Abstract
Unguentaria are ancient vessels for oils, perfumes, ointments, or balms. Glass unguentaria are typically small in size and have long narrow necks to limit the loss of precious contents through spills and evaporation. The vessels may have single or double barrels. This study includes both double and single unguentaria from unprovenanced archaeological contexts. Residues found inside the vessels may reveal the original contents. Gas chromatography-mass spectrometry (GC-MS) and direct analysis in real time-mass spectrometry (DART-MS) were used to identify organic components of the residues, while headspace solid-phase microextraction (HS-SPME) gas chromatography mass spectrometry provided a method to target specifically the volatile aroma compounds. Inorganic compounds in the unguentaria residues were identified by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). The results are consistent with a plant oil base, but few volatile perfume components could be characterized. While the collection of unguentaria may have contained perfumes, these results do not rule out the possibility of other unguents such as cosmetics.
1. Introduction
We can experience the past with our eyes and, occasionally and with great care, our hands, but what of our sense of smell? Understanding the impact of “olfactory heritage” has implications for how we experience cultural objects and spaces [1]. So, what did the ancient world smell like? Though the answers to that question will vary depending upon the location and time period in question, understanding how scent was used in the past provides insight into human behaviors and activities including personal hygiene and fashion. Ritual preparations of the dead involved perfumes and unguents that date back thousands of years. Much previous work on ancient scents and perfumes relies on historical documents, such as the writings of Pliny the Younger, Theophrastus and others. Littman et al. [2] describe the earliest known perfume recipe from Egypt and summarize much of the ancient literature on perfume compositions during the Greco-Roman period. Other centers of perfume manufacture like Paestum and Delos have also been loci of research [3]. Perfumes from this period have long been of interest at the Emory University Museum of Art and Archaeology, now the Michael C. Carlos Museum (MCCM). In 1989, the museum presented the exhibition The Fragrant Past: Perfumes of Cleopatra and Julius Caesar. The exhibition catalog describes various ingredients, recipes, and processes used in the preparation of ancient perfumes [4]. This study seeks to determine if chemical analysis of residues from unguentaria in the MCCM collections, typical of glass unguentaria found throughout the Mediterranean during the Greco-Roman period (1450 BCE–500 AD), can shed light on the previous contents of these vessels.
A recent publication describes how modern science can be used to investigate traces of ancient scent through the characterization of secondary plant metabolites and other molecules [5]. Malik [6] also describes the human aspects of understanding how scent was used and even experienced in the past. Further, the authors address the difficulties of using an analytical chemistry approach for identifying scents in archaeological materials that have oxidized and decomposed over millennia in often less than ideal conditions [5,6]. Organic residue analysis is a popular approach to identify ceramic and glass vessel contents in archaeology, and should be undertaken with caution and skepticism [7]. Those difficulties notwithstanding, much investigation has been undertaken in the attempt to characterize residues of ancient perfumes, unguents, and cosmetics.
Though the perfumes, cosmetics, and unguents themselves are mostly lost to time and the elements, the vessels that once contained them often persist, occasionally with traces of their contents intact. A number of methodologies have been applied to investigate these residues, many of which are summarized by Ribechini et al. [8]. Combining methods of analysis provides the widest amount of information from the samples, including both organic and inorganic components. Cortea and Tentea [9] describe a multi-analytical approach to the analysis of residues recovered from fragments of glass unguentaria from known contexts in Romania. Residues from glass unguentaria from Spain indicated a likely cosmetic composition, being mainly made up of mineral constituents [10]. A recent review describes the ways that pigments have been identified in cosmetic residues [11]. Though dating to the Etruscan period, the residue from an unguentarium from this early context showed similar composition [12]. Unfortunately, some studies of unguentaria residues either have predominantly shown evidence of contamination from handling [13] or may have cross-contaminated the archaeological residues with modern reference materials during the analytical process [14].
The term unguentarium can refer to vessels made of glass, ceramic, or stone (e.g., alabaster or rock crystal) that are thought to have held oils, perfumes, ointments, balms, or cosmetics, more broadly classified as unguents. The most commonly encountered type of glass unguentarium is of a single-barreled design. Less abundant are the more highly decorated and smaller double-barreled unguentaria, which could suggest that they held valuable substances such as perfumes as opposed to mundane medical ointments or balms. This study investigated residues from single- and double-barreled glass unguentaria in the MCCM collections. Replica perfumes prepared in 1989 for the Fragrant Past exhibition were also analyzed to allow direct comparison of known compositions of perfumes with the ancient residues. It is important to note that the unguentaria from the MCCM collections are from unknown archaeological contexts and have been exposed to multiple possible sources of contamination including deposition, excavation, and acquisition into private collections and the museum. Any residues, which would have been complex mixtures in their original form, will have undergone significant change over time and through handling.
Perfumes of the Greco-Roman period generally have two or three main components: the matrix or base, commonly an oil or fat; the aroma derived from herbs, flowers, or other scented materials; and occasionally a fixative to alter the base or preserve the scent [4,15]. The base and aromas require different analytical approaches to sample preparation, though both can be targeted with gas chromatography and mass spectrometry (GC-MS). Free fatty acids are generally not distinct enough to infer a specific lipid source for the base but the heavier molecular weight glycerides, specifically diacylglycerols (DAGs) and triacylglycerides (TAGs), of lipid sources have been previously documented and can be used as markers [16,17]. Any chromatographic method used in residue analysis is destructive as it will require several steps of sample preparation to isolate fractions from other components in the samples as well as derivatization to increase volatility for separation in the gas phase. The separation of the compounds by GC yields greater selectivity than by bulk analysis methods and assists in the characterization of free fatty acids.
Gas chromatography-mass spectrometry (GC-MS) is the standard approach used in the analysis of organic residues on artifacts [18], but direct analysis in real time-mass spectrometry (DART-MS) is a newer technique that can be employed to evaluate its use as a screening tool for the more intensive GC-MS analyses [19]. DART-MS has seen many applications to archaeological and cultural heritage materials since its commercialization in 2005 [20,21,22,23,24,25,26,27,28,29,30]. A recent review of ambient ionization methods in cultural heritage studies describes many more applications of DART-MS along with other related techniques [31].
Headspace-solid-phase microextraction (HS-SPME)-GC-MS has not been widely applied in the study of archaeological materials, though it can be a valuable tool in the identification of resins and other aromatic materials through their volatile components [15,32,33,34]. This technique allows for greater selectivity, as only the volatile components are targeted by the SPME process. Further, the method is essentially non-destructive, which permits multiple runs of the same sample. The volatile terpenes found in resins are not, however, unique to a single source; so the results are more informative than indicative of a specific aroma composition [32].
Any inorganic compounds in the residues were investigated by use of scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). This multi-analytical approach allows for both visual and elemental characterization of the residues.
2. Materials and Methods
2.1. Greco-Roman Unguentaria
Samples for this project were obtained from 11 glass unguentaria in the MCCM collection; not all analyses could be carried out on all of the residues. Table 1 lists the objects that were sampled for this study. The vessels studied lack any records of their excavations, but as described in the Introduction, are consistent with the forms and styles of objects from that time period. Glass deteriorates with time and exposure to burial conditions, resulting in surface delamination and flaking, as well as the formation of characteristic, colorful opalescence. Samples were taken directly from internal residues in six single unguentaria and five double unguentaria. Two additional samples were taken from residues found in the storage bags for two of the double unguentaria. Samples often contain traces of the deteriorated glass. Examples of the two types of unguentaria from which samples were removed for study are shown in Figure 1. Residue samples were acquired by inserting a stainless-steel scalpel into the vessel and applying gentle pressure onto encrusted residues until some cracking or flaking occurred. The vessel was then inverted, and any loosened residue was collected on glassine paper and transferred into a polyethylene microcentrifuge tube. In between sample collections, the blade was wiped clean with a lint-free lab tissue and the glassine paper replaced.

Table 1.
Unguentaria for which residues were sampled and analyzed.

Figure 1.
Examples of the single- (2016.19.19) and double-barreled (2016.19.48) unguentaria from the Michael C. Carlos Museum Greco-Roman Collection.
2.2. Replica Perfumes
In conjunction with The Fragrant Past exhibition, the museum gift shop sold a set of replica perfumes, prepared by Professor Giuseppe Donato, Director Emeritus of the Institute of Applied Technologies, National Research Council of Italy. Samples were obtained from a surviving set of these replica perfumes. Table 2 shows the composition of each of the replica perfume recipes [4]. All of these replica perfumes were prepared using the enfleurage method, wherein the plant materials were mixed with onphacium, oil obtained from unripe olives, and left to soak at “medium temperatures” [4].

Table 2.
Summary of replica perfume recipes [4].
These replica perfumes are complex mixtures and are more than three decades old. The names for some of the ingredients are ambiguous, and some ingredients may serve more than one purpose in the mixture. For example, the rhodinum perfume contains “cinnabar”, which in this case is not the red mercuric sulfide mineral from which natural vermilion pigment is obtained. Instead, cinnabar in the rhodinum perfume refers to dragon’s blood, a dark red resinous material obtained from numerous plants, but likely from Dracaena cinnabaris in Roman times. Dragon’s blood serves the dual purpose of imparting both a red color and a scent to the resulting perfume. The presence of colorants in perfumes has long been established [15].
2.3. DART-MS
A melting point capillary tube was inserted closed end down into the glass vial containing the sample and swirled until some sample remained on the tube when removed. If difficulties arose in maintaining the sample on the capillary tube, a small amount of sample was transferred to a new, clean glass vial and a minimal (3–10 µL) amount of methanol was added to assist adhesion. The capillary tube was then introduced into the space between the DART ionization source (IonSense, Saugus, MA, USA) and Orifice 1 of the AccuTOF mass spectrometer (JEOL USA, Peabody, MA, USA) with the He carrier gas at 350 °C. The voltages of Orifice 1 and Orifice 2 were set to ±30 V and ±5 V, respectively, with the polarity determined by the mode of operation. The AccuToF peaks voltage was set to obtain the maximum intensity for the range of interest, 150–1000 Da. Spectra were collected in both positive and negative ion modes. Before each sample was introduced to the ion source, PEG-600 in methanol was run for calibration of the data. All data files were processed with TSS Pro 3.0 software (Shrader Analytical and Consulting Laboratories, Detroit, MI, USA) and then analyzed by Mass Mountaineer Software (various versions, RBC Software, provided by R.B. Cody).
2.4. HS-SPME-GC-MS
The headspace volatile samples were obtained following the procedure established by Hamm et al. [32]. Approximately 6–10 mg of residue from each unguentarium was placed in a 4 mL glass screw top reaction vial with a silicone septum. For the replica perfumes, 50 µL of each perfume was placed into a 5 mL glass screw top reaction vial with a silicone septum. A 100 μm thick polydimethylsiloxane fiber (PDMS, Supelco Inc., Bellefonte, PA, USA) was conditioned in the injector port of the GC-MS for 30 min at 250 °C and run to measure a background blank and ascertain that no volatiles from previous analyses remained on the fiber. Once a satisfactory blank was achieved, the fiber was then inserted through the septum into the headspace of the archaeological sample in the glass vial and exposed roughly 1 cm above the sample for 40 min (archaeological samples) or 5 min (replica perfumes) at 80 °C. After exposure, the fiber was inserted into the injection port of the GC-MS. A Varian CP-3800 gas chromatograph coupled to a Varian Saturn 2000 ion trap mass spectrometer running in automatic electron impact ionization at 70 eV with a scan range of m/z 50–650 was used to perform all GC-MS analyses. Ultrahigh purity helium gas at a flow rate of 1.5 mL/min was the carrier gas.
The SPME fiber was maintained in the injection port for a minimum of 5 min after beginning the run to assist with thermal desorption before reconditioning of the fiber. A VF-5ms capillary column of 30 m × 0.25 mm and a film thickness of 0.25 μm was utilized for HS-SPME-GC-MS. The oven temperature was held at 40 °C for one minute before increasing 9 °C per minute until 130 °C, then increasing 2 °C per minute until 230 °C.
2.5. GC-MS
The lipid extraction procedure and chromatographic conditions were adapted and modified from Fulcher et al. [35] to obtain the total lipid extracts of the samples. Approximately 5–10 mg of sample was measured into a 1.5 mL microcentrifuge tube, and 1 mL of dichloromethane was added. The tube was sonicated for 20 min at room temperature and centrifuged at 6000 rpm for 20 min. The supernatant was removed and transferred to a 4 mL glass reaction vial. The extraction was repeated twice more and combined in the same glass reaction vial. The extracts were dried down under a gentle stream of nitrogen at room temperature, derivatized with 100 μL N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane (BSTFA/TMCS) and placed in a heat block at 70 °C for 1 h.
After derivatization, the BSTFA/TMCS was dried down under a gentle stream of nitrogen and the sample reconstituted in 50 μL of residue-analysis grade dichloromethane. A 1 μL portion of sample was injected onto the Zebron ZB5-MS column (Phenomenex, 30 m long × 0.25 mm i.d., 0.25 µm film thickness) in splitless mode at 250 °C. The oven temperature was maintained at 50 °C for two minutes before increasing 10 °C per minute until it reached 350 °C and was then maintained for ten minutes. EI mass spectra were collected after a 5 min solvent delay over the range of 50–600 Da.
2.6. SEM-EDS and Optical Microscopy Analysis
Samples were first examined visually with an M26 stereo microscope (Swift Instruments, San Jose, CA, USA) to select particles of interest which were then mounted on an aluminum stub using carbon tape. Data was collected using a JCM-7000 SEM-EDS (JEOL Ltd., Peabody, MA, USA) at a high probe current, a landing voltage of 10 kV, and a working distance of 12.6 mm. Images were collected in both secondary electron image mode (SEM) and backscattered electron image mode (BED) and spectra and/or elemental maps were taken simultaneously. For elemental analysis, both point and area measurements were collected. Due to the variability in measurement with EDS, only qualitative results are reported for the most abundant elements.
3. Results
3.1. DART-MS Results: Fatty Acids
Perfumes require a carrier, the base, to which the aroma compounds are added. Depending on the method of preparation, the aroma may be extracted directly into this base. Historically, plant oils and animal fats are the two primary classes of materials that may have served as carriers in ancient perfumes and cosmetics. Of particular note in much of the literature is the use of onphacium, oil produced from unripe green olives (sometimes even specified as those picked in August [4]). This oil is low in acidity, determined by the ratio of free fatty acids—specifically oleic acid, C18:1—relative to the total amount of oil. Most of the fatty acids are present in the oil as mono-, di-, and triglycerides, forming free fatty acids due to oxidation and photodecomposition. Free fatty acids in the vessel residues and replica perfumes were identified by mass as [M-H]− ions in the negative-ion DART mass spectra with a tolerance of 12 millimass units (0.012 Da) at a minimum relative abundance of 1%. Because compounds are only identified by mass, more than one compound may be represented by a single peak. Some example DART mass spectra are shown in Figure S1 of the Supplementary Materials.
The mass spectral identifications were tabulated and grouped into four distinct classes: saturated fatty acids, unsaturated fatty acids, dioic acids, and hydroxy fatty acids. Saturated fatty acids are more abundant in animal fats, and cholesterol or its oxidation products are also likely to be present if animal fat is present. Dioic acids form from the oxidation of unsaturated fatty acids, which are more prevalent in plant oils. Hydroxy fatty acids tend to form through oxidation and exposure to water. The number of compounds identified from each class was summed and a percentage of the total identified fatty acid classes in each sample was determined. This calculation allowed for an overall comparison of the samples to each other as well as a simple way to compare the ancient residues to the replica perfumes.
Figure 2a shows the relative similarity in fatty acid composition across the seven replica perfume recipes. These results are from single DART-MS analyses of each perfume, so the variation between the classes likely is a measure of the overall variation in the compositions, as the perfume kit was stored as a single unit. Considering that the perfumes were stored in the dark and tightly sealed, the proportion of dioic acids is quite interesting, as is the significant proportion of hydroxy acids. Over the centuries of burial, any perfume residues in the unguentaria would likely lose nearly all of the unsaturated fatty acids, which would result in a higher proportion of dioic acids in the ancient samples.
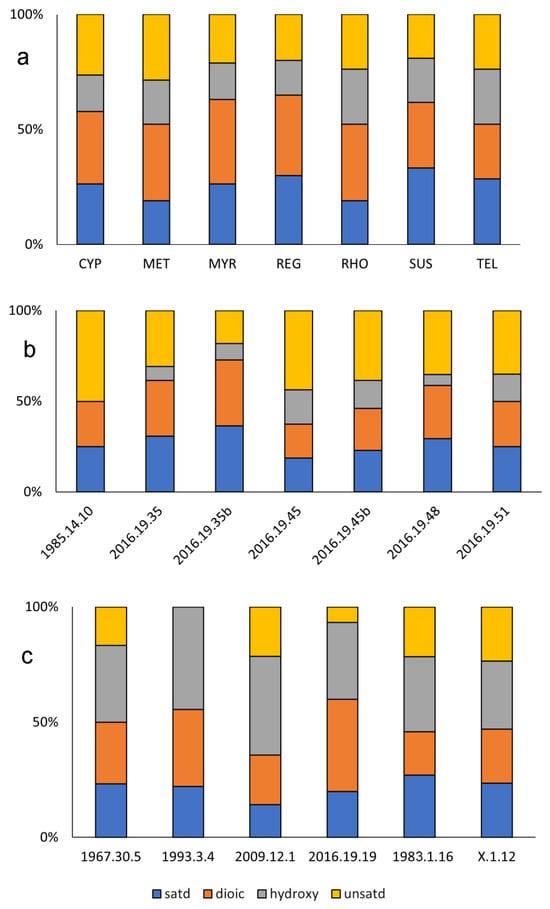
Figure 2.
Distribution of fatty acid components in the (a) replica perfumes, (b) double unguentaria and (c) single unguentaria based on peaks identified by mass in negative-ion DART mass spectra. Only peaks at greater than 1% relative abundance and within 0.012 mass units were identified. See Figure S1 in the Supplementary Information for representative DART mass spectra.
To simplify the graphic presentation, data from the ancient vessel residues are grouped by morphology, single or double barrel. In the associated discussion, both ancient sample groups are compared with the results from the replica perfumes. Figure 2b shows the results of the negative ion DART-MS for the four classes of free fatty acids identified in the residues recovered from the double unguentaria. The residues in the double unguentaria contain a higher percentage of saturated fatty acids as measured against all the identified fatty acids compared to the replica perfumes prepared in onphacium. This disparity may be due in part to the lower number of fatty acids detected and identified overall in the residues from the double unguentaria. Only 13 fatty acids on the selected search list on average (median 14) were identified in the double unguentaria, compared to 21 (both average and median) in the replica perfumes. The list was by no means exhaustive but consisted of the most likely abundant species in both plant and animal fats. No cholestadiene, a degradation product of cholesterol often though not always observed in animal fats by DART-MS, was detected in the double unguentaria residues. Unsaturated and dioic acids were present in approximately equal proportions, and few hydroxy fatty acids were detected in the double unguentaria. As noted in Table 1, two of the vessels (2002.19.35 and 2002.19.45) had loose material in their storage bags (designated “b” in Figure 2b). It was uncertain if the loose material came from within the vessels, but at least from the standpoint of the fatty acids identified, the internal residues and loose materials were indeed consistent. It is important to note that the “residues” may be heavily contaminated by soil from the burial environment, as the most prominent saturated fatty acids were consistent with the m/z for the [M-H]− ions of the ubiquitous palmitic and stearic acids.
The fatty acid compositions of the single-barreled unguentaria, based on the four groups of fatty acids identified in the DART mass spectra, were overall quite similar to the composition of the onphacium oil aged for 30 years (Figure 2c). The number of dioic acids detected was generally consistent relative to the number of unsaturated free fatty acids, as would be expected if the former was produced by the oxidation of the latter. The proportion of saturated fatty acids was also lower than the proportion of unsaturated, as was the case for the replica perfumes. A higher abundance of hydroxy acids likely arises from the markedly different exposure history of the materials, as the replica perfumes were not open to the atmosphere or stored unsealed. The major difference between the double and single unguentaria residues was in the hydroxy fatty acids, which made up a greater percentage of the total fatty acids identified. It is not clear why there is such a difference in the hydroxylated fatty acids. The interpretation of the residues is complicated by the storage history and taphonomic changes that have occurred over the centuries since they were buried. Clearly the fatty acid composition alone cannot provide a complete picture of the residue compositions, particularly when the signal from the unguentaria residues was many orders of magnitude smaller than that of the replica perfumes
3.2. DART-MS Results: “Aroma” Components
The scent (or other ingredient) components in the replica perfumes arise from the numerous materials that were extracted by soaking the plant materials in the carrier, onphacium. A searchable database (as an Excel spreadsheet) was created with the major components expected to be present from the wide variety of scented materials that might have been present in the ancient perfumes. DART produces primarily molecular ions (as the [M-H]− in negative ion mode, or MH+ in positive ion mode) at the conditions used in this study, with a low voltage applied to orifice 1 of the AccuTOF mass spectrometer. In this case, components are identified only by mass, resulting in many compounds with the same molecular formula being grouped together as a single class, e.g., all monoterpenes with the formula C10H16 result in a single peak at m/z 136.125, etc. The search database was thus simplified such that recurring compounds with the same formula were identified only by class, where monoterpenes in the following figures include a variety of compounds including monoterpenoids. All identifications were made with a tolerance of 12 millimass units (0.012 Da) at a minimum relative abundance of 1%.
The aroma components in the replica perfumes are shown by class in Figure 3a. Though some of the materials in these samples were difficult to identify based on the descriptions provided, the detected components were consistent with what was expected. Although tartaric acid would not have imparted a scent to metopium, it was detected in the DART mass spectrum for this replica perfume. The recipe for metopium (Table 2) includes wine, for which tartaric acid is often considered a biomarker [36,37]. The only other replica perfume that purportedly contained wine was the regale unguentum, for which no tartaric acid was detected by DART-MS. The complexity of regale unguentum compared to metopium may be one reason this marker was not detected. The aromatic compositions of all of the replica perfumes are dominated by mono-, sesqui-, and diterpenes and -terpenoids, making up from 55–75% of the identified components. All of the replica perfumes showed significant signals at the m/z values consistent with C8H8O3, which we believe is most likely to be vanillin, and C20H28O2, most likely dehydroabietic acid (DHA). Vanillin may be contributed by any of the woody materials like cinnamon bark; DHA is abundant in oxidized conifer resin (see [38], page 304 for more on the effects of oxidation on pine resins), though it or a compound of the same molecular formula may be present in any of the resinous scent additives. Without the separation of species and fragmentation from EI-MS or CID, identification of specific components is not possible by DART-MS.
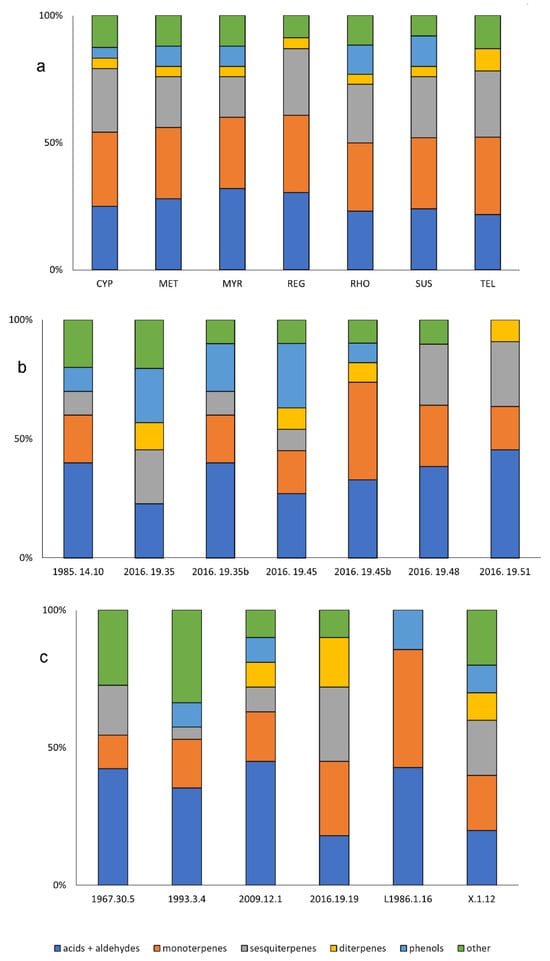
Figure 3.
“Aroma” compound classes detected by DART-MS (identified by mass only) for (a) the replica perfumes, (b) the residues from the double-barreled unguentaria, and (c) the single-barreled unguentaria residues.
The DART-MS results for the aroma compounds in the double-barreled unguentaria residues are summarized in Figure 3b; some example spectra are shown in Figure S2 of the Supplementary Materials. The terpene/terpenoid components make up 30–55% of the identified components in these residues, generally lower than what was observed for the replica perfumes. Given that the residues have likely undergone significant degradation, differences would be expected even if the residues were known to have been the same perfume compositions. Most interesting is the significant differences in the aromatic components detected in the interior and bag residues associated with double unguentaria 2016.19.35 and 2016.19.45. Entire classes of components (diterpenes/diterpenoids in the former, and sesquiterpenes/sesquiterpenoids in the latter) are missing from the material recovered from the bags compared to that collected from the interiors of the vessels. Perhaps these components were absorbed by the plastic storage bag or lost through evaporation. Tartaric acid was identified in 2016.19.51, and vanillin in 1985.14.10. Dehydroabietic acid was observed in four of the six double unguentaria residues: 2016.19.35 (but not in the bag sample), both of the 2016.19.45 residues, and in 2016.19.51. No other clear patterns were observed for the aroma components in the double unguentaria.
The DART-MS results for the single-barreled unguentaria residues are summarized in Figure 3c. The terpene/terpenoid components make up 26–77% of the identified components in these residues, showing much greater variation. Two of the single-barreled unguentaria lacked any phenolic components, and three showed no evidence of diterpenes/diterpenoids. Two-thirds of the residues contained what appears to be DHA; this m/z was not detected in either 1960.30.5 or 1983.1.16 (formerly L1986.1.16). The residue from 1993.3.4 was the only residue from a single unguentarium that indicated the presence of tartaric acid.
There are no clear trends in “aroma” compositions among the unguentaria residues. The general similarities between the compositions of the residues and the aroma components in the replica perfumes suggests that the residues may indeed be from perfumes, though the limitations of DART-MS for identifying specific components rather than general classes based only upon mass requires additional follow-up with methods such as GC-MS.
3.3. HS-SPME-GC-MS: Replica Perfumes and Unguentaria Residues
A total of 94 individual compounds were identified by their EI mass spectra in the headspace SPME of the replica perfumes (for a full list of compounds, see Table S1 and for chromatograms Figure S3, both in the Supplementary Material). This wide range of different compounds was characteristic of the aroma materials, nearly all of which were terpenes and terpenoids, as shown in Figure 4. Many of the monoterpenes, such as α-pinene, camphor, sabinene, linalool oxide, 1-terpinen-4-ol, terpineol acetate and fenchyl acetate, were non-diagnostic, occurring in all of the replica perfumes. Three sesquiterpenes—bourbonene, elemene, and humulene—were also found in all of the replica perfumes. Other compounds were specific to the aroma components used in the preparation of the replicas, such as the identification of rose oxide in the rhodinum replica perfume scented with roses, thujone in the cyprinum that was scented with wormwood oil prepared from Artemesia spp., and myrtyl acetate in the myrtum laurum prepared from myrtle (Myrtus communis L.). Irone was identified in the susinum replica perfume, as would be expected in a lily extract, but was also present in cyprinum and telinum, both of which contained calamus (Acorus calamus L.). Interestingly, no benzaldehyde was detected by HS-SPME-GC-MS in the metopium replica perfume, scented with bitter almonds. The complexity and long ingredient list for regale unguentum did not suggest any specific compounds of interest that should be expected in the HS-SPME-GC-MS.
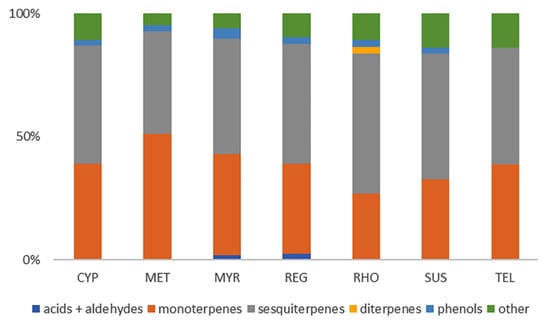
Figure 4.
Composition of the volatile components in the replica perfumes as determined with HS-SPME-GC-MS based on the previously identified classes.
Perhaps not unexpectedly, due to their age, the ancient residues showed a far smaller range of components by HS-SPME-GC-MS. Only four compounds were identified by their mass spectra (Table 3). Camphene, a monoterpene found in many different essential oils and extracts, was identified in three of the double unguentaria residues and the only single unguentarium residue characterized with HS-SPME. Five of the seven replica perfumes also contained camphene (Table 4). Di-epi-α-cedrene was also identified in the same four unguentaria residues, along with one other (1985.14.10); this compound was also identified in the cyprinum replica perfume and may be contributed by several of the plant components. Para-cymene was detected in the residue from 2016.19.51, but not in any of the replica perfumes. No isolongifolene, detected in three of the unguentaria residues, was identified in the replica perfumes, though longifolene was identified in five of the seven. These sesquiterpenes impart a piney odor and may come from a variety of different aroma constituents. Bleton and Tchapla [34] attribute both cedrene and longifolene to plants of the Juniperus genus. Figure 5 shows the chromatograms from the HS-SPME-GC-MS of the two unguentaria residues with the most identified compounds; additional chromatograms are in Figure S3 of the Supplementary Materials.

Table 3.
Compounds identified in the unguentaria residues by HS-SPME gas chromatograms based on mass spectra.

Table 4.
Compounds identified in both the unguentaria residues (shown in Table 3) and replica perfumes by HS-SPME gas chromatograms based on mass spectra.
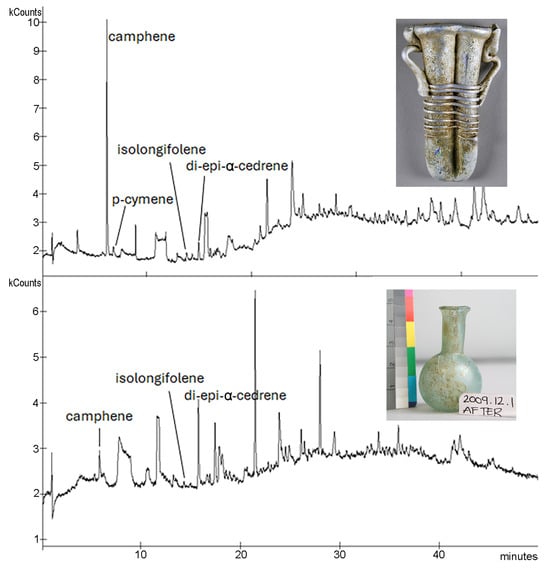
Figure 5.
Chromatograms from HS-SPME-GC-MS of two of the unguentaria residues: top, 2016.19.51 (double-barreled); bottom, 2009.12.1 (single-barreled). Additional chromatograms are provided in the Supplementary Materials.
3.4. GC-MS: Unguentaria and Replica Perfumes
An initial study with high-temperature GC-MS of the unguentaria residues did not show any indications of di- or triglycerides, nor were these compounds observed in the DART mass spectra, even when the ion source was doped with ammonia vapor to give lower detection limits. The chloroform–methanol total lipid extracts of the residues were run under standard GC-MS conditions as described above to focus on the free fatty acid fraction as TMS derivatives. For comparison, two of the replica perfumes were also prepared in the same way for GC-MS analysis; only two were chosen as all were prepared in the same base. The aged onphacium in the replica samples yielded a fatty acid profile similar to what would be expected for olive oil, with a large contribution from oleic acid, C18:1. Azelaic acid (nonanedioic acid, diC9) was also significant in the GC-MS chromatograms of the replica perfume base. Palmitic, stearic and intermediate-chain fatty acids (nonanoic, decanoic, dodecanoic and in cyprinum, tetradecanoic) complete the list of fatty acids identified in the replica perfume base. No aroma compounds were detected in the GC-MS chromatograms of the replica perfumes. This is significantly different from what was observed in the DART mass spectra of the replica perfumes.
Only four fatty acids were identified in the double-barreled unguentaria residues as TMS derivatives, and even then only in low concentrations: C9:0 (nonanoic), C16:0 (palmitic), C18:1 (possibly oleic acid), and C18:0 (stearic). See Table S2 and Figure S4 in the Supplementary Information. The unsaturated C18:1 was only identified in two of the samples, 2016.19.48 and in the bag sample for 2016.19.45 at just a trace amount, but not at all in the material recovered from the interior of the vessel itself. Though far fewer fatty acids were detected in the GC-MS analysis than in the DART-MS, these results are generally consistent with the saturated nature of the fatty acids observed in those spectra. No cholesterol was detected in any of the residues from the double-barreled unguentaria. The diterpene kaurene was identified in the bag material from 2016.19.45, though not in the interior residue, and two compounds that appear to be TMS derivatives of sugars based on the suggested but low-quality matches to the NIST13 mass spectral database were observed in all of the other residues. Though these were briefly considered as possible indicators of the presence of honey, neither was observed in the sample of metopium that did contain honey in its recipe. The residue in 1985.14.10 contains two markers for conifer resin or oil, dehydroabietane and dehydroabietic acid, along with cuparene, a sesquiterpene also found in conifer and other essential oils. However, none of these compounds were observed in the GC-MS chromatogram of the metopium replica perfume that contained balsam and turpentine. The single-barreled unguentaria residues, on the other hand, contained many more components, both fatty acids and aroma components. These are shown in Table 5; Figure 6 shows a chromatogram for the extracted and derivatized residue from 1983.1.16. The TMS sugar derivatives observed in the double-barreled unguentaria residues were again present in some of the residues from the single-barreled unguentaria, but as discussed above, may be contamination. Unlike the DART spectra, GC-MS showed stronger signals for the saturated fatty acids than for the unsaturated in the residues from the single-barreled unguentaria. Dioic acids, which were abundant in the DART mass spectra were nearly absent in the GC-MS chromatograms, with only azelaic acid present in X.1.12, interestingly without any oleic acid present. It may be the case that most of the oleic acid, if it were indeed present as part of a plant oil, has decomposed to form azelaic acid in this residue.

Table 5.
GC-MS results for the residues from the single-barreled unguentaria. All fatty acids were identified as TMS derivatives (dicarboxylic acid = di, as bis-TMS). Other compounds were underivatized unless stated as such. tr = trace (only observed under extracted ion searches), xx = strong signal, dominating chromatogram. Peak column refers to the numbers shown in Figure 6. Results for the double-barreled unguentaria residues are shown in Table S2 of the Supplementary Materials.
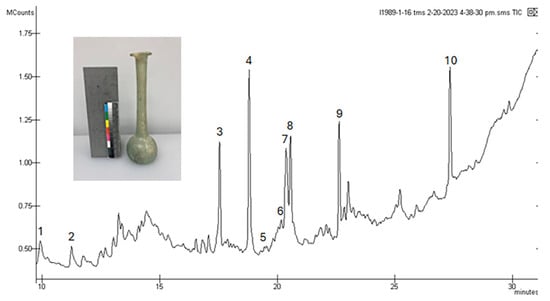
Figure 6.
Chromatogram for the extracted and TMS-derivatized residue from 1983.1.16, a single-barreled unguentarium.
3.5. Microscopy and SEM-EDS Results
Visual examination of the residues under the digital microscope revealed the presence of what may be pigments or other coloring materials, consistent with the addition of colorants as described for the replica perfumes. Figure 7 shows the material provided for analysis from four of the unguentaria, portions of which were selected for SEM-EDS. The residue from 1967.30.4, a single-barreled unguentarium, shown in Figure 7a, is mostly glassy flakes, probably from the vessel itself. The residues from the double-barreled unguentaria (Figure 7b–d) are more varied, showing fragments of colored materials. In the case of 2016.19.48, the residue was nearly all made up of pigmented material. Further characterization of these materials was carried out with SEM-EDS.

Figure 7.
Optical microscope images of a selection of the unguentaria residues: (a) 1967.30.5; (b) 1985.14.10; (c) 2016.19.45; (d) 2016.19.48.
Particles of interest that were identified in the optical microscope images were selected for SEM-EDS, the results of which are provided in Table 6. The elemental composition of the glassy fragments in 1967.30.5 was indeed consistent with glass. The green fragments in 1985.14.10 are generally consistent in elemental composition and color with soil and malachite, a basic copper carbonate mineral used as a pigment during this time period [11]. The other elements suggest soil (Si and Al) or even glass particles. The white fragments may be basic lead carbonate or cerussite. Both of these pigments, the latter of which is synthetic, were known in the ancient world [11,39]. Calcium carbonate, either from shell or limestone, also is suggested by the elemental composition of the white fragments in the 1985.14.10 residue. The presence of charcoal fragments (high in carbon and showing the wood-like structure in the electron micrographs; see Figure S5 in the Supplementary Materials for SEM images, spectra and element maps) may be pigments as well. The pink-red materials in 2016.19.35 and 2016.19.45 may contain hematite (based on the presence of Fe in some fragments) or even a lake pigment where particles appear pink in visible light, but no Fe is detected; a lake pigment with aluminum hydroxide and silicates would be consistent with the observed elemental composition. Phosphorus along with Ca, C, and O in other white fragments also suggests bone white in these residues. The green pigment in 2016.19.48, showing high Cu, C, and O, like the ones in 1985.14.10, was also tested with acid, showing strong reaction and the release of gas, consistent with a carbonate mineral like malachite. The residues from 2016.19.48 and 2016.19.51 both also contained gray particles that are consistent in elemental composition with galena (PbS), the major constituent of kohl eye pigment [40]. All of the pigment materials were observed in the double-barreled unguentaria, though not all of the single-barreled unguentaria residues were examined with this method due to limited access to the JEOL Neoscope.

Table 6.
SEM-EDS results for a selection of the unguentaria residues.
4. Discussion
No one analytical method can answer every question about complex materials like the ones described herein. Each method has its own advantages and disadvantages. Methods like DART-MS are fast, but are of limited use for mixtures, whereas GC-MS requires significant preparation of the samples, which may result in contamination or loss of certain components; but, GC-MS can clearly identify each component based on the EI mass spectrum. The selectivity of the method of choice is also an important consideration, as in the case of solid-phase microextraction and the selection of an appropriate fiber composition that will target the volatile compounds of interest. Finally, we need to consider the divide between what we want to learn and what we can learn from the analysis we undertake of objects that may have been used and reused in antiquity, discarded or lost, recovered, cleaned, stored, handled, displayed, and finally sampled for analysis.
4.1. DART-MS vs. GC-MS
The results of DART and GC-MS for the fatty acids analysis show the advantages and disadvantages of the two methods. DART-MS is rapid and requires no sample preparation but yields only molecular ions that can be compared to expected masses for known compounds (as is true for all direct mass spectrometry methods). Without the separation step of GC-MS, the mass spectral peaks can correlate to multiple compounds, and without the separation of GC and fragmentation of EI-MS, the structures cannot be confirmed. The lengthy sample preparation required for GC-MS analysis, consisting of extraction and derivatization, targets only specific classes of compounds based on solubility and reactivity. Neither step is 100% efficient. Further, the longer and more involved the preparation protocol, the more chances there are for contamination or loss of sample.
By focusing exclusively on four of the most abundant or diagnostic lipid components, the differences between the DART-MS and GC-MS results are clear. A selection of the results for palmitic and stearic (saturated), “oleic” (consistent with C18:1, though where the double bond is located cannot be distinguished by DART-MS), and the dioic acid azelaic acid are shown in Table 7 and Table 8. DART-MS gave the only clear indication of hydroxyacids in the residues.

Table 7.
Comparison of three fatty acids and one dioic acid decomposition product by GC-MS and DART in the double unguentaria.

Table 8.
Comparison of three fatty acids and one dioic acid decomposition product by GC-MS and DART in the single unguentaria.
Exactly why the results for just these four compounds are so variable across the two methods is not clear. DART-MS has proven useful for rapid screening of materials for further study with the lengthier GC-MS preparation and analysis [19]. For the double unguentaria, DART-MS appears to be more sensitive, as everything detected in the GC-MS results was also observed in the DART spectra. This trend is not consistent for the single unguentaria residues and may be the result of poor sample handling during the extraction and derivatization steps for GC-MS or inconsistencies between aliquots of the residue material that was selected for each of the analyses.
Considering both the DART-MS and GC-MS results for all of the fatty acids, can it be determined if the residues are consistent with the use of animal fat or plant oils? According to Cramp and Evershed [41], high concentrations of monounsaturated fatty acids, dicarboxylic acids and dihydroxyacids would suggest the presence of plant oils. For the double-barreled unguentaria residues, the ratios (based on the DART-MS results) range from 1:1.5 to 1:4.3 saturated fatty acids to the others, which is generally consistent with the presence of plant oils, though the GC-MS results were not consistent with this conclusion. The ratios for the single-barreled unguentaria range from 1:2.6 to 1:6.1. A lower abundance of saturated fatty acids overall seems to indicate that the most likely source of the oils in the unguentaria may have been plant oil. However, mixing of materials in antiquity, soil contamination, change over time, and handling of the objects during excavation and storage may have altered the overall composition such that today, the analysis of the residues does not clearly indicate that plant oils persist in any meaningful way in these samples.
4.2. SPME for Volatiles
Solid-phase microextraction is selective for the volatile components in mixtures, and should be ideal for the study of resins and perfumes. However, it is important to consider not only the composition of the fiber coating (ranging from nonpolar PDMS to strongly polar Carbowax mixed with Carboxen and/or divinylbenzene, or some mixture of all of these) and the thickness of the coating (where thicker coatings have a higher capacity), but also the length of time and temperature of the fiber exposure to the headspace above the sample. Depending upon the selected conditions and fiber composition and thickness, different results may be obtained from the headspace analysis. The nonpolar PDMS stationary phase used in this study concentrates the nonpolar compounds present in the headspace more readily than it does the polar components, and the sampling time (5 min for replica perfumes, 40 min for residues) also affects what we expect to observe in the chromatograms. Comparing the results of HS-SPME-GC-MS and DART-MS once again shows the relative advantages and disadvantages of the methods. The DART-MS results are not specific for individual compounds, only for groups of compounds with the same molecular formulas. HS-SPME is more specific, but depending on the fiber and exposure parameters, it yields different results.
4.3. Pigments
When pigments like the ones suggested by the SEM-EDS analysis of the MCCM unguentaria residues have been identified in previous studies [11], the residues have been presumed to be cosmetics. However, we know that organic colorants like alkanet and dragon’s blood resin were included in ancient perfume recipes specifically to yield coloration. Perhaps there was no clear-cut line between perfumes and cosmetics, explaining the presence of these mineral pigments in the unguentaria, or the vessels themselves may have been reused for different substances in antiquity. The archaeological context in which the vessels were found might have provided additional clues, but as is the case with many museum collections, such information is not available. Follow-up studies of the colored material using FTIR or XRD would provide additional information about their molecular composition.
5. Conclusions
The overall trend is that the composition of the residues from all of the unguentaria suggests a possible presence of plant oils over animal fats, though significant changes through time due to oxidation and contamination from soil components make it impossible to say how much the analysis reflects the original composition of the vessel contents. Pigmented materials were only identified in the double-barreled unguentaria; the presence of pigments may suggest a cosmetic use, though ancient perfume recipes did include organic colorants such as alkanet and dragon’s blood. Perhaps the general idea of a “perfume” might include a scented cosmetic as well. The variations in the fatty acid compositions of the residues may be due to reuse, contamination or taphonomic changes from whatever the original residues were to what remains today.
The aroma components identified by DART-MS in the replica perfumes generally fell into the same classes, with some of the replica perfumes showing few if any phenolic components. The phenolic composition of the unguentaria residues also varied. Headspace SPME-GC-MS successfully identified a wide range of volatile terpenes and terpenoid compounds in the replica perfumes, but few if any in the ancient residues. Volatile components would likely be lost over time, though some do appear to remain trapped within the residues. Direct comparisons between the ancient residue aroma components and those identified in the replica perfumes, particularly by GC-MS, show too much variability to say anything concrete about what aroma-bearing ingredients may have once been present in the unguentaria.
The results of this study underscore the difficulty of determining what an ancient residue may have been based on what remains today after burial, excavation, storage, and handling. Comparing ancient residues to modern replicas following ancient recipes may not be fruitful, since much change will have occurred in the intervening centuries. Different analytical methods also yield different, and seemingly inconsistent results; careful consideration must be given to the differences in selectivity and sensitivity of all the steps in each process leading up to the final results. Although the data do not support identification of the how these particular vessels were originally used based on the compounds present in these complex residues, we hope that this research lays a foundation for further study, mindful of the pitfalls and aware of the possibilities to learn from ancient residues of the fragrant past.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/heritage8050170/s1, Figure S1. Negative ion DART mass spectra for (a) cyprinum replica perfume, (b) 2016.19.51 (double) and (c) 2016.19.19 (single) residue, searched for saturated, unsaturated, dioic and hydroxy fatty acids. Compounds identified by mass only as the adduct shown. Note the marked difference in absolute intensity between the replica and the ancient samples; Figure S2. Positive ion DART mass spectra for (a) cyprinum replica perfume, (b) 2016.19.51 (double) and (c) 2016.19.19 (single) residue, searched for “aroma” compounds in replica perfume recipes. Compounds identified by mass only as the adduct shown. Note the difference in absolute intensity between the replica and the ancient samples; Figure S3. HS-SPME chromatograms of the unguentaria residues: (a) 1985.14.10, (b) 2009.12.1, (c) 2016.19.35, (d) 2016.19.35 residue from storage bag, (e) 2016.19.45, (f) 2016.19.45 residue from storage bag, and (g) 2016.19.51; Figure S4. GC-MS chromatograms for TMS derivatized total lipid extracts from (a) two of the replica perfumes, cyprinum and metopium, both prepared in onphacium approximately 30 years ago; and the unguentaria residues (b) 1967.30.5, (c) X.1.12, (d) 1993.3.4, (e) 2016.19.19; Figure S5. SEM-EDS images, spectra and elemental maps: (a) SEM images, EDS element maps and overall spectrum for green pigment fragment in 1985.14.10; (b) backscattered electron image of a charcoal fragment in 1985.14.10; (c) possible bone fragment in 2016.19.45; (d) gray fragment from 2016.19.48; (e) red fragment from 2016.19.45; Table S1. Compounds identified in replica perfumes and unguentaria residues in the HS-SPME gas chromatograms based on mass spectra. Siloxane and phthalate contaminants and unidentified peaks are not shown in this table. Figure S3. HS-SPME chromatograms of the replica perfumes: (a) cyprinum, (b) metopium, (c) myrtum laurum, (d) regale unguentum, (e) rhodinum, and (f) susinum; Table S2. Compounds identified based on mass spectra in the double unguentaria residues in the GC-MS chromatograms based on mass spectra. Siloxane and phthalate contaminants and unidentified peaks are not shown in this table.
Author Contributions
Conceptualization, R.S. and R.A.A.; methodology, S.J.M. and R.A.A.; investigation, S.J.M. and R.A.A.; resources, R.S. and R.A.A.; data curation, R.A.A.; writing—original draft preparation, S.J.M.; writing—review and editing, R.A.A., R.S. and S.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an EMU College of Arts & Sciences Dean’s Faculty Professional Development Award to support SM and by the EMU Chemistry Department. Additional support was provided by an EMU Faculty Research Fellowship to RAA and by the Andrew W. Mellon Foundation. Publication made possible in part by support from Eastern Michigan University’s Faculty Open Access Publishing Fund, administered by the Associate Provost and Associate Vice President for Graduate Studies and Research, with assistance from the EMU Library.
Data Availability Statement
All data are available from the authors by email.
Acknowledgments
Former students Maxine Faass (Emory) and Jennifer Campos Ayala (EMU) helped with the initial DART-MS analyses of the unguentaria residues. Access to the Neoscope SEM-EDS system was made possible by JEOL USA through a short-term equipment loan in August-September 2019.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bembibre, C.; Strlic, M. Smell of heritage: A framework for the identification, analysis and archival of historic odours. Herit. Sci. 2017, 5, 11. [Google Scholar] [CrossRef]
- Littman, R.J.; Silverstein, J.; Goldsmith, D.; Coughlin, S.; Mashaly, H. Eau de Cleopatra Mendesian Perfume and Tell Timai. East. Archaeol. 2021, 84, 216–229. [Google Scholar] [CrossRef]
- Brun, J.P. The production of perfumes in antiquity: The cases of Delos and Paestum. Am. J. Archaeol. 2000, 104, 277–308. [Google Scholar] [CrossRef]
- Donato, G.; Seefried, M. The Fragrant Past. In Perfumes of Cleopatra and Julius Caesar. Emory University Museum of Art and Archaeology Atlanta; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 1989. [Google Scholar]
- Huber, B.; Larsen, T.; Spengler, R.N.; Boivin, N. How to use modern science to reconstruct ancient scents. Nat. Hum. Behav. 2022, 6, 611–614. [Google Scholar] [CrossRef]
- Malik, R. Does Archaeology Stink? Detecting Smell in the Past Using Headspace Sampling Techniques. Int. J. Hist. Archaeol. 2021, 25, 273–296. [Google Scholar] [CrossRef]
- Whelton, H.L.; Hammann, S.; Cramp, L.J.E.; Dunne, J.; Roffet-Salque, M.; Evershed, R.P. A call for caution in the analysis of lipids and other small biomolecules from archaeological contexts. J. Archaeol. Sci. 2021, 132, 105397. [Google Scholar] [CrossRef]
- Ribechini, E.; Modugno, F.; Perez-Arantegui, J.; Colombini, M.P. Discovering the composition of ancient cosmetics and remedies: Analytical techniques and materials. Anal. Bioanal. Chem. 2011, 401, 1727–1738. [Google Scholar] [CrossRef]
- Cortea, I.; Țentea, O. Characterization of residues found within some Roman unguentaria glass artefacts: Preliminary results of a multi-disciplinary approach. Cercet. Arheol. 2023, 30, 345–354. [Google Scholar] [CrossRef]
- PerezArantegui, J.; PazPeralta, J.A.; OritzPalomar, E. Analysis of the products contained in two Roman glass unguentaria from the colony of Celsa. J. Archaeol. Sci. 1996, 23, 649–655. [Google Scholar] [CrossRef]
- Perez-Arantegui, J. Not only wall paintings-pigments for cosmetics. Archaeol. Anthropol. Sci. 2021, 13, 10. [Google Scholar] [CrossRef]
- Colombini, M.P.; Giachi, G.; Iozzo, M.; Ribechini, E. An Etruscan ointment from Chiusi (Tuscany, Italy): Its chemical characterization. J. Archaeol. Sci. 2009, 36, 1488–1495. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Donato, I.D.; Filizzola, F. Identification of organic compounds in fictile unguentaria from two sicilian necropolis of greek age (5th century, b.C.) by GC-MS analysis. Ann. Chim. 2007, 97, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Cosano, D.; Roman, J.M.; Lafont, F.; Arrebola, J.R.R. Archaeometric Identification of a Perfume from Roman Times. Heritage 2023, 6, 4472–4491. [Google Scholar] [CrossRef]
- Castel, C.; Fernandez, X.; Filippi, J.J.; Brun, J.P. Antique perfumery in the Mediterranean area. Actual. Chim. 2012, 359, 42–49. [Google Scholar]
- Brockerhoff, H.; Yurkowski, M. Stereospecific analyses of several vegetable fats. J. Lipid Res. 1966, 7, 62–64. [Google Scholar] [CrossRef]
- Mattson, F.H.; Lutton, E.S. The specific distribution of fatty acids in the glycerides of animal and vegetable fats. J. Biol. Chem. 1958, 233, 868–871. [Google Scholar] [CrossRef]
- Ribechini, E.; Modugno, F.; Colombini, M.P.; Evershed, R.P. Gas chromatographic and mass spectrometric investigations of organic residues from Roman glass unguentaria. J. Chromatogr. A 2008, 1183, 158–169. [Google Scholar] [CrossRef]
- Hopkins, J.; Armitage, R.A. Characterizing Organic Residues on Ceramics by Direct Analysis in Real Time Time-of-Flight Mass Spectrometry. In Collaborative Endeavors in the Chemical Analysis of Art and Cultural Heritage Materials; Lang, P.L., Armitage, R.A., Eds.; ACS Symposium Series 1103; American Chemical Society: Washington, DC, USA, 2012; pp. 131–142. [Google Scholar]
- Armitage, R.A.; Day, C.J.; Jakes, K.A. Identification of Anthraquinone Dye Colorants in Red Fibers from an Ohio Hopewell Burial Mound by Direct Analysis in Real Time Mass Spectrometry. STAR Sci. Technol. Archaeol. Res. 2015, 1, 1–15. [Google Scholar] [CrossRef]
- Armitage, R.A.; Fraser, D.; Degano, I.; Colombini, M.P. The analysis of the Saltzman Collection of Peruvian dyes by high performance liquid chromatography and ambient ionisation mass spectrometry. Herit. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Geiger, J.; Armitage, R.A.; Selvius DeRoo, C. Identification of Organic Dyes by Direct Analysis in Real Time-Time of Flight Mass Spectrometry. In Collaborative Endeavors in the Chemical Analysis of Art and Cultural Heritage Materials; Lang, P.L., Armitage, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 123–129. [Google Scholar]
- Armitage, R.A.; Jakes, K.A.; Day, C.J. Direct Analysis in Real Time-Mass Spectroscopy for Identification of Red Dye Colorants in Paracas Necropolis Textiles. STAR Sci. Technol. Archaeol. Res. 2015, 1, 60–69. [Google Scholar] [CrossRef]
- Day, C.J.; Selvius DeRoo, C.; Armitage, R.A. Developing Direct Analysis in Real Time Time-of-Flight Mass Spectrometric Methods for Identification of Organic Dyes in Historic Wool Textiles. In Archaeological Chemistry VIII; Armitage, R.A., Burton, J.H., Eds.; ACS: Washington, DC, USA, 2013; pp. 69–85. [Google Scholar]
- Alvarez-Martin, A.; Cleland, T.P.; Kavich, G.M.; Janssens, K.; Newsome, G.A. Rapid evaluation of the debromination mechanism of eosin in oil paint by direct analysis in real time and direct infusion-electrospray ionization mass spectrometry. Anal. Chem. 2019, 91, 10856–10863. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martin, A.; George, J.; Kaplan, E.; Osmond, L.; Bright, L.; Newsome, G.A.; Kaczkowski, R.; Vanmeert, F.; Kavich, G.; Heald, S. Identifying VOCs in exhibition cases and efflorescence on museum objects exhibited at Smithsonian’s National Museum of the American Indian-New York. Herit. Sci. 2020, 8, 115. [Google Scholar] [CrossRef]
- Cleland, T.P.; Newsome, G.A.; Hollinger, R.E. Proteomic and direct analysis in real time mass spectrometry analysis of a Native American ceremonial hat. Analyst 2019, 144, 7437–7446. [Google Scholar] [CrossRef]
- Selvius DeRoo, C.; Armitage, R.A. Direct Identification of Dyes in Textiles by Direct Analysis in Real Time-Time of Flight Mass Spectrometry. Anal. Chem. 2011, 83, 6924. [Google Scholar] [CrossRef]
- Manfredi, M.; Robotti, E.; Bearman, G.; France, F.; Barberis, E.; Shor, P.; Marengo, E. Direct analysis in real time mass spectrometry for the nondestructive investigation of conservation treatments of cultural heritage. J. Anal. Methods Chem. 2016, 2016, 6853591. [Google Scholar] [CrossRef]
- Armitage, R.A.; Jakes, K.A. Sequencing Analytical Methods for Small Sample Dating and Dye Identification of Textile Fibers: Application to a Fragment from Seip Mound Group, Ohio. Midcont. J. Archaeol. 2015, 41, 26–40. [Google Scholar] [CrossRef]
- Vettorazzo, C.; Sandström, E.; Troalen, L.G.; Logan Mackay, C.; Hulme, A.N. Heritage science applications of ambient mass spectrometry. Anal. Methods 2025, 17, 3357–3369. [Google Scholar] [CrossRef]
- Hamm, S.; Bleton, J.; Tchapla, A. Headspace solid phase microextraction for screening for the presence of resins in Egyptian archaeological samples. J. Sep. Sci. 2004, 27, 235–243. [Google Scholar] [CrossRef]
- Hamm, S.; Bleton, J.; Connan, J.; Tchapla, A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry 2005, 66, 1499–1514. [Google Scholar] [CrossRef]
- Bleton, J.; Tchapla, A. SPME/GC-MS in the characterisation of terpenic resins. In Organic Mass Spectrometry in Art and Archaeology; Colombini, M.P., Modugno, F., Eds.; Wiley: Berlin, Germany, 2009; pp. 261–302. [Google Scholar]
- Fulcher, K.; Serpico, M.; Taylor, J.H.; Stacey, R. Molecular analysis of black coatings and anointing fluids from ancient Egyptian coffins, mummy cases, and funerary objects. Proc. Natl. Acad. Sci. USA 2021, 118, e2100885118. [Google Scholar] [CrossRef]
- Guasch-Jane, M.R.; Ibern-Gomez, M.; Andres-Lacueva, C.; Jauregui, O.; Lamuela-Raventos, R.M. Liquid chromatography with mass spectrometry in tandem mode applied for the identification of wine markers in residues from ancient Egyptian vessels. Anal. Chem. 2004, 76, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.H.; McGovern, P.E.; Badler, V.R. The first wine & beer. Anal. Chem. 1993, 65, 408A–413A. [Google Scholar]
- Pollard, A.M.; Heron, C.; Armitage, R.A. Archaeological Chemistry, 3rd ed.; Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Riesmeier, M.; Keute, J.; Veall, M.A.; Borschneck, D.; Stevenson, A.; Garnett, A.; Williams, A.; Ragan, M.; Deviese, T. Recipes of Ancient Egyptian kohls more diverse than previously thought. Sci. Rep. 2022, 12, 5932. [Google Scholar] [CrossRef] [PubMed]
- Cramp, L.J.; Evershed, R.P. Reading the residues: The use of chromatographic and mass spectrometric techniques for reconstructing the role of kitchen and other domestic vessels in Roman antiquity. In Ceramics, Cuisine and Culture: The Archaeology and Science of Kitchen Pottery in the Ancient Mediterranean World; Oxbow: Oxford, UK, 2015; pp. 125–140. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).