Sustainability of Managing Archaeological Iron Collections
Abstract
1. Introduction
- Material is apparently stable up to 80% RH.
- Approximately 85% of the unstable material begins to react slightly at 16%, reacts more rapidly above 30% and then dramatically increases in reaction rate somewhere between 48 and 60% depending on the temperature. A small, but as yet not fully elucidated portion of this material (possibly due to storage at high RH values after excavation), can begin to react from 11%.
- Some material is more reactive at lower RH values, with significant reaction just above 20%.
- The final group of material shows no reactivity up to 55 or 65%, but then begins to react after this point.
2. Materials and Methods
2.1. LCA for Conventional Desalination Treatments
2.2. Investigation into Sodium Hydroxide Treatments
2.3. LCA for Preventive Conservation
2.4. Overall LCA
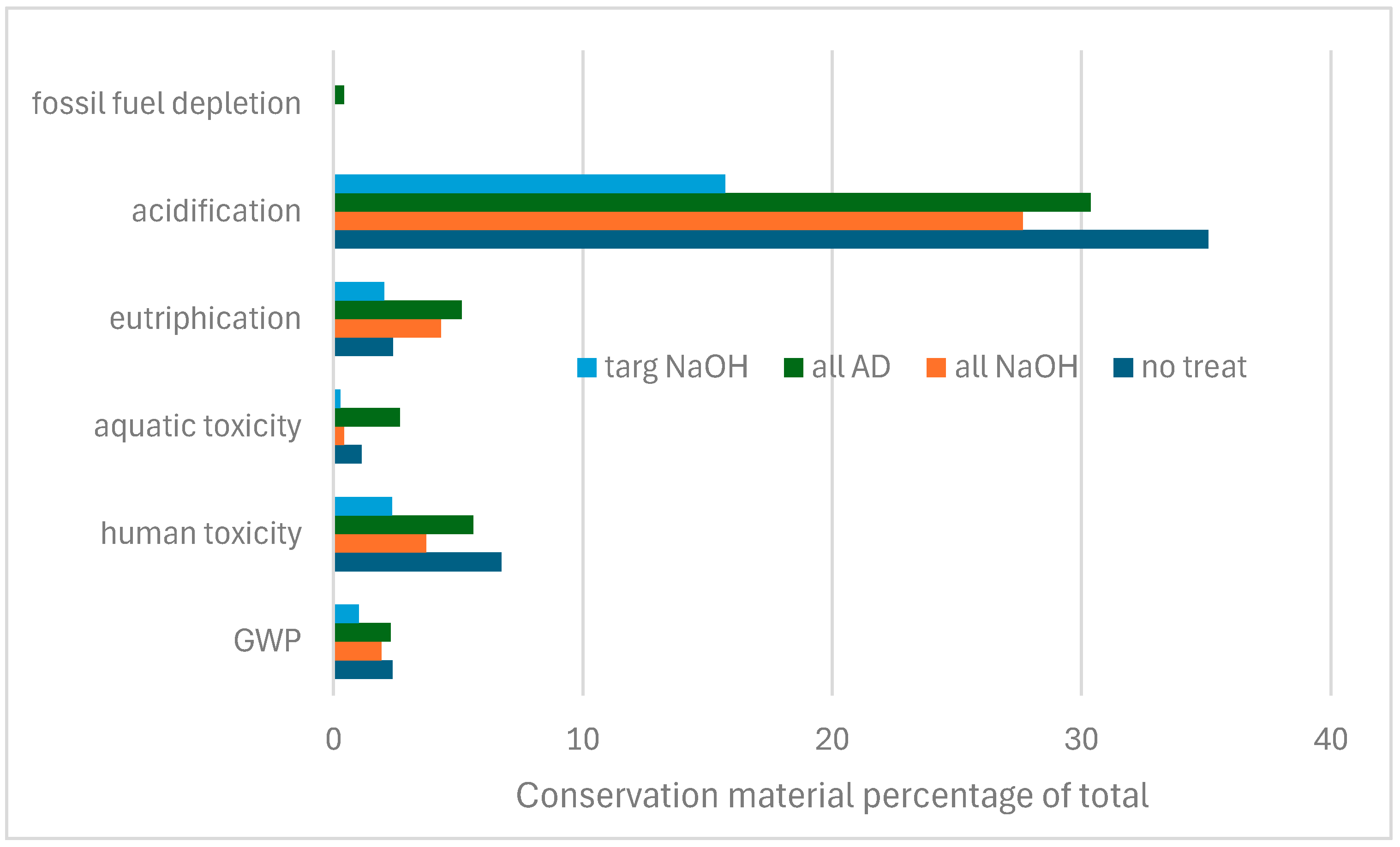
3. Results
3.1. LCA for Conventional Desalination Treatments
3.2. Investigation into Sodium Hydroxide Treatments
3.3. LCA for Preventive Conservation
3.4. LCA Display
3.5. Overall LCA
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCA | Life cycle assessment |
| GWP | Global warming potential |
References
- Pinasco, M.R.; Ienco, M.G.; Piccardo, P.; Pellati, G.; Stagno, E. Metallographic Approach to the Investigation of Metallic Archaeological Objects. Ann. Chim. 2007, 97, 553–574. [Google Scholar] [CrossRef]
- Kutlu, E.; Ali, M.A. A review on conservational methodologies of tangible cultural artifacts from environmental corrosion. Int. J. Environ. Geoinform. (IJEGEO) 2024, 11, 024–029. [Google Scholar] [CrossRef]
- Zouarhi, M. Bibliographical Synthesis on the Corrosion and Protection of Archaeological Iron by Green Inhibitors. Electrochem 2023, 4, 103–122. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Antiquites en Fer et en Bronze, leur Transformation.et leur Conservation; Gyldendalske Boghandels Sortiment: Copenhagen, Denmark, 1917. [Google Scholar]
- De Graaf, M.J.; Severens, R.J.; van IJzendoorn, L.J.; Munnik, F.; Meijers, H.J.M.; Kars, H.; van de Sanden, H.C.M.; Schram, D.C. Cleaning of iron archaeological artefacts by cascaded arc plasma treatment. Surf. Coat. Technol. 1995, 74–75, 351–354. [Google Scholar] [CrossRef][Green Version]
- Dussere, F. Peut-on Concevoir le Plasma comme un Traitement de Masse? In Metal 95, Proceedings of the International Conference on Metals Conservation, Semur en Auxois, France, 25–28 September 1995; James and James (Science Publishers) Ltd.: London, UK, 1997; pp. 138–146. [Google Scholar]
- Schmidt-Ott, K. Plasma-Reduction: Its Potential for Use in the Conservation of Metals. In Metal 04, Proceedings of the International Conference on Metals Conservation, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, Australia, 2004; pp. 235–246. [Google Scholar]
- Drews, M.J.; Gonzalez, N.; de Vivies, P.; Nestor, G.; Mardikian. A study of the analysis and removal of chloride in samples from the Hunley. In Metal 04, Proceedings of the International Conference on Metals Conservation, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; Canberra, National Museum of Australia: Canberra, Australia, 2004; pp. 247–260. [Google Scholar]
- de Vivies, P.; Cook, D.; Drews, M.J.; Gonzalez, N.; Mardikian, P.; Memet, J.B. Transformation of akaganeite in archaeological iron artefacts using subcritical treatment. In Metal 07, Proceedings of the Interim Meeting of the ICOM-CC Metal Working Group; Volume 5: Protection of Metal Artefacts. Amsterdam, The Netherlands, 17–21 September 2007; Degrigny, C., van Langh, R., Joosten, I., Ankersmit, B., Eds.; Rijksmuseum: Amsterdam, The Netherlands, 2007; pp. 26–30. [Google Scholar]
- North, N.A.; Pearson, C. Alkaline sulfite reduction treatment of marine iron. In Proceedings of the ICOM Committee for Conservation 4th Triennial Meeting, Venice, Italy, 13–18 October 1975; International Council of Museums: Paris, France, 1975; Volume 13, pp. 1–14. [Google Scholar]
- North, N.A.; Pearson, C. Washing methods for chloride removal from marine iron artifacts. Stud. Conserv. 1978, 23, 174–186. [Google Scholar] [CrossRef]
- Skinner, T. The treatment of archaeological iron by the alkaline sulphite method. In Conservation of Iron; Bryce, T., Tate, J., Eds.; National Museum of Antiquities of Scotland: Edinburgh, UK, 1982. [Google Scholar]
- Keene, S.; Orton, C. Stability of treated archaeological iron: An assessment. Stud. Conserv. 1985, 30, 136–142. [Google Scholar] [CrossRef]
- Selwyn, L.S.; Argyropoulos, V. Removal of chloride and iron ions from archaeological wrought iron with sodium hydroxide and ethylenediamine solutions. Stud. Conserv. 2005, 50, 81–100. [Google Scholar] [CrossRef]
- Scott, D.; Eggert, G. Iron and Steel in Art Corrosion, Colorants, Conservation; Archeotype: London, UK, 2009. [Google Scholar]
- Eggert, G.; Schmutzler, B. Lasst sich die Konservierung von Eisenfunden ‘auf Standard’ bringen? In kulturGUTerhalten: Standards in der Restaurierungswissenschaft und Denkmalpflege; Staatliche Museen zu Berlin: Berlin, Germany, 2009; pp. 91–95. [Google Scholar]
- Rimmer, M.B. Investigating the Treatment of Chloride-Infested Archaeological Iron Objects. Ph.D. Thesis, University of Cardiff, Cardiff, UK, 2011. [Google Scholar]
- Gilberg, M.R.; Seeley, N.J. The Alkaline Sodium Sulphite Reduction Process for Archaeological Iron: A Closer Look. Stud. Conserv. 1984, 27, 180–184. [Google Scholar] [CrossRef]
- Neff, D.; Dillmann, P.; Bellot-Gurlet, L.; Beranger, G. Corrosion of iron archaeological artefacts in soil: Characterisation of the corrosion system. Corros. Sci. 2005, 47, 515–535. [Google Scholar] [CrossRef]
- Hjelm-Hansen, N.; van Lanschot, J.; Szalkay, C.D.; Turgoose, S. Electrochemical monitoring of archaeological iron artefact during treatment. In Proceedings of the Non Destructive Testing, Microanalytical Methods and Environment Evaluation for Study and Conservation of Works of Art, 3nd International Conference, Viterbo, Italy, 4–8 October 1992; pp. 361–373. [Google Scholar]
- Al-Zahrani, A.A. Chloride Ion Removal from Archaeological Iron and Beta-FeOOH. Ph.D. Thesis, University of Wales, Cardiff, UK, 1999. [Google Scholar]
- Gil, M.L.A.; Santos, A.; Bethencourt, M.; Garcia, T.; Fernandez-Bastero, S.; Velo, A.; Gago-Duport, L. Use of X-ray and other techniques to analyse the phase transformation induced in archaeological cast iron after its stabilisation by the electrolytic method. Anal. Chim. Acta 2003, 494, 245–254. [Google Scholar] [CrossRef]
- Thickett, D. The Formation and Transformation of Akaganéite. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, UK, 16–20 September 2013; pp. 103–110. [Google Scholar]
- Thickett, D. Post Excavation Changes and Preventive Conservation of Archaeological Iron. Ph.D. Thesis, University of London, London, UK, 2012. Available online: https://www.english-heritage.org.uk/siteassets/home/learn/conservation/collections-advice--guidance/thickettthesisfinalversion.pdf (accessed on 6 August 2021).
- Petrasz, P.; Zhioua, S.; James, S.; Bindschedler, S.; Junier, P.; Joseph, E. Green alternatives for archaeological iron stabilization. Stud. Conserv. 2024, 69 (Suppl. 1), 270–280. [Google Scholar] [CrossRef]
- Petrasz, P.; Junier, P.; Joseph, E. Utilization of dead biomass from an extremophilic yeast Meyerozyma sp. as a novel stabilization treatment for archaeological iron artifacts. In Proceedings of the ICOMCC-Metal 2025, Cardiff, UK, 1–5 September 2025. [Google Scholar]
- Tate Harte, A.; Thickett, D. Calculating the Carbon Footprint of Interventive and Preventive Conservation at English Heritage, UK. Stud. Conserv. 2024, 69 (Suppl. 1), 323–332. [Google Scholar] [CrossRef]
- Thickett, D. Oxygen Depletion Testing of Metals. Heritage 2021, 4, 2377–2389. [Google Scholar] [CrossRef]
- Watkinson, D.; Emmerson, N.; Seifert, J. Matching Display Relative Humidity to Corrosion Rate: Quantitative Evidence for Marine Cast Iron Cannon Balls. In Proceedings of the Metal 2016: Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; pp. 195–202. [Google Scholar]
- Rimmer, M.B.; Emmerson, N.J. The Influence of Relative Humidity and Intrinsic Chloride on Post-excavation Corrosion Rates of Archaeological Wrought Iron. Stud. Conserv. 2019, 64, 456–471. [Google Scholar] [CrossRef]
- Thickett, D. Better Use of Showcases for Preservation and Sustainability. Stud. Conserv. 2022, 67 (Suppl. 1), 267–276. [Google Scholar] [CrossRef]
- Errington, E.; Guo, M.; Heng, J.Y.Y. Synthetic amorphous silica: Environmental impacts of current industry and the benefit of biomass-derived silica. Green Chem. 2023, 25, 4244. [Google Scholar] [CrossRef]
- Thomson, G. Stabilisation of RH in Exhibition Case: Hygrometric Half Time. Stud. Conserv. 1977, 22, 85–102. [Google Scholar] [CrossRef]
- EcoInvent Version 3.11. Available online: https://ecoquery.ecoinvent.org/3.11/cutoff (accessed on 29 September 2025).
- UCL LEAF—Laboratory Efficiency Assessment Framework. Available online: https://www.ucl.ac.uk/sustainable/take-action/staff-action/leaf-laboratory-efficiency-assessment-framework (accessed on 29 September 2025).
- BEIS UK Government GHG Conversion Factors, V1.1. 10 June 2025. Available online: https://www.gov.uk/government/publications/greenhouse-gas-reporting-conversion-factors-2024 (accessed on 29 September 2025).
- Thickett, D.; Odlyha, M. Assessment of dry storage microenvironments for archaeological iron. In Proceedings of the Post Prints of the Conservation of Archaeological Materials: Current Trends and Future Directions, Williamsburg, VA, USA, 13–17 November 2005; pp. 187–199. [Google Scholar]
- Thickett, D.; Odlyha, M. Application of Thermo-magnetometry to Corrosion Studies of Archaeological Iron. J. Therm. Anal. Calorim. 2005, 80, 565–571. [Google Scholar] [CrossRef]
- Thickett, D. In situ analysis of metal corrosion. In Metal 2025, Proceedings of International Conference on Metals Conservation, Cardiff, UK, 1–5 September 2025; Emmerson, N., Thunberg, J., Watkinson, D., Eds.; University of Cardiff: Cardiff, UK, 2025; pp. 212–233. [Google Scholar]
- EN ISO 12569:2017; Thermal Performance of Buildings and Materials—Determination of Specific Airflow Rate in Buildings—Tracer Gas Dilution Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- EN ISO 9972:2015; Thermal Performance of Buildings—Determination of Air Permeability of Buildings—Fan Pressurization Method. International Organization for Standardization: Geneva, Switzerland, 2015.
- Munters, MG Dehumidiers. Available online: https://www.munters.com/en-gb/products-cms/dehumidifiers/mg/ (accessed on 29 September 2025).
- Munters, MCS Dehumidiers. Available online: https://www.munters.com/en-gb/products-cms/dehumidifiers/mcs/ (accessed on 29 September 2025).
- Song, X.; Montelius, M.; Carlsson, C. Life Cycle Assessment of Per- and Polyfluoroalkyl Substances (PFAS) Remediation Technologies: A Literature Review. Environments 2024, 11, 203. [Google Scholar] [CrossRef]
- Thickett, D. Sustainable Collections Environments. Estud. Coserv. Rest. 2019, 11, 93–103. [Google Scholar]
- GoGreen. Available online: https://gogreenconservation.eu/ (accessed on 29 September 2025).
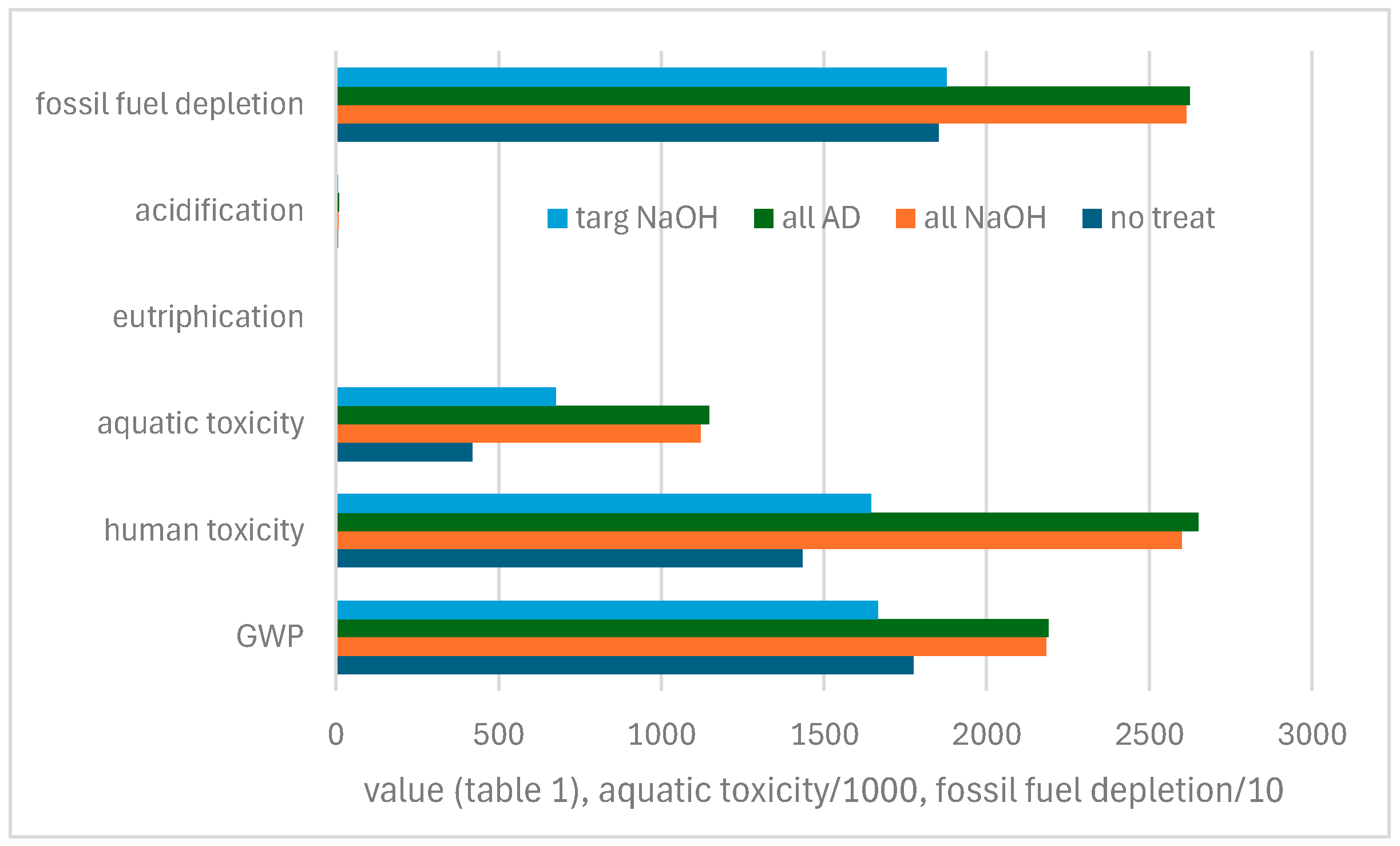
| 1 kg NaOH | 1 kg Na2SO3 | 1 L Water | Disposal 5 L Water | 0.1 MNaOH/ 0.05 MNa2SO3 per kg Iron | 0.1 M NaOH per kg/Iron | |
|---|---|---|---|---|---|---|
| Global warming potential GWP (kg CO2eq) | 0.633 | 1.086 | - | 0.01 | 0.02534 | 0.03566 |
| Human toxicity potential (g) | 0.493 | 6.827 | 0.019 | - | 0.01974 | 0.08461 |
| Aquatic ecotoxity (g) | 1.298 | 3404.3 | 0.95 | - | 0.04047 | 203.4 |
| Eutrophication potential (PO4 equivalent, g) | 0.035 | 0.003 | - | - | 0.02238 | 0.5640 |
| Acidification potential (SOx equivalent, g) | 0.706 | 0.042 | - | - | 0.02496 | 0.02422 |
| Fossil Energy Resource consumption (MJ) | 3.5 | 13.2 | 0.004 | 0 | 0.01380 | 0.2779 |
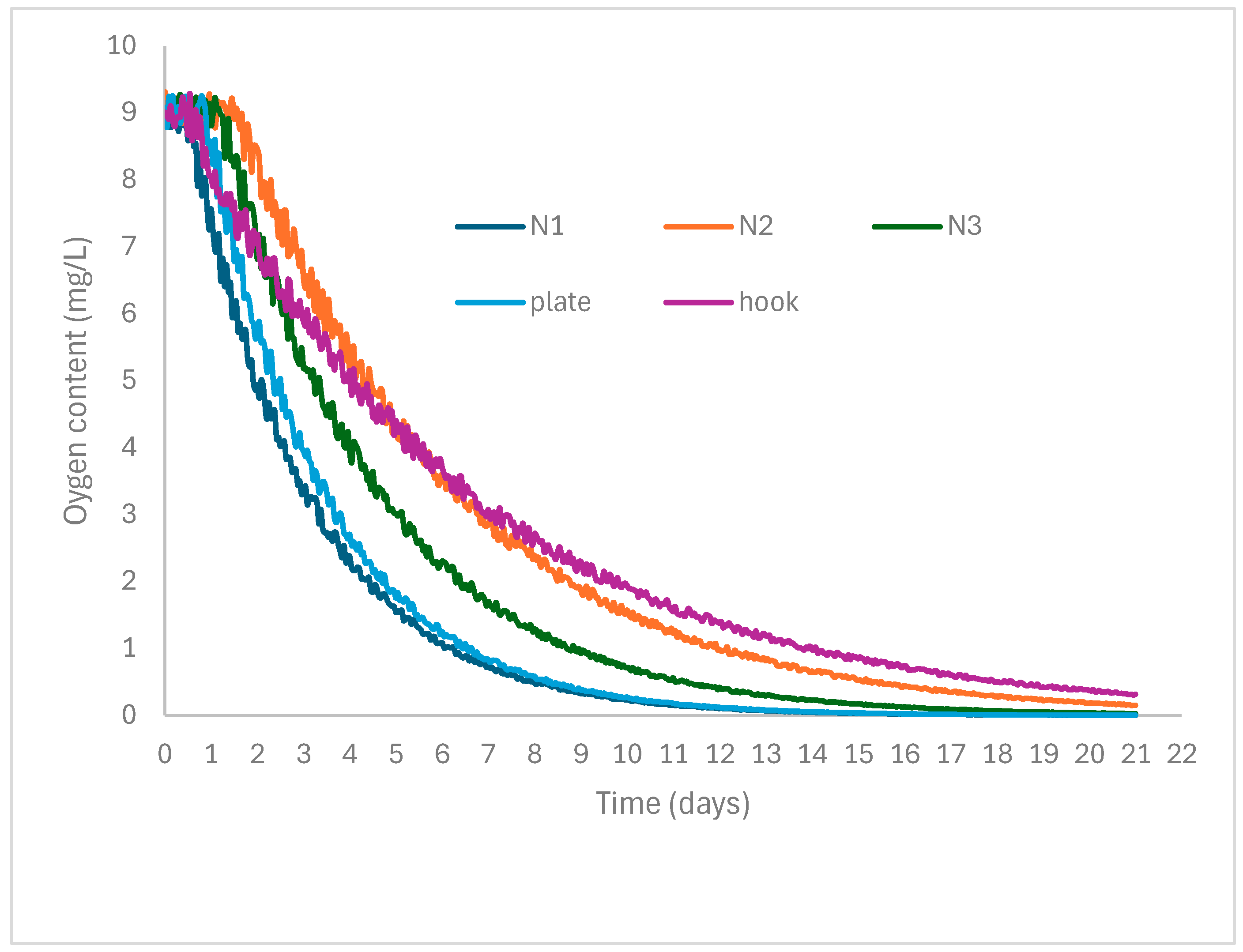
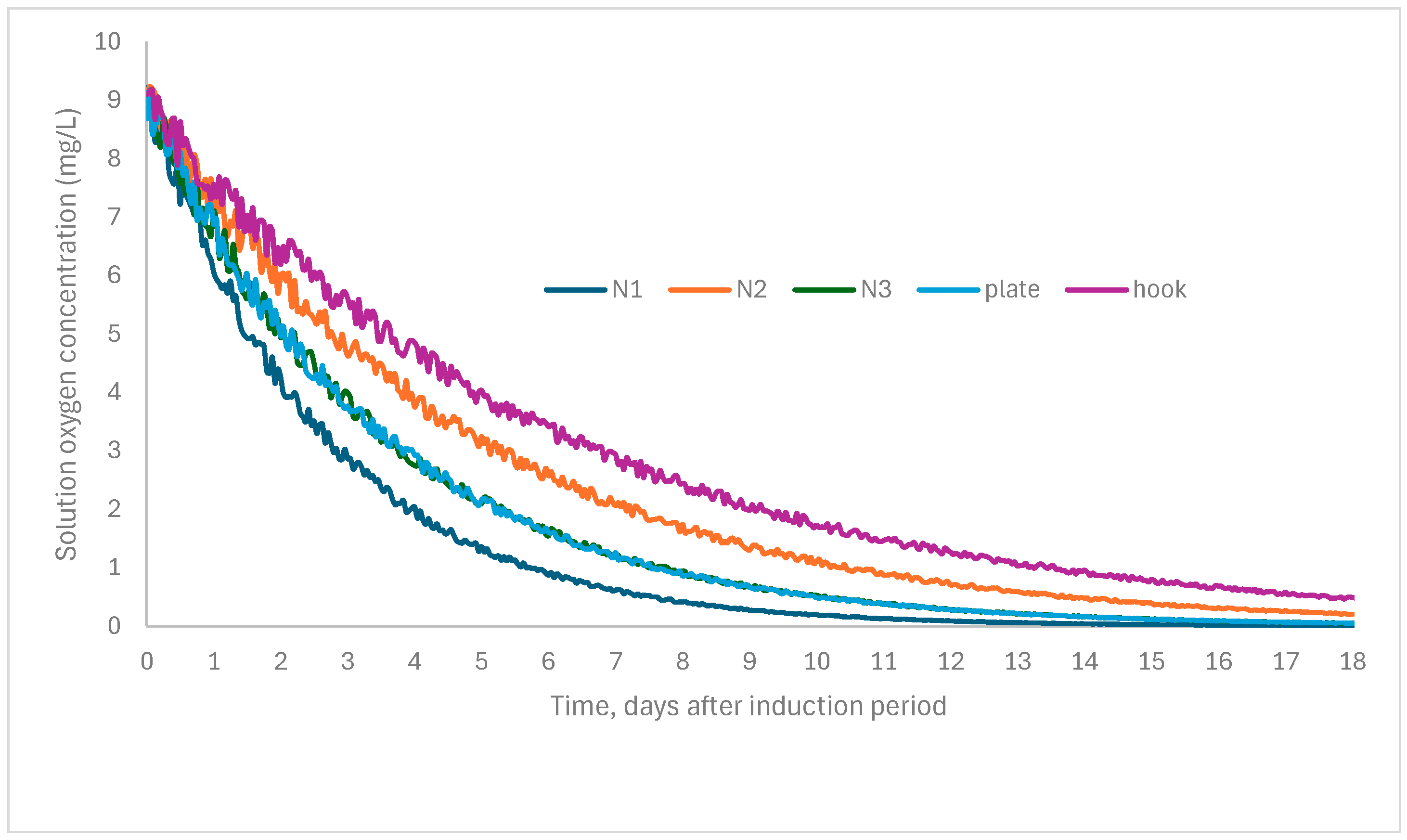
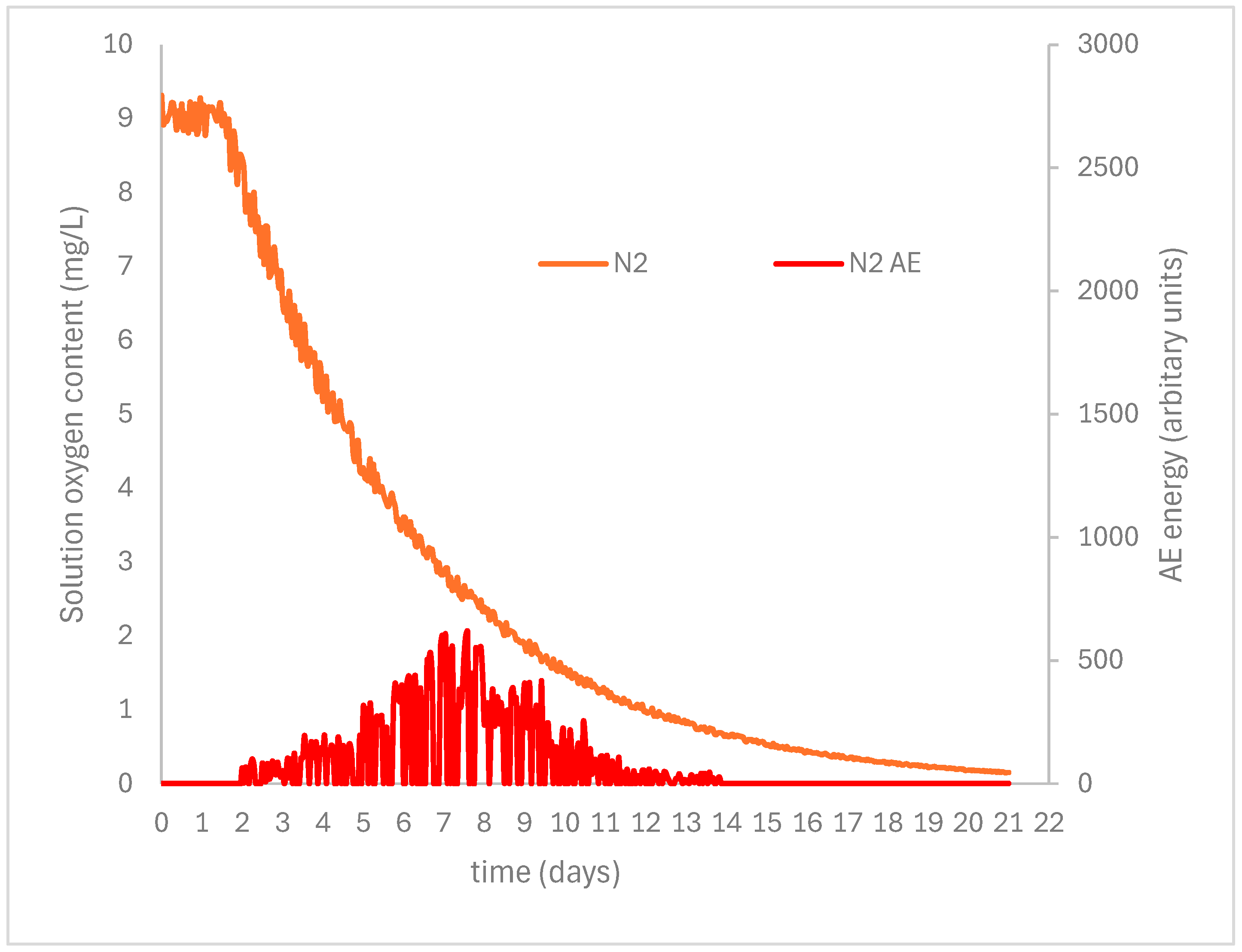
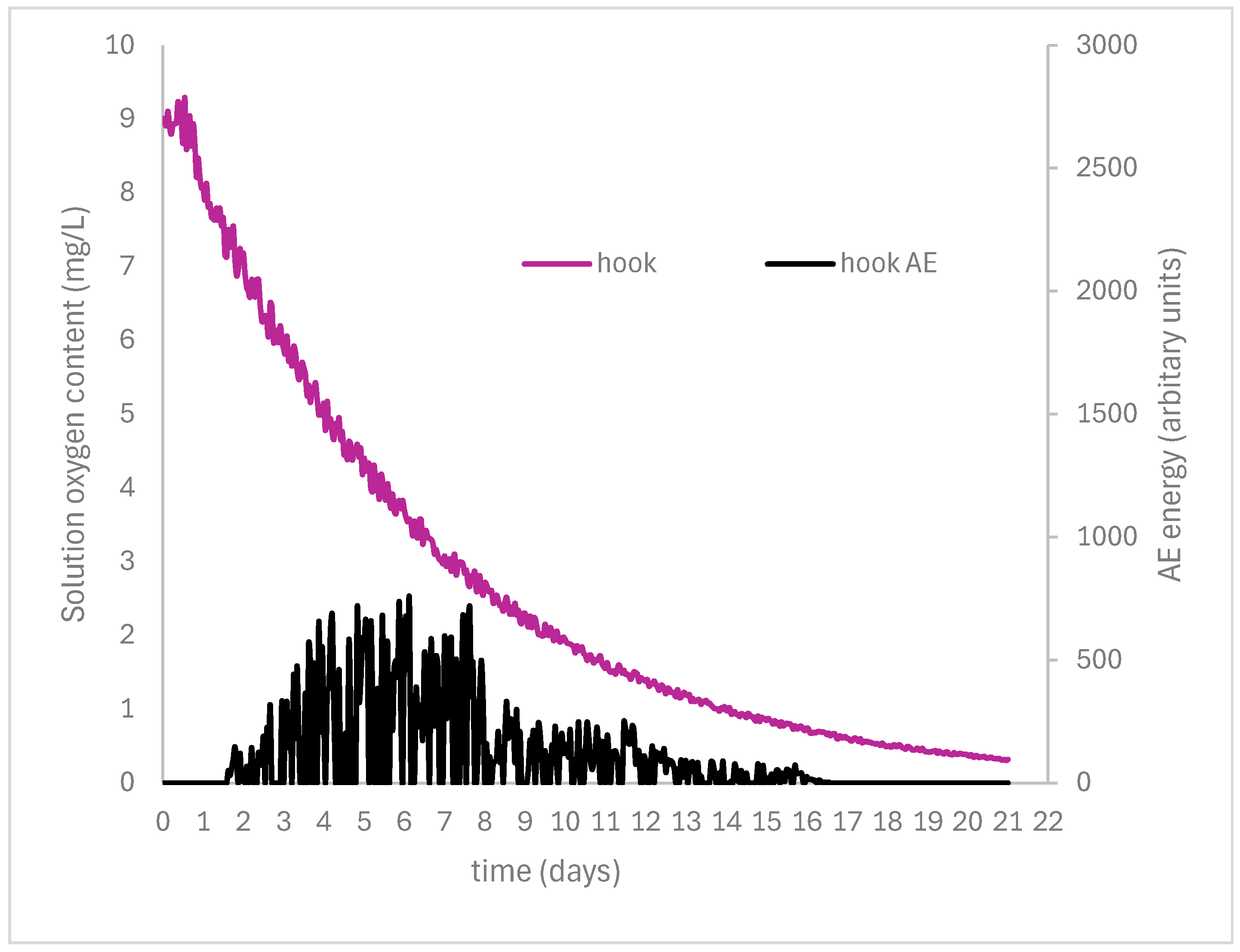
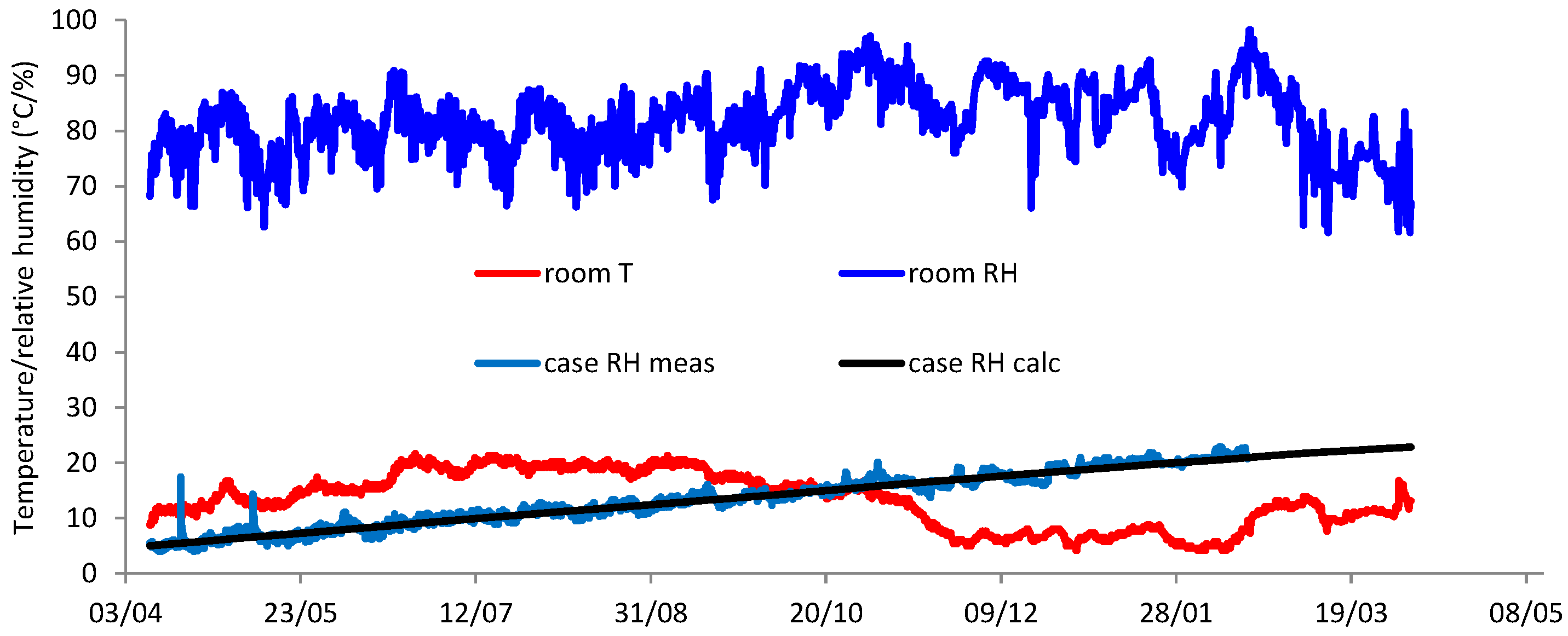
| Number | Object | Oxygen Depletion (%) at 50% RH | Thermomagnetometry (Mass %) | |||
|---|---|---|---|---|---|---|
| iron | magnetite | goethite | akaganeite | |||
| 1 | A | 6.19 | 16.06 | 30.14 | 53.81 | 0.00 |
| 2 | C | 5.99 | 16.86 | 14.97 | 66.66 | 1.51 |
| 3 | B | 5.85 | 13.88 | 18.78 | 65.91 | 1.43 |
| 4 | A | 5.26 | 17.4 | 20.57 | 60.76 | 1.27 |
| 5 | E | 5.14 | 15.60 | 14.68 | 69.72 | 0.00 |
| 6 | D | 4.65 | 9.44 | 29.21 | 60.41 | 0.94 |
| 7 | B | 4.12 | 14.46 | 51.74 | 33.27 | 0.54 |
| 8 | D | 4 | 6.11 | 16.56 | 76.48 | 0.84 |
| 9 | B | 3.89 | 27.69 | 38.52 | 33.28 | 0.51 |
| 10 | C | 3.44 | 10.68 | 10.43 | 77.91 | 0.98 |
| 11 | E | 3.32 | 23.61 | 38.89 | 36.84 | 0.66 |
| 12 | D | 3.18 | 19.90 | 23.53 | 55.41 | 1.16 |
| 13 | C | 3.08 | 19.05 | 45.23 | 34.88 | 0.83 |
| 14 | C | 2.97 | 11.62 | 49.04 | 39.31 | 0.03 |
| 15 | E | 2.72 | 3.14 | 27.69 | 68.14 | 1.04 |
| 16 | D | 2.19 | 29.08 | 38.53 | 31.87 | 0.51 |
| 17 | A | 1.91 | 8.91 | 35.91 | 54.29 | 0.89 |
| 18 | A | 1.5 | 22.55 | 12.67 | 64.4 | 0.39 |
| 19 | B | 1.24 | 0.56 | 50.61 | 48.63 | 0.21 |
| 20 | A | 1.17 | 2.32 | 16.83 | 80.75 | 0.1 |
| 21 | E | 0.89 | 3.16 | 51.57 | 45.25 | 0.03 |
| 22 | D | 0.78 | 8.28 | 45.08 | 46.64 | 0 |
| 23 | E | 0.43 | 18.21 | 51.49 | 30.3 | 0 |
| 24 | C | 0.14 | 17.62 | 29.95 | 51.11 | 1.32 |
| 25 | D | 0 | 20.73 | 40.04 | 39.16 | 0.07 |
| 26 | E | 0 | 1.48 | 10.31 | 88.03 | 0.18 |
| 27 | F | 0 | 18.53 | 19.26 | 61.99 | 0.22 |
| 28 | F | 0 | 10.44 | 26.52 | 62.60 | 0.44 |
| 29 | F | 0 | 7.62 | 46.02 | 45.95 | 0.42 |
| 30 | F | 0 | 28.67 | 47.78 | 22.14 | 1.40 |
| 31 | G | 0 | 24.93 | 50.16 | 23.62 | 1.29 |
| 32 | G | 0 | 26.34 | 26.71 | 45.78 | 1.16 |
| 33 | G | 0 | 14.52 | 32.84 | 51.13 | 1.51 |
| 34 | G | 0 | 27.04 | 36.16 | 35.76 | 1.04 |
| Plate | 0 | 1.87 | 25.83 | 36.83 | 35.22 | 2.12 |
| hook | 0 | 5.32 | 29.43 | 21.75 | 44.85 | 3.97 |
| RH Akaganeite Sample Formed at (%) | Akaganeite (%) | Goethite (%) |
|---|---|---|
| 60 | 94 | 6 |
| 70 | 89 | 11 |
| 80 | 86 | 14 |
| 90 | 85 | 15 |
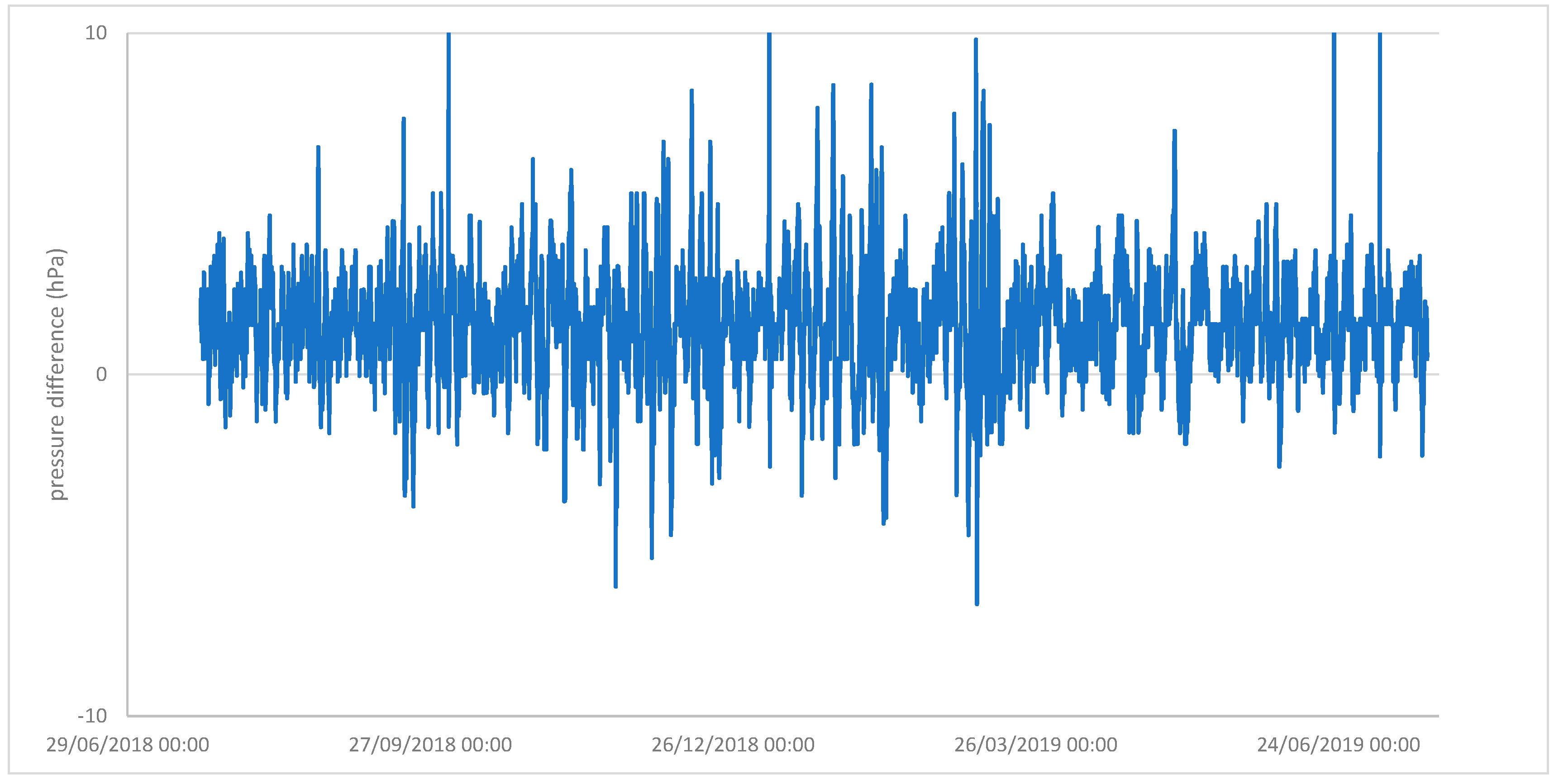
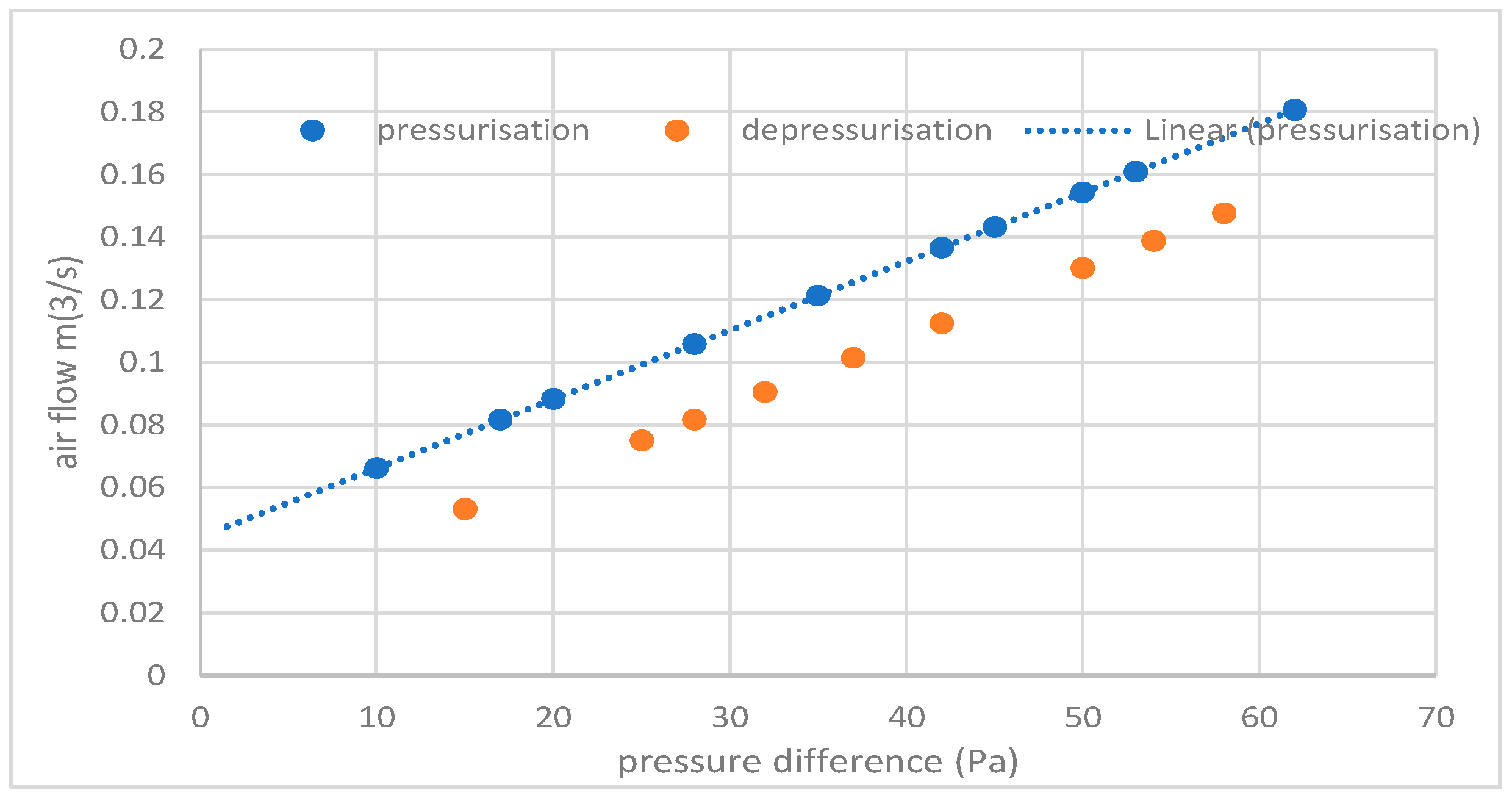
| External Wall, Percentage Area of Windows | Floor | Ceiling | Volume m3 | AER (Tracer Gas) | AER (Pressurisation) | Dehumidifier Type | Calculated Energy Use (kWh) | Measured Energy Use (kWh) | |
|---|---|---|---|---|---|---|---|---|---|
| Wrest Park Small Finds Store | N | con | 226 | 0.72 | 0.95 | MCS | 280 | 339 | |
| Wrest Park Main Store | Y | con | 6060 | 1.03 | 1.20 | none | Nd | Nd | |
| Temple cloud Store | Y metal clad | con | con | 157 | 0.81 | 1.10 | MCS | 431 | 401 |
| Dover | Y, 25 | wood | Plast | 45.6 | 2.87 | Nd | MG50 | 437 | 402 |
| Dover | Y, 22 | Wood | Plast | 37.8 | 2.45 | Nd | MG50 | 278 | 245 |
| Dover | Y, 18 | Wood | Plast | 33.6 | 1.63 | Nd | MG50 | 167 | 131 |
| Rangers | Y, 25 | Wood | Plast | 27.1 | 3.28 | Nd | MG50 | 204 | 212 |
| Rangers windows sealed | Y, 25 (0) | Wood | Plast | 27.1 | 0.59 | Nd | none | Nd | Nd |
| Rangers floor sealed | Y, 25 | Al foil | Plast | 27.1 | 2.89 | Nd | none | Nd | Nd |
| Rangers ceiling sealed | Y, 25 | Wood | Al foil | 27.1 | 3.12 | Nd | none | Nd | Nd |
| Rangers door sealed | Y,25 | Wood | Plast | 27.1 | 2.98 | Nd | none | Nd | Nd |
| Atcham container | N | steel | Steel | 27.6 | 0.34 | Nd | MG50 | 52 | 50 |
| Atcham container | N | steel | Steel | 27.6 | 0.21 | Nd | MG50 | 46 | 47 |
| Energy | Embedded | ||||||
|---|---|---|---|---|---|---|---|
| UK per kW | Ecoinvent (Europe) per kWh | Drying Silica Gel per kg | Dehumidifier | Filter Replacement | Boxes | Silica Gel per kg | |
| Global warming potential (kg) | 0.22 | 0.4514 | 0.66 | 93 | 0.35 | 2.20 | 2.18 |
| Human toxicity potential (g) | - | 0.8251 | - | 0.4454 | 0.2593 | 1.63 | - |
| Aquatic ecotoxicity (g) | - | 408.1 | - | 538.3 | 0.1406 | 0.884 | 0.230 |
| Eutrophication potential (PO4 equivalent, g) | - | 0.000293 | - | 1.068 | 0.0004947 | 0.00311 | 0.00023 |
| Acidification potential (SOx equivalent, g) | - | 0.00098 | - | 0.8562 | 0.0008097 | 0.00509 | 0.0106 |
| Fossil Energy Resource (MJ) | - | 6.785 | - | 1051 | 11.343 | 71.3 | - |
| Glass (m2 = 13 kg) | Steel (kg) | MDF (kg) | |
|---|---|---|---|
| Global warming potential (kg CO2eq) | 1.815 | 1.73 | 0.7707 |
| Human toxicity potential (g) | 2.554 | 0.9213 | |
| Aquatic ecotoxicity (g) | 272.3 | 4.97 | 0.5173 |
| Eutrophication potential (PO4 equivalent, g) | 0.003846 | 0.00358 | 0.04347 |
| Acidification potential (SOx equivalent, g) | 0.01231 | 0.00477 | 0.003733 |
| Fossil Energy Resource (MJ) | 20.69 | 16.9 | 12.49 |
| UK recycling (%) | 74.2 | 96 | 25 of fibres (estimated by 2030) |
| Disposal incineration (kg CO2eq) | 0 | 0 | 0.0641 |
| Disposal land fill (kg CO2eq) | 0 | 0.00123 | 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thickett, D.; Petrasz, P.; Joseph, E. Sustainability of Managing Archaeological Iron Collections. Heritage 2025, 8, 502. https://doi.org/10.3390/heritage8120502
Thickett D, Petrasz P, Joseph E. Sustainability of Managing Archaeological Iron Collections. Heritage. 2025; 8(12):502. https://doi.org/10.3390/heritage8120502
Chicago/Turabian StyleThickett, David, Patrycja Petrasz, and Edith Joseph. 2025. "Sustainability of Managing Archaeological Iron Collections" Heritage 8, no. 12: 502. https://doi.org/10.3390/heritage8120502
APA StyleThickett, D., Petrasz, P., & Joseph, E. (2025). Sustainability of Managing Archaeological Iron Collections. Heritage, 8(12), 502. https://doi.org/10.3390/heritage8120502






