Sequencing Analysis and Radiocarbon Dating of Yarn Fragments from Six Paracas Mantles from Bundle WK12-382
Abstract
1. Introduction
2. Materials and Methods
2.1. WK12-382 Textiles
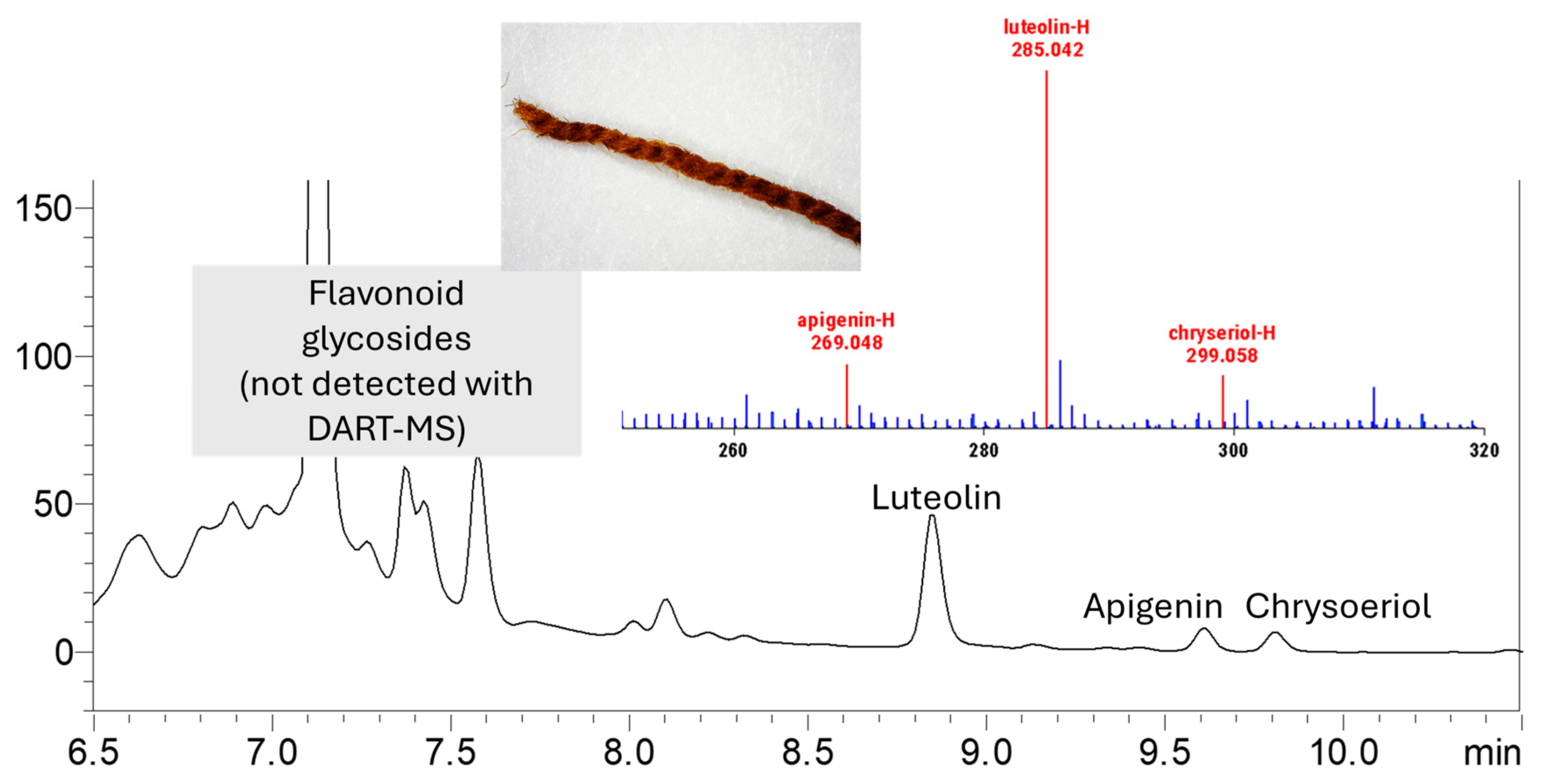
2.2. DART-MS
2.3. HPLC-DAD
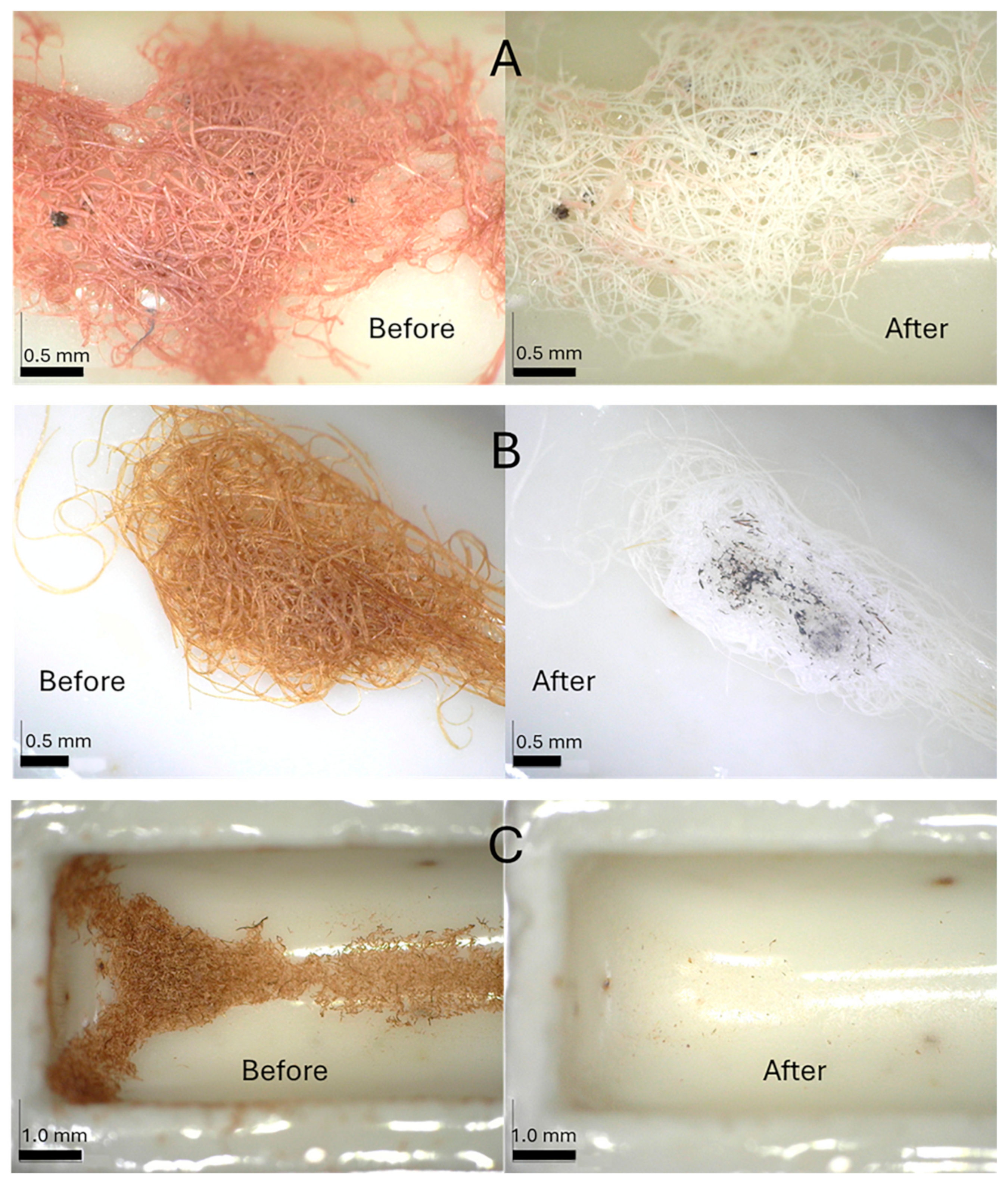
2.4. Plasma Oxidation and AMS Radiocarbon Dating
2.5. Stable Isotope Analysis
2.6. Colorimetric Analysis
3. Results
3.1. Dye Analysis by DART-MS and HPLC-DAD
| Mantle | Color | Part of the Mantle | Anthraquinones | Yellows | Indigoids |
|---|---|---|---|---|---|
| 4 | Blue | Emb. thread | None detected | None detected | Indigotin/indirubin |
| Red-brown | Ground cloth | Purpurin, xanthopurpurin, rubiadin, munjistin, lucidin | 269, 283 (likely anthraquinones, not yellows), HMBA, HBA | None detected | |
| Black | Border fringe | Trace of xanthopurpurin | None detected | Indigotin/indirubin | |
| Green | Border fringe | None detected | 285, 269, 299, (likely luteolin, apigenin, chrysoeriol) | Indigotin/indirubin | |
| Orange | Border fringe | Rubiadin, purpurin | 299, HMBA, HBA | None detected | |
| Gold | Border fringe | None detected | HBA, DHBA, HMBA (with FA, 283, 271, 287, 269, likely apigenin ME, butein, okanin, and apigenin) | Indigotin/indirubin | |
| Red | Border fringe | Purpurin, xanthopurpurin, munjistin, lucidin | HBA | None detected | |
| 5 | Purple-black | Emb. thread | None detected | HBA | Indigotin/indirubin |
| Red | Emb. thread | Purpurin, munjistin | HBA, DHBA, HMBA, 299 (possibly pseudopurpurin?) | None detected | |
| Black | Mantle warp | Purpurin | 299 (possibly pseudopurpurin?) | Indigotin/indirubin | |
| Red | Fringe | Purpurin, xanthopurpurin, munjistin | HBA, HMBA | None detected | |
| 6 | Red | Fringe | Purpurin, rubiadin | HBA | None detected |
| Yellow | Ground cloth | None detected | 285, 269, 299 (luteolin, apigenin, chrysoeriol) | None detected | |
| Yellow-brown | Mantle warp | Traces of lucidin, rubiadin, purpurin | 285, 269, 299, DHBA, DMBA, HBA (luteolin, apigenin, chrysoeriol) | None detected | |
| Brown | Mantle weft | Traces of lucidin, rubiadin, purpurin, munjistin | 285, 269, 299, DHBA, DMBA, HBA (luteolin, apigenin, chrysoeriol) | Trace indigotin/indirubin | |
| 7 | Red weft | Mantle weft | Pupurin, rubiadin | HBA | None detected |
| Red warp | Mantle warp | Purpurin | None detected | None detected | |
| Red | Fringe | Traces of xanthopurpurin, purpurin, rubiadin | None detected | Trace indigotin/indirubin | |
| Green | Fringe | None detected | 329 (rhamnazin?) | Indigotin/indirubin | |
| Purple-brown | Fringe | Traces of purpurin and xanthopurpurin, only with formic acid treatment | DHBA, HBA, 329 (rhamnazin?) | Indigotin/indirubin, DBI? | |
| Yellow | Fringe | Possible trace xanthopurpurin | DHBA, HBA, 285, 283 (luteolin, apigenin methyl ether/genkwanin) | None detected | |
| 9 | Black | Mantle warp | None detected | HBA, DHBA, 299 only with FA (chrysoeriol plus other luteolin MEs) | Indigotin/indirubin, DBI? |
| Black | Mantle weft | None detected | 299, flavonoid degradation products (chrysoeriol plus other luteolin MEs) | Indigotin/indirubin, DBI? | |
| Green | Border background | None detected | 299, DHBA, HBA (as above) | Indigotin/indirubin | |
| Brown | Emb. thread | Traces of munjistin and rubiadin | 285, 299, 315, DHBA, HBA (luteolin, luteolin MEs, rhamnetin?) | None detected | |
| Red | Emb. thread | Purpurin, munjistin, rubiadin, pseudopurpurin, lucidin | None detected | None detected | |
| Brown fringe | Fringe | None detected | 329, 299, 343, HBA, DHBA, HMBA (rhamnazin, chrysoeriol/luteolin MEs, trimethylquercetin?) | Indigotin/indirubin | |
| Blue/purple | Thread | None detected | None detected | Indigotin/indirubin | |
| 10 | purple | Loose threads | None detected | 299, HBA (chrysoeriol/luteolin MEs) | Indigotin/indirubin |
| green | Loose threads | None detected | 285, 269, 299, DHBA, HMBA, HBA (luteolin, apigenin, chrysoeriol) | Indigotin/indirubin, DBI? | |
| green-brown | Border groundcloth | None detected | 285, DHBA, 269, 299, HBA, 329 (chrysoeriol/luteolin MEs, luteolin, apigenin, rhamnazin) | Indigotin/indirubin |
| Mantle | Color | Part of the Mantle | Compounds Identified in | Compounds Identified in | Compounds Identified in |
|---|---|---|---|---|---|
| MeOH-OxA | DMSO | HCl-MeOH | |||
| 4 | Blue | Emb. thread | 350 nm: caffeoylquinic acid, gallic acid, indirubin | Indigotin, indirubin | Not run |
| Brown | Warp | Xanthopurpurin, purpurin | Not run | Not run | |
| Red-brown | Ground cloth | Xanthopurpurin, purpurin | No compounds observed | Purpurin | |
| Black | Border fringe | 350 nm: caffeoylquinic acids (6), luteolin, luteolin methyl ether, luteolin-like compounds (methyl ethers), indirubin | Indigotin, indirubin, traces of yellow luteolin-like compounds (methyl ethers?) | 350 nm: chrysoeriol, luteolin-like compounds (MEs) indirubin | |
| Green | Border fringe | Indirubin | Indigotin, indirubin | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, genkwanin (apigenin methyl ether), luteolin methyl ether, other flavonoid glycosides and methyl ethers, cafeoylquinic acid, indirubin | |
| Orange | Border fringe | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, genkwanin (apigenin methyl ether); 450 nm: traces of xanthopurpurin and rubiadin. | Not run | 450 nm: Purpurin, rubiadin, xanthopurpurin, indigotin (contamination?); 350 nm: luteolin, xanthopurpurin | |
| Gold | Border fringe | 350 nm: rutin?, lutelin 7-O-glycoside, luteolin; 500 nm: indirubin, traces of indigo | Not run | 350 nm: luteolin | |
| Red | Border fringe | Xanthopurpurin, purpurin | No compounds detected | Xanthopurpurin, purpurin | |
| 5 | Purple-black | Emb. thread | Indirubin, indigotin | Indirubin, indigotin; 350 nm: luteolin, chrysoeriol, indirubin | 350 nm: Indigotin, indirubin |
| Red | Emb. thread | Xanthopurpurin, purpurin | Not run | Purpurin | |
| Black (33) | Mantle warp | Indirubin | Indigotin (tr), indirubin | Indigotin, indirubin, luteolin | |
| Red | Fringe | No compounds detected | Not run | Purpurin | |
| 6 | Red | Fringe | No compounds detected | Not run | purpurin |
| Yellow | Ground cloth | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, chrysoeriol, apigenin glycoside, luteolin glycosides, luteolin-like compounds (methyl ethers) | Not run | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, chrysoeriol, apigenin glycoside, luteolin glycosides (2), luteolin-like compounds (methyl ethers) | |
| Yellow-brown | Mantle warp | 350 nm: luteolin 7-O-glucoside, apigenin glycoside, chrysoeriol glycosides?, luteolin, apigenin, chrysoeriol, rhamnetin; 450 nm: Xanthopurpurin | Not run | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, chrysoeriol, apigenin glycosides (2), luteolin glycosides (3) | |
| Brown | Mantle weft | 350 nm: luteolin 7-O-glucoside, apigenin glycoside, chrysoeriol glycosides (2)?, luteolin glycoside, luteolin, apigenin, chrysoeriol | 350 nm: luteolin 7-O-glucoside, apigenin glycoside, luteolin glycoside, luteolin, apigenin, chrysoeriol | 350 nm: Luteolin 7-O-glucoside, luteolin, apigenin, chrysoeriol, apigenin glycoside, ellagic acid, luteolin glycosides (2), luteolin-like | |
| 7 | Red weft | Mantle weft (39) | Purpurin | Not run | Purpurin, xanthopurpurin, possible luteolin glucoside |
| Red warp | Mantle warp (41) | No compounds identified. | Not run | Not run, too little material | |
| Red | Fringe (43) | Xanthopupurin | Not run | 350 nm: Purpurin, xanthopurpurin, ellagic acid, possible luteolin; 450 nm: purpurin, xanthopurpurin, other unidentified anthraquinones | |
| Green | Fringe | 350 nm: quinic acid-like, caffeic acid, rutin, caffeoylquinic acids, quercetin glycoside?, chrysoeriol-like (5), isorhamnetin, indirubin | Indigotin; 350 nm: luteolin?, unknown luteolin-like compounds, indigotin, indirubin | 350 nm: luteolin, chrysoeriol, indigotin, indirubin | |
| Purple | Fringe | 350 nm: indirubin; 450 nm: Indirubin, purpurin | 350 nm: Indirubin, indigotin, luteolin, luteolin 7-O-glucoside; 598 nm: dibromoindigotin. | 350 nm: luteolin-7-O-glucoside, purpurin, indirubin; 450 nm: purpurin, indirubin | |
| yellow | Fringe | 350 nm: Quinic acid-like compound, chrysoeriol glycosides? (2), Luteolin-7-O-glucoside, luteolin | Not run | Luteolin-7-O-glucoside, luteolin, apigenin, chrysoeriol | |
| 9 | Black | Mantle warp | Indirubin | 538/606 nm: indigotin, indirubin, pseudoindirubin; 598 nm: monobromoindigo, dibromoindigo | 350 nm: luteolin, indigotin, indirubin, caffeoylquinic acid |
| Black | Mantle weft | Indirubin, cafeoylquinic acid-like compound | 538/606 nm: indigotin, indirubin, pseudoindirubin; 598 nm: dibromoindigo | 350 nm: indigotin, indirubin, no significant yellows | |
| Green | Border background | 350 nm: dihydrobenzoic acid, caffeic acid, cafeoylquinic acid-like compounds, luteolin, flavonoid methyl ethers | indigotin, indirubin | 350 nm: caffeic acid derivatives, luteolin, luteolin methyl ether, indigotin, indirubin, many luteolin-like compounds (probably methyl ethers, check MS); 450 nm: indigotin, indirubin | |
| Brown | Emb. thread | 350 nm: luteolin, luteolin 4-methyl ether, other favonoid methyl ethers | indigotin, indirubin | 350 nm: quinic acid derivatives, luteolin and other flavonoid methyl ethers; 606 nm: trace indigotin; 538 nm: trace indirubin | |
| Red | Emb. thread | xanthopurpurin, purpurin | Not run | purpurin, xanthopurpurin, pseudopurpurin? | |
| Brown fringe | Fringe | 350 nm: cafeoylquinic acids (5), luteolin, luteolin-like, rutin-like, flavonoid methyl ethers | indigotin, indirubin, luteolin methyl ethers | 350 nm: luteolin, luteolin 4-methyl ether, luteolin methyl ethers, genkwanin, indigotin | |
| Blue/purple | Thread | 350 nm: quinic acid-like compounds (3), rutin-like and luteolin-like late-eluting compounds (methyl ethers), indirubin; 450 nm: pseudoindirubins, indirubin | indigotin, indirubin | Not run | |
| 10 | Purple | Loose threads | Indirubin, ellagic acid @ 350 nm | 350 nm: genkwanin, indirubin, caffeic acid-like, and quinic acid-like compounds; 538 nm: indirubin; 606 nm: trace indigotin; 598 nm: dibromoindigo | 450 nm: purpurin, indigotin, indirubin |
| Green | Loose threads | 350 nm: luteolin 7-O-glucoside, luteolin, indirubin | 350 nm: Indigotin, indirubin, luteolin 7-O-glucoside; 598 nm: dibromoindigo | 350 nm: luteolin 7-O-glucoside; 450 nm: indigotin, indirubin | |
| Green-brown | Border ground cloth | No dyes detected | 350 nm: Indigotin, indirubin, luteolin 7-O-glucoside | 350 nm: quinic acid-like compounds; 538 nm: indirubin; 606 nm: indigotin |
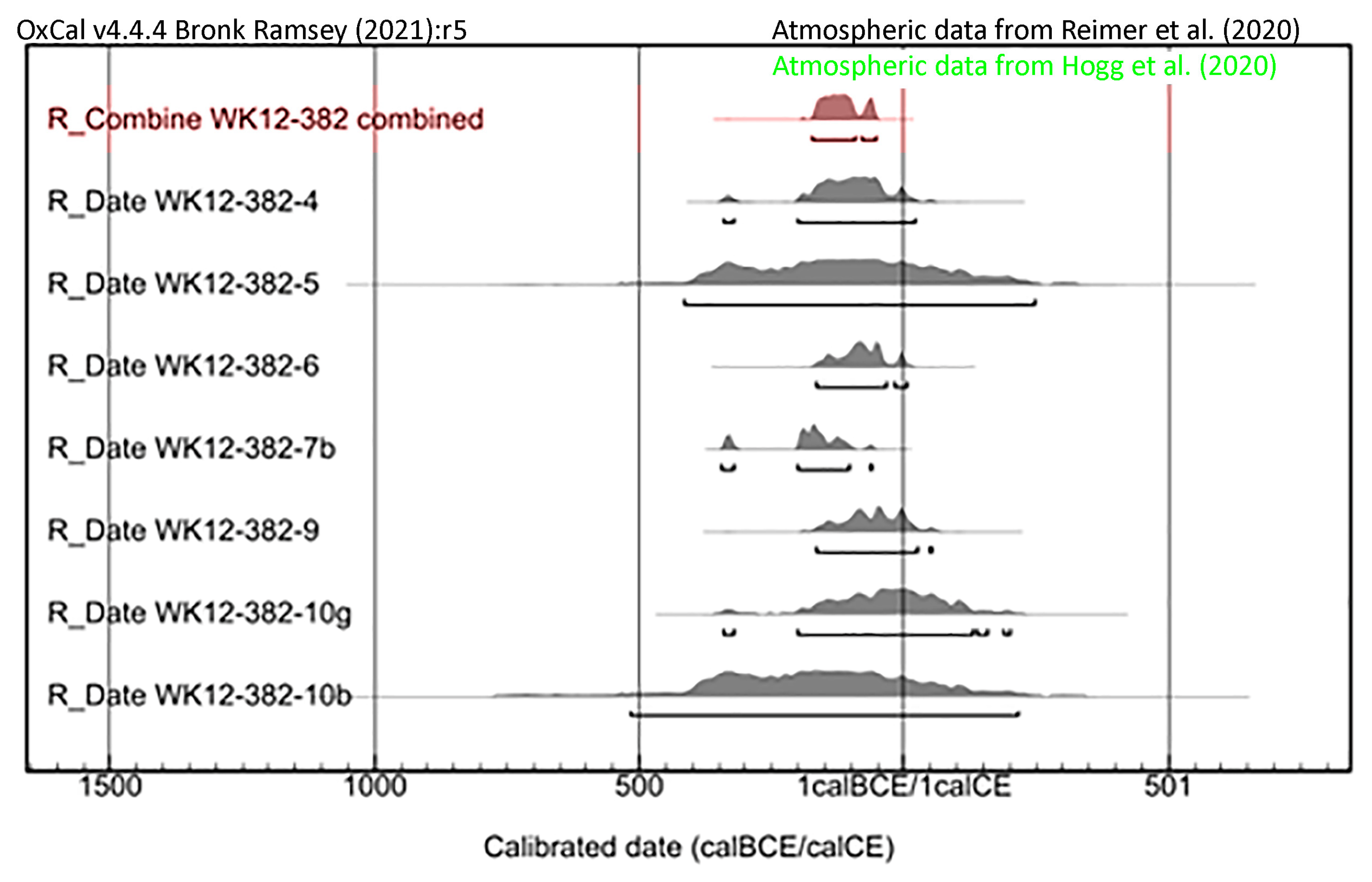
3.2. Plasma Oxidation and AMS Radiocarbon Dating
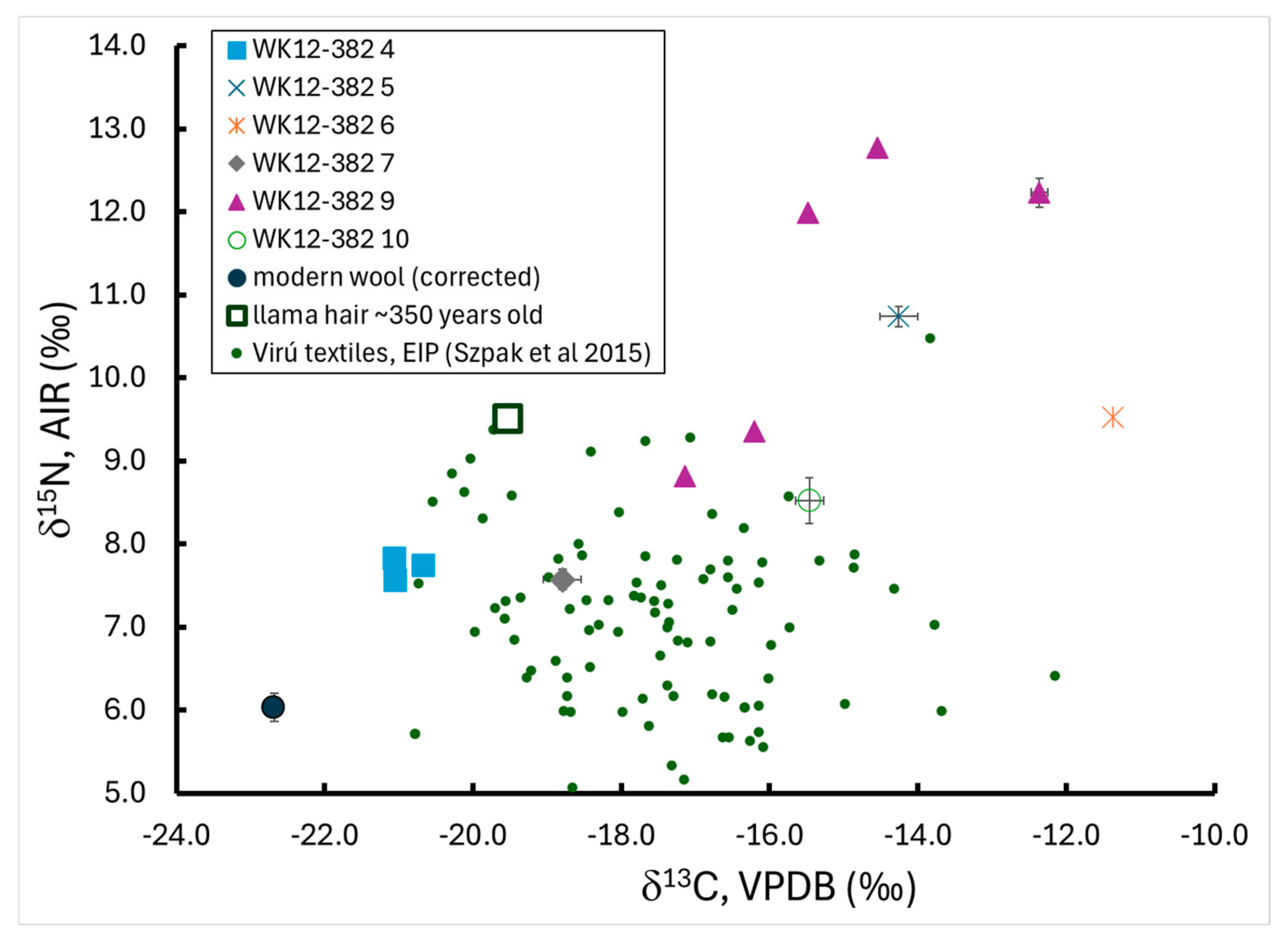
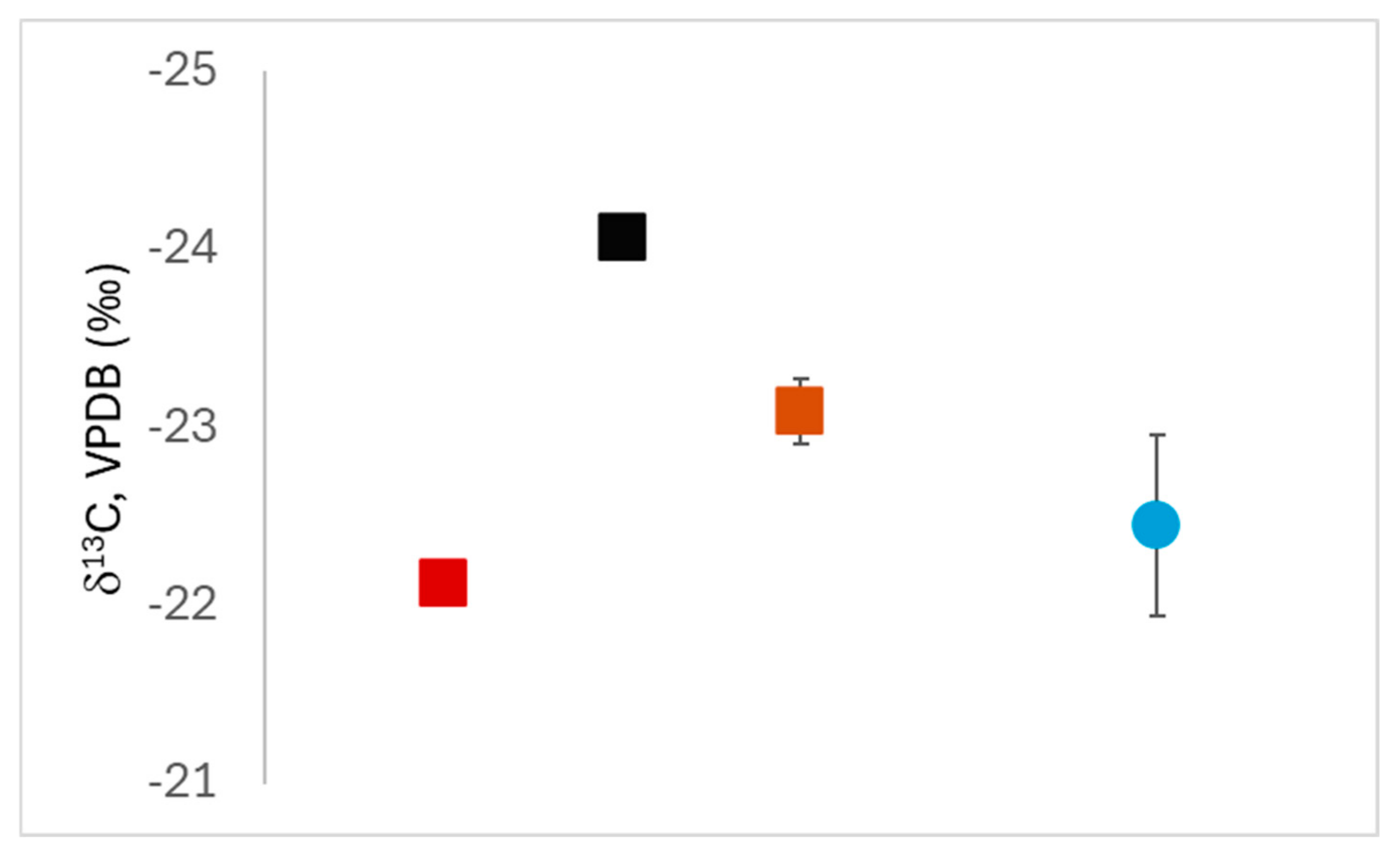
3.3. Light Stable Isotope Results
4. Discussion
“Consequently, several factors can modify the dye composition of a botanical species: the age of the plant, its growth phase, the natural environment in which it grows (soil type, altitude), the season (climate), its condition (infestation [as with fungi in the case of Baccharis]) and preparation, the dyeing process, etc. It is therefore difficult to distinguish in a single analysis all the dye molecules that a single species can produce. If one or more of these criteria is/are not identical to those that prevailed for the [ancient] dyers, it is possible that the chromatographic results obtained on our dye samples do not correspond, or only imperfectly, to the results from the archeological fabrics.”
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Fester, G.; Cruellas, J. Colorantes de Paracas. Rev. Mus. Nac. 1934, 3, 154–156. [Google Scholar]
- Dulanto, J. Puerto Nuevo: Redes de intercambio a larga distancia durante la primera mitad del primer mileno antes de nuestra era. Boletín Arqueol. PUCP 2013, 17, 103–132. [Google Scholar] [CrossRef]
- Van Gijseghem, H.; Vaughn, K.J.; Whalen, V.H.; Grados, M.L.; Canales, J.O. Economic, social, and ritual aspects of copper mining in ancient Peru: An upper Ica valley case study. In Mining and Quarrying in the Ancient Andes: Sociopolitical, Economic, and Symbolic Dimensions; Springer: New York, NY, USA, 2012; pp. 275–298. [Google Scholar]
- Vaughn, K.J.; Van Gijseghem, H.; Linares Grados, M.; Eerkens, J.W. Minería de hematita en la costa sur del Perú: Investigaciones arqueológicas en Mina Primavera. Chungará 2013, 45, 131–142. [Google Scholar] [CrossRef]
- Velarde, M.I.; de la Mata, P.C. Transición Paracas-Nasca, continuidad e innovación en los metales. Boletín Arqueol. PUCP 2018, 135–145. [Google Scholar]
- DeLeonardis, L.; Glascock, M.D. From Queshqa to Callango: A Paracas obsidian assemblage from the lower Ica Valley, Peru. Ñawpa Pacha 2013, 33, 163–192. [Google Scholar] [CrossRef]
- Beresford-Jones, D.G.; Mader, C.; Lane, K.J.; Cadwallader, L.; Gräfingholt, B.; Chauca, G.; Grant, J.; Hölzl, S.; Coll, L.V.J.; Lang, M.; et al. Beyond Inca roads: Archaeological mobilities from the high Andes to the Pacific in southern Peru. Antiquity 2023, 97, 194–212. [Google Scholar] [CrossRef]
- Chauca, G.E.; Arce, S.; Beresford-Jones, D.G. El Spondylus del Periodo Intermedio Tardío (ca. 1000–1470 d.C.) del valle de Ica: Rutas de abastecimiento. In Actas del VIII Congreso Nacional de Arqueología; Ministerio de Cultura Peru: Lima, Peru, 2022. [Google Scholar]
- Yacovleff, E. Arte plumaria entre los antiguos peruanos. Rev. Mus. Nac. 1933, 2, 2. [Google Scholar]
- Peters, A. Dressing the Leader, Dressing the Ancestor: The longue durée in the South Central Andes. In Proceedings of the Textile Society of America Symposium Proceedings, Los Angeles, CA, USA, 10–14 September 2014. [Google Scholar]
- Peters, A. Two-headed serpents and rayed heads: Precedents and reinterpretations in Paracas Necropolis imagery. In PreColumbian Textile Conference VIII/Jornadas de Textiles PreColombinos VIII; Bjerregaard, L., Peters, A.H., Eds.; Zea Books: Lincoln, NE, USA, 2020; pp. 23–49. [Google Scholar]
- Gómez Mejía, J. Qualidade de vida e dinâmicas de conflito na população da península de Paracas, costa sul do Peru durante o final do Horizonte Temprano (400 aC–100 dC). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2016. [Google Scholar]
- Verano, J. Holes in the Head: The Art and Archaeology of Trepanation in Ancient Peru; Dumbarton Oaks: Washington, DC, USA, 2016. [Google Scholar]
- Tello, J.C. Paracas: Primera Parte; Institute of Andean Research: Lima, Peru, 1959. [Google Scholar]
- Tello, J.C.; Xesspe, T.M. Paracas, Segunda Parte: Cavernas y Necrópolis; Universidad Nacional Mayor de San Marcos y Institute of Andean Research: Lima, Peru, 1979. [Google Scholar]
- Paul, A. Paracas Ritual Attire; University of Oklahoma Press: Norman, OK, USA, 1990. [Google Scholar]
- Paul, A. L’utilisation de la couleur dans les textiles Paracas-Necrópolis: Que nous apprend-elle sur l’organisation de la teinture, les procédés de création, et la société Paracas? In Paracas: Trésors inédits du Pérou ancien; Lavallée, D., Ed.; Musée du Quai Branly and Flammarion: Paris, France, 2008. [Google Scholar]
- Tello, J.C. Antiguo Perú: Primera Época; Comisión Organizadora del Segundo Congreso Sudamericano de Turismo: Lima, Peru, 1929. [Google Scholar]
- Carrión Cachot, R. La indumentaria en la antigua cultura de Paracas. Wira Kocha Rev. Peru. Estud. Antropológicos 1931, 1, 37–86. [Google Scholar]
- O’Neale, L.M. Textile periods in ancient Peru II: Paracas Cavernas and the Grand Necropolis. Univ. Calif. Publ. Am. Archaeol. Ethnol. 1945, 39, 143–202. [Google Scholar]
- Carrión Cachot, R. Paracas, Cultural Elements; Corporación Nacional de Turismo: Lima, Peru, 1949. [Google Scholar]
- Sotelo, C. (Ed.) Paracas Cavernas. Lima: Museo de Arqueología y Antropología, Universidad Nacional Mayor de San Marcos; Cuaderno del Archivo Tello 7; Museo de Arqueología y Antropología, Universidad Nacional Mayor de San Marcos: Lima, Peru, 2009. [Google Scholar]
- Sotelo, C. (Ed.) Wari Kayan; Cuaderno del Archivo Tello 9; Museo de Arqueología y Antropología, Universidad Nacional Mayor de San Marcos: Lima, Peru, 2012. [Google Scholar]
- Yacovleff, E.; Muelle, J.C. Un fardo funerario de Paracas. Rev. Mus. Nac. 1934, 3, 63–138. [Google Scholar]
- Museo Nacional de Antropología, Arqueología e Historia del Peru (MNAAHP). Arqueológicas; Ministerio de Cultura Peru: Lima, Peru, 2016.
- Dwyer, J.P. The Chronology and Iconography of Paracas-style Textiles. In The Junius B. Bird Pre-Columbian Textile Conferenc; Rowe, A.P., Benson, E.B., Schaffer, A.-L., Eds.; Textile Museum and Dumbarton Oaks: Washington, DC, USA, 1979; pp. 105–128. [Google Scholar]
- Paul, A. The chronological relationship of the Linear, Block Color, and Broad Line Styles of Paracas embroidered images. In Pre-Columbian Art History: Selected Readings; Alana, C.-C., Ed.; Peek Publications: Palo Alto, CA, USA, 1982; pp. 255–277. [Google Scholar]
- Paul, A. Radiocarbon dates for Paracas. In Paracas Art and Architecture: Object and Context in South Coastal Peru; Paul, A., Ed.; University of Iowa Press: Iowa City, IA, USA, 1991; pp. 22–53. [Google Scholar]
- León Canales, E. Cronología de los fardos funerarios de Wari Kayan, Paracas Necrópolis. In Hilos del Pasado: El Aporte Frances. al Legado Textil Paracas; Instituto Nacional de Cultura: Lima, Peru, 2007; pp. 33–47. [Google Scholar]
- Paul, A. Alte Textilen aus den Anden als Spiegel del kulturellen Entwicklung und Ein Mumienbündel aus Paracas Necrópolis. In Nasca, Geheimnisvolle Zeichen im Alten Peru; Rickenbach, J., Ed.; Museum Reitberg: Zurich, Switzerland, 1999; pp. 17–58. [Google Scholar]
- Paul, A. Color patterns on Paracas necropolis weavings: A combinatorial language on ancient cloth. Tech. Culture. Rev. Semest. D’anthropologie Tech. 1998, 113–153. [Google Scholar] [CrossRef]
- Paul, A. Protective perimeters: The symbolism of borders on Paracas textiles. RES Anthropol. Aesthet. 2000, 38, 144–167. [Google Scholar] [CrossRef]
- Paul, A.; Niles, S.A. Identifying hands at work on a Paracas mantle. Text. Mus. J. 1985, 23, 5–15. [Google Scholar]
- Martoglio, P.A.; Bouffard, S.P.; Sommer, A.J.; Katon, J.E.; Jakes, K.A. Unlocking the secrets of the past: The analysis of archaeological textiles and dyes. Anal. Chem. 1990, 62, 1123A–1128A. [Google Scholar] [CrossRef]
- Saltzman, M. The identification of dyes in archaeological and ethnographic textiles. In Archaeological Chemistry II; Carter, G., Ed.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1978; pp. 172–185. [Google Scholar]
- Frame, M. Structure, image, and abstraction: Paracas Necropolis headbands as system templates. In Paracas Art and Architecture; Paul, A., Ed.; University of Iowa Press: Iowa City, IA, USA, 1991; pp. 110–171. ISBN 0-87745-327-6. [Google Scholar]
- Frame, M. Las prendas bordadas de la necropolis de Wari Kayan. Hilos Pasado Aporte Fr. Legado Paracas 2007, 65–73. [Google Scholar]
- Frame, M. Representaciones de género, jerarquía y otras relaciones en los bordados Paracas Necrópolis. Arqueol. Soc. 2008, 241–264. [Google Scholar] [CrossRef]
- Aponte, D. Presentación de los materiales del fardo funerario 290 de Wari Kayán, Paracas Necrópolis. Arqueológicas 2006, 27, 9–99. [Google Scholar]
- Peters, A.H. Paracas Necropolis: Communities of textile production, exchange networks, and social boundaries in the Central Andes, 150 BC to AD 250. In Textiles, Technical Practice and Power in the Andes; Dransart, P., Arnold, D.Y., Eds.; Archetype: London, UK, 2014; pp. 109–139. [Google Scholar]
- Peters, A.H. The cemetery of Paracas Necropolis: Mortuary practice and social network. In Tres Ensayos Sobre Paracas Necrópolis. Historia de la Investigación, Tecnologías Textiles y Prácticas Mortuorias; Sinclaire, C., Torre, A., Berenguer, J., Eds.; Arte Encuentro; Museo Chileno de Arte Precolombino: Santiago, Chile, 2016; pp. 43–66. [Google Scholar]
- Peters, A.H. El testimonio de una tumba: La presencia Nasca en Paracas; “Fardo WK 382”, “Fardo WK 378” y “Fardo WK 319” en Anexo: Paracas y Nasca en Wari Kayan. In Nasca; Pardo, C., Fux, P., Eds.; Museo de Arte de Lima y Museum Reitburg: Lima, Peru, 2017; pp. 62–69, 296–327. [Google Scholar]
- Peters, A.H. Paracas Necropolis: La estructura del fardo y teorías de la práctica mortuoria. In El Estudio del Mundo Andino; Marco, C.P., Ed.; Pontificia Universidad Católica del Perú: Lima, Peru, 2019; pp. 59–72. [Google Scholar]
- Peters, A.H. Emblematic and material color in the Paracas-Nasca Transition. Textiles Amérindiens. Regards Croisés sur les Couleurs, Desrosiers, S., Nuñez-Regueiro, P., Eds.; Mundo Nuevo—Nuevos Mundos, Colloques. 2016. [Google Scholar] [CrossRef]
- Peters, A.H.; Tomasto-Cagigao, E. Masculinities and femininities: Forms and expressions of power in the Paracas Necropolis. In Dressing the Part: Power, Dress, Gender, and Representation in the Pre-Columbian Americas; Sarah, E.M.S., Billie, J.A.F., Eds.; University Press of Florida: Gainesville, FL, USA, 2017; pp. 371–449. [Google Scholar]
- Fehren-Schmitz, L.; Reindel, M.; Cagigao, E.T.; Hummel, S.; Herrmann, B. Pre-Columbian population dynamics in coastal southern Peru: A diachronic investigation of mtDNA patterns in the Palpa region by ancient DNA analysis. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2010, 141, 208–221. [Google Scholar] [CrossRef]
- Tomasto Cacigao, E.; Lund, M.; Garcia, U.; Knudson, K.; Lombardi, G. Análisis de los materiales asociados: Restos humanos. Arqueológicas 2016, 30, 121–136. [Google Scholar]
- Tomasto Cacigao, E.; Reindel, M.; Isla, J. Paracas funerary practices in Palpa, south coast of Peru. Funer. Pract. Models Anc. Andes 2015, 69–86. [Google Scholar]
- Knudson, K.J.; Peters, A.H.; Cagigao, E.T. Paleodiet in the Paracas Necropolis of Wari Kayan: Carbon and nitrogen isotope analysis of keratin samples from the south coast of Peru. J. Archaeol. Sci. 2015, 55, 231–243. [Google Scholar] [CrossRef]
- Pezo-Lanfranco, L.; Aponte, D.; Eggers, S. Aproximación a la dieta de las sociedades formativas tardías del litoral de Paracas (Costa sur del Perú): Evidencias bioarqueológicas e isotópicas. Ñawpa Pacha 2015, 35, 23–55. [Google Scholar] [CrossRef]
- Gómez-Mejía, J.; Aponte, D.; Pezo-Lanfranco, L.; Eggers, S. Intentional cranial modification as a marker of identity in Paracas Cavernas, South-Central Coast of Peru. J. Archaeol. Sci. Rep. 2022, 41, 103264. [Google Scholar] [CrossRef]
- Cagigao, E.T.; Peters, A.; Lund, M.; Ayarza, A. Body modification at Paracas Necropolis (South Coast of Peru ca. 2000 BP). Zur. Stud. Archaeol. 2013, 9, 49–58. [Google Scholar]
- Dulanto, J.; Accinelli, A. Disco Verde: 50 años después de Frédéric Engel: La primera temporada de excavaciones del Proyecto de Investigaciónes Arqueológicas Paracas en el sitio. Boletín Arqueol. PUCP 2013, 133–150. [Google Scholar] [CrossRef]
- Druc, I.; Dulanto, J.; de Castro, A.R.; Guadalupe, E. Análisis de la composición mineral de las vasijas de cerámica de Puerto Nuevo: Algunas consideraciones preliminares sobre su producción y procedencia. Boletín Arqueol. PUCP 2017, 133–157. [Google Scholar] [CrossRef]
- Lévy Contreras, J.; Peters, A. Las warakas (hondas) de Arena Blanca, Cerro Colorado y Wari Kayan, península de Paracas: Nuevos datos e interpretaciones. In PreColumbian and Amerindian Textiles Conference/Jornadas de Textiles Precolombinos y ameríndios IX, Milán 2022; Orsini, C., Villa, F., Eds.; Zea Books: Lincoln, NE, USA, 2024. [Google Scholar]
- Tantaleán, H.; Stanish, C. Cerro del Gentil: Un Sitio Paracas en el Valle de Chincha, Costa sur del Perú; Programa Arqueológico Chincha (PACH): Lima, Peru, 2017. [Google Scholar]
- Stanish, C.; Tantaleán, H.; Knudson, K. Feasting and the evolution of cooperative social organizations circa 2300 BP in Paracas culture, southern Peru. Proc. Natl. Acad. Sci. USA 2018, 115, E6716–E6721. [Google Scholar] [CrossRef]
- Nigra, B.T. Huaca Soto and the evolution of Paracas communities in the Chincha Valley, Peru; University of California: Los Angeles, CA, USA, 2017; ISBN 1-369-86633-X. [Google Scholar]
- Weinberg, C.; Osborn, J.; Espino Huaman, R. Marine shellfish exploitation as a means of reducing vulnerability to resource uncertainty in southern coastal Peru (200 BCE–150 CE). Holocene 2022, 32, 1503–1517. [Google Scholar] [CrossRef]
- Orccosupa, B.; Tantaleán, H.; Stanish, C. The Topará ceramic style: A perspective from Pozuelo in the Lower Chincha Valley. Chungará 2023, 55, 229–260. [Google Scholar] [CrossRef]
- Osborn, J.; Hundman, B.; Weinberg, C.; Huaman, R.E. Reassessing the chronology of Topará emergence and Paracas decline on the Peruvian south coast: A bayesian approach. Radiocarbon 2023, 65, 930–952. [Google Scholar] [CrossRef]
- Balbuena-Cotlear, L. Evidencias paracas en los valles de Pisco y Mala. Boletín Arqueol. PUCP 2013, 57–75. [Google Scholar] [CrossRef]
- Peters, A. Topará en Pisco: Patrón de asentamiento y paisaje. Boletín Arqueol. PUCP 2013, 77–101. [Google Scholar] [CrossRef]
- De La Torre Zevallos, J.C.; Lapi, B.; Manrique, D.D. Proyecto Arqueológico Pisco Temprano: Hacia la interpretación de los espacios arquitectónicos en los conjuntos monumentales de Chongos (siglos III ANE y II DNE). In Proceedings of the Actas del IV Congreso Nacional de Arqueología, 8–11 August 2017; Ministerio de Cultura Peru: Lima, Peru, 2019; pp. 133–142. [Google Scholar]
- Young, M. De la montaña al mar: Intercambio entre la sierra centro-sur y la costa sur durante el Horizonte Temprano. Boletín Arqueol. PUCP 2017, 9–34. [Google Scholar] [CrossRef]
- Splitstoser, J.C. Practice and meaning in spiral-wrapped batons and cords from Cerrillos, a Late Paracas Site in the Ica Valley, Peru. In Textile, Technical Practice and Power in the Andes; Denise, Y.A., Penelope, D., Eds.; Archetype: London, UK, 2014; pp. 46–82. [Google Scholar]
- DeLeonardis, L. Early Paracas Cultural Contexts: New Evidence from Callango. Andean Past 2005, 7, 7. [Google Scholar]
- Bacha, A.B. El Edificio de los Frisos de Ánimas Altas. Ser paracas en el valle bajo de Ica. Boletín Arqueol. PUCP 2017, 191–225. [Google Scholar] [CrossRef][Green Version]
- Beresford-Jones, D.; Alarcón, C.; Arce, S.; Chepstow-Lusty, A.; Whaley, O.; Sturt, F.; Gorriti, M.; Portocarrero, O.; Cadwallader, L. Ocupación y subsistencia del Horizonte Temprano en el contexto de cambios ecológicos de largo plazo en las cuencas de Samaca y Ullujaya, valle bajo de Ica. Boletín Arqueol. PUCP 2009, 237–257. [Google Scholar] [CrossRef]
- Massey, S.A. Social and political leadership in the lower Ica Valley: Ocucaje Phases 8 and 9. In Paracas Art and Architecture; Paul, A., Ed.; University of Iowa Press: Iowa City, IA, USA, 1991; pp. 315–348. ISBN 0-87745-327-6. [Google Scholar]
- Massey, S. Tajahuana: New Insights into a Familiar Paracas Site. Oral presentation at the Society for American Archaeology National Meeting, Chicago, IL, USA, 14–18 April 2021.
- Lane, K.; Huaman, O.; Coll, L.; Pullen, A.; Beresford-Jones, D.; French, C. De fronteras y enclaves: La presencia Nasca en la sierra de Ica (260 a.C.–640 d.C.). Boletín Arqueol. PUCP 2017, 117–132. [Google Scholar] [CrossRef][Green Version]
- Kaulicke, P.; Fehren-Schmitz, L.; Kolp-Godoy, M.; Landa, P.; Loyola, Ó.; Palma, M.; Tomasto, E.; Vergel, C.; Vogt, B. Implicancias de un área funeraria del Periodo Formativo Tardío en el departamento de Ica. Boletín Arqueol. PUCP 2009, 289–322. [Google Scholar] [CrossRef]
- Isla Cuadrado, J. From hunters to regional lords: Funerary practices in Palpa, Peru. In New Technologies for Archaeology: Multidisciplinary Investigations in Palpa and Nasca, Peru; Springer: Berlin/Heidelberg, Germany, 2009; pp. 119–139. [Google Scholar]
- Reindel, M.; Isla, J. De Paracas a Nasca: Nuevas evidencias desde la vertiente occidental de la sierra de Lucanas, Ayacucho. Boletín Arqueol. PUCP 2018, 229–254. [Google Scholar] [CrossRef]
- Mader, C. Sea Shells in the Mountains and Llamas on the Coast: The Economy of the Paracas Culture (800 to 200 BC) in Southern Peru; Harrassowitz Verlag: Wiesbaden, Germany, 2019; ISBN 3-447-11327-8. [Google Scholar]
- Orefici, G. Nuevo enfoques sobre la transición Paracas-Nasaca en Cahuachi (Perú). Andes Boletín Misión Arqueol. Andin. 1996, 1, 173–198. [Google Scholar]
- Lasaponara, R.; Masini, N.; Orefici, G. The Ancient Nasca World: New Insights from Science and Archaeology; Springer: Cham, Switzerland, 2017; ISBN 3-319-47052-3. [Google Scholar]
- Vaughn, K.J.; Conlee, C.A.; Neff, H.; Schreiber, K. A Compositional Analysis of Nasca Pigments: Implications for Craft Production on the Prehispanic South Coast of Peru. Laser Ablation ICP-MS in Archaeological Research; University of New Mexico Press: Albuquerque, NM, USA, 2005; pp. 139–153. [Google Scholar]
- Hajdas, I.; Christi, C.; Bonani, G.; Maurer, M. Textiles and Radiocarbon Dating. Radiocarbon 2014, 56, 637–643. [Google Scholar] [CrossRef]
- Bronk, C.R.; Hedges, R.E.M. A gaseous ion source for routine AMS radiocarbon dating. Nucl. Instrum. Methods Phys. Res. B 1990, 52, 322–326. [Google Scholar] [CrossRef]
- Russ, J.; Hyman, M.; Shafer, H.J.; Rowe, M.W. Radiocarbon dating of prehistoric rock paintings by selective oxidation of organic carbon. Nature 1990, 348, 710–711. [Google Scholar] [CrossRef]
- Steelman, K.L.; Rowe, M.W. Potential for virtually nondestructive radiocarbon and stable carbon isotopic analyses on perishable archaeological artifacts. In Archaeological Chemistry: Materials, Methods, and Meaning; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; pp. 8–21. [Google Scholar]
- Armitage, R.A.; Ellis, M.E.; Merrell, C. New Developments in the “Nondestructive” Dating of Perishable Artifacts Using Plasma-Chemical Oxidation. In Collaborative Endeavors in the Chemical Analysis of Art and Cultural Heritage Materials; Lang, P.L., Armitage, R.A., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1103, pp. 143–154. [Google Scholar]
- Selvius DeRoo, C.; Armitage, R.A. Direct Identification of Dyes in Textiles by Direct Analysis in Real Time-Time of Flight Mass Spectrometry. Anal. Chem. 2011, 83, 6924. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.A.; Jakes, K.A.; Day, C.J. Direct Analysis in Real Time-Mass Spectroscopy for Identification of Red Dye Colorants in Paracas Necropolis Textiles. Sci. Technol. Archaeol. Res. 2015, 1, 60–69. [Google Scholar] [CrossRef]
- Day, C.J.; Selvius DeRoo, C.; Armitage, R.A. Developing Direct Analysis in Real Time Time-of-Flight Mass Spectrometric Methods for Identification of Organic Dyes in Historic Wool Textiles. In Archaeological Chemistry VIII; Armitage, R.A., Burton, J.H., Eds.; ACS: Washington, DC, USA, 2013; pp. 69–85. [Google Scholar]
- Lackner, R.M.; Ferron, S.; Boustie, J.; Le Devehat, F.; Lumbsch, H.T.; Shibayama, N. Unraveling a Historical Mystery: Identification of a Lichen Dye Source in a Fifteenth Century Medieval Tapestry. Heritage 2024, 7, 2370–2384. [Google Scholar] [CrossRef]
- Campos Ayala, J.; Mahan, S.; Wilson, B.; Antúnez de Mayolo, K.; Jakes, K.; Stein, R.; Armitage, R.A. Characterizing the Dyes of Pre-Columbian Andean Textiles: Comparison of Ambient Ionization Mass Spectrometry and HPLC-DAD. Heritage 2021, 4, 1639–1659. [Google Scholar] [CrossRef]
- Degano, I.; Colombini, M.P. Multi-analytical techniques for the study of pre-Columbian mummies and related funerary materials. J. Archaeol. Sci. 2009, 36, 1783–1790. [Google Scholar] [CrossRef]
- Sabatini, F.; Bacigalupo, M.; Degano, I.; Javér, A.; Hacke, M. Revealing the organic dye and mordant composition of Paracas textiles by a combined analytical approach. Herit. Sci. 2020, 8, 122. [Google Scholar] [CrossRef]
- Armitage, R.A.; Fraser, D.; Degano, I.; Colombini, M.P. The analysis of the Saltzman Collection of Peruvian dyes by high performance liquid chromatography and ambient ionisation mass spectrometry. Herit. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Smith, G.D.; Chen, V.J.; Holden, A.; Haghipour, N.; Hendriks, L. Combined, sequential dye analysis and radiocarbon dating of single ancient textile yarns from a Nazca tunic. Herit. Sci. 2022, 10, 179. [Google Scholar] [CrossRef]
- Armitage, R.A.; Jakes, K.A. Sequencing Analytical Methods for Small Sample Dating and Dye Identification of Textile Fibers: Application to a Fragment from Seip Mound Group, Ohio. Midcont. J. Archaeol. 2015, 41, 26–40. [Google Scholar] [CrossRef]
- Jakes, K.A. Physical and Chemical Analysis of Paracas Fibers. In Paracas Art and Architecture; Paul, A., Ed.; University of Iowa Press: Iowa City, IA, USA, 1991; pp. 222–239. [Google Scholar]
- Jakes, K.A.; Katon, J.E.; Martoglio, P.A. Identification of dyes and characterization of fibers by infrared and visible microspectroscopy: Application to Paracas textiles. In Archaeometry ’90; Wagner, G.A., Pernicka, E., Eds.; Birkhäuser: Basel, Switzerland, 1991. [Google Scholar]
- Daggett, R.E. The Paracas Mummy Bundles of the Great Necropolis of Wari Kayan: A History. Andean Past 1994, 4, 7. [Google Scholar]
- Rowe, A.P. The linear mode revisited. Ñawpa Pacha 2015, 35, 237–258. [Google Scholar] [CrossRef]
- Szpak, P.; Millaire, J.F.; White, C.D.; Donnan, C.B.; Longstaffe, F.J. Stable Isotope Sourcing of Wool from Textiles at Pacatnamu. Archaeometry 2018, 60, 612–627. [Google Scholar] [CrossRef]
- Szpak, P.; Millaire, J.-F.; White, C.D.; Lau, G.F.; Surette, F.; Longstaffe, F.J. Origins of prehispanic camelid wool textiles from the north and central coasts of Peru traced by carbon and nitrogen isotopic analyses. Curr. Anthropol. 2015, 56, 449–459. [Google Scholar] [CrossRef]
- Wouters, J.; Rosario-Chirinos, N. Dye analysis of pre-Columbian Peruvian textiles with high-performance liquid chromatography and diode-array detection. J. Am. Inst. Conserv. 1992, 31, 237–255. [Google Scholar] [CrossRef]
- Boucherie, N.; Nowik, W.; Cardon, D. La producción tintórea Nasca: Nuevos datos analíticos obtenidos sobre textiles recientemente descubiertos en excavaciones. Nuevo Mundo Mundos Nuevos 2016. [Google Scholar] [CrossRef]
- Manhita, A.; Balcaen, L.; Vanhaecke, F.; Ferreira, T.; Candeias, A.; Dias, C.B. Unveiling the colour palette of Arraiolos carpets: Material study of carpets from the 17th to 19th century period by HPLC-DAD-MS and ICP-MS. J. Cult. Herit. 2014, 15, 292–299. [Google Scholar] [CrossRef]
- Manhita, A.; Ferreira, T.; Candeias, A.; Dias, C.B. Extracting natural dyes from wool—An evaluation of extraction methods. Anal. Bioanal. Chem. 2011, 400, 1501–1514. [Google Scholar] [CrossRef]
- Santos, G.M.; Southon, J.; Griffin, S.; Beaupre, S.R.; Druffel, E.R. Ultra small-mass 14C-AMS sample preparation and analysis at the KCCAMS Faclitity. Nucl. Instrum. Methods Phys. Res. 2007, B259, 293–302. [Google Scholar] [CrossRef]
- Southon, J.; Santos, G.; Druffel-Rodriguez, K.; Druffel, E.; Trumbore, S.; Xu, X.; Griffin, S.; Ali, S.; Mazon, M. The Keck Carbon Cycle AMS laboratory, University of California, Irvine: Initial operation and a background surprise. Radiocarbon 2004, 46, 41–49. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Groning, M.; Meijer, H.A.; Toman, B.; Verkouteren, R.M. Guest Editorial After two decades a second anchor for the VPDB d 13 C scale y. Rapid Commun. Mass. Spectrom. 2006, 20, 3165–3166. [Google Scholar] [CrossRef] [PubMed]
- Boehlke, J.K.; Coplen, T.B. Interlaboratory Comparison of Reference Materials for Nitrogen-Isotope-Ratio Measurements; International Atomic Energy Agency: Vienna, Austria, 1995. [Google Scholar]
- Tello, J.C.; van Dalen Luna, P.D. Paracas: Necrópolis de wari kayan; Museo de Arqueología y Antropología, Universidad Nacional Mayor de San Marcos: Lima, Peru, 2012. [Google Scholar]
- Cody, R.B.; Laramee, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- Diaz-Granados, K.S.; Bergemann, L.J.; Ballard, M.; Newsome, G.A.; Kavich, G.M.; Caldwell, J.D.; Cleland, T.P. Investigation of Natural Dyes and Taxonomic Identification of Fibers Used in Chancay Textiles by Vibrational Spectroscopy and Mass Spectrometry. J. Proteome Res. 2025, 24, 710–728. [Google Scholar] [CrossRef]
- Boucherie, N. La Couleur dans la Civilisation Nasca: Production Tinctoriale et Picturale. Ph.D. Thesis, Université Lumière Lyon 2, Lyon, France, 2014. [Google Scholar]
- Zhang, X.; Boytner, R.; Cabrera, J.L.; Laursen, R. Identification of Yellow Dye Types in Pre-Columbian Andean Textiles. Anal. Chem. 2007, 79, 1575–1582. [Google Scholar] [CrossRef]
- Antúnez de Mayolo, K.K. Report on the Collection of Peruvian Dye Plants; Unpublished Report; Smithsonian Museum: Chantilly, VA, USA, 1977; p. 43. [Google Scholar]
- Schmeda-Hirschmann, G.; Theoduloz, C.; Jiménez-Aspee, F.; Echeverría, J. Bioactive Constituents from South American Prosopis and their Use and Toxicity. Curr. Pharm. Des. 2020, 26, 542–555. [Google Scholar] [CrossRef]
- Bronk Ramsey, C.; Lee, S. Recent and Planned Developments of the Program OxCal. Radiocarbon 2013, 55, 720–730. [Google Scholar] [CrossRef]
- Conlee, C.A.; Contreras, D.A.; Peters, A.H.; Vaughn, K.J. Reconsidering chronologies and cultural change on the south coast of Peru: A compilation and analysis of radiocarbon dates from Nasca, Ica, and Paracas. Quat. Int. 2024, 703, 97–111. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Hogg, A.G.; Heaton, T.J.; Hua, Q.; Palmer, J.G.; Turney, C.S.; Southon, J.; Bayliss, A.; Blackwell, P.G.; Boswijk, G.; Ramsey, C.B.; et al. SHCal20 Southern Hemisphere Calibration, 0–55,000 Years cal BP. Radiocarbon 2020, 62, 759–778. [Google Scholar] [CrossRef]
- Santana-Sagredo, F.; Schulting, R.J.; Méndez-Quiros, P.; Vidal-Elgueta, A.; Uribe, M.; Loyola, R.; Maturana-Fernández, A.; Díaz, F.P.; Latorre, C.; McRostie, V.B.; et al. ‘White gold’ guano fertilizer drove agricultural intensification in the Atacama Desert from AD 1000. Nat. Plants 2021, 7, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Santana-Sagredo, F.; Schulting, R.; Lee-Thorp, J.; Agüero, C.; Uribe, M.; Lemp, C. Paired Radiocarbon Dating on Human Samples and Camelid Fibers and Textiles from Northern Chile: The Case of Pica 8 (Tarapacá). Radiocarbon 2017, 59, 1195–1213. [Google Scholar] [CrossRef]
- Dantas, M.; Figueroa, G.G.; Laguens, A. Llamas in the Cornfield: Prehispanic Agro-Pastoral System in the Southern Andes. Int. J. Osteoarchaeol. 2014, 24, 149–165. [Google Scholar] [CrossRef]
- Cárcamo-Vega, J.J.; Sepúlveda, M.; Casanova-González, E.; Gutiérrez, S.; Mitrani, A.; Dauelsberg, E.J.; Aliaga, Á.E.; Lemp, C.; Ruvalcaba-Sil, J.L. New insights into archaeological textiles (1000–1450AD) from the coastal region of the Atacama Desert: Preliminary evidence of a cochineal and shellfish purple dye combination. PLoS ONE 2025, 20, e0325623. [Google Scholar] [CrossRef]
- Wallert, A.; Boytner, R. Dyes from the Tumilaca and Chiribaya Cultures, South Coast of Peru. J. Archaeol. Sci. 1996, 23, 853–861. [Google Scholar] [CrossRef]
- Michel, R.H.; Lazar, J.; McGovern, P.E. Indigoid dyes in Peruvian and Coptic textiles of The University Museum of Archaeology and Anthropology. Archaeomaterials 1992, 6, 69–83. [Google Scholar]
- King, M.E. Textiles and Basketry of the Paracas Period, Ica Valley, Peru. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1965. [Google Scholar]
- Saltzman, M.; Keay, A.M.; Christensen, J. The identification of colorants in ancient textiles. Dyestuffs 1963, 44, 241–251. [Google Scholar]
- Price, K.E.; Higgitt, C.; Devièse, T.; McEwan, C.; Sillar, B. Tools for eternity: Pre-Columbian workbaskets as textile production toolkits and grave offerings. Br. Mus. Tech. Res. Bull. 2015, 9, 65–86. [Google Scholar]
- Trentelman, K.; Bouchard, M.; Ganio, M.; Namowicz, C.; Patterson, C.S.; Walton, M. The examination of works of art using in situ XRF line and area scans. X-Ray Spectrom. 2010, 39, 159–166. [Google Scholar] [CrossRef]
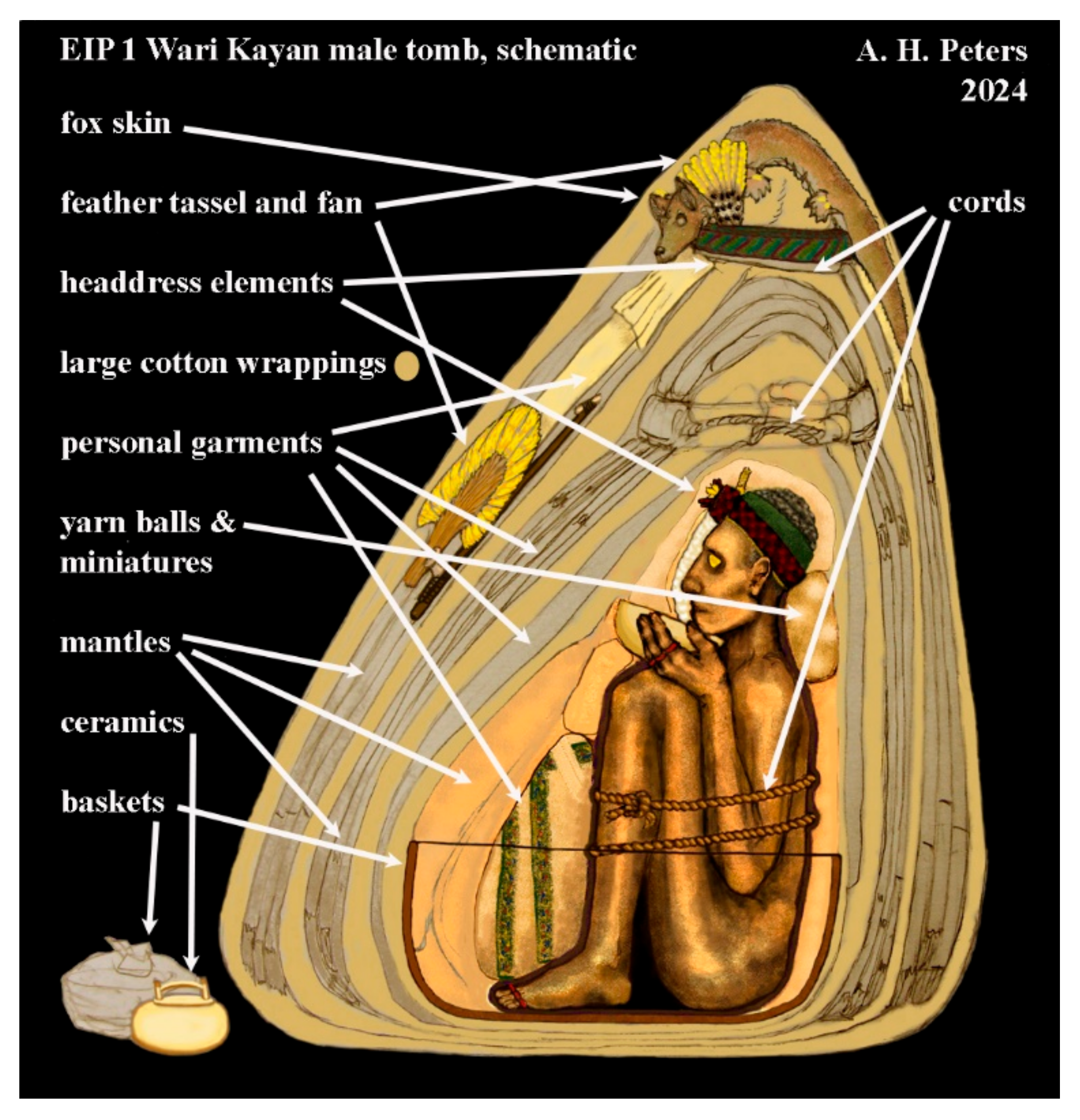


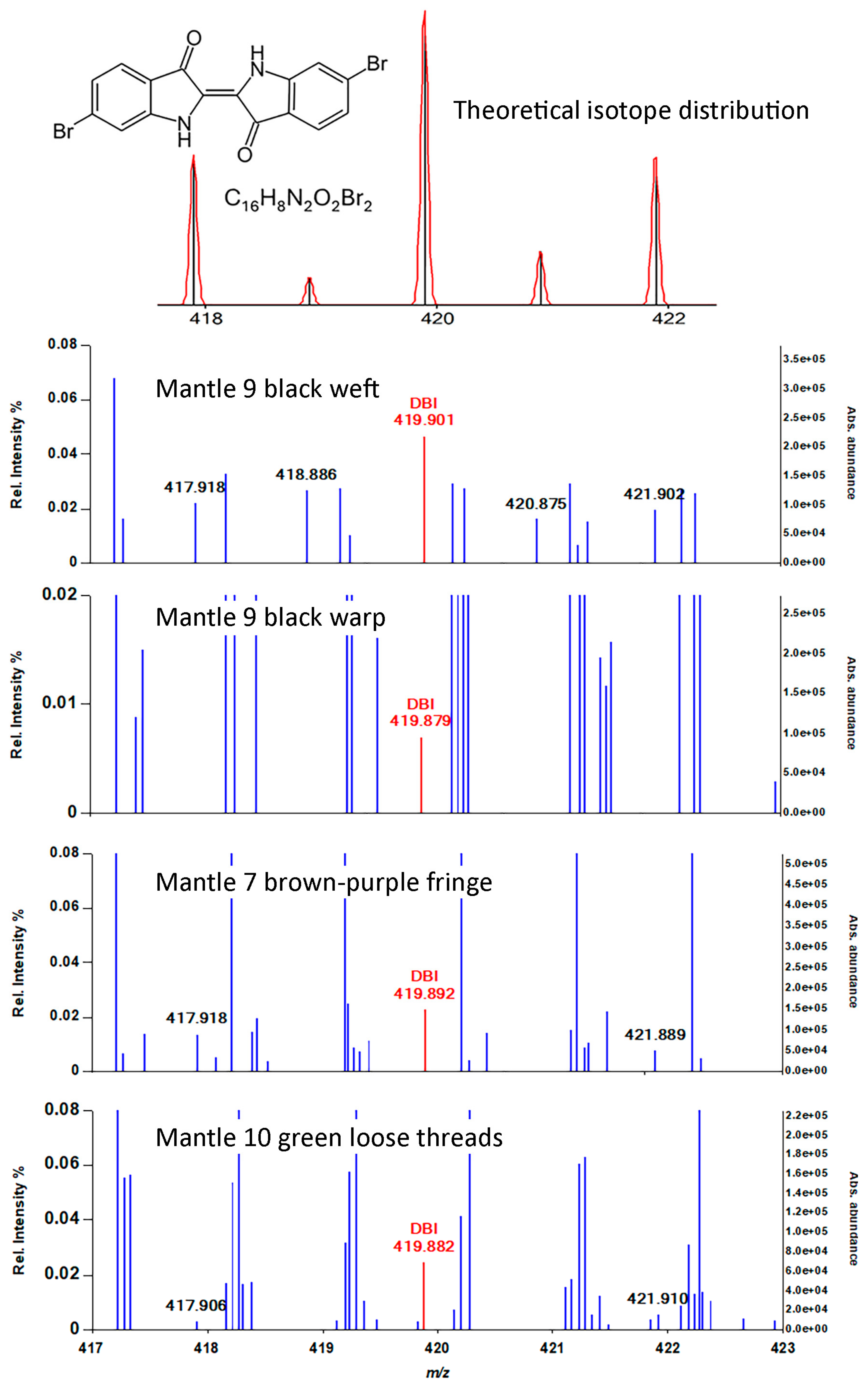
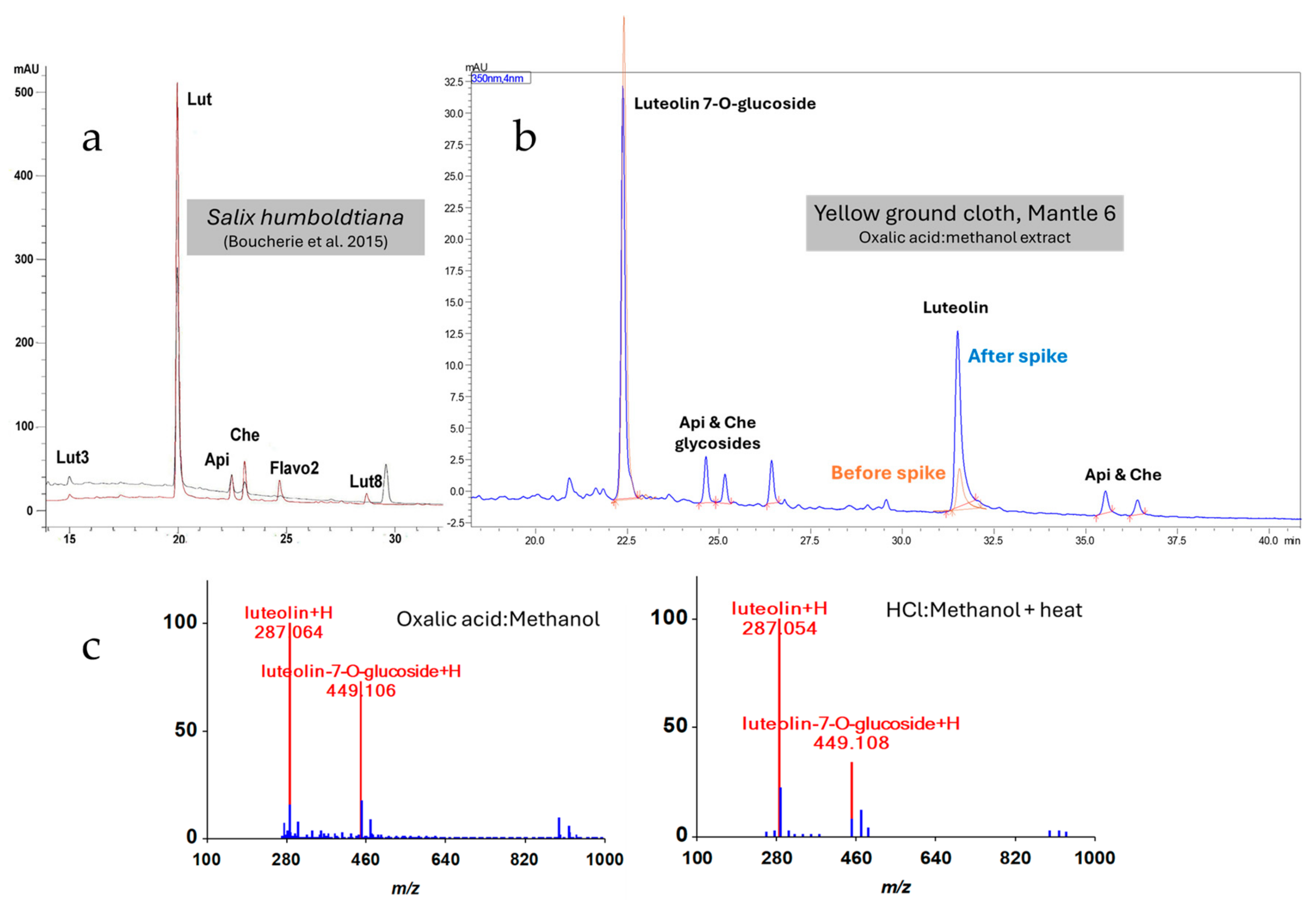
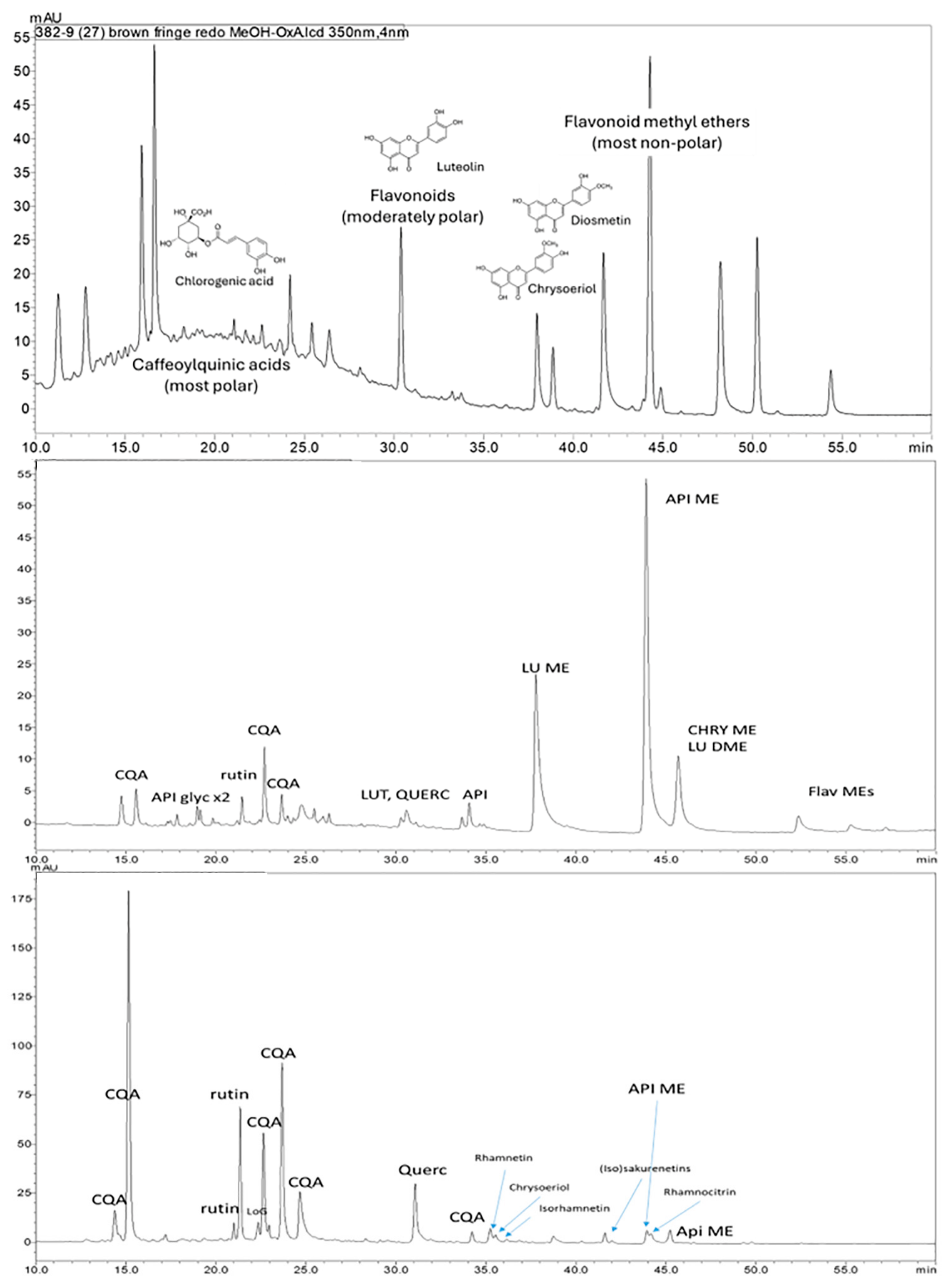

| Object Number | Style | Description | Samples Collected by Dr. Paul in 1985 |
|---|---|---|---|
| 382-4 | Linear | Mantle: light red plain weave, embroidered borders and transverse bands, med. green ground; interlocking feline motifs in 5 colors; yarn fringe | Brown–red ground cloth (woven fragments and loose strands, including some identified as warp and weft) |
| Blue embroidery thread | |||
| Border fringe and weft yarns in red, green, orange, gold, and black | |||
| 382-5 | Linear | Mantle: dark blue plain weave; embroidered borders and center panels, med. red ground; standing warrior motifs in 3 colors; yarn fringe | Black yarns (2 warp, 1 weft) |
| Embroidery threads, loose, in black, reds (2) | |||
| Red fringe | |||
| 382-6 | Linear | Mantle: ochre plain weave; embroidered borders and center panels, med. red ground; horizontal figures with serpentine appendages in 3+ colors; yarn fringe | Yellow–orange ground cloth (unidentified strands, plus some designated warp and weft) |
| Red fringe | |||
| 382-7 | Block | Mantle: red/ochre plain weave; embroidered borders and center figures, dark purple ground; standing warrior motifs in 9 colors; yarn fringe | Red–orange yarns identified as warp and weft |
| Fringe yarns in purple–brown, green, red, and yellow | |||
| 382-9 | Block | Mantle: dark blue plain weave, embroidered borders and center figures, med. green ground; horizontal figures with streaming hair in 10 colors; yarn fringe | Black yarns identified as warp and weft |
| Green yarn from border background | |||
| Embroidery yarns in orange, red, and brown | |||
| Brown fringe yarns (were green) | |||
| Blue yarn | |||
| 382-10 | Block | Mantle: plain weave bands in dark blue, yellow, green, red; embroidered borders, med. green ground; standing warrior motifs in 11 colors; tabs embroidered head motifs; yarn fringe | Green–brown disintegrating fibers from border ground cloth |
| Loose threads in purple and green |
| Mantle | Sample | Oxygen Plasma Conditions | Mass, µg C | EMU Sample ID | Sample Consumed? |
|---|---|---|---|---|---|
| 4 | Red ground cloth fragment | 50 W, 7 min | 30 | 3P187 | Yes |
| 5 | Red embroidery yarn | 40 W, 15 min | 10 | 3P193 | No |
| 6 | Yellow-orange warp | 40 W, 20 min | 67 | 3P188 | Yes |
| 7 | Red mantle weft | 40 W, 15 min | 8 | 3P189 | Yes |
| 7b | Green emb. thread | 50 W, 18 min | 160 | 3P207 | No |
| 9 | Brown fringe | 50 W, 15 min | 46 | 3P191 | Mostly |
| 10g | Green-brown border ground cloth | 60 W, 20 min | 21 | 3P195 | Mostly |
| 10b | Green-brown border ground cloth | 30 W, 12 min | 10 | 3P186 | Mostly |
| UCIAMS# | EMU Sample ID | Mantle | Mass, µg C | Radiocarbon Years Before Present, Uncalibrated | Calibrated Age Range |
|---|---|---|---|---|---|
| 297733 | 3P187 | 4 | 30 | 2085 ± 45 | 343 BCE–21 CE |
| 297739 | 3P193 | 5 | 10 | 2090 ± 140 | 416 BCE–247 CE |
| 297734 | 3P188 | 6 | 67 | 2070 ± 25 | 167 BCE–7 CE |
| 297735 | 3P189 | 7 | 8 | 4420 ± 300 | Not calibrated |
| 303849 | 3P207 | 7b | 160 | 2140 ± 15 | 344-61 BCE |
| 297737 | 3P191 | 9 | 46 | 2055 ± 35 | 168 BCE–55 CE |
| 297732 | 3P186 | 10b | 10 | 2130 ± 140 | 515 BCE–219 CE |
| 297741 | 3P195 | 10g | 21 | 2030 ± 70 | 343 BCE–201 CE |
| Object | Style | Source of Yarns | Dyes Identified |
|---|---|---|---|
| 382-4 | Linear | Brown-red ground cloth | Relbunium |
| Blue embroidery thread | Indigo + tannin? | ||
| Border fringe and weft yarns in red, green, orange, gold, and black | Red: Relbunium; Green and black: indigo + yellow 2; Orange: Relbunium + yellow 1; Gold: Yellow 1 | ||
| 382-5 | Linear | Black yarns from ground cloth | Indigo + yellow trace? |
| Black and red embroidery threads | Black: Indigo + yellow 1; Red: Relbunium | ||
| Red fringe | Relbunium | ||
| 382-6 | Linear | Yellow-orange ground cloth | Yellow 1 |
| Red fringe | Relbunium | ||
| 382-7 | Block | Red-orange yarns from ground cloth | Relbunium |
| Fringe yarns in purple-brown, green, red, and yellow | Red: Relbunium; Green: indigo + yellow 2; Purple: indigo + relbunium + possible yellow 1? + shellfish purple; Yellow: Yellow 1 | ||
| 382-9 | Block | Black yarns from ground cloth | Indigo + possible tannin or yellow 2 + shellfish purple |
| Green yarn from border background | Indigo + yellow 2 | ||
| Embroidery yarns in orange/red and brown | Red: Relbunium; Brown: Yellow 2 + indigo | ||
| Brown fringe yarns | Yellow 2 + indigo | ||
| Blue loose yarn | Indigo + yellow 2 | ||
| 382-10 | Block | Green-brown disintegrating fibers from border ground cloth | Indigo + possible yellow? |
| Loose threads in purple and green | Purple: Indigo + tannin? + Relbunium; Green: Indigo + yellow 2; both contain shellfish purple |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, J.; Dragun, A.; Shehab, M.; Peterkin, I.; Peters, A.H.; Jakes, K.; Southon, J.; Sauter, C.; Moran, J.; Armitage, R.A. Sequencing Analysis and Radiocarbon Dating of Yarn Fragments from Six Paracas Mantles from Bundle WK12-382. Heritage 2025, 8, 398. https://doi.org/10.3390/heritage8100398
Williams J, Dragun A, Shehab M, Peterkin I, Peters AH, Jakes K, Southon J, Sauter C, Moran J, Armitage RA. Sequencing Analysis and Radiocarbon Dating of Yarn Fragments from Six Paracas Mantles from Bundle WK12-382. Heritage. 2025; 8(10):398. https://doi.org/10.3390/heritage8100398
Chicago/Turabian StyleWilliams, Jaime, Avi Dragun, Malak Shehab, Imani Peterkin, Ann H. Peters, Kathryn Jakes, John Southon, Collin Sauter, James Moran, and Ruth Ann Armitage. 2025. "Sequencing Analysis and Radiocarbon Dating of Yarn Fragments from Six Paracas Mantles from Bundle WK12-382" Heritage 8, no. 10: 398. https://doi.org/10.3390/heritage8100398
APA StyleWilliams, J., Dragun, A., Shehab, M., Peterkin, I., Peters, A. H., Jakes, K., Southon, J., Sauter, C., Moran, J., & Armitage, R. A. (2025). Sequencing Analysis and Radiocarbon Dating of Yarn Fragments from Six Paracas Mantles from Bundle WK12-382. Heritage, 8(10), 398. https://doi.org/10.3390/heritage8100398










