Rare Earth Elements to Control Bone Diagenesis Processes at Rozafa Castle (Albania)
Abstract
1. Introduction
Archaeological Background
2. Materials and Methods
2.1. Samples
2.2. Analytical Methodology
2.3. Rare Earth Elements Data Processing
2.4. Statistical Analysis
2.5. Paleodiet Indexes
3. Results
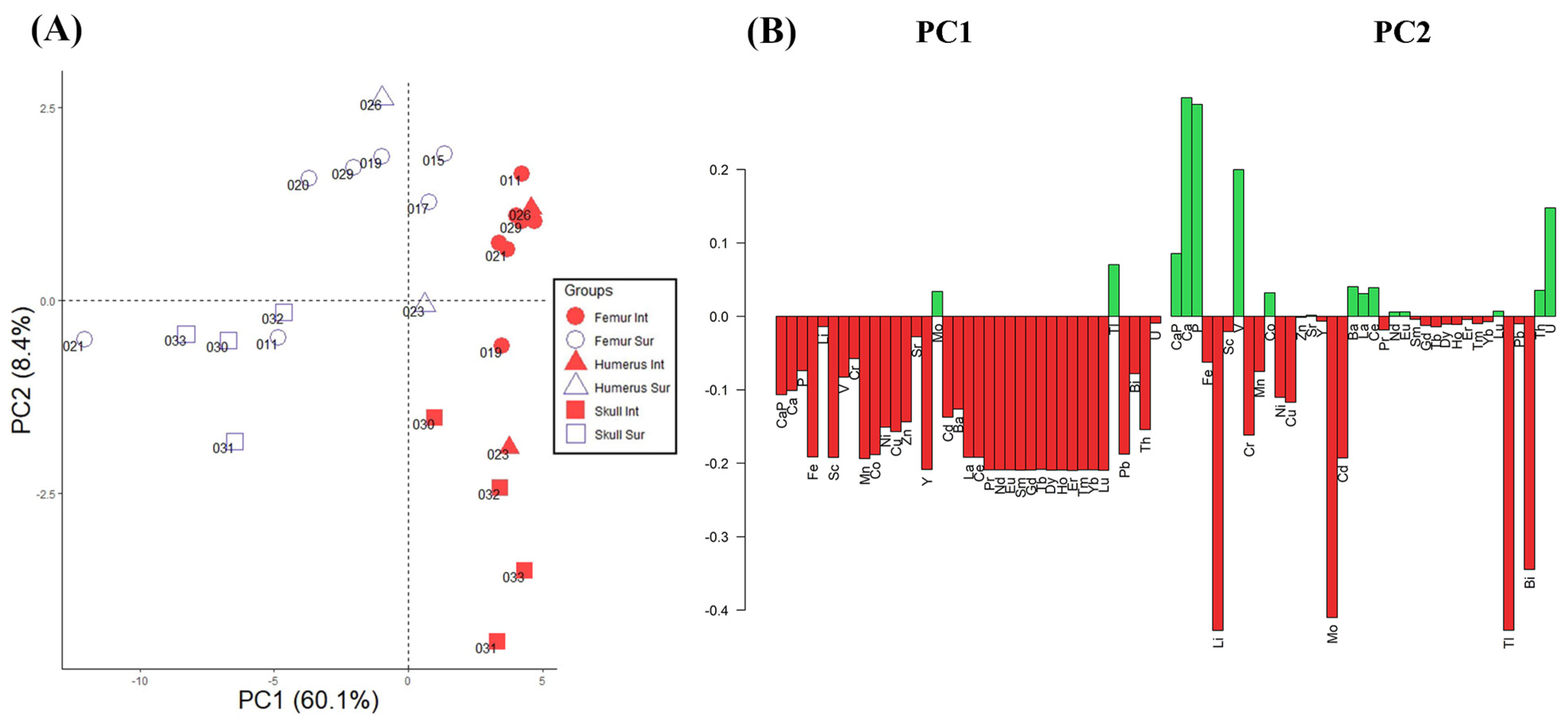
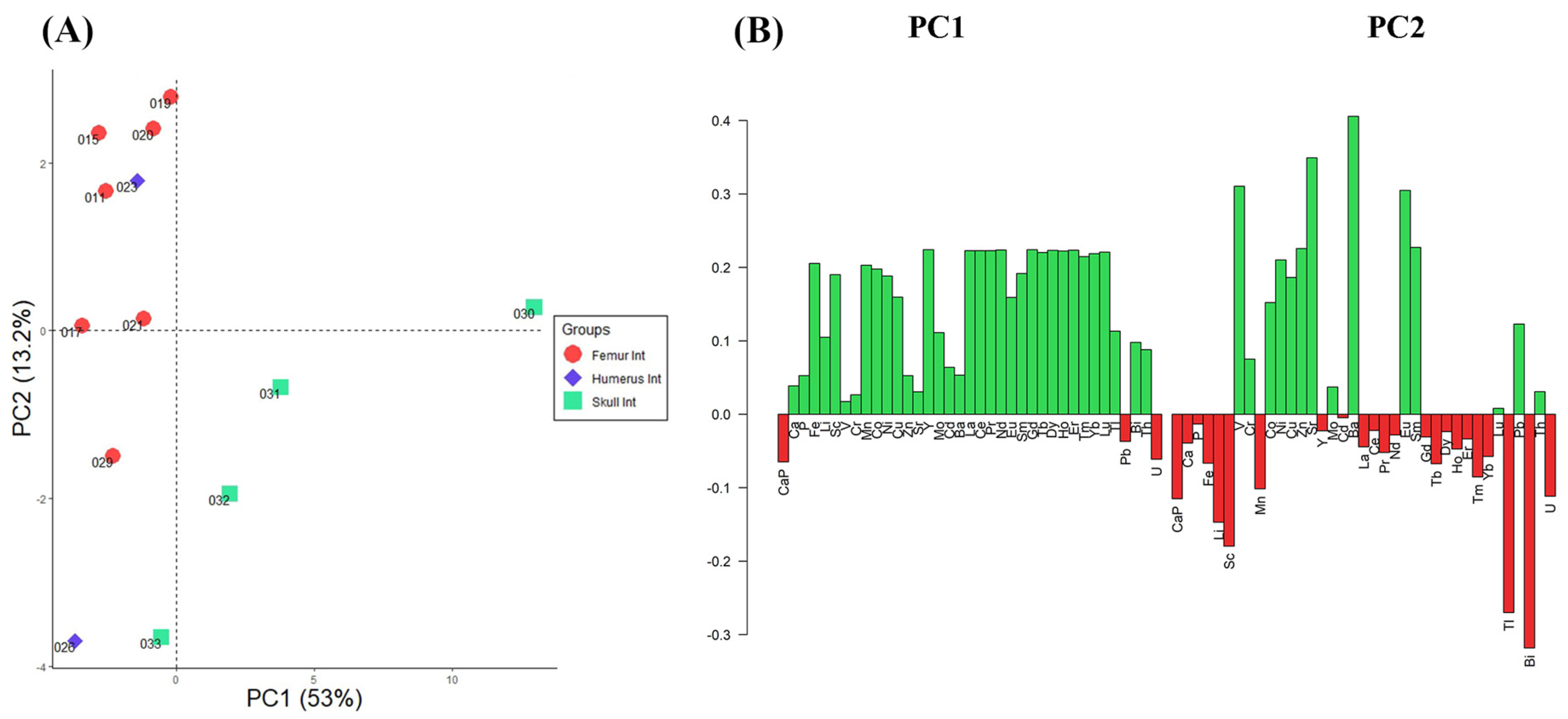
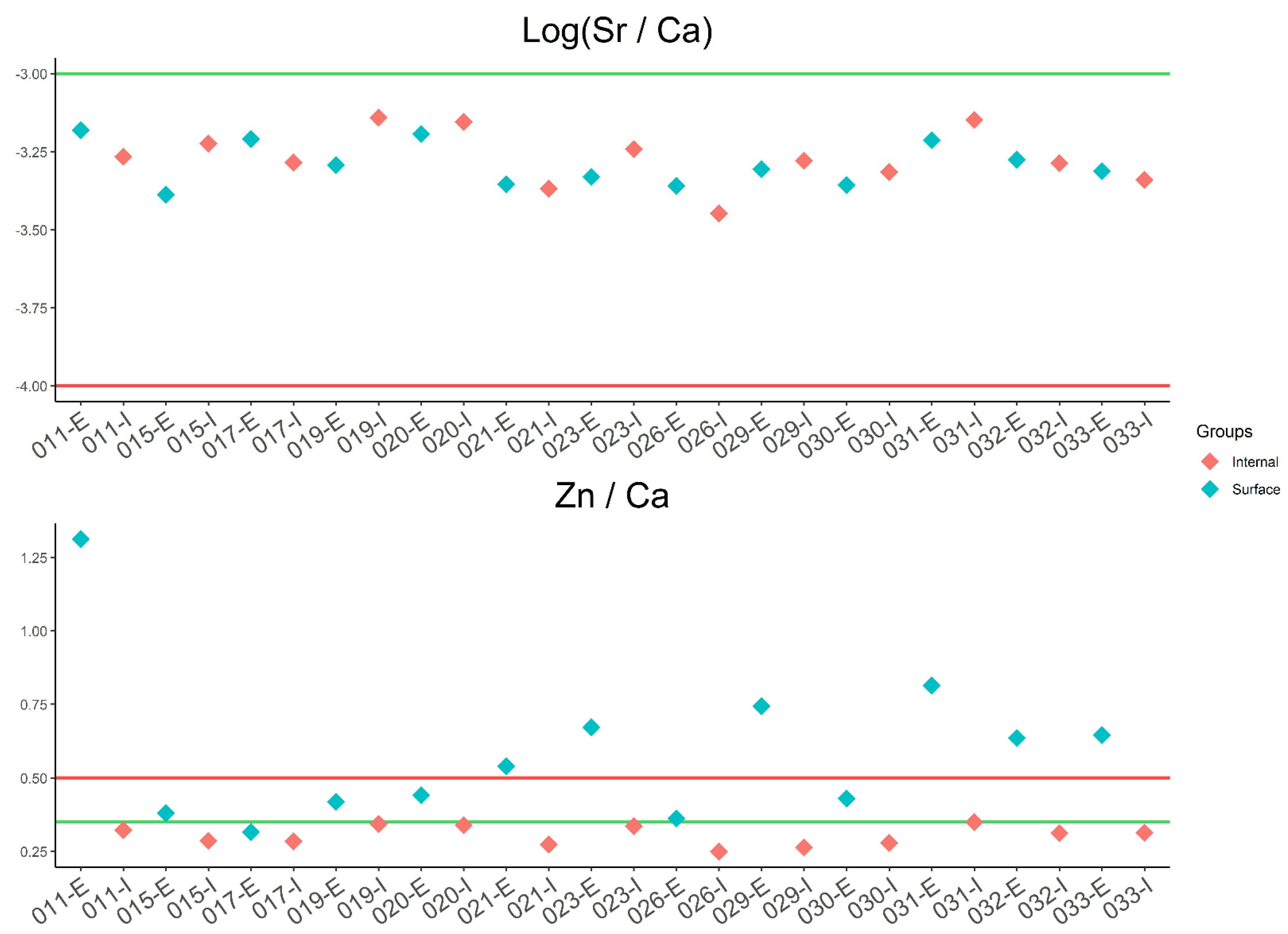
3.1. Elemental Composition and Statistical Analysis
3.2. Testing a Paleodiet Reconstruction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bala, Y.; Farlay, D.; Boivin, G. Bone mineralization: From tissue to crystal in normal and pathological contexts. Osteoporos. Int. 2012, 24, 2153–2166. [Google Scholar] [CrossRef]
- Keenan, S.W.; Engel, A.S. Early diagénesis and recrystallization of bone. Geochim. Cosmochim. Acta 2017, 196, 209–223. [Google Scholar] [CrossRef]
- Vidaud, C.; Bourgeois, D.; Meyer, D. Bone as target organ for metals: The case of f-elements. Chem. Res. Toxicol. 2012, 25, 1161–1175. [Google Scholar] [CrossRef]
- Dyczek, P.; Shpuza, S. From Antiquity to Modernity, 1st ed.; University of Warsaw: Warsaw, Poland, 2020; pp. 7–401. [Google Scholar]
- Alapont, L.; Gallello, G.; Martinón-Torres, M.; Osanna, M.; Amoretti, V.; Chenery, S.; Ramacciotti, M.; Jiménez, J.L.; Rubio, Á.M.; Cervera, M.L.; et al. The casts of Pompeii: Post-depositional methodological insights. PLoS ONE 2023, 18, e0289378. [Google Scholar] [CrossRef]
- Toots, H.; Voorhies, M.R. Strontium in Fossil Bones and the Reconstruction of Food Chains. Science 1965, 149, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Sillen, A.; Kavanagh, M. Strontium and paleodietary research: A review. Am. J. Phys. Anthropol. 1982, 25, 67–90. [Google Scholar] [CrossRef]
- Gallello, G.; Kuligowski, J.; Pastor, A.; Diez, A.; Bernabeu, J. Chemical element levels as a methodological tool in Forensic Science. J. Forensic Res. 2015, 6, 1. [Google Scholar] [CrossRef]
- Winter, N.J.; Snoeck, C.; Claeys, P. Seasonal cyclicity in trace elements and stable isotopes of modern horse enamel. PLoS ONE 2016, 11, e0166678. [Google Scholar] [CrossRef]
- Gallello, G.; Cilli, E.; Bartoli, F.; Andretta, M.; Calcagnile, L.; Pastor, A.; de la Guardia, M.; Serventi, P.; Marino, A.; Benazzi, S.; et al. Poisoning histories in the Italian renaissance: The case of Pico Della Mirandola and Angelo Poliziano. J. Forensic Leg. Med. 2018, 56, 83–89. [Google Scholar] [CrossRef]
- López-Costas, O.; Lantes-Suarez, O.; Cortizas, M.A. Chemical compositional changes in archaeological human bones due to diagenesis: Type of bone vs soil environment. J. Archaeol. Sci. 2016, 67, 43–51. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Boldsen, J.L.; Kristensen, H.K.; Skytte, L.; Hansen, K.L.; Mølholm, L.; Grootes, P.M.; Nadeau, M.J.; Eriksen, K.M.F. Mercury levels in Danish medieval human bones. J. Archaeol. Sci. 2008, 35, 2295–2306. [Google Scholar] [CrossRef]
- Scharlotta, I.; Goriunova, O.I.; Weber, A. Micro-sampling of human bones for mobility studies: Diagenetic impacts and potentials for elemental and isotopic research. J. Archaeol. Sci. 2013, 40, 4509–4527. [Google Scholar] [CrossRef]
- Balter, V.; Braga, J.; Télouk, P.; Thackeray, J.F. Evidence for dietary change but not landscape use in South African early hominins. Nature 2012, 489, 558–560. [Google Scholar] [CrossRef]
- Gallello, G. Western Mediterranean Archaeology: Chemical Elements Levels in Archaeology Materials as a Methodological Tool. Ph.D. Dissertation, Universidad de Valencia, Valencia, Spain, 2014. [Google Scholar]
- Suarez, C.A.; Suarez, M.B.; Terry, D.O.; Grandstaff, J.R.; Grandstaff, D.E. Rare earth element geochemistry and taphonomy of terrestrial vertebrate assemblages. Palaios 2007, 22, 500–512. [Google Scholar] [CrossRef]
- Trueman, C.N.; Behrensmeyer, A.K.; Tuross, N.; Weiner, S. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: Diagenetic mechanisms and the role of sediment pore fluids. J. Archaeol. Sci. 2004, 31, 721–739. [Google Scholar] [CrossRef]
- Karaiskaj, G. Die Spätantiken und Mittelalterlichen Wehranlagen in Albanien; Verlag Dr. Kovac: Hamburg, Germany, 2010; pp. 9–249. [Google Scholar]
- Lemke, M. Fieldwork at Scodra 2013. Światowit XI (LII)/A; University of Warsaw: Warsaw, Poland, 2014; pp. 189–196. [Google Scholar]
- Dyczek, P. Terra Incognita: Results of Polish Excavations In Albania And Montenegro. Stud. Eur. Gnes. 2017, 16, 351–369. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Milner, G.R.; Skytte, L.; Lynnerup, N.; Lange Thomsen, J.; Lier Boldsen, J. Mapping diagenesis in archaeological human bones. Herit. Sci. 2019, 7, 41. [Google Scholar] [CrossRef]
- Harkness, S.H.; Darrah, T.H. From the crust to the cortical: The geochemistry of trace elements in human bone. Geochim. Cosmochim. Acta 2019, 249, 76–94. [Google Scholar] [CrossRef]
- Zapata, J.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Tovar, P. Diagenesis, not biogénesis: Two late Roman skeletal examples. Sci. Total Environ. 2006, 369, 357–368. [Google Scholar] [CrossRef]
- Hancock, R.G.V.; Grynpas, M.D.; Åkesson, K.; Obrant, J.T.; Kessler, M.J. Baselines and variabilities for major and trace elements in bone. In Prehistoric Human Bone: Archaeology at the Molecular Level; Lambert, J.B., Grupe, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 189–201. [Google Scholar] [CrossRef]
- Gallello, G.; Kuligowski, J.; Pastor, A.; Diez, A.; Bernabeu, J. Biological mineral content in Iberian skeletal cremains for control of diagenetic factors employing multivariate statistics. J. Archaeol. Sci. 2013, 40, 2477–2484. [Google Scholar] [CrossRef]
- Reymann, C.R.; Filzmoser, P.; Garrett, G.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-98581-6. [Google Scholar]
- Gallello, G.; Scopa, S.; Kuligowski, J.; Bartoli, F.; Mallegni, F.; Pastor, A. Variación química intraesquelética relacionada con la diagenesis en los restos óseos de C/en Gil (Valencia). Saguntum 2015, 47, 175–186. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 28 November 2022).
- Rasmussen, K.L.; Milner, G.R.; Delbey, T.; Skytte, L.; Lynnerup, N.; Thomsen, J.L.; Schiavone, S.; Torino, M.; Larsen, L.A.; Boldsen, J.L. Trace element distribution in human cortical bone microstructure: The potential for unravelling diet social status in archaeological bones. Herit. Sci. 2020, 8, 111. [Google Scholar] [CrossRef]
- Scharlotta, I.; Bazaliiskii, V.I.; Kusaka, S.; Weber, A. Diet or mobility? Multi-isotopic (C, N, and Sr) dietary modeling at Shamanka II, Cis-Baikal, Siberia. Archaeol. Res. Asia 2022, 29, 100340. [Google Scholar] [CrossRef]
- Lambert, J.B.; Vlasak, S.M.; Thometz, A.C.; Buikstra, J.E. A comparative study of the chemical analysis of ribs and femurs in Woodland population. Am. J. Phys. Anthropol. 1982, 59, 289–294. [Google Scholar] [CrossRef]
- Comar, C.L.; Russell, R.S.; Wasserman, R.H. Strontium-calcium movement from soil to man. Science 1957, 126, 485–492. [Google Scholar] [CrossRef]
- Reynard, B.; Balter, V. Trace elements and their isotopes in bones and teeth: Diet, environments, diagenesis, and dating of archaeological and paleontological samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 4–16. [Google Scholar] [CrossRef]
- Burton, J.; Wright, L. Nonlinearity in the relationship between bone Sr/Ca and diet: Paleodietary implications. Am. J. Phys. Anthropol. 1995, 96, 273–282. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary factors influencing Zinc absorption. J. Nutr. 2000, 130, 1378–1383. [Google Scholar] [CrossRef]
- Kohn, M.J.; Morris, J.; Olin, P. Trace element concentrations in teeth—A modern Idaho baseline with implications for archaeometry, forensics, and palaeontology. J. Archaeol. Sci. 2013, 40, 1689–1699. [Google Scholar] [CrossRef]
- Burton, J. Bone chemistry and trace element analysis. In Biological Anthropology of the Human Skeleton, 2nd ed.; Katzenberg, M.A., Shelley, S.R., Eds.; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2008; Chapter 14. [Google Scholar] [CrossRef]
- Hedges, R.E.M.; Millard, A.R.; Pike, A.W.G. Measurements and relationships of diagenetic alteration of bone from three archaeological sites. J. Archaeol. Sci. 1995, 22, 201–209. [Google Scholar] [CrossRef]
- Fabig, A.; Herrmann, B. Trace elements in buried human bones: Intra-population variability of Sr/Ca and Ba/Ca ratios—Diet or diagenesis? Naturwissenschaften 2002, 89, 115–119. [Google Scholar] [CrossRef]
- Trueman, C.N.; Benton, M.J. A geochemical method to trace the taphonomic history of reworked bones in sedimentary settings. Geology 1997, 25, 263–266. [Google Scholar] [CrossRef]
- Trueman, C.N.G.; Behrensmeyer, A.K.; Potts, R.; Tuross, N. High-resolution records of location and stratigraphic provenance from the rare earth element composition of fossil bones. Geochim. Cosmochim. Acta GCA 2006, 70, 4343–4355. [Google Scholar] [CrossRef]
- Trueman, C.N.; Palmer, M.R.; Field, J.; Privat, K.; Ludgate, N.; Chavagnac, V.; Eberth, D.A.; Cifelli, R.; Rogers, R.R. Comparing rates of recrystallisation and the potential for preservation of biomolecules from the distribution of trace elements in fossil bones. Comptes Rendus Palevol 2008, 7, 145–158. [Google Scholar] [CrossRef]
- Wang, S.; Huang, T.D.; Yan, R.; Chen, H.; Zhang, S.; Li, X.; Kótai, L.; Reisz, R.R. Rare Earth Elements in Dinosaur Bones Across the Embryo-Adult Spectrum. Front. Earth Sci. 2020, 8, 230. [Google Scholar] [CrossRef]
- Williams, C.T.; Henderson, P.; Marlow, C.A.; Molleson, T.I. The environment of deposition indicated by the distribution of rare earth elements in fossil bones from Olduvai Gorge, Tanzania. Appl. Geochem. 1997, 12, 537–547. [Google Scholar] [CrossRef]
- Trueman, C.N. Forensic geology of bone mineral: Geochemical tracers for post-mortem movement of bone remains. In Forensic Geosciences, Principles, Techniques and Applications; Pye, K., Croft, D.J., Eds.; The Geological Society of London: London, UK, 2004; pp. 249–256. [Google Scholar]
- Mazzi, M.S. Consumi Alimentari e Malattie nel Basso Medioevo. In Archeologia Medievale: Cultura Materiale, Insediamenti, Territorio: VIII; All’insegna del Giglio: Firenze, Italy, 1981; Volume 8, pp. 321–336. Available online: http://digital.casalini.it/10.1400/245696 (accessed on 9 October 2023).
- Slaus, M.; Bedíc, Z.; Sikanjic, P.R.; Vodanovic, M.; Kunic, A.D. Dental health at the transition from the late antique to the early medieval period on Croatia’s eastern Adriatic coast. Int. J. Osteoarchaeol. 2010, 21, 577–590. [Google Scholar] [CrossRef]
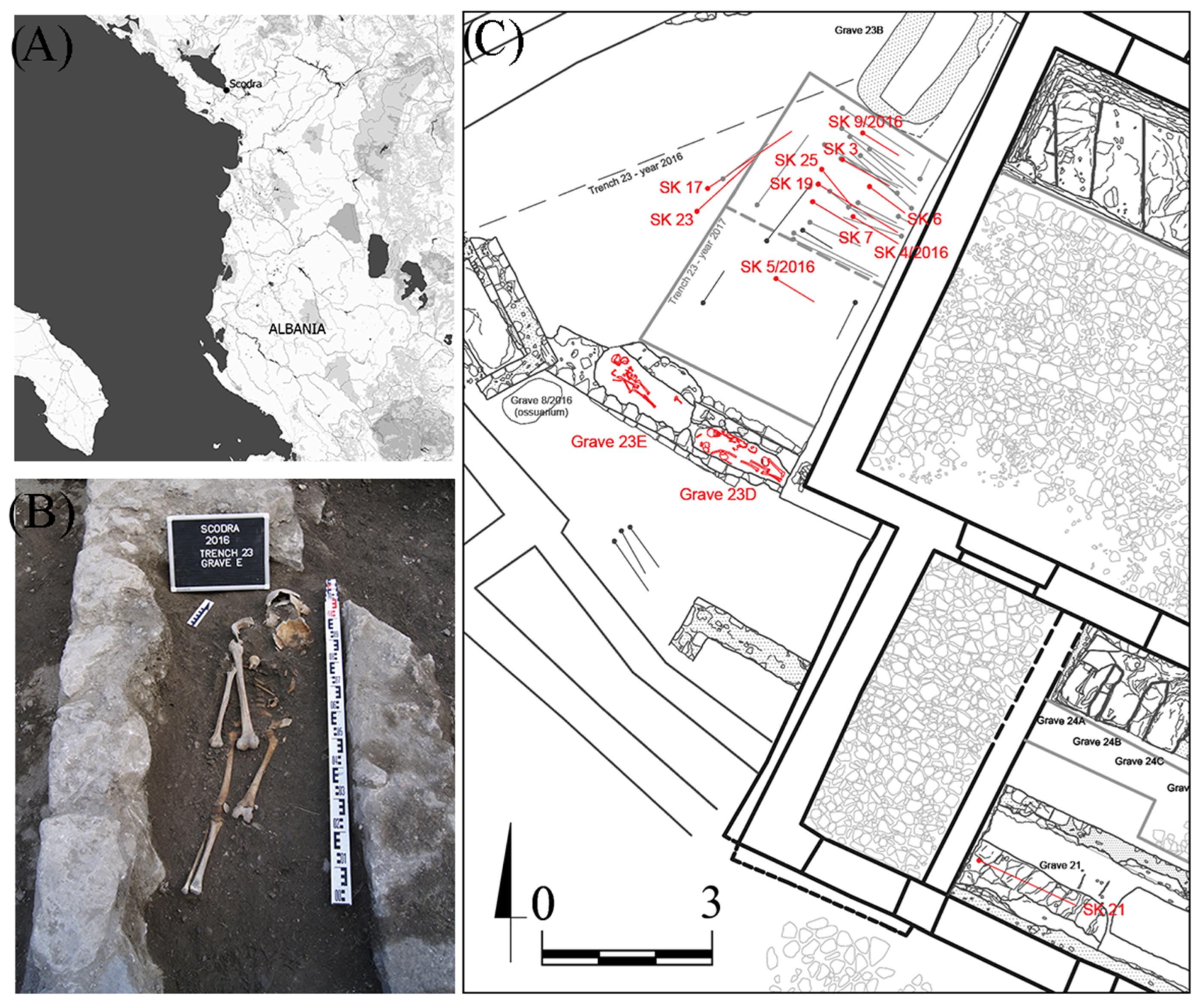
| Lab ID | Grave | Sex | Age | Bone | Type | Sample ID | Bone | Type | Sample ID |
|---|---|---|---|---|---|---|---|---|---|
| SHK 033 | SK4/2016 | Unknown | Adult | Skull | Internal | 033-I | Skull | Surface | 033-S |
| SHK 032 | SK5/2016 | Female | 25–29 | Skull | Internal | 032-I | Skull | Surface | 032-S |
| SHK031 | SK9/2016 | Male | 18–25 | Skull | Internal | 031-I | Skull | Surface | 031-S |
| SHK 030 | SK17 | Unknown | 18–21 | Skull | Internal | 030-I | Skull | Surface | 030-S |
| SHK 029 | SK19 | Female | 50+ | Femur | Internal | 029-I | Femur | Surface | 029-S |
| SHK 026 | SK21 | Immature | 10–12 | Humerus | Internal | 026-I | Humerus | Surface | 026-S |
| SHK 023 | SK21 | Unknown | Adult | Humerus | Internal | 023-I | Humerus | Surface | 023-S |
| SHK 021 | SK23 | Male | 30–39 | Femur | Internal | 021-I | Femur | Surface | 021-S |
| SHK 020 | SK23D | Male | Adult (30–39) | Femur | Internal | 020-I | Femur | Surface | 020-S |
| SHK 019 | SK23E | Unknown | Adult | Femur | Internal | 019-I | Femur | Surface | 019-S |
| SHK 017 | SK3 | Male | 20–29 | Femur | Internal | 017-I | Femur | Surface | 017-S |
| SHK 015 | SK6 | Female | 20–25 | Femur | Internal | 015-I | Femur | Surface | 015-S |
| SHK 011 | SK7 | Male | 20–29 | Femur | Internal | 011-I | Femur | Surface | 011-S |
| REE | Internal | Surface | Majors and Trace | Internal | Surface | Majors and Trace | Internal | Surface |
|---|---|---|---|---|---|---|---|---|
| La | 26.6 ± 32.9 a | 442 ± 289 b | Ca/P | 2.25 ± 0.04 a | 2.29 ± 0.05 b | Bi | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Ce | 49.4 ± 54.4 a | 607 ± 355 b | Ca | 359 ± 26.5 a | 399 ± 16.9 b | Cd | 0.22 ± 0.14 | 0.54 ± 0.43 |
| Pr | 6.09 ± 7.51 a | 79.5 ± 48.2 b | P | 160 ± 11.9 a | 174 ± 9.04 b | Ba | 48.6 ± 11.2 a | 60.9 ± 11.4 b |
| Nd | 30.2 ± 37.9 a | 404 ± 222 b | Fe | 0.48 ± 0.10 a | 1.05 ± 0.43 b | Tl | 0.05 ± 0.03 b | 0.02 ± 0.01 a |
| Eu | 8.46 ± 2.52 a | 29.9 ± 12.8 b | Li | 1.24 ± 0.53 | 1.21 ± 0.32 | Pb | 3.42 ± 2.07 a | 15.0 ± 9.30 b |
| Sm | 29.3 ± 9.14 a | 107± 48.5 b | Th | 0.09 ± 0.02 a | 0.16 ± 0.05 b | |||
| Gd | 7.60 ± 8.30 a | 94.8 ± 55.8 b | V | 39.8 ± 5.66 a | 62.9 ± 24.5 b | |||
| Tb | 0.92 ± 1.18 a | 13.5 ± 8.16 b | Cr | 13.9 ± 3.53 | 16.4 ± 4.17 | |||
| Dy | 5.45 ± 6.83 a | 78.4 ± 45.4 b | Mn | 6.28 ± 8.08 a | 45.6 ± 33.7 b | |||
| Ho | 1.04 ± 1.30 a | 15.8 ± 9.27 b | Co | 0.90 ± 0.17 a | 1.39 ± 0.28 b | |||
| Er | 2.81 ± 3.24 a | 43.1 ± 24.9 b | Ni | 12.9 ± 3.76 a | 17.3 ± 3.60 b | |||
| Tm | 0.50 ± 0.45 a | 5.86 ± 3.45 b | Cu | 14.7 ± 8.88 | 22.9 ± 10.8 | |||
| Yb | 2.81 ± 2.32 a | 34.9 ± 20.2 b | Zn | 109 ± 9.53 a | 234 ± 92.9 b | |||
| Lu | 0.49 ± 0.35 a | 5.43 ± 2.93 b | Sr | 196 ± 32.2 | 207 ± 31.6 | |||
| Sc | 409 ± 36.2 a | 557 ± 115 b | U | 0.73 ± 0.65 | 0.82 ± 0.61 | |||
| Y | 46.3 ± 43.3 a | 670 ± 401 b | Mo | 1.52 ± 0.69 | 1.21 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, D.R.; Gallello, G.; Recław, J.; Panzarino, G.; Cervera, M.L.; Pastor, A. Rare Earth Elements to Control Bone Diagenesis Processes at Rozafa Castle (Albania). Heritage 2024, 7, 5800-5813. https://doi.org/10.3390/heritage7100273
Navarro DR, Gallello G, Recław J, Panzarino G, Cervera ML, Pastor A. Rare Earth Elements to Control Bone Diagenesis Processes at Rozafa Castle (Albania). Heritage. 2024; 7(10):5800-5813. https://doi.org/10.3390/heritage7100273
Chicago/Turabian StyleNavarro, Daniel Román, Gianni Gallello, Janusz Recław, Ginevra Panzarino, M. Luisa Cervera, and Agustín Pastor. 2024. "Rare Earth Elements to Control Bone Diagenesis Processes at Rozafa Castle (Albania)" Heritage 7, no. 10: 5800-5813. https://doi.org/10.3390/heritage7100273
APA StyleNavarro, D. R., Gallello, G., Recław, J., Panzarino, G., Cervera, M. L., & Pastor, A. (2024). Rare Earth Elements to Control Bone Diagenesis Processes at Rozafa Castle (Albania). Heritage, 7(10), 5800-5813. https://doi.org/10.3390/heritage7100273






