Molecular Diagnostics and Determining of Biodeterioration Risk for the 16th Century Icon “Descent into Hell” from the State Tretyakov Gallery
Abstract
1. Introduction
2. Materials and Methods
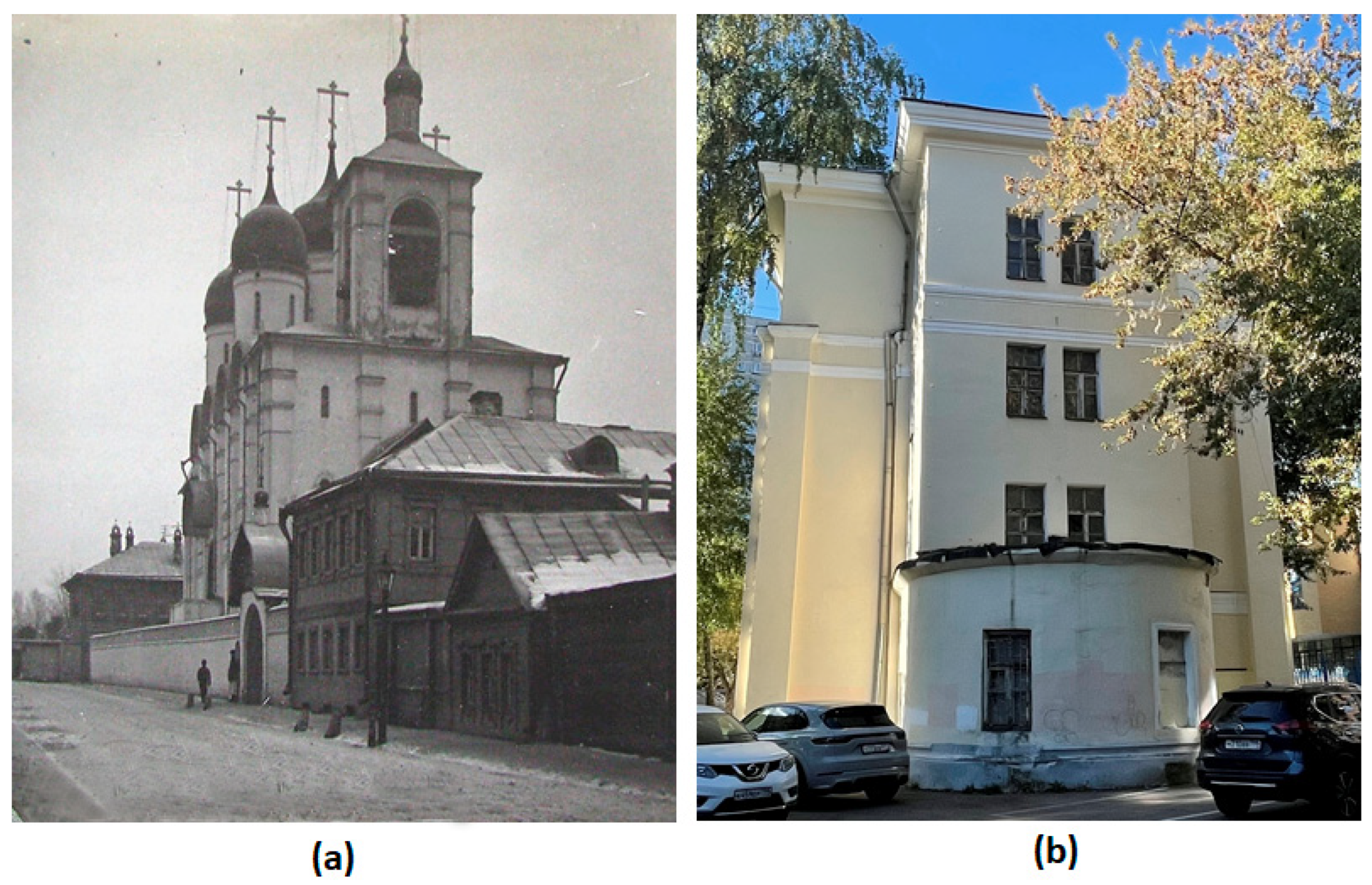
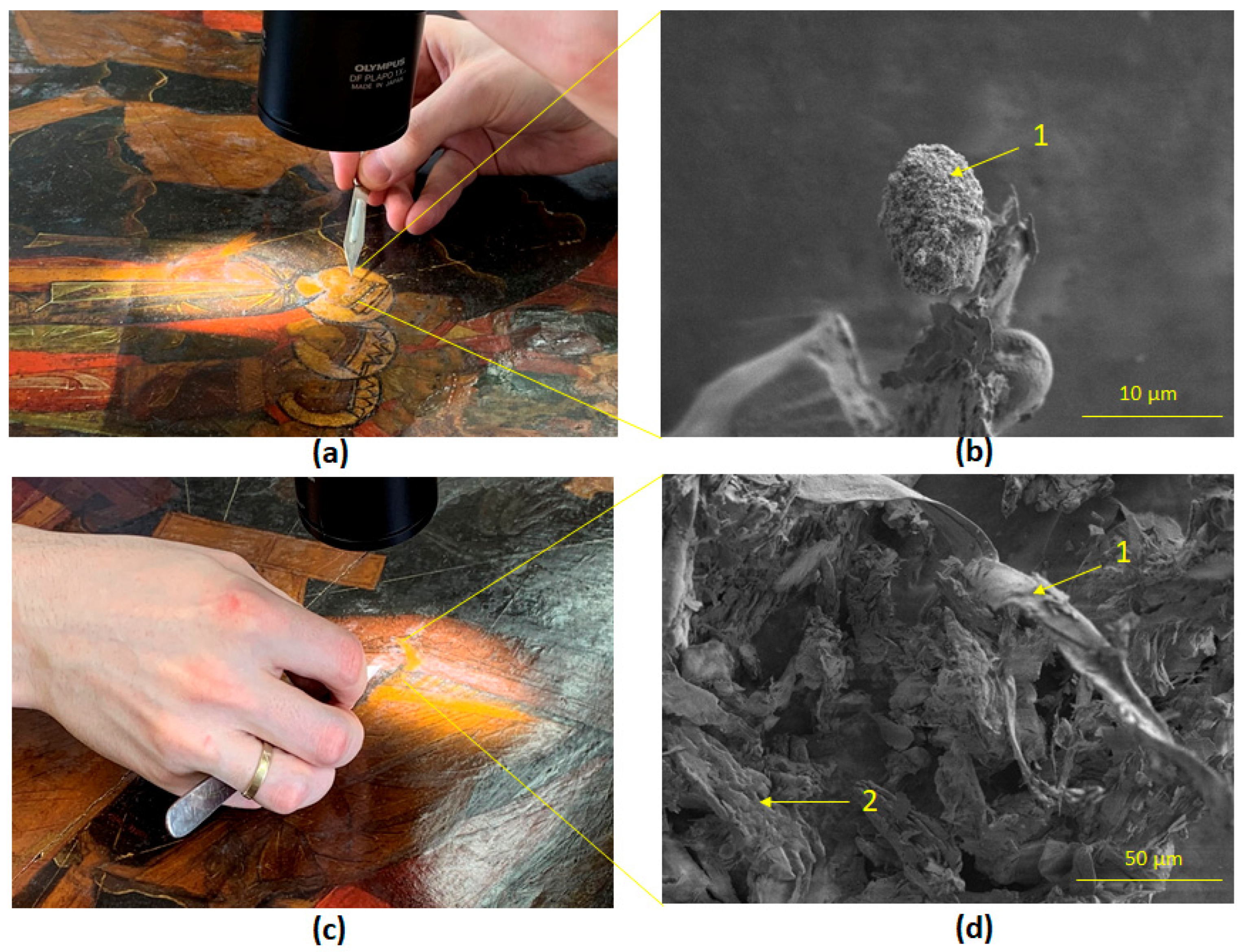
2.1. The Object of the Study
2.2. Isolation and Growth of Fungi Strains
2.3. Phenotypic Characterization of Fungi Strains
2.3.1. Light and Fluorescence Microscopy
2.3.2. Scanning Electron Microscopy
2.4. Genomic DNA Extraction
2.5. PCR Amplification
2.6. Sequencing
2.7. Mock Layer Design and Inoculation
3. Results
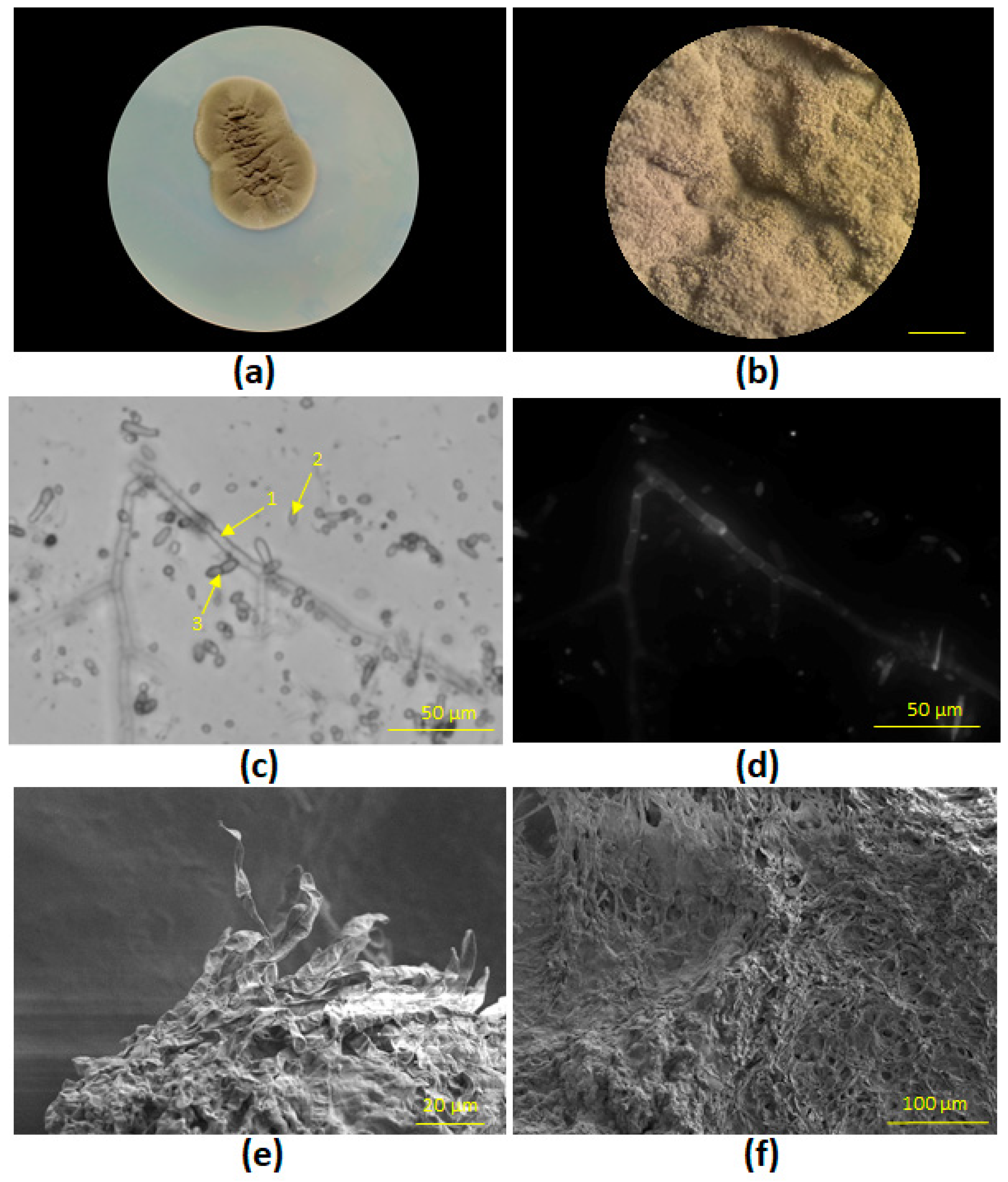
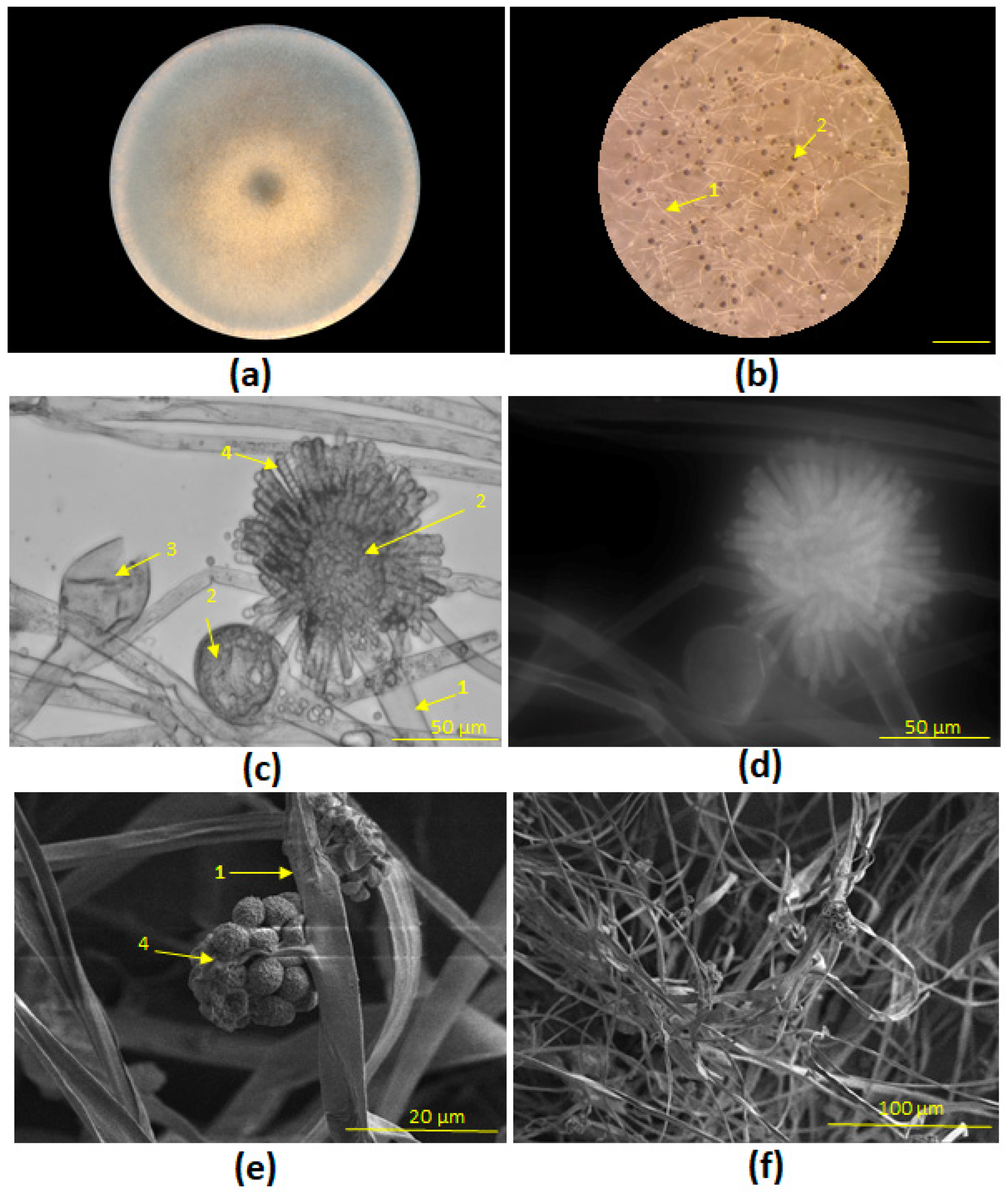
3.1. Phylogenetic Identification and Morphological Characterization of Fungal Strains STG-160 and STG-161
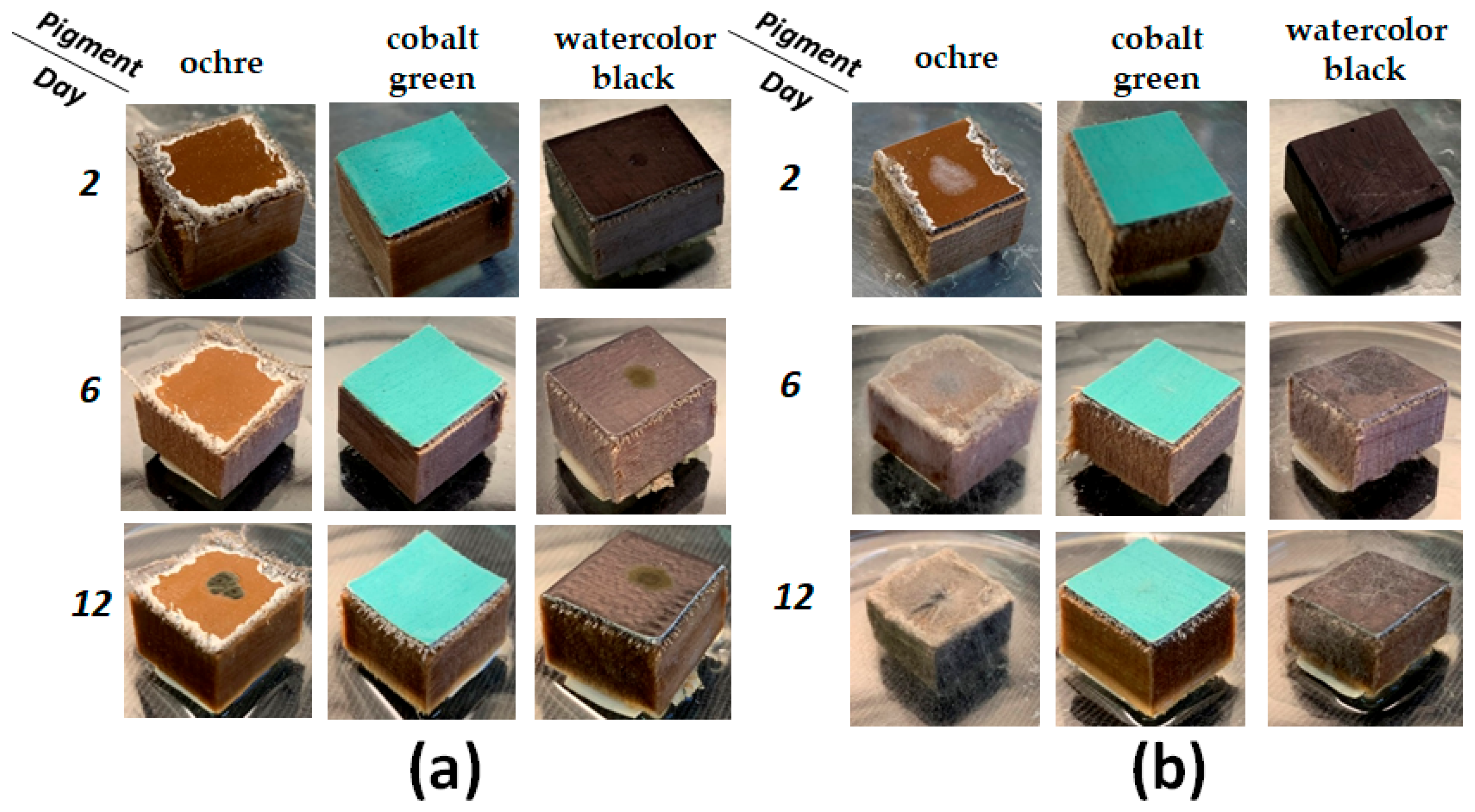
3.2. Mock Layers Biodeterioration
4. Discussion
4.1. Detection of Destructive Fungi in Tempera Paintings
4.2. Dynamics of Paint Layer Biodeterioration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zmeu, C.N.; Bosch-Roig, P. Risk analysis of biodeterioration in contemporary art collections: The poly-material challenge. J. Cult. Herit. 2022, 58, 33–48. [Google Scholar] [CrossRef]
- Caselli, E.; Pancaldi, S.; Baldisserotto, C.; Petrucci, F.; Impallaria, A.; Volpe, L.; D’Accolti, M.; Soffritti, I.; Coccagna, M.; Sassu, G.; et al. Characterization of biodegradation in a 17th century easel painting and potential for a biological approach. PLoS ONE 2018, 13, e0207630. [Google Scholar] [CrossRef]
- Salvador, C.; Bordalo, R.; Silva, M.; Rosado, T.; Candeias, A.; Caldeira, A.T. On the Conservation of Easel Paintings: Evaluation of Microbial Contamination and Artists Materials. Appl. Phys. A Mater. Sci. Process. 2017, 123, 80. [Google Scholar] [CrossRef]
- Giuffrida, M.G.; Mazzoli, R.; Pessione, E. Back to the Past: Deciphering Cultural Heritage Secrets by Protein Identification. Appl. Microbiol. Biotechnol. 2018, 102, 5445–5455. [Google Scholar] [CrossRef]
- Castrillón Rivera, L.E.; Palma Ramos, A.; Castañeda Sánchez, J.I.; Drago Serrano, M.E. Origin and Control Strategies of Biofilms in the Cultural Heritage. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar]
- Paiva de Carvalho, H.; Mesquita, N.; Trovão, J.; Fernández Rodríguez, S.; Pinheiro, A.C.; Gomes, V.; Alcoforado, A.; Gil, F.; Portugal, A. Fungal contamination of paintings and wooden sculptures inside the storage room of a museum: Are current norms and reference values adequate? J. Cult. Herit. 2018, 34, 268–276. [Google Scholar] [CrossRef]
- Zalar, P.; Graf Hriberšek, D.; Gostinčar, C.; Breskvar, M.; Džeroski, S.; Matul, M.; Novak Babič, M.; Čremožnik Zupančič, J.; Kujović, A.; Gunde-Cimerman, N.; et al. Xerophilic fungi contaminating historically valuable easel paintings from Slovenia. Front. Microbiol. 2023, 14, 1258670. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Chovanová, K.; Simonovicová, A.; Ferianc, P. Investigation of microbial community isolated from indoor artworks and air environment: Identification, biodegradative abilities, and DNA typing. Can. J. Microbiol. 2009, 55, 277–287. [Google Scholar] [CrossRef]
- Caldeira, A.T. Green Mitigation Strategy for Cultural Heritage Using Bacterial Biocides. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; pp. 137–154. ISBN 978-3-030-69410-4. [Google Scholar]
- Rosado, T.; Gil, M.; Mirão, J.; Candeias, A.; Caldeira, A.T. Oxalate Biofilm Formation in Mural Paintings due to Microorganisms—A Comprehensive Study. Int. Biodeterior. Biodegrad. 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Zhgun, A.; Avdanina, D.; Shumikhin, K.; Simonenko, N.; Lyubavskaya, E.; Volkov, I.; Ivanov, V. Detection of Potential Biodeterioration Risks for Tempera Painting in 16th Century Exhibits from State Tretyakov Gallery. PLoS ONE 2020, 15, e0230591. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Bastholm, C.J.; Madsen, A.M.; Frisvad, J. Xerophilic Fungi in Museum Repositories Challenge Our Perception of Healthy Buildings and the Preservation of Cultural Heritage. In Healthy Buildings; SINTEF Academic Press: Oslo, Norway, 2021; pp. 260–268. Available online: https://www.researchgate.net/publication/359168951_Xerophilic_fungi_in_museum_repositories_challenge_our_perception_of_healthy_buildings_and_the_preservation_of_cultural_heritage (accessed on 29 May 2025).
- Avdanina, D.A.; Ermolyuk, A.A.; Bashkirova, K.Y.; Vorobyova, O.B.; Simonenko, N.P.; Shitov, M.V.; Zhgun, A.A. Determining the Source of Biodeterioration of the 16th Century Icon Deesis Tier of 13 Figures from the State Tretyakov Gallery. Microbiology 2024, 93, S87–S92. [Google Scholar] [CrossRef]
- Kavkler, K.; Humar, M.; Kržišnik, D.; Turk, M.; Tavzes, Č.; Gostinčar, C.; Džeroski, S.; Popov, S.; Penko, A.; Gunde-Cimerman, N.; et al. A multidisciplinary study of biodeteriorated Celje Ceiling, a tempera painting on canvas. Int. Biodeterior. Biodegrad. 2022, 170, 105389. [Google Scholar] [CrossRef]
- Climaco, G.; Oliva, G.; Fiore, P.; Tedesco, C.; Castiglione, S.; Vigliotta, G. Biodeterioration of canvas paintings: Microbial role and development of sustainable treatments for biocontrol. Appl. Microbiol. Biotechnol. 2025, 109. [Google Scholar] [CrossRef]
- Ali, M.F.; Mansour, M.M.A.; Badr, N.M.; Salem, M.Z.M. A Study of Biodeterioration and Chromatic Alterations of Painted and Gilded Mummy Cartonnage at the Saqqara Museum Storeroom, Egypt. Archaeometry 2018, 60, 845–858. [Google Scholar] [CrossRef]
- Ermolyuk, A.A.; Avdanina, D.A.; Koblov, F.S.; Kalinin, S.G.; Vasilieva, B.F.; Demiankova, M.V.; Efremenkova, O.V.; Zhgun, A.A. Fungi-Destructors of Painting Materials Isolated in the State Tretyakov Gallery as Novel Promising Producers of Antimicrobial Compounds. Appl. Biochem. Microbiol. 2025, 1–14. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 315–322. [Google Scholar] [CrossRef]
- Hong, S.B.; Go, S.J.; Shin, H.D.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 2019, 91, 553–556. [Google Scholar] [CrossRef]
- Tippmann, H.F. Analysis for free: Comparing programs for sequence analysis. Brief. Bioinform. 2004, 5, 82–87. [Google Scholar] [CrossRef]
- Lu, G.; Moriyama, E.N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 2004, 5, 378–388. [Google Scholar] [CrossRef]
- Klokova, G.S.; Demina, O.V.; Eremina, I.M.; Osipov, Y.A.; Posternak, O.P.; Pershin, D.S.; Pershina, D.S.; Rebrikova, N.L.; Toskina, I.N.; Fedorova, I.V.; et al. Restoration of Easel Tempera Paintings: A Study Guide for Higher Education Institutions; Orthodox St. Tikhon’s University for the Humanities: Moscow, Russia, 2021; ISBN 978-5-7429-1400-6. [Google Scholar]
- Kosolapov, A.I. Natural Scientific Methods in the Examination of Art Works; State Herm.: St. Petersburg, Russia, 2015; ISBN 978-5-93572-636-2. [Google Scholar]
- Fulcher, K. Evidence for the use of madder as a pigment in Nubia. Sudan Nubia 2017, 21, 113–116. [Google Scholar]
- Ware, M. Prussian Blue: Artists’ Pigment and Chemists’ Sponge. J. Chem. Educ. 2008, 85, 612–620. [Google Scholar] [CrossRef]
- Owen, L. Fire and Paper: An Examination of the Materials and Techniques of Lee Bontecou’s Soot Drawings. In Book and Paper Group session, AIC 36th Annual Meeting; Book and Paper Group: Philadelphia, PA, USA, 2008. [Google Scholar]
- Ibrahim, A.B.M.; Mahmoud, G.A.E.; Cordes, D.B.; Slawin, A.M.Z. Pb (II) and Hg (II) thiosemicarbazones for inhibiting the broad-spectrum pathogen Cladosporium sphaerospermum ASU18 (MK387875) and altering its antioxidant system. Appl. Organomet. Chem. 2022, 36, e6798. [Google Scholar] [CrossRef]
- Garg, N.; Prakash, O. Biodegradation of mango kernel by Syncephalastrum racemosum and its biological control. BioControl 2006, 51, 353–361. [Google Scholar] [CrossRef]
- Rahmawati; Eltivitasari, A.; Romadhonsyah, F.; Gemantari, B.M.; Nurrochmad, A.; Wahyuono, S.; Astuti, P. Effect of light exposure on secondary metabolite production and bioactivities of Syncephalastrum racemosum endophyte. Trop. J. Nat. Prod. Res. 2021, 5, 312–318. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Solovey, V.R.; Shumikhin, K.V.; Lizunova, A.A.; Lisovskii, S.V.; Liubavskaya, E.A.; Seregina, T.V.; Basova, I.G.; Dyakonova, Y.B.; Simonenko, T.L.; et al. A study of “The Portrait of F.P. Makerovsky in a Masquerade Costume” by Dmitry Levitsky from the collection of the State Tretyakov Gallery. Herit. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Ferreira, D.N.; Melo, V.d.F.; Testoni, S.A.; Vidal-Torrado, P.; de Oliveira, J.C., Jr. Origin and properties of kaolinites from soils of a toposequence in Southern Brazil. Rev. Bras. Cienc. Solo 2024, 48. [Google Scholar] [CrossRef]
- Prasad, C.R.V.; Reddy, P.H.P.; Murthy, V.R.; Sivapullaiah, P.V. Swelling characteristics of soils subjected to acid contamination. Soils Found. 2018, 58, 110–121. [Google Scholar] [CrossRef]
- Viegas, C.; Cervantes, R.; Dias, M.; Gomes, B.; Pena, P.; Carolino, E.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S.; et al. Unveiling the Occupational Exposure to Microbial Contamination in Conservation–Restoration Settings. Microorganisms 2022, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, H.M.; Cavaliere, A.R. Artworks, Drawings, Prints, and Documents—Fungi Eat Them All! In Art, Biology, and Conservation; Koestler, R.J., Koestler, V.H., Charola, A.E., Nieto-Fernandez, F.E., Eds.; The Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 128–151. [Google Scholar]
- Amatya, R.; Khanal, B.; Rijal, A. Syncephalastrum species producing mycetoma-like lesions. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 284–286. [Google Scholar] [CrossRef]
- Garg, N.; Prakash, O.; Pandey, B.K.; Singh, B.P.; Pandey, G. First Report of Black Soft Rot of Indian Gooseberry Caused by Syncephalastrum racemosum. Plant Dis. 2004, 88, 575. [Google Scholar] [CrossRef]
- Baby, S.; Ramya, T.G.; Geetha, R.K. Onychomycosis by Syncephalastrum racemosum: Case report from kerala, India. Dermatol. Rep. 2015, 7, 5527. [Google Scholar] [CrossRef]
- Irshad, M.; Nasir, N.; Hashmi, U.H.; Farooqi, J.; Mahmood, S.F. Invasive pulmonary infection by Syncephalastrum species: Two case reports and review of literature. IDCases 2020, 21. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, P.; Siddall, R. Pigment Compendium—A Dictionary of Historical Pigments; Elsevier: Oxford, UK, 2004; ISBN 0 7506 57499. [Google Scholar]
- Babich, H.; Stotzky, G. Toxicity of zinc to fungi, bacteria, and coliphages: Influence of chloride ions. Appl. Environ. Microbiol. 1978, 36, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Klimek, B.; Niklińska, M. Zinc and copper toxicity to soil bacteria and fungi from zinc polluted and unpolluted soils: A comparative study with different types of biolog plates. Bull. Environ. Contam. Toxicol. 2007, 78, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. Redox cycling in iron uptake, efflux, and trafficking. J. Biol. Chem. 2010, 285, 26729–26735. [Google Scholar] [CrossRef]
- Tamayo, E.; Gómez-Gallego, T.; Azcón-Aguilar, C.; Ferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 5, 113084. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdanina, D.; Ermolyuk, A.; Simonenko, N.; Troyan, E.; Shitov, M.; Zhgun, A. Molecular Diagnostics and Determining of Biodeterioration Risk for the 16th Century Icon “Descent into Hell” from the State Tretyakov Gallery. Heritage 2025, 8, 498. https://doi.org/10.3390/heritage8120498
Avdanina D, Ermolyuk A, Simonenko N, Troyan E, Shitov M, Zhgun A. Molecular Diagnostics and Determining of Biodeterioration Risk for the 16th Century Icon “Descent into Hell” from the State Tretyakov Gallery. Heritage. 2025; 8(12):498. https://doi.org/10.3390/heritage8120498
Chicago/Turabian StyleAvdanina, Daria, Anna Ermolyuk, Nikolay Simonenko, Egor Troyan, Michael Shitov, and Alexander Zhgun. 2025. "Molecular Diagnostics and Determining of Biodeterioration Risk for the 16th Century Icon “Descent into Hell” from the State Tretyakov Gallery" Heritage 8, no. 12: 498. https://doi.org/10.3390/heritage8120498
APA StyleAvdanina, D., Ermolyuk, A., Simonenko, N., Troyan, E., Shitov, M., & Zhgun, A. (2025). Molecular Diagnostics and Determining of Biodeterioration Risk for the 16th Century Icon “Descent into Hell” from the State Tretyakov Gallery. Heritage, 8(12), 498. https://doi.org/10.3390/heritage8120498









