On the Identification of the a fresco or a secco Preparative Technique of Wall Paintings
Abstract
1. Introduction
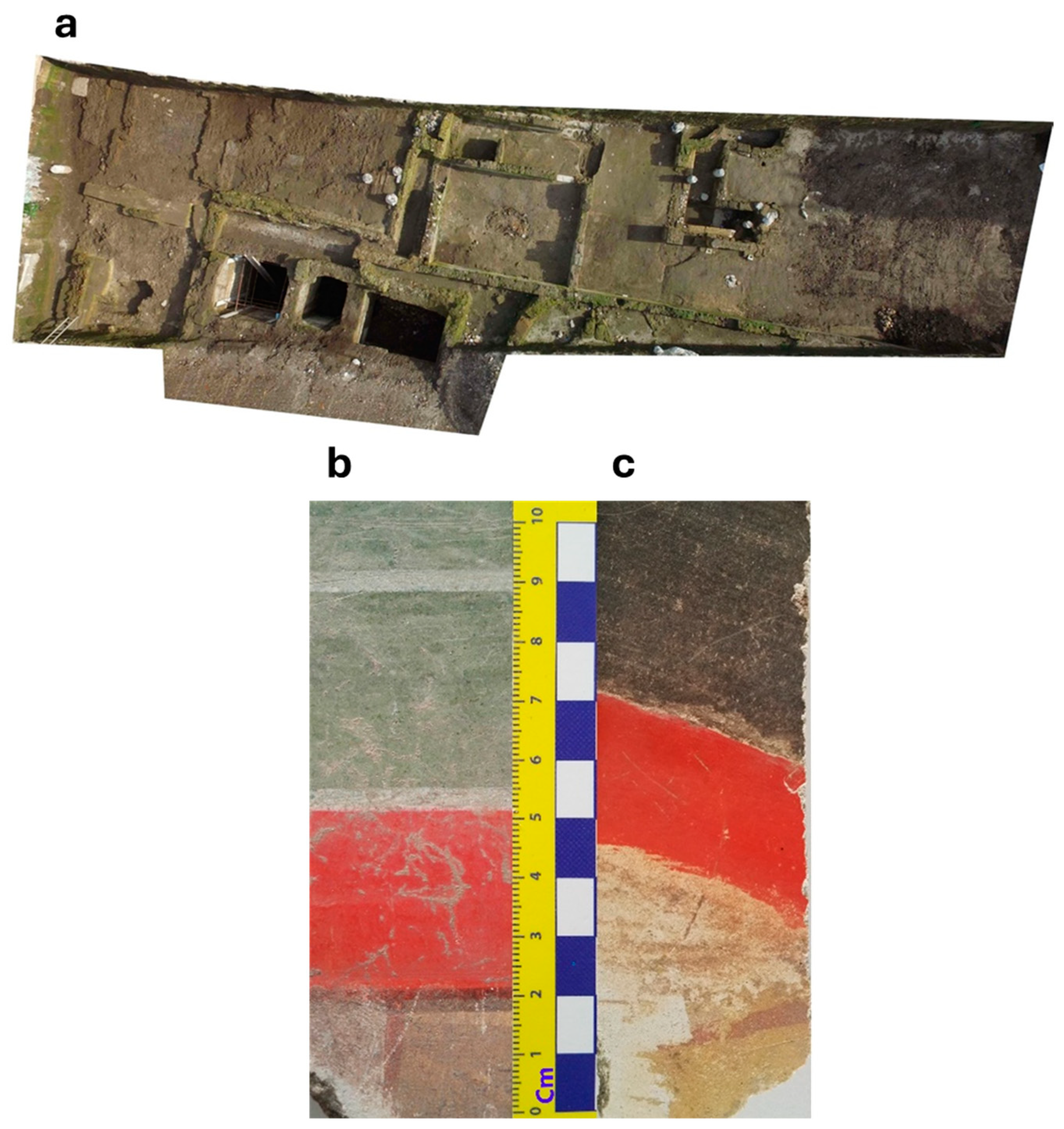
2. The Archaeological Site
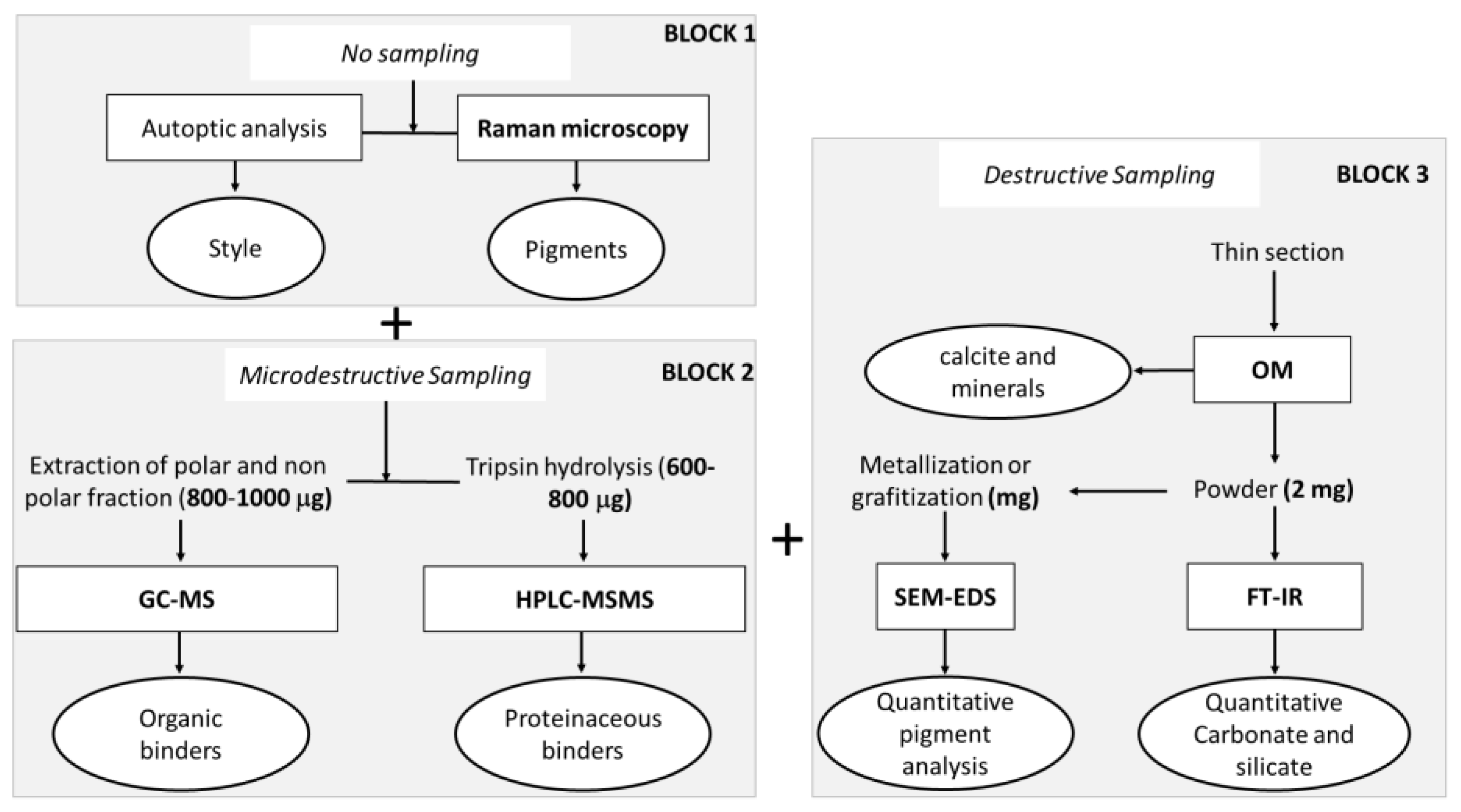
3. Materials and Methods
4. Results and Discussion
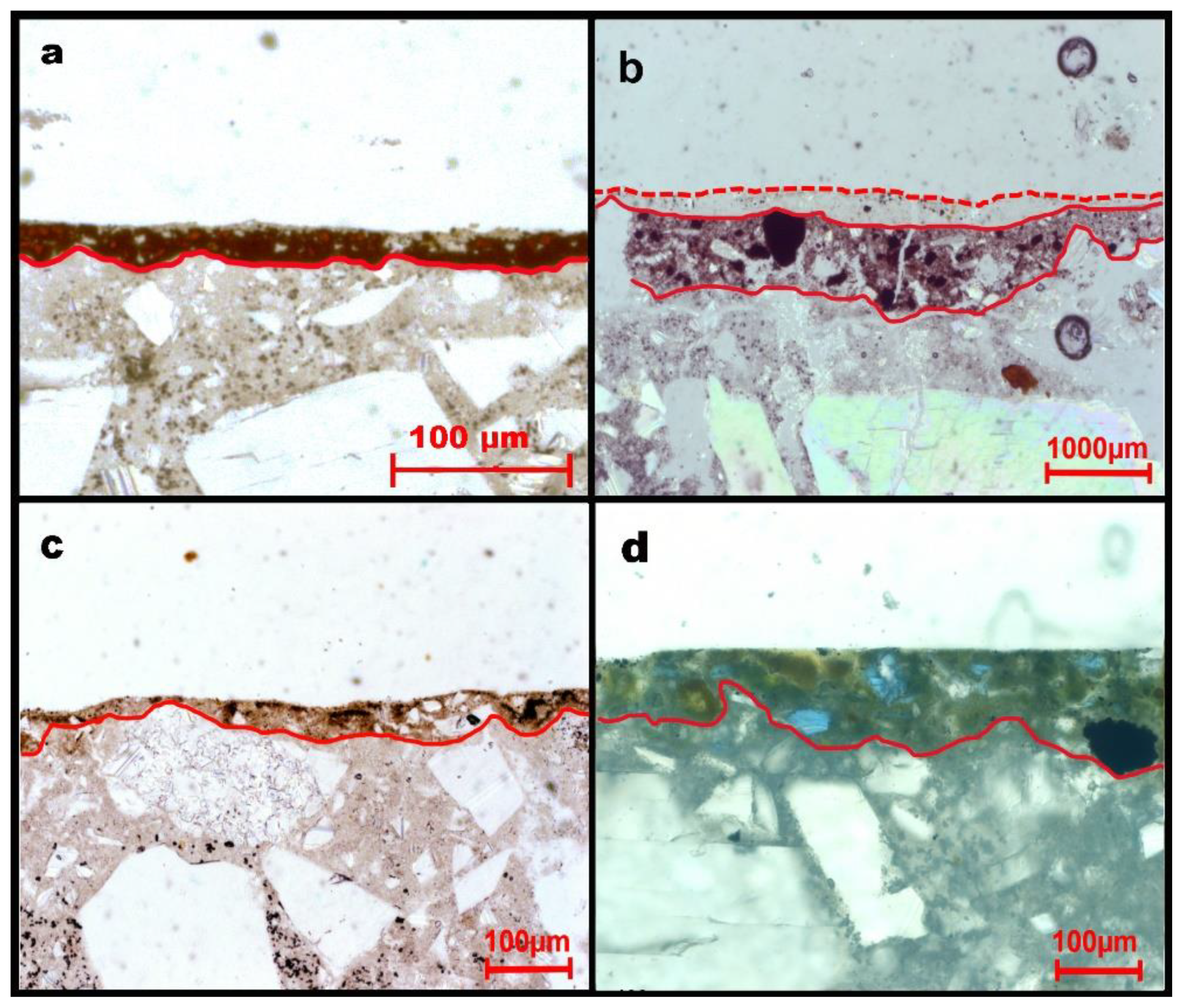
4.1. Optical, Electron Microscopy, and Quantitative Chemical Analysis
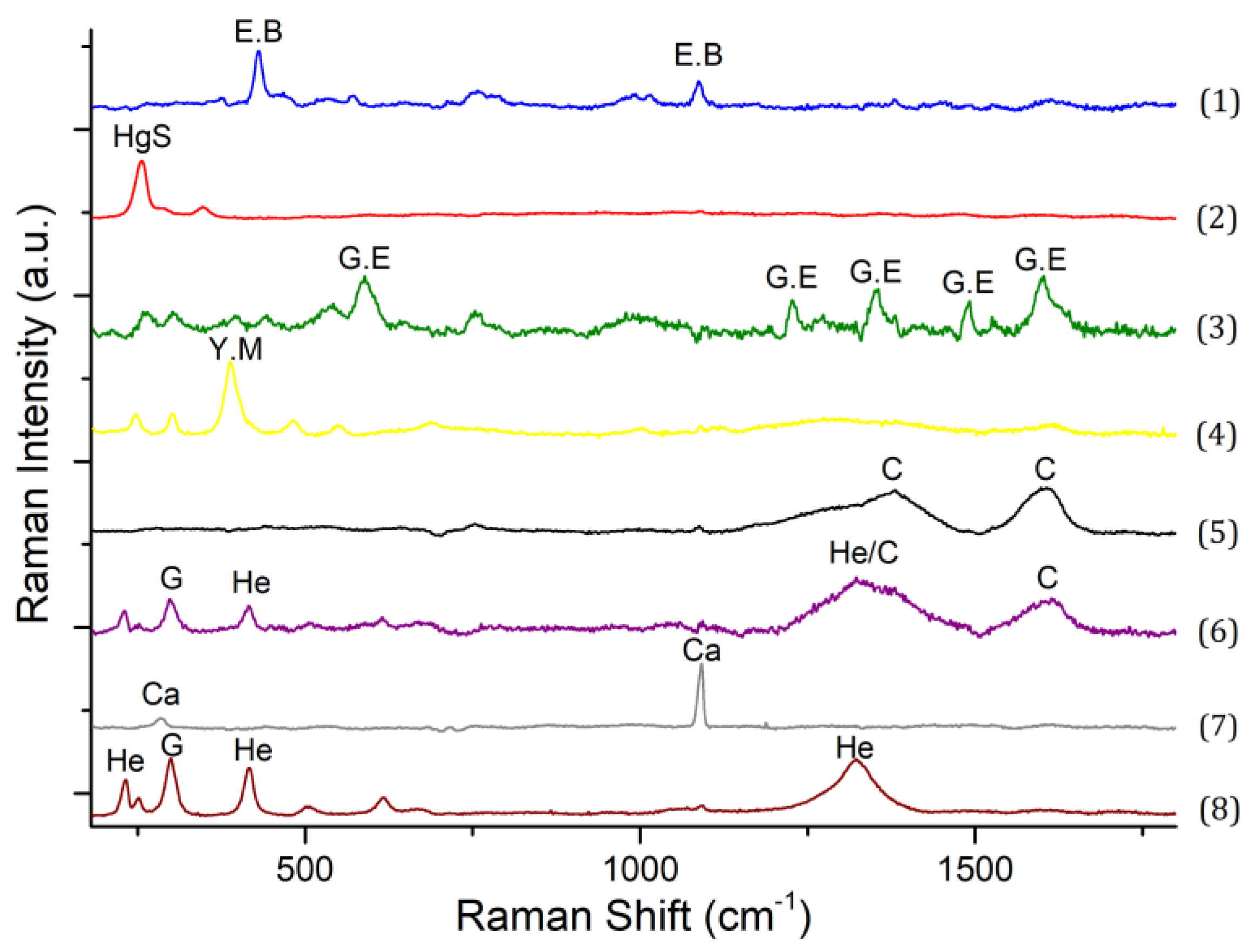
4.2. Raman Micro-Spectroscopy
4.3. GC-MS: Analysis of Organic Binders
4.4. LC-MS/MS: Analysis of Proteinaceous Binders
4.5. Distinguishing Different Preparative Techniques of Wall Paintings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuní, J. What do we know of Roman wall painting technique? Potential confounding factors in ancient paint media analysis. Herit. Sci. 2016, 4, 44. [Google Scholar] [CrossRef]
- Angelini, I.; Asscher, Y.; Secco, M.; Parisatto, M.; Artioli, G. The pigments of the frigidarium in the Sarno Baths, Pompeii: Identification, stratigraphy and weathering. J. Cult. Herit. 2019, 40, 309–316. [Google Scholar] [CrossRef]
- Bergamonti, L.; Cirlini, M.; Graiff, C.; Lottici, P.P.; Palla, G.; Casoli, A. Characterization of Waxes in the Roman Wall Paintings of the Herculaneum Site (Italy). Appl. Sci. 2022, 12, 11264. [Google Scholar] [CrossRef]
- Cuní, J.; Cuní, P.; Eisen, B.; Savizky, R.; Bové, J. Characterization of the binding medium used in Roman encaustic paintings on wall and wood. Anal. Methods 2012, 4, 659. [Google Scholar] [CrossRef]
- Omarini, S. (Ed.) Encausto: Storia, Tecniche e Ricerche = Encaustic: History, Technique and Research; Nardini: Firenze, Italy, 2012; ISBN 978-88-404-4216-7. [Google Scholar]
- Dilaria, S.; Sbrolli, C.; Mosimann, F.S.; Favero, A.; Secco, M.; Santello, L.; Salvadori, M. Production technique and multi-analytical characterization of a paint-plastered ceiling from the Late Antique villa of Negrar (Verona, Italy). Archaeol. Anthr. Sci. 2024, 16, 74. [Google Scholar] [CrossRef]
- Brecoulaki, H.; Verri, G.; Kalaitzi, M.; Maniatis, Y.; Lilimpaki-Akamati, M. Investigating Colors and Techniques on the Wall Paintings of the ‘Tomb of the Philosophers’, an Early Hellenistic Macedonian Monumental Cist Tomb in Pella (Macedonia, Greece). Heritage 2023, 6, 5619–5647. [Google Scholar] [CrossRef]
- Conti, C.; Botteon, A.; Colombo, C.; Pinna, D.; Realini, M.; Matousek, P. Advances in Raman spectroscopy for the non-destructive subsurface analysis of artworks: Micro-SORS. J. Cult. Herit. 2020, 43, 319–328. [Google Scholar] [CrossRef]
- Carlomagno, I.; Drnec, J.; Scaparro, A.M.; Cicia, S.; Vlaic, S.; Felici, R.; Meneghini, C. Co-Ir interface alloying induced by thermal annealing. J. Appl. Phys. 2016, 120, 195302. [Google Scholar] [CrossRef]
- Mateos, L.D.; Esquivel, D.; Cosano, D.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Micro-Raman analysis of mortars and wallpaintings in the Roman villa of Fuente Alamo (Puente Genil, Spain) and identification of the application technique. Sens. Actuators A Phys. 2018, 281, 15–23. [Google Scholar] [CrossRef]
- Casoli, A. Research on the Organic Binders in Archaeological Wall Paintings. Appl. Sci. 2021, 11, 9179. [Google Scholar] [CrossRef]
- Smoluch, M.; Sobczyk, J.; Szewczyk, I.; Karaszkiewicz, P.; Silberring, J. Mass spectrometry in art conservation—With focus on paintings. Mass. Spectrom. Rev. 2023, 42, 1625–1646. [Google Scholar] [CrossRef]
- Vinciguerra, R.; De Chiaro, A.; Pucci, P.; Marino, G.; Birolo, L. Proteomic strategies for cultural heritage: From bones to paintings. Microchem. J. 2016, 126, 341–348. [Google Scholar] [CrossRef]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Gautier, G.; Modugno, F.; Ribechini, E. Combined GC/MS Analytical Procedure for the Characterization of Glycerolipid, Waxy, Resinous, and Proteinaceous Materials in a Unique Paint Microsample. Anal. Chem. 2006, 78, 4490–4500. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Tomeo, A. Capua—La Seconda Roma: Nuovi Studi e Ricerche; Soprintendenza ABAP Caserta/Benevento: Caserta, Italy, 2021; ISBN 978-88-943027-9-0. [Google Scholar]
- Gelzo, M.; Grimaldi, M.; Vergara, A.; Severino, V.; Chambery, A.; Dello Russo, A.; Piccioli, C.; Corso, G.; Arcari, P. Comparison of binder compositions in Pompeian wall painting styles from Insula Occidentalis. Chem. Cent. J. 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corso, A.D.; Pignataro, L.; Belvisi, L.; Gennari, C. Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem.—A Eur. J. 2019, 25, 14740–14757. [Google Scholar] [CrossRef] [PubMed]
- Prisco, G. Su Alcune Particolarità Tecniche delle Officine Addette alla Decorazione della Domus Vettiorum. In Nuove Ricerche Archeologiche a Pompei ed Ercolano; Atti del Convegno Internazionale: Roma, Italy, 2002; pp. 355–366. [Google Scholar]
- Vergara, A.; Vitagliano, L.; Merlino, A.; Sica, F.; Marino, K.; Verde, C.; di Prisco, G.; Mazzarella, L. An Order-Disorder Transition Plays a Role in Switching Off the Root Effect in Fish Hemoglobins. J. Biol. Chem. 2010, 285, 32568–32575. [Google Scholar] [CrossRef]
- Nesse, W.D. Introduction to Mineralogy, 2nd ed.; Oxford University Press: New York, NY, USA, 2012; ISBN 978-0-19-982738-1. [Google Scholar]
- Dana, J.D. Manual of Mineralogy: After James D. Dana, 20th ed.; Klein, C., Hurlbut, C.S., Eds.; Wiley: New York, NY, USA, 1985; ISBN 978-0-471-80580-9. [Google Scholar]
- Pouchou, J.-L.; Pichoir, F. Quantitative Analysis of Homogeneous or Stratified Microvolumes Applying the Model “PAP.” In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Springer: Boston, MA, USA, 1991; pp. 31–75. ISBN 978-1-4899-2619-7. [Google Scholar] [CrossRef]
- Anthony, J.W. (Ed.) Handbook of Mineralogy; Mineral Data Pub: Tucson, AZ, USA, 1990; ISBN 978-0-9622097-0-3. [Google Scholar]
- Melchiorre, C.; Dello Ioio, L.; Ntasi, G.; Birolo, L.; Trojsi, G.; Cennamo, P.; Barone Lumaga, M.R.; Fatigati, G.; Amoresano, A.; Carpentieri, A. A multi disciplinary assessment to investigate a XXII dynasty wooden coffin. Int. J. Conserv. Sci. 2020, 11, 25–38. [Google Scholar]
- Boccalon, E.; Rosi, F.; Vagnini, M.; Romani, A. Multitechnique approach for unveiling the technological evolution in building materials during the Roman Imperial Age: The Atrium Vestae in Rome. Eur. Phys. J. Plus 2019, 134, 528. [Google Scholar] [CrossRef]
- Birolo, L.; Rossi, M.; Alberico, M.; De Riso, N.; Ntasi, G.; Tomeo, A.; Vergara, A. Inorganic, organic and biochemical characterization of wall paintings from a Roman domus. In Proceedings of the 2022 IMEKO TC4 International Conference on Metrology for Archaeology and Cultural Heritage, Calabria, Italy, 19–21 October 2022; IMEKO: Rome, Italy, 2023; pp. 55–59. [Google Scholar]
- Zezza, U. La Petrografia Micoscopica; La Goliardica Pavese: Pavia, Italy, 1976; ISBN 978-88-7830-088-0. [Google Scholar]
- Peccerillo, A.; Perugini, D. Introduzione alla Petrografia Ottica; Morlacchi: Perugia, Italy, 2003; ISBN 978-88-88778-27-3. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Mahmoud, H.H.M. Investigations by Raman microscopy, ESEM and FTIR-ATR of wall paintings from Qasr el-Ghuieta temple, Kharga Oasis, Egypt. Herit. Sci. 2014, 2, 18. [Google Scholar] [CrossRef]
- Baraldi, P.; Baraldi, C.; Curina, R.; Tassi, L.; Zannini, P. A micro-Raman archaeometric approach to Roman wall paintings. Vib. Spectrosc. 2007, 43, 420–426. [Google Scholar] [CrossRef]
- Clark, R.J.; Gibbs, P.J. Non-Destructive In Situ Study of Ancient Egyptian Faience by Microscopy. J. Raman. Spectr. 1997, 28, 99–103. [Google Scholar] [CrossRef]
- Smith, D.K. Opal, cristobalite, and tridymite: Noncrystallinity versus crystallinity, nomenclature of the silica minerals and bibliography. Powder Diffr. 1998, 13, 2–19. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Halac, E.B.; Reinoso, M.; Di Liscia, E.J.; Maier, M.S. Micro-Raman spectroscopy of carbon-based black pigments. J. Raman Spectrosc. 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Lalla, E.A.; Lopez-Reyes, G.; Sansano, A.; Sanz-Arranz, A.; Martínez-Frías, J.; Medina, J.; Rull-Pérez, F. Raman-IR vibrational and XRD characterization of ancient and modern mineralogy from volcanic eruption in Tenerife Island: Implication for Mars. Geosci. Front. 2016, 7, 673–681. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar] [CrossRef]
- Bedarida, F.; Flamini, F.; Pedamonte, G.M. Hematite to goethite surface weathering. Scanning Electron Microsc. 1971, 58, 7–8. [Google Scholar]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre- ≈ 1850 AD). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Osterrothová, K.; Novotná, J.; Nedbalová, L.; Kopecký, J.; Němec, I.; Oren, A. Potential and limits of Raman spectroscopy for carotenoid detection in microorganisms: Implications for astrobiology. Phil. Trans. R. Soc. A. 2014, 372, 20140199. [Google Scholar] [CrossRef]
- Azemard, C.; Menager, M.; Vieillescazes, C. Analysis of diterpenic compounds by GC-MS/MS: Contribution to the identification of main conifer resins. Anal. Bioanal. Chem. 2016, 408, 6599–6612. [Google Scholar] [CrossRef] [PubMed]
- Beltran, V.; Salvadó, N.; Butí, S.; Pradell, T. Ageing of resin from Pinus species assessed by infrared spectroscopy. Anal. Bioanal. Chem. 2016, 408, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, G.; Theodoridis, G.; Dabalis, T. Determination of anabolic steroids in bovine urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, J.-M. Hasubanan Type Alkaloids from Stephania longa. J. Nat. Prod. 2005, 68, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Ranchana, P.; Ganga, M. Investigation of Volatile Compounds from the Concrete of Jasminum auriculatum Flowers. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1525–1531. [Google Scholar] [CrossRef]
- Blaško, J.; Kubinec, R.; Husová, B.; Přikryl, P.; Pacáková, V.; Štulík, K.; Hradilová, J. Gas chromatography/mass spectrometry of oils and oil binders in paintings. J. Sep. Sci. 2008, 31, 1067–1073. [Google Scholar] [CrossRef]
- Piovesan, R.; Siddall, R.; Mazzoli, C.; Nodari, L. The Temple of Venus (Pompeii): A study of the pigments and painting techniques. J. Archaeol. Sci. 2011, 38, 2633–2643. [Google Scholar] [CrossRef]

| Pictorial Layer Colors | Sample | Mineral Amounts | Mineral Composition | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Fe2O3 | PbO | MnO | Hg | S | H2O | Tot | |||||||||||||||
| Bright Red | 151 | 90% Cin | - | - | - | - | 86.40 (65) | 12.62 (70) | - | 99.02 | ||||||||||||
| 10% Hem | 0.49 (32) | 97.93 (78) | - | 0.27 (51) | - | - | - | 98.69 | ||||||||||||||
| 151C | 90% Cin | - | - | - | - | 84.19 (52) | 14.09 (88) | - | 98.28 | |||||||||||||
| 10% Hem | 1.08 (74) | 98.56 (29) | - | 0.25 (62) | - | - | - | 99.89 | ||||||||||||||
| Ochre yellow | 151 | 5% Pb rich-Gth | - | 58.48 (56) | 33.66 (62) | - | - | - | 7.94 | 100.00 | ||||||||||||
| 95% Pb-Gth | - | 87.19 (2.1) | 3.41 (2.5) | - | - | - | 9.40 | 100.00 | ||||||||||||||
| 151C | 100% Pb-Gth | - | 86.35 (55) | 3.38 (12) | 0.23 (10) | - | - | 10.03 | 100.00 | |||||||||||||
| Reddish/brown | 151 | 20% Hem | 1.12 (10) | 96.93 (46) | - | 0.80 (12) | - | - | 98.85 | |||||||||||||
| 80% Gth | 0.29 (6) | 88.97 (24) | - | 0.15 (12) | - | - | 10.59 | 100.00 | ||||||||||||||
| 151C | 10% Hem | 0.81 (10) | 98.33 (56) | - | 0.52 (16) | - | - | 99.66 | ||||||||||||||
| 90% Gth | 0.38 (22) | 89.91 (70) | - | - | - | 9.72 | 100.00 | |||||||||||||||
| 151B | 10% Hem | 0.51 (21) | 97.83 (77) | 0.45 (10) | - | - | - | 98.79 | ||||||||||||||
| 90% Gth | 2.78 (10) | 86.94 (86) | 1.46 (30) | - | - | - | 8.82 | 100.00 | ||||||||||||||
| Pictorial Layer Color | Sample | Mineral Amounts | Mineral Composition | |||||||||||||||||||
| SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | CuO | K2O | H2O | Tot | |||||||||||
| Green | 151 | Glt 47% | 46.94 (92) | 5.87 (21) | 23.48 (30) | 3.45 (16) | 0.63 (32) | 6.90 (20) | 12.54 | 100.00 | ||||||||||||
| Cuv 35% | 63.67 (70) | 15.04 (35) | 21.30 (21) | 100.01 | ||||||||||||||||||
| Gth 3% | 0.73 (45) | 87.67 (50) | 0.34 (35) | 1.10 (12) | 10.16 | 100.00 | ||||||||||||||||
| Hem 4% | 100.24 (15) | 100.24 | ||||||||||||||||||||
| Uspl 2% | 19.82 (25) | 2.75 (90) | 9.56 (51) | 65.44 (81) | 0.97 (12) | 1.14 (54) | 99.70 | |||||||||||||||
| (a) | ||||||
| Sample | RT (min) | Name | ||||
| 151A | 6132 | Cholest-5-en-19-al,3ß-hydroxy-,cyclic ethylene mercaptal, acetate | ||||
| 17.77 | 10,18-bisnorabieta-8,11,13-triene | |||||
| 22.12 | Diglycidyl bisphenol A | |||||
| 23,225 | Hexestrol, di-TMS | |||||
| 151B | 6383 | Cholest-5-en-19-al,3ß-hydroxy-,cyclic ethylene mercaptal, acetate | ||||
| 10,434 | Dianhydro-2-deoxy-ß-d-ribo-hexopyranose | |||||
| 13,563 | 2-Methyl-7-hydroxy-8-allyl-isoflavone | |||||
| 14,097 | 3-Heptadecenal | |||||
| 21,473 | Oxalic acid, hexadecyl isohexylester | |||||
| 17.77 | 10,18-bisnorabieta-8,11,13-triene | |||||
| 22.12 | Diglycidyl bisphenol A | |||||
| 151C | 17.04 | Stephaboline | ||||
| 17.77 | 10,18-bisnorabieta-8,11,13-triene | |||||
| 22.12 | Diglycidyl bisphenol A | |||||
| 187 | 17.77 | 10,18-bisnorabieta-8,11,13-triene | ||||
| 22.12 | Diglycidyl bisphenol A | |||||
| 21,284 | 1-Dodecanol, 3,7,11-trimethyl- | |||||
| 21,356 | 7-Hexadecenal, (Z)- | |||||
| 21,452 | Octadecane, 1-(ethenyloxy)- | |||||
| 21,555 | 1-Dodecanol, 3,7,11-trimethyl- | |||||
| 21,808 | 7-Hexadecenal, (Z)- | |||||
| 22,253 | 1-Dodecanol, 3,7,11-trimethyl- | |||||
| (b) | ||||||
| RT (min) | Name | C:N * | 151A | 151B | 151C | 187 |
| 12.88 | Myristic acid | C14:0 | 1.60% | 1.93% | 9.25% | |
| 14.71 | Pentadecanoic acid | C15:0 | 3.44% | 3.45% | ||
| 16.25 | Palmitic acid | C16:0 | 35.73% | 28.17% | 26.25% | 45.52% |
| 17.52 | Margaric acid | C17:0 | 4.57% | 4.63% | ||
| 18.36 | 16-octadecenoic acid | C18:1 | 43.30% | 49.80% | 10.90% | |
| 18.63 | Stearic acid | C18:0 | 14.80% | 12.03% | 62.85% | 41.78% |
| Palmitic/Stearic | C16:0/C18:0 | 2.41 | 2.34 | 0.42 | 1.09 | |
| (a) | |||
| Protein Name (Uniprot Entry) | Sequence Coverage % | m/z | Peptide |
| Collagen alpha-1(I) chain (P02453) | 7 | 314,677 | R,GLPGER,G |
| 322,673 | R,GLPGER,G + Hydroxylation (P) | ||
| 392,223 | R,GAAGLPGPK,G + Hydroxylation (P) | ||
| 426,218 | R,GFSGLDGAK,G | ||
| 449,756 | R,GVVGLPGQR,G + Hydroxylation (P) | ||
| 450,251 | R,GVVGLPGQR,G + Deamidated (NQ); Hydroxylation (P) | ||
| 553,286 | R,GVQGPPGPAGPR,G + Hydroxylation (P) | ||
| 553,779 | R,GVQGPPGPAGPR,G + Deamidated (NQ); Hydroxylation (P) | ||
| 589,776 | R,GQAGVMGFPGPK,G + Oxidation (M); Deamidated (NQ); Hydroxylation (K) | ||
| 781,888 | K,DGLNGLPGPIGPPGPR,G + Deamidated (NQ); 3 Hydroxylation (P) | ||
| 941,451 | K,GDTGAKGEPGPAGVQGPPGPAGEEGKRGAR,G + Deamidated (NQ); 4 Hydroxylation (P) | ||
| Collagen alpha-2(I) chain (P02465) | 5% | 314,677 | R,GLPGER,G |
| 322,673 | R,GLPGER,G + Hydroxylation (P) | ||
| 379,696 | R,GLPGADGR,A + Hydroxylation (P) | ||
| 393,220 | R,GATGPAGVR,G | ||
| 420,739 | R,GVVGPQGAR,G | ||
| 421,234 | R,GVVGPQGAR,G + Deamidated (NQ) | ||
| 434,735 | R,VGAPGPAGAR,G + Hydroxylation (P) | ||
| 459,726 | R,AGVMGPAGSR,G + Oxidation (M) | ||
| 596,838 | R,IGQPGAVGPAGIR,G | ||
| 634,339 | R,GIPGPVGAAGATGAR,G + Hydroxylation (P) | ||
| (b) | |||
| Protein Name (Uniprot Entry) | Sequence Coverage % | m/z | Peptide |
| Collagen alpha-1(I) chain (P02453) | 7% | 449,760 | R,GVVGLPGQR,G + Hydroxylation (P) |
| 450,254 | R,GVVGLPGQR,G + Deamidated (NQ); Hydroxylation (P) | ||
| 544,777 | R,GFPGADGVAGPK,G + Hydroxylation (P) | ||
| 553,788 | R,GVQGPPGPAGPR,G + Deamidated (NQ); Hydroxylation (P) | ||
| 589,783 | R,GQAGVMGFPGPK,G + Oxidation (M); Deamidated (NQ); Hydroxylation (K) | ||
| 629,800 | K,GLTGSPGSPGPDGK,T + 2 Hydroxylation (P) | ||
| 730,351 | R,GSAGPPGATGFPGAAGR,V + 2 Hydroxylation (P) | ||
| 781,900 | K,DGLNGLPGPIGPPGPR,G + Deamidated (NQ); 3 Hydroxylation (P) | ||
| 793,884 | K,GANGAPGIAGAPGFPGAR,G + Deamidated (NQ); 3 Hydroxylation (P) | ||
| Collagen alpha-2(I) chain (P02465) | 4% | 367,185 | K,GPSGDPGKAGEK,G |
| 596,845 | R,IGQPGAVGPAGIR,G | ||
| 597,334 | R,IGQPGAVGPAGIR,G + Deamidated (NQ) | ||
| 601,296 | R,GEPGNIGFPGPK,G + Hydroxylation (P); Hydroxylation (K) | ||
| 604,844 | R,IGQPGAVGPAGIR,G + Hydroxylation (P) | ||
| 634,342 | R,GIPGPVGAAGATGAR,G + Hydroxylation (P) | ||
| 714,369 | R,GIPGEFGLPGPAGAR,G + 2 Hydroxylation (P) | ||
| Sample | Color | Raman Micro-Spectroscopy | SEM-EDS | GC-MS | LC-MS |
|---|---|---|---|---|---|
| 151A | White | Calcite | Calcite | / | / |
| Yellow | Iron Hydroxide | Limonite | |||
| Blue | Egyptian Blue | Cuprorivaite | Pinaceous resin + Animal fat | Collagen | |
| Green | Green Earths | Glauconite | |||
| Red | Cinnabar | Cinnabar | |||
| 151B | Red | Hemtite + Goethite + Carbon | Hematite + Goethite | Pinaceous resin + Animal fat | Collagen |
| 151C | Red | Hemtite + Goethite | Hematite + Goethite | Pinaceous resin + Vegetable fatty acids | / |
| 187 | Blue | Traces of carotenoids | / | Pinaceous resin + Vegetable fatty acids | / |
| Green | |||||
| 63 | Blue | Traces of carotenoids | / | / | / |
| Green | |||||
| 139 | Red | Hemtite + Goethite | / | / | |
| 146 | Blue | Egyptian Blue | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntasi, G.; Rossi, M.; Alberico, M.; Tomeo, A.; Birolo, L.; Vergara, A. On the Identification of the a fresco or a secco Preparative Technique of Wall Paintings. Heritage 2024, 7, 3902-3918. https://doi.org/10.3390/heritage7080184
Ntasi G, Rossi M, Alberico M, Tomeo A, Birolo L, Vergara A. On the Identification of the a fresco or a secco Preparative Technique of Wall Paintings. Heritage. 2024; 7(8):3902-3918. https://doi.org/10.3390/heritage7080184
Chicago/Turabian StyleNtasi, Georgia, Manuela Rossi, Miriam Alberico, Antonella Tomeo, Leila Birolo, and Alessandro Vergara. 2024. "On the Identification of the a fresco or a secco Preparative Technique of Wall Paintings" Heritage 7, no. 8: 3902-3918. https://doi.org/10.3390/heritage7080184
APA StyleNtasi, G., Rossi, M., Alberico, M., Tomeo, A., Birolo, L., & Vergara, A. (2024). On the Identification of the a fresco or a secco Preparative Technique of Wall Paintings. Heritage, 7(8), 3902-3918. https://doi.org/10.3390/heritage7080184








