Abstract
Desiccated microclimates offer an effective method of managing safe storage of archaeological metals. They utilise simple hardware that can produce low relative humidity (RH) environments on a small scale to control post-excavation change in objects. Previous studies have highlighted the complexity of decision-making when setting up desiccated microclimates involving many factors that can impact on their performance. These include the design of the container used to house the microclimate, the ambient external atmosphere, the target internal RH, the amount of silica gel used and its regeneration schedule. This paper builds on that understanding by replicating reported sector-wide variations in how silica gel is used within desiccated microclimates. The desiccation efficacy, rate of change in RH and response to short-term fluctuations have been examined by monitoring the RH in polypropylene containers when silica gel is used loose, in polythene bags and Tyvek® bags. The effect of variables in the use of polythene bags to hold silica gel, including the distribution of bags and the number and size of holes in bags has also been investigated. Results indicate that these variables impact rates of change in RH and how effective the desiccated microclimate is at buffering external RH fluctuations. All tests reinforce the importance of airflow between the silica gel and the microclimate. Where airflow is restricted, the ability of the microclimate to desiccate the environment below lowest known corrosion thresholds (15% RH) is compromised. The practical implications of the results have been discussed to support decision-making and guidance is offered on best practice.
1. Introduction
The safekeeping of archaeological metals in storage and on display presents significant management challenges for heritage sector professionals. Electrochemical corrosion occurring in the post-excavation environment can result in loss of metallic cores and formation of voluminous corrosion compounds that threaten the physical integrity of objects [1,2]. The primary aim of management of archaeological metal objects is to prevent loss of metal collections by controlling post-excavation corrosion. By identifying the mechanisms driving corrosion of archaeological metals, it is possible to develop methods of controlling unwanted change in objects [3]. A substantial body of research has collectively created a good understanding of the variables responsible for corrosion once objects are excavated [4,5,6,7]. From these, distinct or combined methods have been developed and adopted in practice, targeting the intrinsic and extrinsic variables responsible for corrosion.
For iron and copper alloys, chloride and chloride bearing compounds resulting from their corrosion in the burial environment are particularly problematic. They support a range of corrosion mechanisms that influence and lower the RH corrosion threshold. Strategies to prevent corrosion include removal of chloride or chloride compounds within the complex corrosion profiles of objects [8,9,10,11] or preventing interaction between the object and atmospheric oxygen or water by application of coatings or inhibitors [12,13,14,15] or by controlling the environment around the object. A quantitative relationship between corrosion rate and the amount of atmospheric water, measured as relative humidity (RH), has been proven [11,16,17,18,19] and controlling RH offers a readily achievable technique for controlling corrosion. Desiccating the environment can reduce the rates at which post-excavation corrosion occurs [7,11,17,18] and when the RH falls below critical thresholds, electrolytic corrosion cannot be supported [20]. These thresholds are different for different metals but must be considered low. For example, corrosion has been measured at 15% RH and 28% RH when simulating post-excavation corrosion mechanisms for archaeological iron and copper alloys containing chlorides and/or chloride compounds [17,18,21]. An effective management system that uses desiccation to control corrosion should achieve and retain a low RH over long periods of time. It should also offer protection against events that may affect the RH around objects, such as long-and short-term fluctuations of external environments. This simple concept can, in reality, be challenging to achieve [22]. Nearly 30% of museums worldwide struggle to maintain adequate hygrometric levels for their collections, and almost one in six museums (16%) report that objects have been damaged due to difficulties of achieving and maintaining environmental parameters, including temperature and humidity [23].
2. Desiccated Microclimates in the Heritage Sector
Desiccated microclimates are commonly used in the heritage sector to control RH around objects immediately post-excavation and during longer-term storage and display [16]. They offer a cost-effective and sustainable solution where large quantities of objects are reliant on RH control for their preservation without the need for structural climate control units to be installed. By utilising relatively cheap and reusable materials they can avoid the need for interventive treatment of objects. They are a “greener” alternative than many chemical treatments and have a smaller carbon footprint than climate-controlled stores. The hardware required to set up a desiccated microclimate is an airtight enclosure, a desiccant and a device to monitor the RH within the enclosure [16,24] (Figure 1). Each of these play an important part in the overall success of a desiccated microclimate.
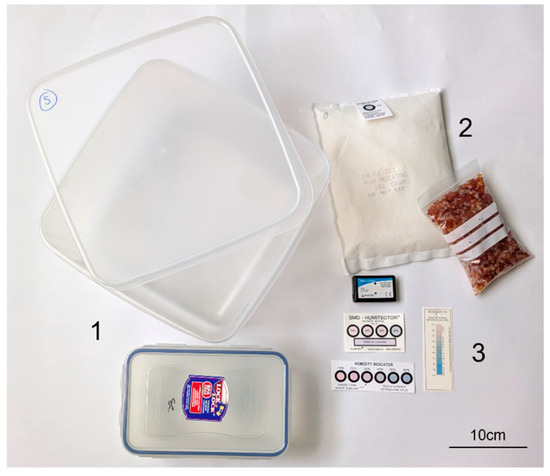
Figure 1.
Components of a desiccated microclimate. Examples of (1) airtight enclosure (2) desiccant and (3) RH monitoring devices.
2.1. Components of Desiccated Microclimates
An enclosure acts as a host in which desiccation can be achieved on a small scale. Enclosures used in practice include display cases, metal cabinets and polyolefin containers, amongst others. To retain a desiccated environment, the airtightness of an enclosure is critical. No enclosure can provide a hermetic seal as imperfections in materials and design allow movement of air and moisture vapour between the interior of enclosures and the external environment [16,25,26,27,28,29]. Diffusion is expected to take place whenever there is a difference between the internal and ambient environment of an enclosure [16,30]. In the case of desiccated microclimates, the internal enclosure RH is usually lower than the external RH. This causes diffusion-driven net movement of water vapour into the enclosure, which increases the internal RH as it strives to equilibrate with the incoming damp air. RH below corrosion thresholds is more likely to be achieved in enclosures with good airtightness and this will prolong the time between maintenance of the desiccated microclimates. Because of the important role of airtightness, much of the research conducted on microclimates in display cases and storage has focused on airtightness of enclosures and how to evaluate this (e.g., [16,26,27,28,29,30,31,32,33,34]).
A desiccant is placed in an enclosure to lower the RH below corrosion thresholds and to counteract changes in RH caused by diffusion of water vapour into the microclimate from the external environment. Regular density (RD) silica gel is commonly used as a desiccant [35,36]. It is an amorphous silicon dioxide which has an affinity to hydrogen bond with water molecules via a large surface network of polar silanol groups (Si-OH) [37,38,39]. It will adsorb water molecules in layers until saturation is achieved, when the amount of adsorbate is up to 40% of the weight of silica gel [37,40]. The adsorption capacity of silica gel makes it an excellent material for desiccating microclimates below corrosion thresholds even where small quantities of gel are used. A significant advantage of using silica gel as a desiccant is its ability to be reused. Heating the desiccant in elevated temperatures will break the bonds between water and the silica gel surface, allowing evaporation of water molecules. This frees up the silanol sites for further adsorption of water vapour.
The large capacity of silica gel to hold water vapour also decreases the rate at which RH changes within an enclosed desiccated microclimate due to inward movement of water vapour. As with any adsorbent material, silica gel will respond to an increase in RH by adsorbing water vapour until equilibrium is achieved [35,36]. This buffering effect offsets features that compromise the airtightness of enclosures and reduces the rate at which the RH changes [35,41]. As silica gel absorbs moisture its capacity to desiccate gradually diminishes and the equilibrium RH it maintains in the enclosed space it is controlling rises. Therefore, it must be replaced with fresh gel before its equilibrium RH exceeds corrosion thresholds. Increasing the amount of silica gel within microclimates reduces the rate at which RH changes by providing a bigger reservoir of adsorbency, which offers more consistent performance between microclimates, thereby offsetting variations in airtightness of enclosures [16]. To manage the performance of the silica gel within the enclosure, a monitoring device is essential to ensure that the RH is maintained below target RH thresholds. Accurate operation and user interpretation of these has significant impact on the successful use of desiccated microclimates as they show whether desiccation below corrosion thresholds is achieved and indicate when the desiccant requires regeneration [24,28,42,43,44].
While the components of a desiccated microclimate are straightforward and the role of each part is clear, the use of each component involves several decisions which each affect the overall success of the desiccated microclimate. Enclosures utilise different materials and designs that impact their airtightness [16,26,27,33], monitoring devices have different practical implications in their use and accuracy [28,42] and the efficacy of silica gel has been shown to be affected by factors such as the amount included in the enclosure and whether damp objects are present [16]. This demonstrates the importance of examining both what hardware is used and how it is used to allow development of best-practice standards.
Despite these multifactor variables that influence performance of microclimates, their use in the heritage sector is still largely led by anecdotal success and institutional habit. Efforts are made to standardise practice, but evidence is still limited to prove and effectively communicate which variables drive the success of desiccated microclimates and how different practices affect their performance. Where nearly one in three museums (30%) report that staff have not received adequate training for managing collections held in storage [23] this presents risk to objects. The limited application of evidence-based practice is evident in the varied practices employed for desiccated microclimates for archaeological metals [16].
2.2. Use of Silica Gel in Desiccated Microclimates
Although it is widely known that silica gel should be used to achieve a low RH environment in a desiccated microclimate, few guidelines offer any recommendations for how silica gel should be employed. This has led to considerable variation in the use of silica gel within desiccated microclimates across the sector. Many do not control or know the amount of silica gel used in microclimates and regenerate the silica gel on an ad-hoc basis [16]. A survey revealed that silica gel appears to be mainly held in some form of bag, either polythene bags (41%) or in Tyvek® or cotton bags where silica gel is pre-weighed (46%). Fewer practitioners (14%) use silica gel loose in the enclosure [45]. Where polythene bags are used to hold silica gel, these are most often pierced before use. A variety of tools appear to be used to pierce bags; from needles, pins and paperclips to bamboo skewers and hole punches. Most respondents reported piercing holes randomly, while others pierce between a single hole to two thirds of the bag area [45]. Some indicate that they do not pierce any holes in polythene bags (Table 1).

Table 1.
Preparation methods of polythene bags. Based on 90 responses from a sector-wide study [45].
The effect of practical variations like these has only been studied to a limited extent. They may affect successful use of desiccated microclimates by impacting the effectiveness of desiccation and the rate and range of RH change within the enclosure. These are important parameters for understanding whether RH levels below corrosion thresholds are achieved and how often maintenance of the microclimate is required. Previous studies have established a quantitative relationship between the amount of silica gel used and rate of change in RH within polyolefin containers [16]. This paper aims to refine procedural use of silica gel in desiccated microclimates by examining how different methods of packing equal quantities of silica gel within enclosures affects their performance. This was achieved by monitoring the RH within polypropylene containers where:
- the size and number of holes in polythene bags containing silica gel were incrementally increased
- silica gel was loose or distributed across one or several polythene bags
- silica gel was held in bags made of polythene sheet and spun polythene fibres (Tyvek®)
3. Methods
3.1. Desiccation of Silica Gel and Datalogger Calibration
A mixture of 50% regular density (RD) indicating silica gel (<0.2% methyl violet orange-green) and 50% regular density non-indicating silica gel (Gee-Jay Chemicals) was desiccated using a Snol 60/300 LFN (+10−300 °C, ±0.3 °C) oven with temperature controller VC7 ProWIC at 105 °C. Full desiccation was ensured by establishing a constant weight using a Kern precision balance EW 2200–2 NM (±0.01 g) indicating that the gel did not desorb any more moisture at this temperature, and by enclosing the desiccated gel within a container and monitoring its RH using a MadgeTech 101 RH temp data logger (±1.1% RH at 20% RH and 20 °C) for a minimum of 24 h. For use in the experiment, a total of 84 MadgeTech 101A RH temp loggers were two-point calibrated at 80% RH and 20% RH at 20 °C Binder KBF720 (±1.5% RH ±0.1 °C) climate chamber generating an error between ±1.1% RH and 1 ± 1.9% RH depending on the humidity.
3.2. Microclimates
The method of establishing microclimates varied with the purpose of the test.
To investigate desiccation capacity of silica gel according to the initial RH at which the box was closed when setting up the microclimate:
The relationship between the initial RH established within a polypropylene container used as the microclimate enclosure space and the desiccation process within it was examined. Six 1 L Lock and Lock containers had their lids removed and were placed in a Binder KBF720 (±1.5% RH ±0.1 °C) climate chamber at 20 °C and 80% RH for 24 h to equilibrate. After this time, the chamber was opened and silica gel was placed in the containers. In three containers, 88 g of silica gel was placed in two self-sealing mini-grip bags (44 g in each bag) (Table 2). The amount of silica gel was chosen to align the method to previous studies [16]. The seals on the bags were closed and their top half was folded over before placing each bag against either end of the containers (Figure 2) [16]. Each polythene bag was pierced a total of 30 times using 1 mm pins in 30 mm × 20 mm intervals. In the three other containers, 88 g of loose silica gel was included. A MadgeTech RHTemp101A data logger programmed to record RH and temperature at 10-min intervals over the test period was placed inside each container. Containers were closed with their lids and the ambient environment was set to 20 °C and 50% RH in the climatic chamber. The RH within all containers was recorded for 13 days as it has been shown that full desiccation capacity can be achieved within this timeframe when 88 g of silica gel is employed in 1 L Lock & Lock boxes [16]. This process was repeated for an initial equilibration RH of 50% and 30% RH, in each case with the closed containers being enclosed in a 50% RH and 20 °C environment for 13 days.

Table 2.
Summary of hardware use throughout the study. Examined variables highlighted in grey. PE: polyethylene.

Figure 2.
Setup of 1 L Lock and Lock containers illustrating the distribution of 88 g silica gel over two bags in either end of the container and placement of datalogger.
To investigate the effect of piercing polythene bags:
The impact of piercing polythene bags containing silica gel on a microclimate was studied by setting up 40 1 L Lock and Lock containers with bags pierced between 0 to 60 times (Table 2 and Table 3). Each bag was pierced on both sides with 1 mm pins at different intervals, depending on the number of holes. 88 g of silica gel was placed in two self-sealing mini-grip bags (44 g in each bag), their seals were closed and the top half of each bag was folded over before placing them against either end of the containers (Figure 2). A MadgeTech RHTemp101A data logger programmed to record RH and temperature at 10-min intervals over the test period was placed inside each container. The ambient environment was controlled to 20 °C and 50% RH in a Binder KBF720 (±1.5% RH ±0.1 °C). The RH within all containers was recorded for 250 days.

Table 3.
Containers used in conducted tests and the amount of silica gel used for each size. PP: polypropylene, Si: silicone.
To investigate the effect of hole dimensions in pierced polythene bags:
The effect of hole dimensions was examined across 30 1 L Lock & Lock containers. Polythene bags were pierced with pins, needles or bamboo skewers to create different hole dimensions. Each bag was pierced on both sides a total of 30 times at 20 mm × 30 mm intervals (Table 2 and Table 3). 88 g of silica gel was placed in two mini-grip bags (44 g in each bag) (Figure 2). The seals on the bags were closed and their top half was folded over before placing each bag placed against either end of the containers (Figure 2). A MadgeTech RHTemp101A data logger programmed to record RH and temperature at 10-min intervals over the test period was placed inside each container. The ambient environment was controlled to 20 °C and 50% RH in a Binder KBF720 (±1.5% RH ± 0.1 °C). The RH within all containers was recorded for 150 days.
To investigate the effect of distribution of silica gel within a desiccated microclimate:
The effect of distribution of silica gel was examined by placing 88 g of silica gel, either loose or contained across one, two or four bags, in 40 1 L Lock & Lock containers (Table 2 and Table 3). Bag sizes were chosen according to the amount of silica gel to be used so that each bag was approximately half full and a constant silica gel/bag ratio could therefore be achieved (Table 2). Each mini-grip bag was pierced on both sides with a pin at 20 mm × 30 mm intervals between 18 and 30 times to maintain a consistent ratio of holes in bags. The seals on each bag were closed and their top half folded over before placing each bag against opposite ends of the containers (Table 2). A MadgeTech RHTemp101A data logger programmed to record RH and temperature at 10-min intervals over the test period was placed inside each container. The ambient environment was controlled to 20 °C and 50% RH in a Binder KBF720 (±1.5% RH ±0.1 °C). The RH within all containers was recorded for 270 days.
To investigate the buffering capacity of containers and silica gel when placed either in polythene or Tyvek® bags:
To evaluate the buffering capacity during short-term RH fluctuations (Table 4) when silica gel is used in polythene or Tyvek® bags, 500 g of silica gel was placed in 12 containers of different brands (Table 3). 500 g of silica gel was used as this is understood to be a standard amount that is commonly used within desiccated microclimates. Six containers (two of each brand) contained one pierced (Table 2) mini-grip polythene bag with 500 g of mixed RD silica gel and six containers (two of each brand) contained one Tyvek® bag of 500 g non-indicating RD silica gel (Gee-Jay chemicals), desiccated as per method above. Three empty containers (one of each brand) acted as a control (Table 2). A MadgeTech RHTemp101A data logger programmed to record RH and temperature at 10-min intervals over the test period was placed inside each container.

Table 4.
Fluctuation patterns during 24 h. Each cycle was programmed to run for seven days.
Differences between the resulting microclimates according to silica gel bag material were examined by exposing the closed containers to short-term fluctuating RH cycles (Table 4). A Binder KBF240 (±1.5% RH ±0.1 K) was used to produce high RH events of differing lengths at 20 °C. Each cycle was run for seven days. Before and between each cycle, ambient conditions were set to 20 °C and 50% RH for 24 h as a baseline. The test was run for a total of 32 days.
4. Results
4.1. Influence of Initial RH
No relationship is evident between the initial RH and the desiccation capacity of the silica gel. All containers within an identical setup display a similar low RH regardless of the RH in a container when first closed. The RH at the time of closing the container affects the time taken to achieve full desiccation. It takes longer to achieve minimum RH when the RH at the time of closing a container is higher (Table 5). The desiccation behaviour varies depending on whether silica gel is loose or within polythene bags. Containers with loose silica gel are desiccated more rapidly and achieve an overall lower RH than containers containing silica gel in pierced polythene bags (Figure 3). Plotting the rate of desiccation against the square root of time illustrates that the adsorption of water vapour during the initial desiccation of the microclimates is mainly a diffusion process, and shows that the differences in desiccation rates are more pronounced when silica gel is held in polythene bags (Figure 3, Table 5). All containers tested here achieved an internal RH below 15% RH within 7 h (Figure 3).

Table 5.
Minimum RH and time to reach minimum RH in 1 L Lock & Lock containers where 88 g of silica gel is loose or placed in polythene bags at different RH environments.
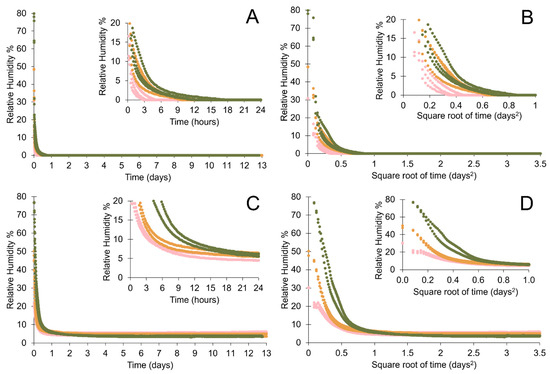
Figure 3.
Desiccation rates of 88 g silica gel in 1 L Lock & Lock containers when (A,B) loose and (C,D) in polythene bags where the initial RH values vary between 30% RH and 80% RH. Linear timelines (A,C) are plotted against square root of time (B,D) to highlight differences in desiccation rates. Insets show detail of the first 24 h.
4.2. Influence of Piercing Polythene Bags
Silica gel held in bags that have not been pierced shows a different desiccation behaviour and change in RH compared to where silica gel is placed in pierced bags (Figure 4). The ranges of the RH in containers with silica gel in pierced bags show considerable overlap but examination of the averages evidences general trends (Table 6).
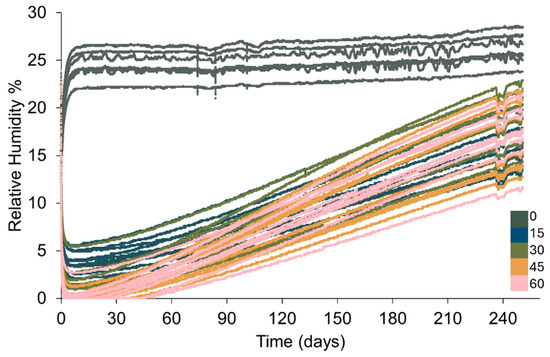
Figure 4.
Change in RH within 1 L Lock & Lock containers where 88 g of silica gel is placed in polythene bags pierced a varying number of times. Key indicates how many times each bag is pierced.

Table 6.
Minimum RH, time to reach minimum RH and the RH at 250 days when 88 g of silica gel is placed in polythene bags with different piercing intervals in 1 L Lock & Lock containers.
The RH in all containers decreases when silica gel is placed within them (Figure 4). In containers with bags that have been pierced, RH levels below corrosion thresholds (15% RH) are readily achieved. A compromised desiccation capacity of silica gel in bags that have not been pierced is evident as a significantly slower initial desiccation rate and a higher minimum RH (16 ± 1.8% RH) (Figure 4 and Figure 5, Table 6) than any setup where bags of silica gel have been pierced. The consistency in performance between containers and initial desiccation rates generally improve with increasing number of holes (Figure 5), although no meaningful difference can be shown between silica gel in bags that have been pierced between 30 and 60 times (Figure 6, Table 6).
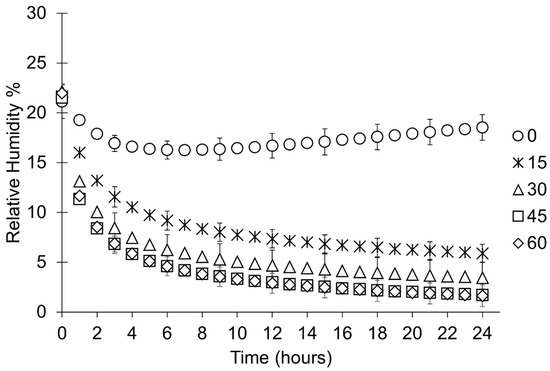
Figure 5.
Rate of desiccation during the first 24 h of the study where mean data from each sample set is presented in one-hour intervals. Error bars represent the standard deviation of that datapoint. Key indicates how many times each bag is pierced.
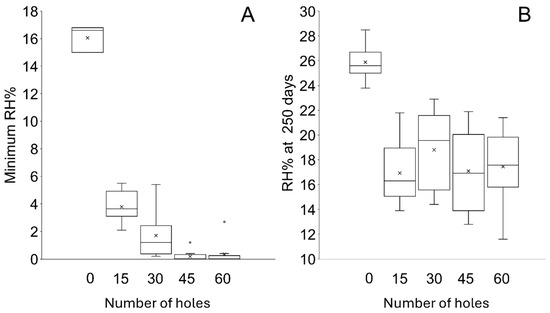
Figure 6.
Boxplot of the (A) minimum RH and (B) RH at 250 days showing the spread of data. In all boxplots presented here, the box represents the interquartile range, the horizontal line within the box denotes the median, the x shows the mean and the upper and lower whiskers represent the maximum and minimum values. A circle shows an outlier, defined as being between 1.5 and 3 times the interquartile range from the upper or lower quartile.
After the minimum RH is reached, the RH within all containers increases. The rate of change in RH is slowest in containers with bags containing no holes, followed by containers holding bags with 15 holes (Figure 4 and Figure 7). The rate of change in RH in containers with bags pierced between 30–60 times is similar throughout the study (Figure 4 and Figure 7). The range of performances and standard deviation of the mean in each setup increase as the RH within the containers increases towards the ambient RH (Figure 7, Table 6). At 250 days, the RH in containers with bags that have not been pierced is on average a minimum of 7.3% RH higher than any other setup (Figure 6 and Figure 7, Table 6). There is no meaningful difference between the end RH achieved where bags have been pierced.
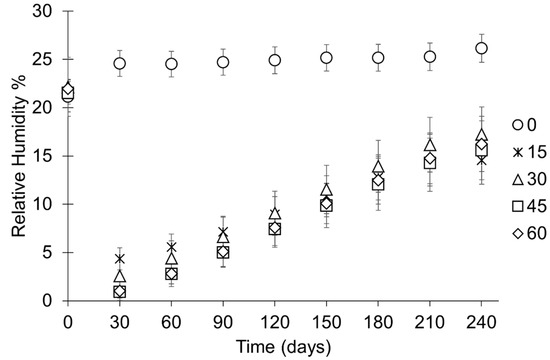
Figure 7.
Rate of change in RH throughout the period of study presented as the mean of each sample set at 30-day intervals. Error bars represent the standard deviation of that datapoint. Key indicates how many times each bag is pierced.
4.3. Influence of Size of Holes
Plotting all data shows an overlap between RH values for containers with different hole sizes in polythene bags and with loose silica gel (Figure 8, Table 7). Loose silica gel produces the lowest RH, followed by silica gel in bags pierced with Ø3 mm holes. Ø2 mm and Ø1 mm holes produce a similar minimum RH (Table 7), although some overlap can be seen between all containers with silica gel in polythene bags (Figure 9). Loose silica gel displays the fastest initial desiccation rate, followed by silica gel in bags pierced with Ø3 mm holes. Ø2 mm and Ø1 mm holes produce a similar rate of desiccation (Figure 10). All setups achieve an RH below accepted corrosion thresholds (15% RH).
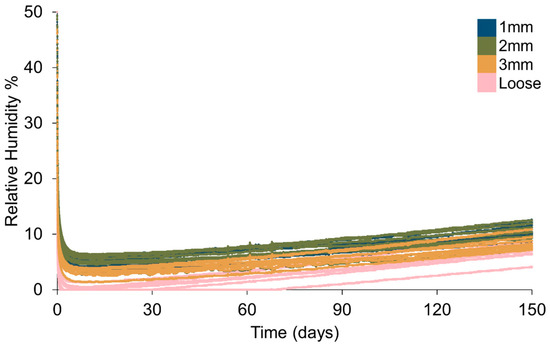
Figure 8.
Change in RH within 1 L Lock & Lock containers where 88 g of silica gel is placed in polythene bags with different diameter holes. Key indicates the size of each hole in diameter (mm).

Table 7.
Minimum RH, time to reach minimum RH and the RH at 150 days when 88 g of silica gel is placed in polythene bags with different piercing intervals in 1 L Lock & Lock containers.
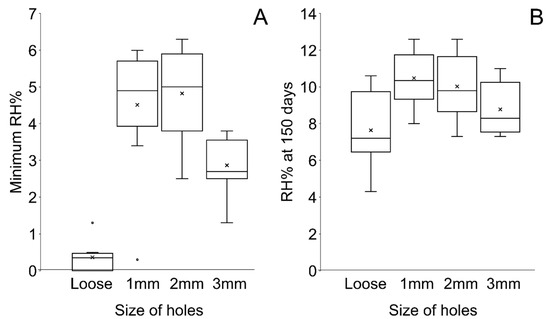
Figure 9.
Boxplot of (A) the minimum RH and (B) RH at 150 days (B) showing the spread of data.
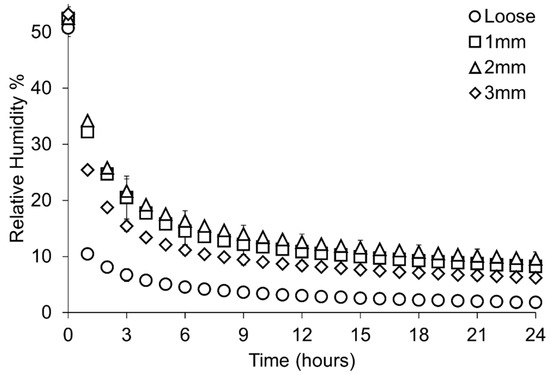
Figure 10.
Rate of desiccation during the first 24 h of the study where mean data from each sample set is presented in one-hour intervals. Error bars represent the standard deviation of that datapoint. Key indicates the size of each hole in diameter (mm).
After the minimum RH is reached, the RH increases in all containers. Loose silica gel demonstrates the highest rate of change in RH, while the rate is similar in all containers where silica gel is held in polythene bags (Figure 11, Table 7). At the end of the study, the mean RH in containers with loose silica gel is lower than other containers (Table 7) but there is an overlap in data between all setup types (Figure 9). The range and standard deviation in all setups increase as the RH increases within the microclimates towards the ambient external RH (Table 7).
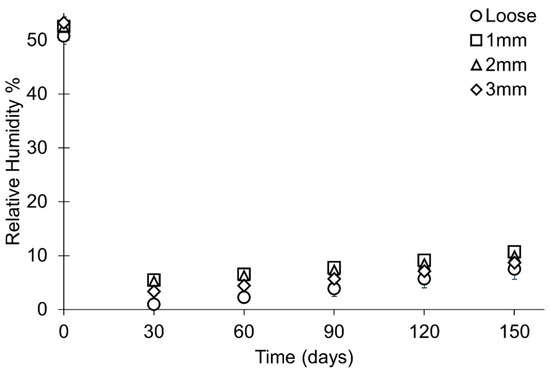
Figure 11.
Rate of change in RH throughout the period of study presented as the mean of each sample set at 30-day intervals. Error bars represent the standard deviation of that datapoint.
4.4. Influence of the Distribution of Silica Gel
Plotting all data shows an overlap between the RH in containers with different distributions of silica gel, however the RH is higher overall where silica gel has been packed in one bag (Figure 12). All setups achieve an RH below accepted corrosion thresholds (15% RH). Silica gel placed in two or four bags desiccates the microclimate similarly and achieves a lower minimum RH than silica gel placed in one bag (Figure 13, Table 8). The initial rate of desiccation is faster where silica gel is placed in two and four bags than silica gel in one bag, however silica gel in four bags reaches the minimum RH before silica gel placed in two bags (Figure 14, Table 8). Examination of the data ranges suggests that the minimum RH achieved is more consistent when silica gel is placed in two or four bags than silica gel in one bag (Figure 14).
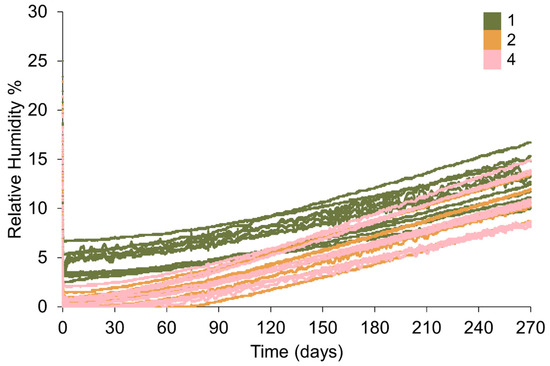
Figure 12.
Change in RH within 1 L Lock & Lock containers where 88 g of silica gel is placed in varying numbers of polythene bags. Key indicates the number of bags silica gel is distributed over.
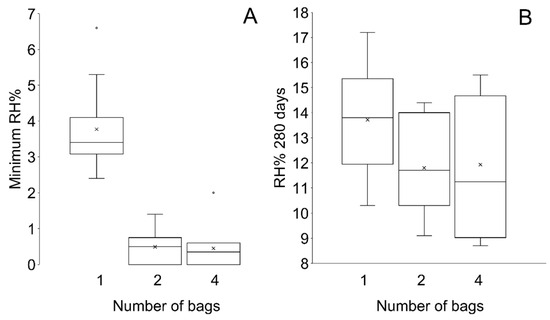
Figure 13.
Boxplot of (A) the minimum RH and (B) RH at 270 days showing the spread of data.

Table 8.
Minimum RH, time to reach minimum RH and the RH at 270 days when 88 g of silica gel is placed in varying numbers of polythene bags in 1 L Lock & Lock containers.
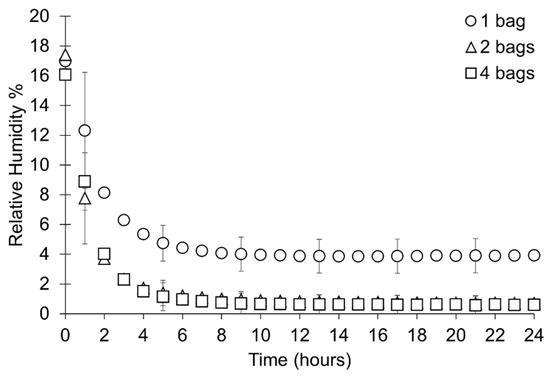
Figure 14.
Rate of desiccation during the first 24 h of the study where mean data from each sample set is presented in one-hour intervals. Error bars represent the standard deviation of that datapoint. Key indicates the number of bags silica gel is distributed over.
When the minimum RH has been reached, the RH increases in all setups. The rate of change in RH is slower where silica gel is placed in one bag than any other setup (Figure 12 and Figure 15). No significant rate differences are evident between two and four bags. The RH in all containers at 270 days shows no significant difference and an overlap in RH values is evident between setups (Figure 13, Table 8). Overall, the range and standard deviation increases as the RH changes within the microclimates towards the ambient RH (Figure 13, Table 8).
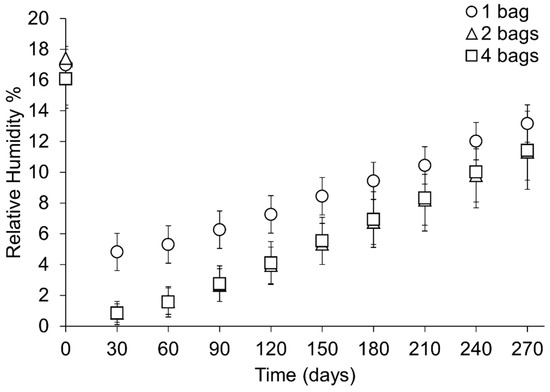
Figure 15.
Rate of change in RH throughout the period of study presented as the mean of each sample set at 30-day intervals. Error bars represent the standard deviation of that datapoint. Key indicates the number of bags silica gel is distributed over.
4.5. Responses to Short-Term Fluctuations
The climatic chamber did not readily achieve 80% RH during short fluctuation events but often remained between 70–75% RH during periods of high RH. At the end of the study the chamber failed (Figure 16). The amount of silica gel used desiccated each container with little difference between container brands and all setups achieve an RH below accepted corrosion thresholds (15% RH). The minimum RH is higher in containers with silica gel in polythene bags, although these values are within or near the error of the dataloggers. Silica gel in Tyvek® bags desiccates containers more rapidly and to a lower RH than in polythene bags. Silica gel in Tyvek® bags achieve full desiccation capacity at 10 and 23 h, as compared to silica gel in polythene bags which required 225 and 233 h (Table 9). After minimum RH is achieved it begins to increase in containers with silica gel in polythene bags. The minimum RH is retained for the duration of the test when silica gel is used in Tyvek® bags (Table 9).
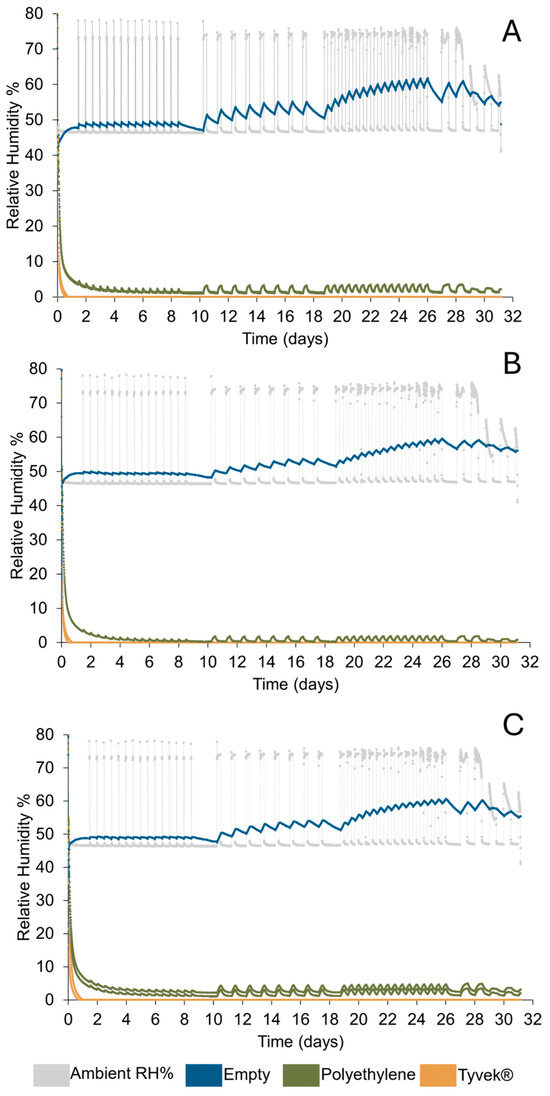
Figure 16.
Response to short-term fluctuations within 5–6.5 l containers that are either empty or 500 g of silica gel is placed in polythene or Tyvek® bags. (A) Stewart Gastronorm (B) Stewart Sealfresh (C) Lock & Lock.

Table 9.
Minimum RH, time to reach minimum RH and the RH at 32 days when 500 g of silica gel is placed in polythene or Tyvek® bags in 5–6.5 L containers of different brands.
Empty containers show some buffering capacity. Their response is smallest in cycle 1, where high RH fluctuations are the shortest. During these short fluctuations, empty containers can resume their initial RH between fluctuations. The response of empty containers became more significant with the length of each high RH fluctuation. Empty containers demonstrate a gradual rise in RH over time (Figure 16) as there is insufficient time between fluctuation cycles for these containers to resume 50% RH. Small differences in responses can be seen depending on the type of container, with Stewart Sealfresh demonstrating the least response in internal environment when subjected to ambient short-term fluctuations (Figure 16).
Containers with silica gel in polythene bags respond to all fluctuation cycles similarly to empty containers during short-term fluctuations (cycles 1–3) although the RH is overall lower (Figure 16). Some variation in response can be seen within container brands but not outside the error of the datalogger and is therefore not reported. Responses to fluctuations are shown immediately as the ambient RH is raised within each container and the RH can be seen to continue to rise during the length of time the ambient RH is high. It decreases immediately after the ambient RH falls.
In containers with silica gel in polythene bags, desiccation had not been completed within the first 24 h, and the continued decrease in RH is more significant than the RH increase during cycle 1 (Table 10). During six to 12 h of periods of high ambient RH in cycles 2–4 the RH rises but resumes the RH it was before the fluctuation event. Within these containers, it takes around 12 h for the RH to resume the value before the start of the fluctuation event (Table 10).

Table 10.
The average increase and decrease in RH between containers during and between fluctuation events. * The decrease in RH becomes smaller as RH approaches the full desiccation capacity.
5. Discussion
Consistent standardisation was attempted when conducting all parts of the study in order to identify trends in datasets. Despite this, variations in results using identical setups are evident throughout the study. Differences in desiccation rates are likely due to variations in RH when closing containers at the beginning of a test. Other variations, such as the minimum RH achieved and the rate of RH change is likely an effect of the containers that the tests are conducted in. Over 80 1 L Lock & Lock containers have been used within this study. These have been acquired in sets at different times. Differences in airtightness caused by small inconsistencies in design [16], use or age are likely to have affected the seal and consequently the ability of microclimates to perform consistently. Variation in airtightness between containers within the same sample set is evident as differences in rates of change in RH over time. This explains why the range and standard deviation of RH within sample sets is seen to increase, as silica gel desiccates the microclimate uniformly, but the subsequent rate of change is largely dependent on the airtightness of the container. The effect of airtightness is further exaggerated by the small amount of silica gel used, which has been shown to be insufficient to effectively reduce the effect of variation in airtightness between enclosures [16]. Similar spread in performances and rate of change in RH have been seen in previous test using 88 g of silica gel [16].
With this in mind, results such as the minimum RH and maximum RH achieved are only helpful to compare general performance trends. Further variation in the results can be expected if, for example, brand, design and volume of containers differ. The results achieved here confirm the important role of airtightness of containers and illustrate the intrinsic links between all variables driving successful performance of microclimates.
5.1. Ambient Conditions When Setting Up Desiccated Microclimates
In practice, microclimates are set up in a range of different climates, from dry to humid environments depending on, for example, geographical location, seasonal fluctuations and weather patterns. It is therefore reasonable to assume that the amount of atmospheric moisture that the silica gel needs to adsorb varies depending on the environment in which the containers are closed. Theoretically, silica gel is able to adsorb a significant amount of water vapour relative to its weight. Assuming that RD silica gel can desiccate up to 40% of its own weight, microclimates using 88 g of silica gel within this study should theoretically be able to remove 35.2 g of water vapour and 500 g up to 200 g of water vapour.
It is possible to calculate hypothetically the absolute quantity of water vapour in the air within a container, using the ideal gas law:
where P is pressure in atmospheres, V is volume in litres, n is the amount of substance in mol, R is the ideal gas constant and T is temperature in Kelvin. Assuming that the container is hermetically sealed, that air is perfectly mixed, the temperature is consistent at 20 °C and water vapour behaves like an ideal gas, the mass of water within the containers used in this study is significantly below the saturation threshold for the amount of silica gel included within the containers when silica gel is dry (Table 11).

Table 11.
The theoretical amount of water vapour in containers used within this study at different RH when the temperature is 20 °C.
The results of this study confirm that the amount of silica gel used in all containers is sufficient to fully desiccate the microclimate consistently at different initial RH when silica gel is loose or held in Tyvek® bags (Figure 3, Figure 8 and Figure 16). The main effect of varying RH when closing the container is on the rate of desiccation. As more water vapour is required to be adsorbed by the silica gel in higher RH, the time to reach maximum desiccation capacity (minimum RH) is extended (Figure 3).
Despite the capacity of silica gel to fully desiccate the microclimate, full desiccation does not consistently occur where silica gel is held in polythene bags and rate of desiccation is slower than when silica gel is loose or held in Tyvek® bags. Reasons for this and variables affecting desiccation efficacy of silica gel in polythene bags is explored below.
5.2. Variables Affecting Desiccation Efficacy
Effective adsorption of any molecule on an adsorbate requires collision to take place between the adsorbate molecule and the adsorbant. In the context of this study, water molecules need to strike the surface of the silica gel for adsorption, and consequently desiccation, to occur [46]. The most effective and consistent adsorption of water vapour therefore occurs in setups where access between silica gel and water vapour is best facilitated. This is evident by comparing the RH in containers with loose silica gel to containers with silica gel held in bags. Desiccation rates are faster and full desiccation consistently occurs when silica gel is loose within containers. Comparing this to setups where water adsorption is prevented by a barrier, such as unpierced polythene bags, provides a stark example of this effect. The difference in desiccation capacity and rate of desiccation between loose silica gel and silica gel held in polythene bags suggest that the polythene bags present a barrier, preventing or delaying adsorption of water vapour. Grip seal polythene bags are typically made with low density polyethylene (LDPE) sheet material. Although not completely impermeable due to their branched polymer configuration, LDPE exhibits relatively low water vapour transmission properties [47,48], making it an extensively used material where low water vapour transmission is desirable, such as food and medical packaging and as a barrier material in construction applications. These properties prevent efficient diffusion of water vapour between the container and the bag which is necessary for adsorption to occur. Silica gel in Tyvek® bags was shown to desiccate the environment to a greater extend and at a faster rate than silica gel in polythene bags. Although both Tyvek® and polyethylene grip seal bags are made of polyethylene (PE), their manufacturing processes are different. Tyvek® is a spun high density polyethylene fibre (HDPE) and designed to allow diffusion of water vapour between the filaments [47,49].
Manipulating polythene bags to improve their diffusion properties has been demonstrated to be essential for allowing microclimates to achieve desiccation below corrosion thresholds. Piercing as little as 0.05% of the total surface area of a polythene bag using 1 mm implements offers sufficient airflow for desiccation to take place. Increasing the number of times a bag is pierced, or increasing the hole sizes, provides improved opportunity for adsorption. This allows for more rapid desiccation and could offer a more consistent minimum RH between containers. Increasing piercing intervals or hole sizes may achieve lower overall minimum RH, but no statistically significant differences were achieved in this study. Manipulating bags in the manner examined here did not offer any significant advantage to benefit the longevity of the silica gel before maintenance is required, as the RH was similar at the end of each test. Regardless of how 88 g of silica gel was used, the ratio of silica gel to volume of container (approximately 5% of total volume (cm2)) would require annual maintenance if the acceptable threshold is 15% RH when the external RH is 50% RH. More significant differences in results may be found where the hole intervals or size intervals are greater.
Similarly, distributing silica gel across two or more bags allows more effective and consistent adsorption of water vapour within containers than holding it in one bag. Spreading the silica gel increases the likelihood of adsorption occurring, as access to silica gel surface area increases. Within this study, this was shown in more rapid desiccation rates and more consistent minimum RH achieved when the same amount of silica gel was distributed over two or four bags. Distributing the silica gel over several bags could, however, not offer a demonstrable benefit to the longevity of silica gel and time to regeneration of silica gel, as the RH was similar in all containers at the end of the study. It is easier and more time-effective to access silica gel in two bags for regeneration than four.
Variations in gradients suggest that the rate of change in RH is mainly driven by the difference between the internal and ambient external RH when the quantity of silica gel is constant. This explains the comparable RH reached at the end of each test, regardless of how the variables within the test changed, and is consistent with results achieved in empty containers [16]. Increasing the amount of silica gel could decrease this effect and prolong the time between maintenance of the silica gel.
5.3. Silica Gel as a Buffer Against Short-Term Fluctuations
When compared to Tyvek®, the barrier to water vapour presented by polythene means that when silica gel is contained in polythene bags, it is not as effective a buffer against short-term fluctuations in RH as when it is contained in Tyvek® bags. In these scenarios, the container itself will be the primary buffer against RH fluctuations. The RH remains below corrosion threshold during short-term fluctuations in this study. Provided there is enough recovery time between short fluctuation events, the desired RH within the microclimate can be maintained. With longer fluctuations, more significant changes in RH the containers can be seen (Figure 16). Over time, the RH is expected to increase by diffusion in a similar pattern as seen in other tests in either setup.
The silica gel distributed in the polyethene bags is a mixture of indicating and clear silica gel while the silica gel in Tyvek® bags is clear silica gel. Both types are regular density (RD) type silica gels and therefore have similar properties. It is, however, acknowledged that indicating and clear silica gel have slightly different hysteresis and therefore different specific moisture reservoir (MH) values. The difference has been measured by others to be around 0.56 g/kg for a 1% RH change at 20 °C between 20–30% RH [41]. This value is considered small and to have a negligible impact on the results. A repeat of the test using either indicating or clear silica gel can confirm this.
5.4. Practical Considerations
All setups examined here allowed for desiccation of the microclimates below accepted corrosion thresholds. The ambient environment and the initial RH of the container when microclimates are set up has no significant implications for silica gel achieving an RH below corrosion thresholds when sufficient silica gel is used.
Containing silica gel loose within a container can produce lower RH values and more rapid desiccation but has practical implications. Guidelines discourage direct contact of objects with silica gel [50,51] and when regeneration is required, all objects must be removed from the container to access silica gel. It is further not known how placing objects on top of loose silica gel affects its desiccation properties. The potential impact of this should be examined.
Silica gel in Tyvek® bags shows superior desiccation properties and response to fluctuations as compared to silica gel in polythene bags. Tyvek® is generally a more expensive material than polythene bags, both as pre-weighed polythene bags or sheet material. Acquiring pre-weighed Tyvek® bags offer an initial advantage in setting-up time but the range of weights available is limited. Customisation of bags can be achieved using Tyvek® fabric but is more labour intensive than using polythene grip-seal bags and may not be practical in some contexts, such as when desiccated microclimates are employed on archaeological site immediately after excavation. As Tyvek® is opaque, it rules out the use of indicating silica gel to monitor RH within microclimates. It is likely that due to the nature of material, more consistent performance can be expected from Tyvek® than polythene bags by removing discrepancies caused by inconsistent methods of piercing bags, but this needs further examination. Tyvek® has a higher melting point (135 °C [52] than LDPE (106–112 °C [53]). Silica gel can therefore be reconditioned in bags and may offer a time-saving measure compared to LDPE bags where silica gel is typically removed from the bags before reconditioning. Note that recommended temperatures for reconditioning silica gel varies between 65 °C and 150 °C [35,41,50,54,55] and that neither bag is suitable to use in some of the higher recommended regeneration temperatures. 95% of adsorbed water vapour has been shown to be removed at 90 °C [37] and can therefore be recommended a minimum temperature for conditioning silica gel until the results of further examination of effective regeneration procedures are made available.
Containing silica gel in polythene bags is a cost-effective option and can effectively achieve desiccation below corrosion thresholds if pierced a minimum of 0.05% of the surface area of the bag used with implements that are a minimum 1 mm in diameter. Further manipulation of the bag to increase the surface area covered by holes, such as increasing hole size or number of holes, or distributing silica gel over several bags can offer advantages of faster desiccation rates and lower minimum RH achieved, but offers no demonstrable benefit in the rate of change in RH and silica gel maintenance intervals for microclimates where the amount of silica gel is constant.
A significant benefit for practice is that increasing size or number of holes in bags and distributing silica gel further within a container can offer more consistent performance between microclimates. Predictability allows for better understanding of when silica gel requires regeneration and is therefore important to produce effective management regimes. Recognising the significance of predictability, users of microclimates should, as far as possible, be consistent in their setup. Where larger surface area of hole coverage is sought, larger implements offer a significant benefit where the same surface area can be achieved with fewer number of holes as compared to smaller implements (Figure 17). As there is no apparent cost-implication or significant time-implications, using any tool that achieves a minimum of Ø3 mm holes in bags covering at least 0.15% of the total surface area of the bag used can be recommended.
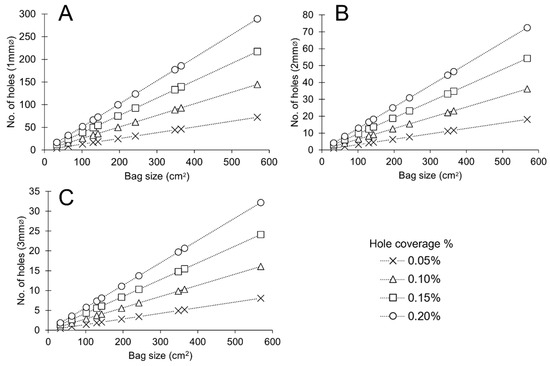
Figure 17.
Number of holes required in standard PA-sized bags to achieve different hole coverage of total surface area of bags. (A) Ø1 mm implements (B) Ø2 mm implements (C) Ø 3 mm implements.
5.5. Guidelines for Use of Desiccated Microclimates for Heritage Metals
In combination with previous studies [16], it is now possible to refine the effective use of desiccants in desiccated microclimates:
- Never use desiccant to attempt to dry damp terrestrial archaeological metals
- Choose an amount of silica gel to suit your management regimes. If no corrosion thresholds (15% RH) is desirable in an ambient environment of 50% RH (note that these recommendations only apply to a proven airtight enclosure [16]:
- ○
- 44 g of silica gel per L of enclosure can be expected to need maintenance four times per year
- ○
- 88 g of silica gel per L of enclosure can be expected to need maintenance biannually
- ○
- 176 g of silica gel per L of enclosure can be expected to need maintenance annually
- ○
- 352 g of silica gel per L of enclosure can be expected to need maintenance every four years
- If storage environments are uncontrolled and include periods of high humidity, it is appropriate to double these values to achieve the same lifespan
- Use permeable materials, such as Tyvek® bags, to contain silica gel in order to achieve full desiccation of a microclimate effectively and to counteract short-term RH fluctuations
- ○
- Polythene bags may offer a cost-effective option for temporary storage and customised use
- ○
- A monitoring device is essential as indicating silica gel cannot be seen within Tyvek® bags
- Polythene bags must be pierced
- ○
- Implements used to pierce holes should be ≥ Ø3 mm
- ○
- A minimum of 0.15% of the bag area should be pierced with holes
- ○
- Holes should be distributed across bags to achieve good airflow between the desiccant and the enclosure
- Distribute silica gel over a minimum of two bags within a container
- Be consistent in setup (amount of silica gel/bag size/hole size/number of holes/distribution) to offer predictability of desiccant performance
This guidance can be applied according to the means and aims of different collections with known implications of the efficacy of desiccated microclimates.
6. Conclusions
The implications of using silica gel in a range of ways that simulate reported practices have been examined for the first time. The outcomes demonstrate which variables affect adsorption of water vapour by silica gel and how internal RH of containers changes depending on how silica gel is used. Comparison between different methods of setting up desiccated microclimates shows that materials chosen and practices used to prepare silica gel for use in desiccated microclimates can influence its success as a desiccant. Work is ongoing to further evaluate variables affecting successful use of desiccated microclimates, including:
- Continuing investigation of the airtightness of commonly used containers
- Examining the effect of ambient temperature and RH on airtightness of containers
- Evaluating the effect of packing density on the rate of humidity change within containers
- Assessing the performance of low-permeability Escal bags for use as an enclosure material for desiccated microclimates
- Evaluating how methods of stacking containers with different seal mechanisms affect their performance
- Cost-benefit appraisal of regeneration procedures for silica gel
- Establishing successful tools for monitoring the environment within desiccated microclimates
This work is performed with the goal of creating transparent, cost-effective, sustainable and predictable management strategies to ensure long-term preservation of archaeological metals.
Author Contributions
Conceptualization, J.T., N.E. and D.W.; methodology, J.T., N.E. and D.W.; formal analysis, J.T.; investigation, J.T.; data curation, J.T.; writing—original draft preparation, J.T.; writing—review and editing, N.E. and D.W.; visualization, J.T.; supervision, N.E. and D.W.; funding acquisition, J.T., N.E. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Arts and Humanities Research Council, South West and Wales Doctoral Training Partnership Studentship.
Data Availability Statement
The datasets presented in this article are not readily available because the data is part of an ongoing study. Requests to access the datasets should be directed to J.T.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- Wang, Q. An Investigation of Deterioration of Archaeological Iron. Stud. Conserv. 2007, 52, 125–134. [Google Scholar] [CrossRef]
- Watkinson, D. Conservation Science in Practice. In Handbook of Archaeological Sciences, 2nd ed.; Pollard, A.M., Armitage, R.A., Makarewicz, C.A., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2023; pp. 1063–1073. [Google Scholar]
- Réguer, S.; Dillmann, P.; Mirambet, F. Buried iron archaeological artefacts: Corrosion mechanisms related to the presence of Cl-containing phases. Corros. Sci. 2007, 49, 2726–2744. [Google Scholar] [CrossRef]
- Remazeilles, C.; Refait, P. On the formation of β-FeOOH (akaganeite) in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- MacLeod, I. Bronze disease: An electrochemical explanation. ICCM Bull. 1981, 7, 16–26. [Google Scholar] [CrossRef]
- Scott, D.A. Bronze Disease: A Review of Some Chemical Problems and the Role of Relative Humidity. J. Am. Inst. Conserv. 1990, 29, 193–206. [Google Scholar] [CrossRef]
- MacLeod, I.D. Conservation of Corroded Copper Alloys: A Comparison of New and Traditional Methods for Removing Chloride Ions. Stud. Conserv. 1987, 32, 25–40. [Google Scholar] [CrossRef]
- Giovanelli, G.; D’Urzo, L.; Maggiulli, G.; Natali, S.; Pagliara, C.; Sgura, I.; Bozzini, B. Cathodic chloride extraction treatment of a late Bronze Age artifact affected by bronze disease in room-temperature ionic-liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesylfonyl)imide (EMI-TFSI). J. Solid State Electrochem. 2010, 14, 479–494. [Google Scholar] [CrossRef]
- Watkinson, D.E.; Emmerson, N.J. The impact of aqueous washing on the ability of βFeOOH to corrode iron. Environ. Sci. Pollut. Res. 2017, 24, 2138–2149. [Google Scholar] [CrossRef]
- Watkinson, D.E.; Rimmer, M.B.; Emmerson, N.J. The Influence of Relative Humidity and Intrinsic Chloride on Post-excavation Corrosion Rates of Archaeological Wrought Iron. Stud. Conserv. 2019, 64, 456–471. [Google Scholar] [CrossRef]
- Molina, T.; Cano, E.; Ramirez-Barat, B. Protective coatings for metallic heritage conservation: A review. J. Cult. Herit. 2023, 62, 99–113. [Google Scholar] [CrossRef]
- Mirambet, F.; Reuger, S.; Rocca, E.; Hollner, S.; Testemale, D. A complementary set of electrochemical and X-ray synchrotron techniques to determine the passivation mechanism of iron treated in a new corrosion inhibitor solution specifically developed for the preservation of metallic artefacts. Appl. Phys. A 2010, 99, 341–349. [Google Scholar] [CrossRef]
- Golfomitsou, S.; Merkel, J.F. Synergistic effects of corrosion inhibitors for copper and copper alloy archaeological artefacts. In Metal 04, Proceeding of the International Conference on Metals Conservation, National Museum of Australia, Canberra, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, ACT, Australia, 2004; pp. 344–368. [Google Scholar]
- Abu-Baker, A.N.; Al-Qudah, M.A. A novel dioxime compound for protecting copper in neutral chloride solutions and to treat bronze disease in archaeological artefacts. AICCM Bull. 2017, 38, 94–102. [Google Scholar] [CrossRef]
- Thunberg, J.; Watkinson, D.; Emmerson, N. Desiccated microclimates for heritage metals: Creation and management. Stud. Conserv. 2021, 66, 127–153. [Google Scholar] [CrossRef]
- Thickett, D. Critical Relative Humidity Levels and Carbonyl Pollution Concentrations for Archaeological Copper Alloys. In Proceedings of the ICOM-CC Metals Working Group New Delhi 2016, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; International Council of Museums Committee for Conservation and Indira Ghandi National Centre for the Arts: New Delhi, India, 2016; pp. 180–187. [Google Scholar]
- Watkinson, D.; Lewis, M.R.T. Desiccated storage of chloride-contaminated archaeological iron objects. Stud. Conserv. 2005, 50, 241–252. [Google Scholar] [CrossRef]
- Thunberg, J.; Emmerson, N.J.; Watkinson, D. Title of Unpublished Work. Heritage, 2025; submitted manuscript. [Google Scholar]
- Leygraf, C.; Graedel, T. Atmospheric Corrosion; Wiley: Chichester, UK, 2000. [Google Scholar]
- Thickett, D.; Odlyha, M. The formation and transformation of akaganeite. In Proceedings of the Metal 2013 Interim Meeting of the International Council of Museums Committee for Conservation Metal Working Group, Edinburgh, Scotland, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; ICOM-CC, Historic Scotland: Paris, France; pp. 103–109. [Google Scholar]
- Ganiaris, H.; Barham, L.; Goodman, L. Great expectations: A review of iron from waterlogged contexts from London sites excavated in the 1980s and 1990s. J. Inst. Conserv. 2012, 35, 3–13. [Google Scholar] [CrossRef]
- ICOM. Museum Storage Around the World; Mairesse, F., Théobault, M., Eds.; ICOM: Paris, France, 2024; Available online: https://icom.museum/en/news/museum-storage-around-the-world/ (accessed on 1 March 2025).
- Thunberg, J.C. Large Collections of Small Metal Objects: Managing their preservation via desiccated microclimates. In Bridging the Gap: Corrosion Science for Heritage Contexts; Neff, D., Grassini, S., Watkinson, D., Emmerson, N., Eds.; European Federation of Corrosion; Woodhead Publishing: London, UK, 2025. [Google Scholar]
- Michalski, S. Leakage prediction for buildings, cases, bags and bottles. Stud. Conserv. 1994, 39, 169–186. [Google Scholar] [CrossRef]
- Senge, K.D. Testing and Implementation of Microclimate Storage Containers. In Objects Speciality Group Postprints, Volume Twenty-One; The American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 2014; pp. 123–140. [Google Scholar]
- Larkin, N.R.; Makridou, E.; Comerford, G. Plastic storage containers: A comparison. Conservator 1998, 22, 81–87. [Google Scholar] [CrossRef]
- Lankester, P.; Thickett, D.; Johnson, S. Long-Term Provision of Stable Environments for Metals Conservation. In Metal 2022, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Helsinki, Finland, 5–9 September 2022; Mardikian, P., Näsänen, L., Arponen, A., Eds.; ICOM-CC: Paris, France; National Museum of Finland: Helsinki, Finland, 2022; pp. 261–270. [Google Scholar]
- Thickett, D.; Luxford, N. Air exchange rate—the dominant parameter for preventive conservation? Conservator 2005, 29, 19–34. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Ramer, B. Museum display cases and the exchange of water vapour. Stud. Conserv. 1983, 28, 179–188. [Google Scholar] [CrossRef]
- Tétreault, J.; Hagan, E. Airtightness Measurement of Display Cases and Other Enclosures; Technical Bulletin 38; Canadian Conservation Institute: Ottawa, ON, Canada, 2022. Available online: https://www.canada.ca/en/conservation-institute/services/conservation-preservation-publications/technical-bulletins/airtightness-measurement-display-cases.html#a8 (accessed on 3 February 2025).
- Calver, A.; Holbrook, A.; Thickett, D.; Weintraub, S. Simple methods to measure air exchange rates and reduce the ingress of externally generated pollutants and particulates. In Proceedings of the ICOM 14th Triennial Meeting; The Hague Preprints, The Hague, The Netherlands, 12–16 September 2005; Committee for Conservation. Verger, I., Ed.; Preprints. James and James: London, UK, 2005; Volume II, pp. 597–609. [Google Scholar]
- Thickett, D.; Odlyha, M. Assessment of dry storage microenvironments for archaeological iron. In The Conservation of Archaeological Materials: Current Trends and Future Directions, Williamsburg, VA, USA, 13–17 November 2010; Peachey, C., Williams, E., Eds.; Postprints; British Archaeological Reports Publishing: Oxford, UK, 2010; pp. 187–199. [Google Scholar]
- Thomson, G. Stabilisation of RH in exhibition cases: Hygrometric half-time. Stud. Conserv. 1977, 22, 85–102. [Google Scholar] [CrossRef]
- Weintraub, S. Demystifying Silica Gel. In Object Speciality Group Postprints; American Institute for Conservation: Washington, DC, USA, 2002; Volume 9, Available online: http://resources.conservation-us.org/osg-postprints/wp-content/uploads/sites/8/2015/02/osg009-12.pdf (accessed on 24 January 2020).
- Yu, D.; Klein, S.A.; Reindl, D.T. An evaluation of silica gel for humidity control in display cases. WAAC Newsl. 2001, 23, 14–19. [Google Scholar]
- Ng, K.C.; Chua, H.T.; Chung, C.Y.; Loke, C.H.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Experimental investigation of the silica gel-water adsorption isotherm characteristics. Appl. Therm. Eng. 2001, 21, 1631–1642. [Google Scholar] [CrossRef]
- Zhuralev, L.T. Concentration of Hydroxyl Groups on the Surface of Amorphous Silicas. Langmuir 1987, 3, 316–318. [Google Scholar] [CrossRef]
- Zhuralev, L.T. The surface chemistry of amorphous silica. Zhuralev Model. Colloids Surf. A Physicochem. Eng. Asp 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Christy, A.A. New insights into the surface functionalities and adsorption evolution of water molecules on silica gel surface: A study by second derivative near infrared spectroscopy. Vibr. Spectrosc. 2010, 54, 42–49. [Google Scholar] [CrossRef]
- Tétreault, J.; Bégin, P. Silica Gel: Passive Control of Relative Humidity; Technical Bulletin 33; Government of Canada, Canadian Conservation Institute: Ottawa, ON, Canada, 2023; Available online: https://www.canada.ca/en/conservation-institute/services/conservation-preservation-publications/technical-bulletins/silica-gel-relative-humidity.html (accessed on 18 April 2025).
- Boff, R.; Daniels, V.; Ward, S. Humidial Corporation Humidity Indicating Card 6203-BB, a note on its use. UKIC Conserv. News 1981, 16, 10–11. [Google Scholar]
- Daniels, V.D.; Wilthew, S.E. An investigation into the use of cobalt salt impregnated papers for the measurement of relative humidity. Stud. Conserv. 1983, 28, 80–84. [Google Scholar] [CrossRef]
- Thickett, D. Post-Excavation Changes and Preventive Conservation of Archaeological Iron. Ph.D. Thesis, University of London, London, UK, 19 March 2012. Available online: https://www.english-heritage.org.uk/link/072d116ec6ad4b889d665ae665463ba4.aspx (accessed on 24 January 2020).
- Whitehead, S. Comparison of Current Practices in the Storage of Archaeological Metals. Master’s Thesis, Cardiff University, Cardiff, UK, September 2018. [Google Scholar]
- Li, X.; Li, Z.; Xia, Q.; Xi, H. Effects of pore sizes of porous silica gels on desorption activation energy of water vapour. App. Therm. Eng. 2007, 27, 869–876. [Google Scholar] [CrossRef]
- Dowan, K.; Seo, J. A review: Breathable films for packaging applications. Trends Food Sci. Technol. 2018, 76, 15–27. [Google Scholar]
- McKeen, L.W. Polyolefins, Polyvinyls, and Acrylics. In Permeability Properties of Plastics and Elastomers, 4th ed.; Plastics Design Library; Elsevier: Amsterdam, The Netherlands; William Andrew: Norwich, NY, USA, 2017; pp. 157–207. [Google Scholar]
- DuPont. What is Tyvek? 2025. Available online: https://www.dupont.com/what-is-tyvek.html (accessed on 14 February 2025).
- Boyle, H.; Rawden, A. (Eds.) Standards and Guidance in the Care of Archaeological Collections; Society for Museum Archaeology: Shrewsbury, UK, 2020; Available online: https://socmusarch.org.uk/training/smart-project/ (accessed on 20 March 2025).
- Senge, K.D. Creating a Microclimate Box for Metal Storage. In Conserve-O-Gram; number 4/16; National Park Service: Washington, DC, USA, 2011. Available online: https://www.nps.gov/museum/publications/conserveogram/04-16.pdf (accessed on 24 January 2020).
- DuPont. Article Information Sheet: DuPont™ Tyvek® Spunbond Polyethylene. 2024. Available online: https://www.3eonline.com/EeeOnlinePortal/DesktopDefault.aspx?tabid=90 (accessed on 15 February 2025).
- Prasad, A. A quantitative Analysis of Low Density Polyethylene and Linear Low Density Polyethylene Blends by Differential Scanning Calorimetry and Fourier Transform Infrared Spectroscopic Methods. Polym. Eng. Sci. 1998, 38, 1716–1728. [Google Scholar] [CrossRef]
- National Park Service. Using Silica Gel in Microenvironments; Conserve O Gram 1/8; National Park Service: Washington, DC, USA, 1999. Available online: https://www.nps.gov/museum/publications/conserveogram/01-08.pdf (accessed on 12 December 2024).
- Watkinson, D.; Neal, V. First Aid for Finds. Practical Guide for Archaeologists, 3rd ed.; Institute for Conservation of Historic & Artistic Works, Archaeology Section: London, UK, 1998. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).