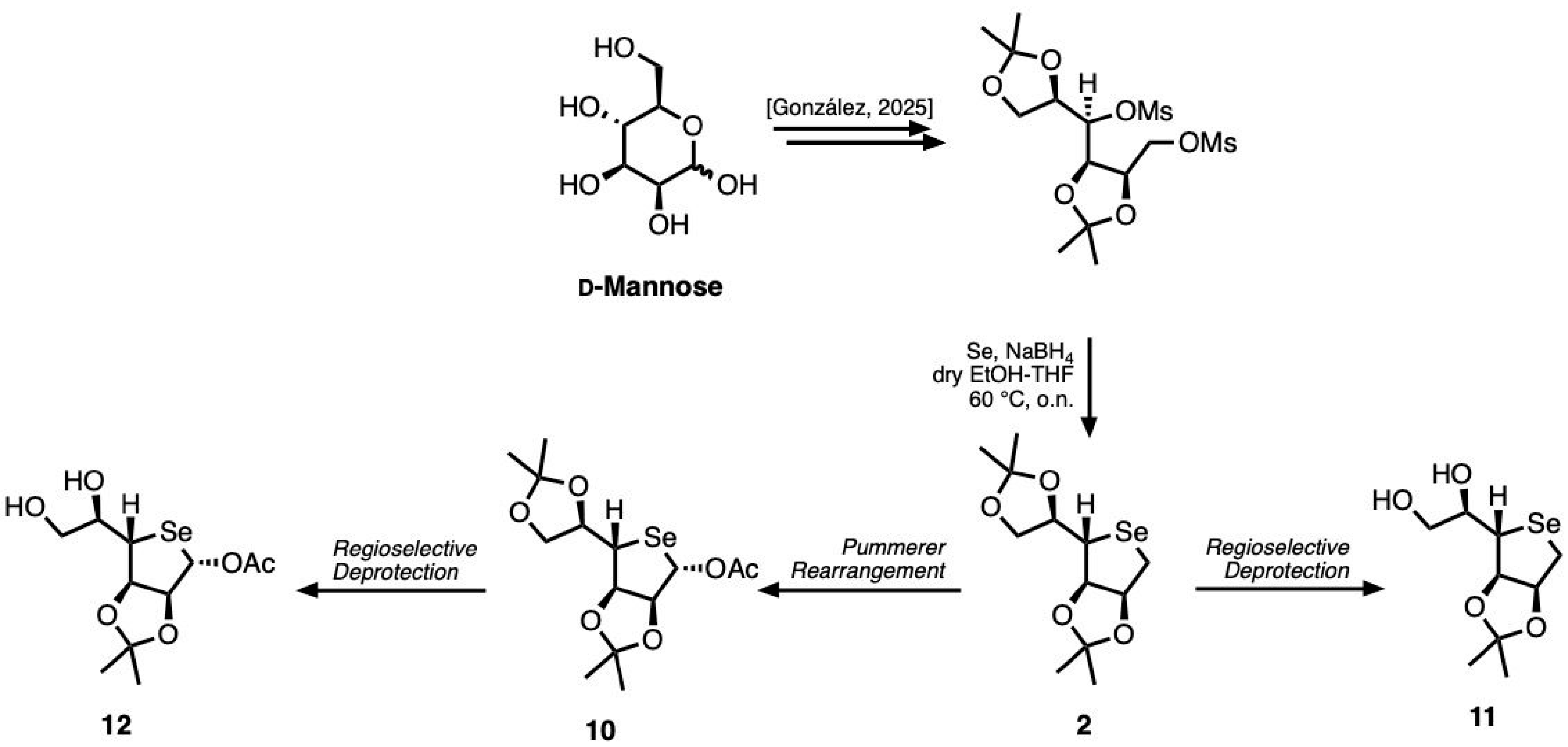
Likewise, to synthesize the homologues of compounds
7 and
8, we employed a strategy previously described in the literature, starting from commercially available
d-mannose (
Scheme 2) [
28].
In this case, the protection of the hydroxyl groups at the C-2/C-3 and C-4/C-5 positions of d-mannose was first achieved simultaneously as isopropylidene acetals. The resulting intermediate was then converted in high yield into the desired d-deoxytalose seleno-sugar 2 using the same reaction sequence described for the previous scaffold.
The introduction of an acetyl group in compound 2 was accomplished using the same methodology employed for the lower homolog. Selective removal of the protecting group at the C-5 and C-6 positiond in compounds 2 and 10 was finally achieved using bismuth(III) chloride as Lewis acid, thereby affording compounds 11 and 12, respectively.
2.2. NMR
Nuclear magnetic resonance spectra were acquired on Bruker DRX (400 MHz) and Varian Inova Marker (500 MHz) spectrometers in a deuterated chloroform (CDCl3) solution, unless otherwise indicated. Chemical shifts are reported in parts per million (ppm) and the coupling constants in Hertz (Hz).
2.3. GC-MS
Samples were derivatized to reduce the polarity of the free hydroxyl groups. Derivatization occurred with 50 µL N,O-bis-(trimethylsilyl)acetamide (TMS; Supelco 15222) for 45 min at 90 °C.
Analysis and characterization were conducted using an Agilent GC 7820A coupled with a 5975 MS detector. Samples were solubilized in hexane (50 mg/L) and 1µ of solution was injected. Each analysis was conducted in triplicate.
The carrier gas used in all the methods was helium. A method involving a mass range of 30 to 600 Dalton (Da) with a solvent delay of 5–50 min was used. The initial temperature was 70 °C, for about 2 min, with a temperature of 230 °C reached with an increase of 20 °C/min, followed by 240 °C and 270 °C. The maximum temperature reached by the instrument was 325 °C.
2.4. Biocidal Activity Assay
A toxicological evaluation of synthetic seleno-sugar compounds was carried out following the OECD Test Guideline No. 201 (Freshwater Algae and Cyanobacteria, Growth Inhibition Test), using the unicellular green microalga
Raphidocelis subcapitata as the model species due to its consistent and standardized sensitivity to chemical perturbations [
29]. Unialgal, non-axenic cultures of
R. subcapitata were obtained from the Algal Collection of the University of Naples Federico II (A.C.U.F. strain number 228;
https://www.acuf.net/ (accessed on 8 September 2025)) and propagated in Bold’s Basal Medium (BBM) under tightly controlled conditions (24 ± 0.1 °C, 280 rpm orbital agitation, constant illumination at 20 μmol photons m
−2 s
−1) to reach the exponential growth phase prior to treatment.
Each seleno-sugar was solubilized in an aqueous vehicle containing 5% (v/v) DMSO and subsequently tested across a concentration gradient consisting of five levels (40, 20, 10, 5, and 2.5 mg L−1). Experimental exposures were performed in 1 mL volumes distributed across six biological replicates per concentration. The experimental design also incorporated three control conditions—untreated culture medium, solvent control (5% DMSO), and a positive control using Biotin T—all prepared in parallel with six biological replicates each. Exposure was carried out in 24-well microplates maintained under the same incubation parameters for a duration of 72 h. Each group of biological replicates (six for each of the aforementioned conditions) was inoculated along one of the horizontal lines of the 24-well microplate, from A to D.
Following incubation, algal physiological status was assessed via spectrophotometric and spectrofluorometric endpoints. Cell density and pigment concentrations (chlorophyll a, chlorophyll b, and carotenoids) were determined at 750 nm, 450 nm, 490 nm, and 531 nm, respectively, using a Victor multilabel plate reader after 30 s of orbital shaking. Fluorescence emissions specific to chlorophyll a and b were additionally quantified using the instrument’s fluorescence detection mode. All readouts were conducted in technical triplicates to ensure analytical robustness. Averaged values were subsequently employed to construct concentration–response profiles for each compound. For substance SD3, the dose–response curve was fitted using a non-linear four-parameter logistic regression model (non-linear least squares fit; scipy.optimize.curve_fit) to derive the IC
50 value (defined as the concentration required to inhibit 50% of algal growth) [
30].
3. Results and Discussion
In order to evaluate the synthesis with a high degree of certainty, structural characterization by NMR and GC-MS was performed on each sample as described in the Materials and Methods section. Our results (reported in the
Supplementary Materials) indicate the correct structure of each analyzed molecule. For each spectrum, all fragments related to TMS and its derivatives, as well as the fragmentation of the molecule under investigation that contains selenium, were identified.
Growth Inhibition Test—In Vitro Assay
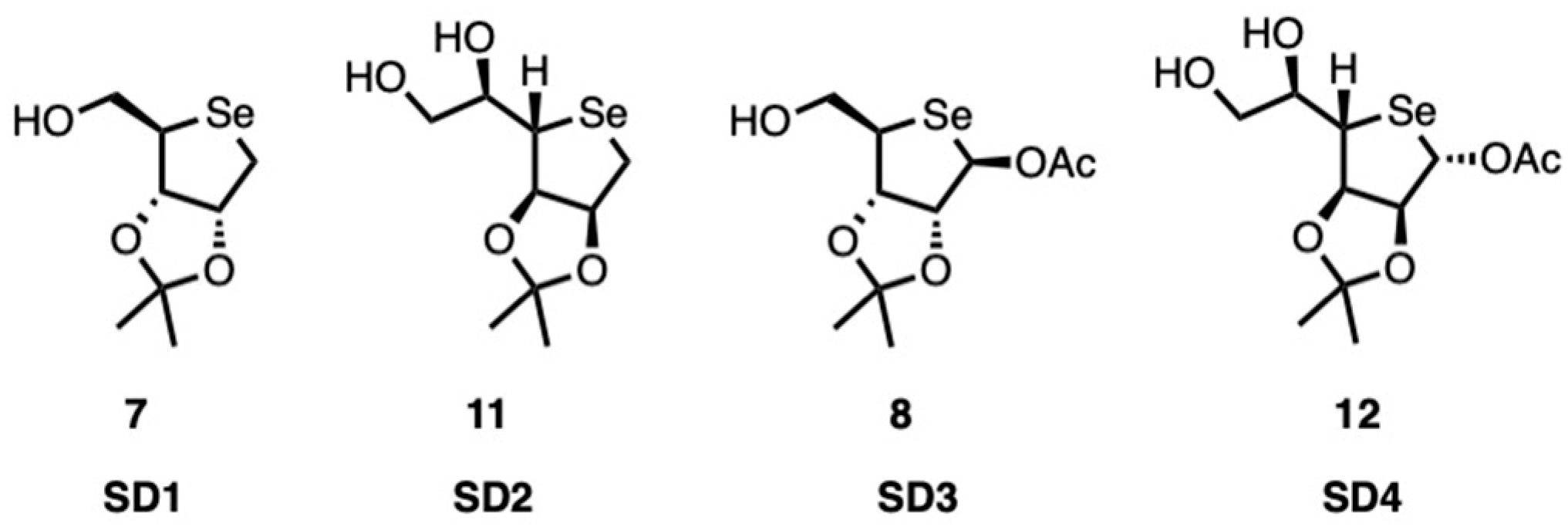
The effect of four different substances (denoted as
SD1,
SD2,
SD3, and
SD4;
Figure 2) was evaluated by ecotoxicological testing according to OECD 201 guidelines.
This involves monitoring algal growth in the presence of potentially toxic substances for a period of 72 h. The measurements comprised physiological and biochemical parameters, including cell density (750 nm), pigment content (carotenoids, chlorophyll a and b), and the fluorescence of chlorophyll a. All substances negatively affected algal growth to a greater or lesser extent.
The biocidal efficacy of the substances was evaluated by calculating the percentage of inhibition compared with the mean values obtained from the controls. The results indicate that the substances analyzed exhibit distinct behaviors. The graphs presented are based on overall averages, calculated from biological averages and technical repeat averages, derived from six replicates for each assay, each measured in triplicate.
In the case of SD1, as shown in
Figure 3, growth is increasingly inhibited from 2.5 mg/L to a maximum at 10 mg/L. However, a marked dose-dependent effect is not observed. The absence of a dose-dependent response indicates that the substance exhibits low biocidal efficacy or has already reached a state of “saturation” at lower dosages.
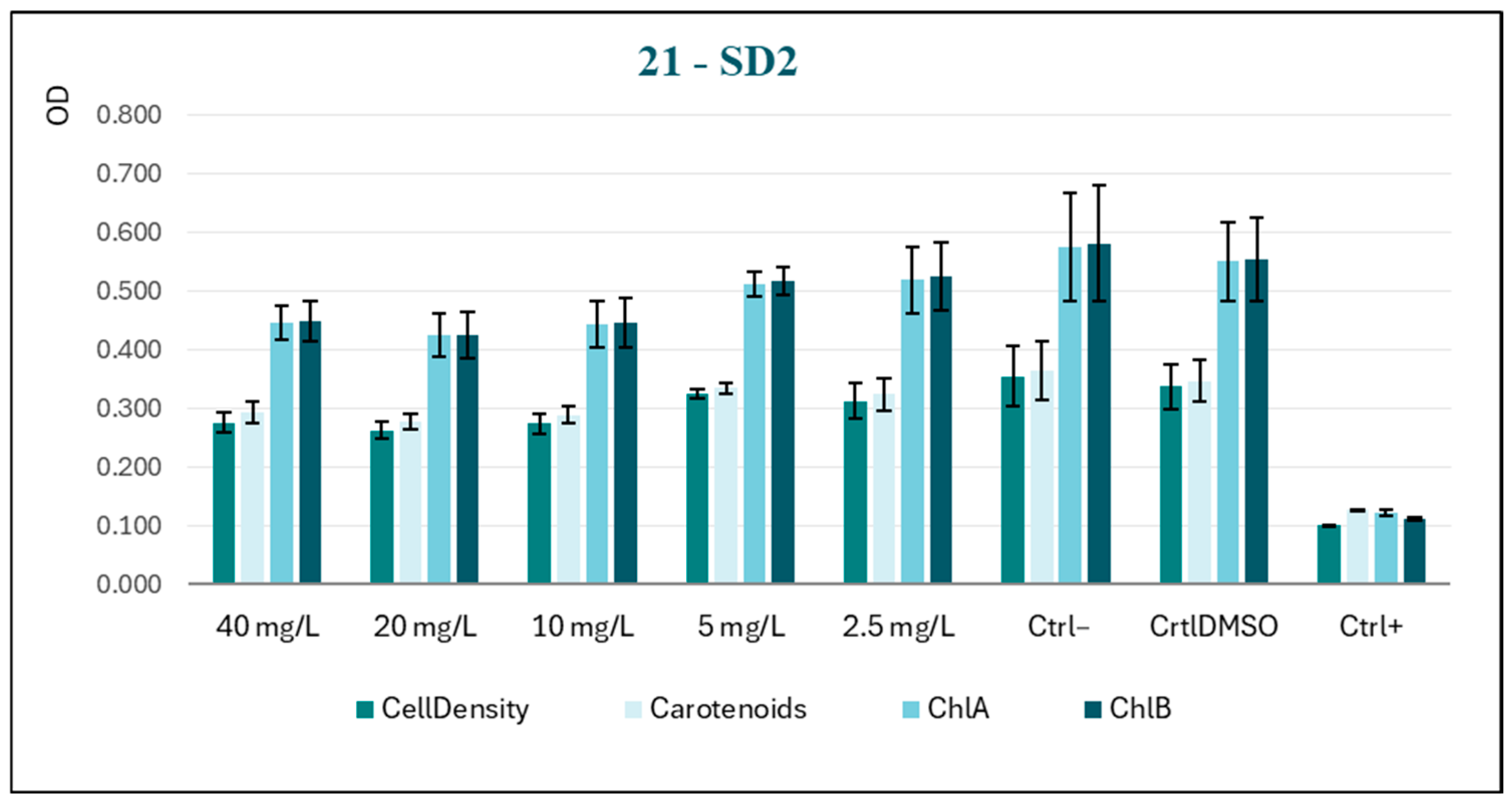
A comparison of the behavior of SD2 and SD1 reveals a lack of a discernible dose–response trend (
Figure 4), suggesting that the two subjects exhibit similar responses to the experimental conditions. In this case, the photosynthetic pigments appear to be less impacted by the substance, which provides further confirmation of low biocidal activity.
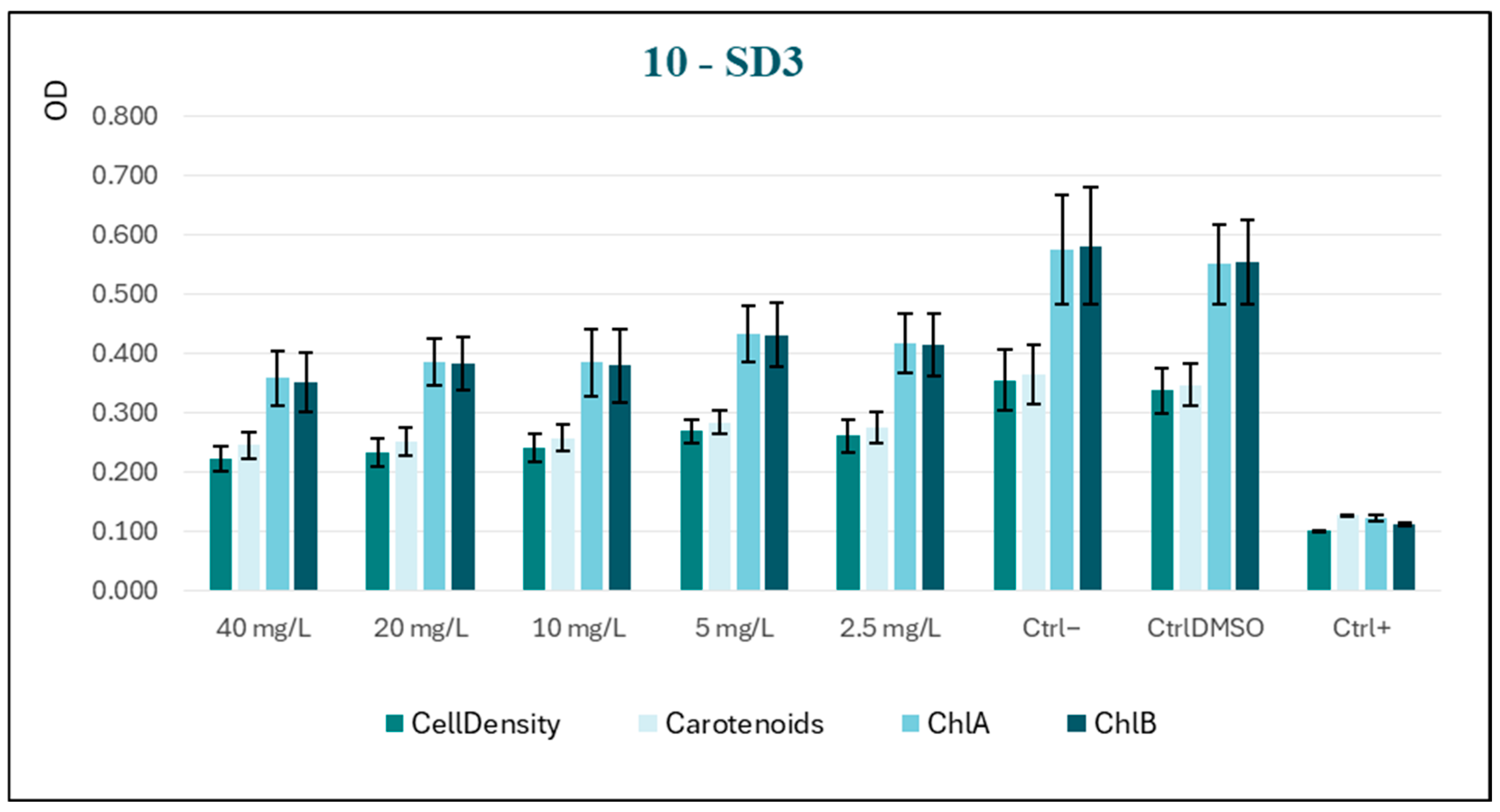
Conversely, the substance SD3 has been observed to induce significant growth inhibition at as low a concentration as 5 mg/L, as highlighted in
Figure 5. However, this inhibition does not become more pronounced with increasing concentration. The trend of the curve suggests modest toxic activity, which is useful for our purposes.
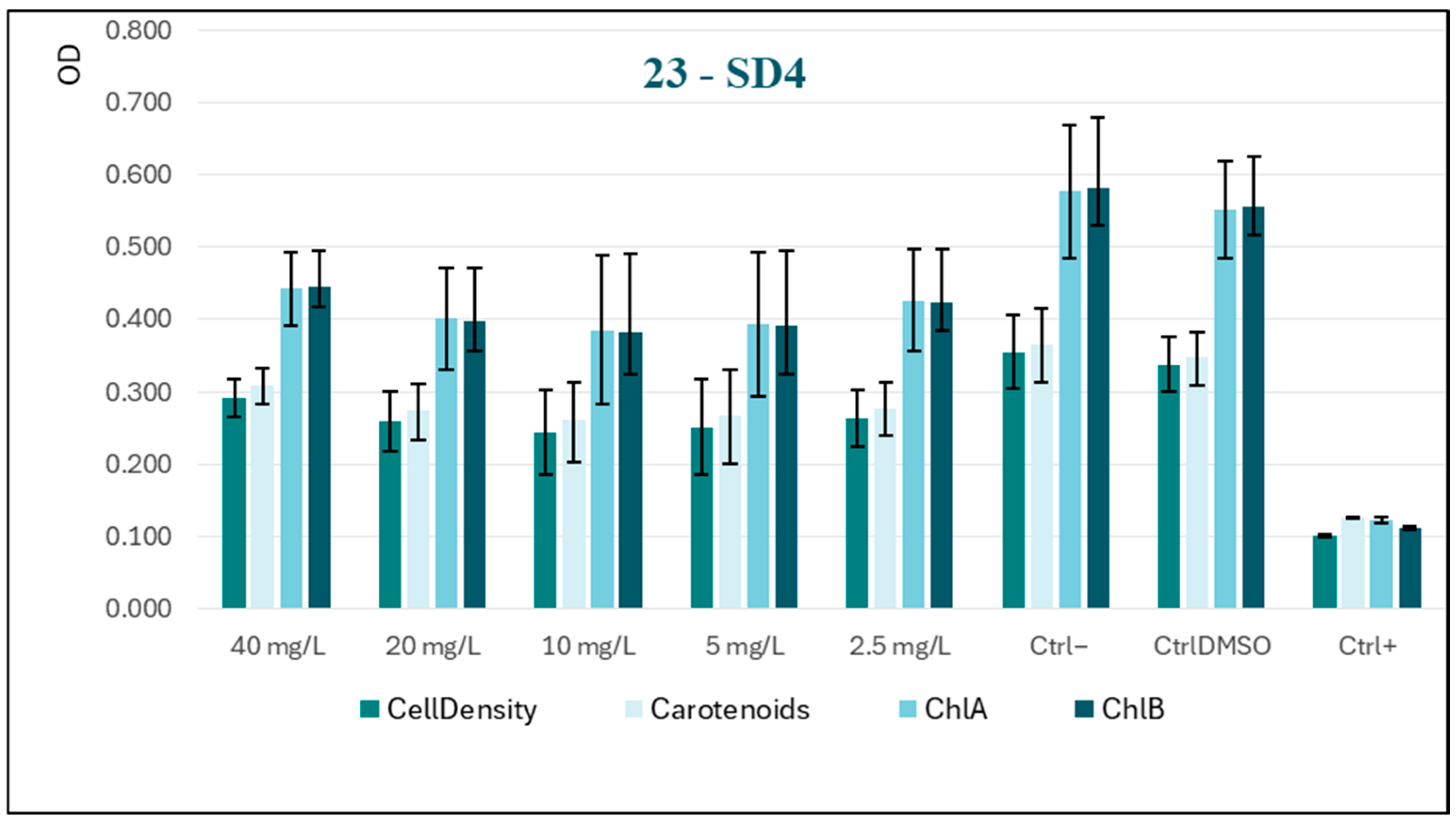
Among all tested substances, SD4 exhibited (at higher concentrations) behavior that was consistently the opposite of the desired effect (
Figure 6). This profile, highlighting an almost stimulatory action, indicates that SD4 is not only ineffective as a biocide, but may potentially promote algal growth. This may be attributable to SD4 acting as a source of nutrients or by stimulating photosynthetic activity. Substances exhibiting such characteristics are contraindicated for utilization as biocidal agents.
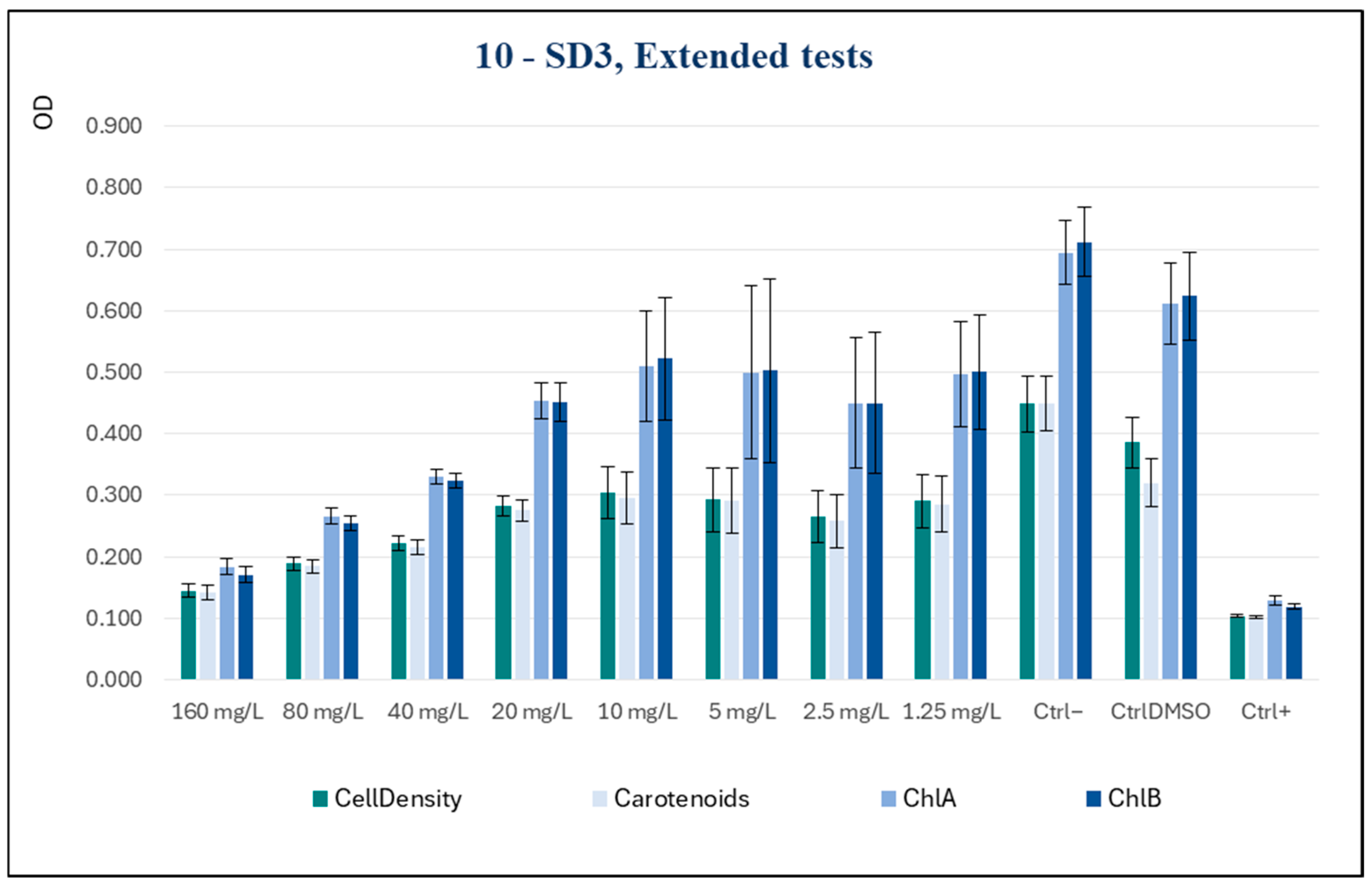
Subsequently, to inquire about the potentialities of SD3 as an active biocide, the spectrum of tested concentrations was widened, extending it to higher concentrations, specifically of 160 and 80 mg/L, and to a lower one of 1.25 mg/L.
The new series of measurements, shown in
Figure 7, demonstrated a clear dose-dependent effect, indicating that as the concentration increased, there was a progressive and marked reduction in all the parameters tested. The inhibitory effect of the substance was observed to be significant at concentrations as low as 40 mg/L, with these levels being comparable to those of the positive control at the highest concentration tested (160 mg/L).
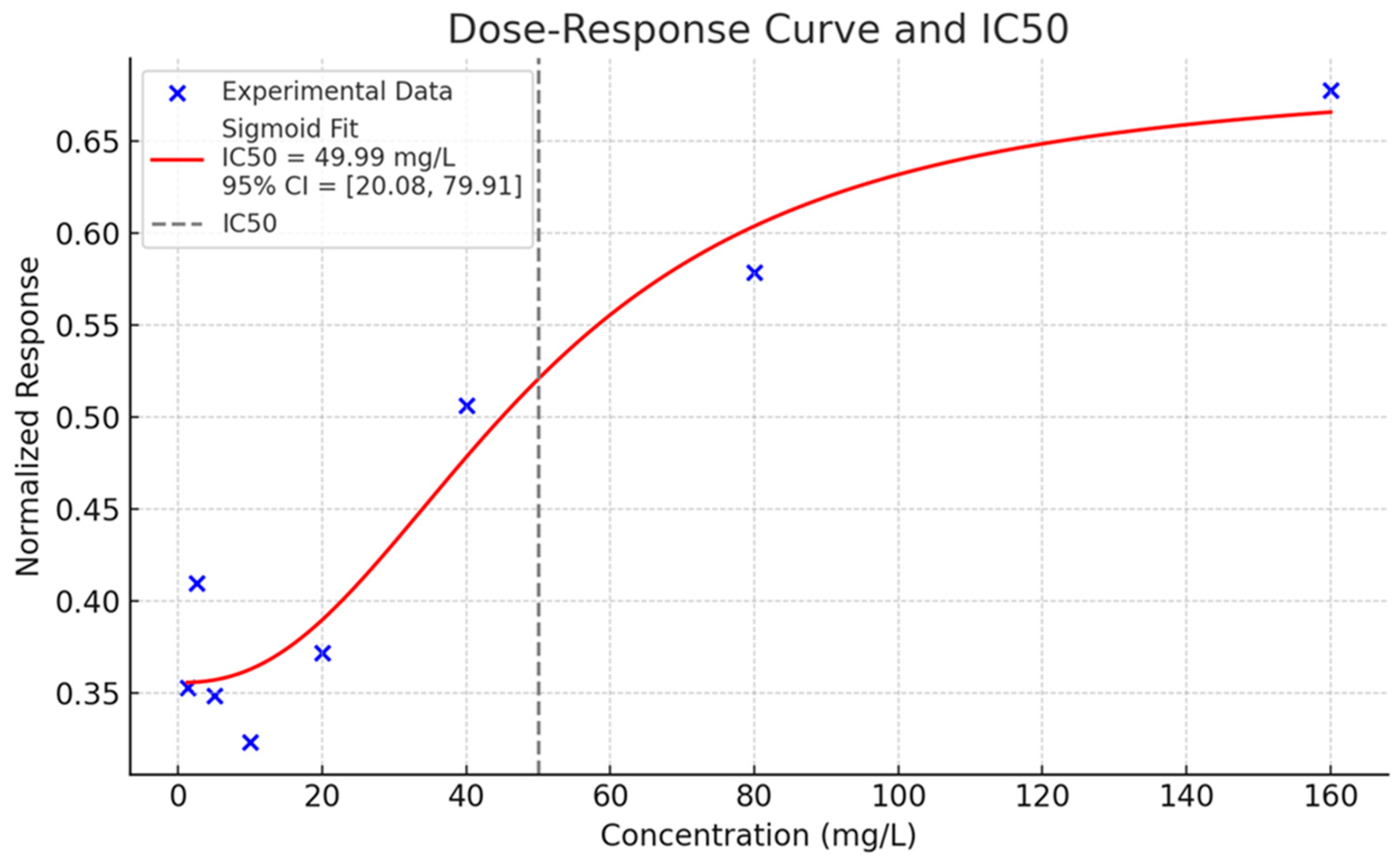
In view of the consistent biological response exhibited by substance SD3, the dose–response curve and related IC
50 value, shown in
Figure 8, were preliminarily estimated in order to characterize its toxicological profile.
The sigmoid fit exhibits a high degree of correlation with the experimental points, demonstrating an increase in inhibition with increasing concentration. The IC50 was estimated as 49.99 mg/L (±15.26), and the associated p-value was 0.031, indicating a statistically significant deviation from zero.
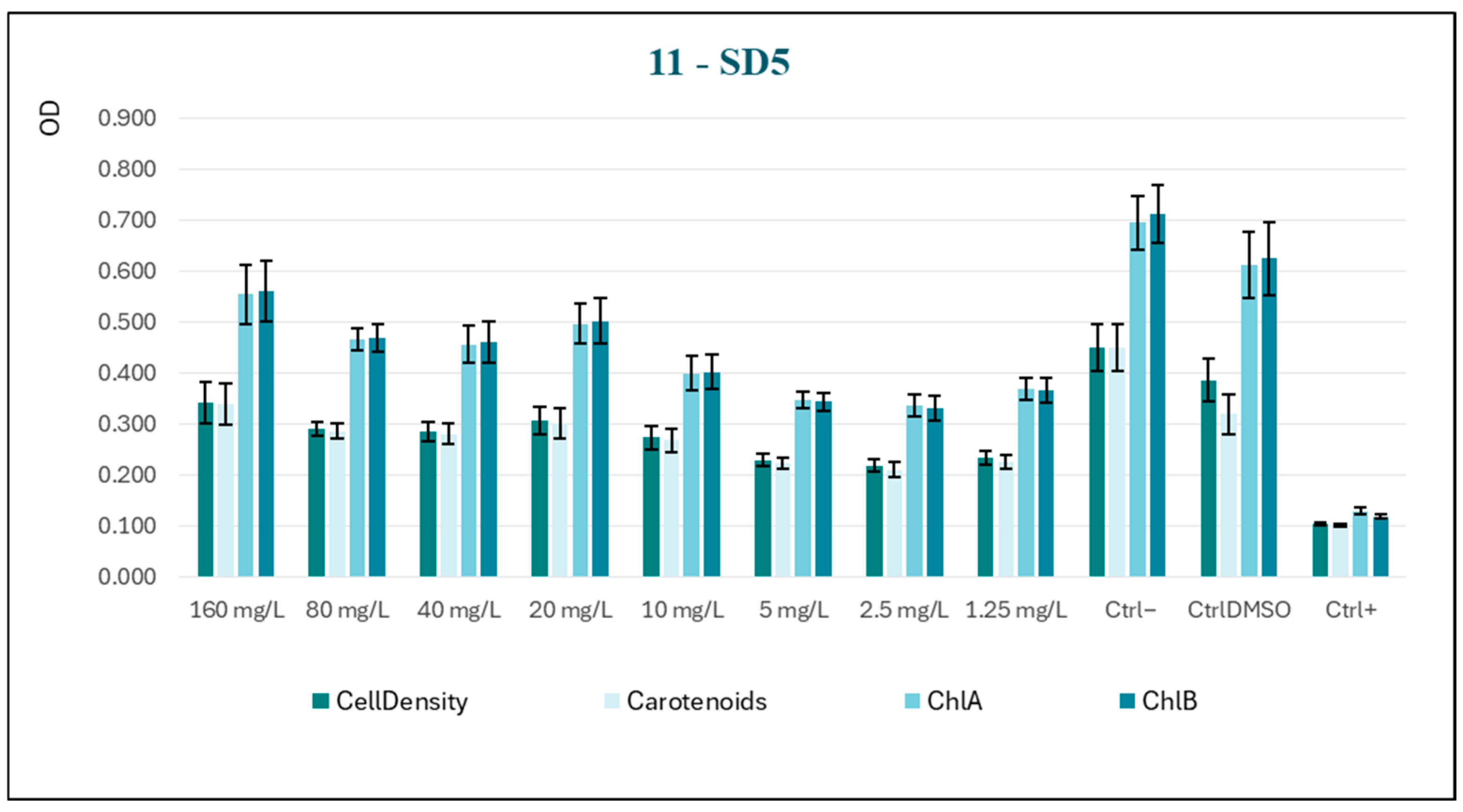
In light of the findings, it was determined that the analysis of a fifth substance, designated SD5-11 (
Figure 9), would be undertaken.
The substance under study was selected based on its structural similarity to the molecule previously analyzed. A notable distinction between the two is the presence of an isopropylidene-protecting group in the former. In comparison with SD3, this molecule constitutes a narrower analog of ribose, further characterized by the presence of an acetyl group, a factor that endows it with greater cell permeability.
Once more, the decision was made to proceed with a progression of concentrations from 1.25 mg/L up to 160 mg/L. The data trend (
Figure 10) manifests an evident increase in algal growth as the concentration of the substance rises, a phenomenon previously observed with substance SD4; the progressive increase in treated samples was, however, never comparable to the vitality shown by both negative controls. This finding indicates that the test substance may be partially assimilated and utilized by the model organism as a nutrient source.
4. Conclusions
This paper presents the design, synthesis, and biological evaluation of a novel class of selenium-based biocides developed to protect cultural heritage materials from bio-deterioration while remaining safe for use by restorers. Microbial colonization by bacteria, fungi, and algae poses a significant threat to historical artifacts and architectural surfaces, often resulting in irreversible chemical and physical degradation. Addressing this challenge requires innovative and sustainable preservation strategies. Selenium, owing to its unique redox chemistry and known biological activity, was explored in this study as a key component in the development of new biocidal agents. Efforts were made to minimize the environmental impact of the synthetic approach by using stoichiometric amounts of solvents and naturally occurring monosaccharides, such as D-mannose and D-ribose, as starting materials. These sugars were functionalized to produce a series of seleno-sugar derivatives designed to mimic natural substrates while incorporating selenium atoms to confer oxidative antimicrobial activity. The resulting compounds were comprehensively characterized using Nuclear Magnetic Resonance (NMR) spectroscopy and Gas Chromatography–Mass Spectrometry (GC-MS), confirming their structural integrity and purity. A central aspect of the molecular design involved converting the starting monosaccharides into their isopropylidene derivatives, which altered the polarity of the sugar cores and enhanced their cellular uptake. Experimental results in fact indicated that compound SD5 exhibits lower toxicity compared to SD3, likely due to structural differences—specifically, the presence of the isopropylidene group in SD3. Upon cellular uptake, the redox properties of selenium contributed to the destruction of test microorganisms, primarily through the generation of reactive oxygen species (ROS). To evaluate the biological efficacy, the synthesized compounds were tested using the OECD 201 algal growth inhibition assay—a standardized protocol for assessing the toxicity of chemicals to aquatic primary producers. The freshwater green microalga Raphidocelis subcapitata was selected as the model organism due to its sensitivity to toxicants and its relevance in eco-toxicological studies. The assay provided IC50 values (half-maximal effective concentrations) as quantitative indicators of biocidal activity. The bioassay results revealed that one of the synthesized compounds exhibited significant algicidal activity, characterized by a clear dose–response relationship and an IC50 value indicative of strong inhibitory effects. The bioassay results revealed that one of the synthesized compounds, i.e., SD3, exhibited significant algicidal activity, characterized by a clear dose–response relationship and an IC50 value of 49.99 mg/L (p-value: 0.031), indicative of potent inhibitory effects. Interestingly, SD4 exhibited biphasic, concentration-dependent effects: it stimulated algal growth at lower concentrations but inhibited it at higher concentrations. This hormetic behavior suggests a more complex mechanism of action, potentially involving sub-lethal oxidative stress responses or metabolic activation pathways. These findings highlight both the potential and the complexity of using selenium-based compounds in biocidal applications. Further studies are warranted to elucidate the underlying mechanisms of action, evaluate long-term environmental impacts (unpublished results will be available shortly) and refine the chemical structures for targeted application in the conservation of cultural heritage. In conclusion, this study reports the successful synthesis and validation of the antimicrobial activity of selenium-functionalized sugars, laying the groundwork for their further development as operator-safe biocides. Taking into account the highlighted toxicity of SD3 to the algal model organism, it is reasonable to expect a similar response from taxonomically related aeroterrestrial strains. The next steps will focus on the evaluation of dose-effect response from previously isolated algal strains involved in the biodeterioration of mosaics and plasters in the Archaeological Park of Baia’s Thermal Baths; analyses of environmental samples and taxonomic annotation of algal strains such as Stichococcus bacillaris and Jenufa aeroterrestrica are discussed in another article currently under review. The results contribute to the growing body of research aimed at integrating advanced chemical strategies into the sustainable preservation of vulnerable cultural assets.