Preserving Intangible Heritage through Tangible Finds: The “Skull with Ears”—St. Luciella ai Librai’s Church (Naples, Italy)
Abstract
1. Introduction
Ethical Statement
2. Materials and Methods
2.1. Definition of Conservation State
- A Madatec multispectral system consisting of a Samsung NX500 28.2 MP BSI CMOS camera.
- Ultraviolet fluorescence by using Madatec ultraviolet (UV) spotlights (365 nm), a HOYA UV-Infrared (IR) filter cut 52, and a Yellow 495, 52 mm F-PRO MRC 022.
- Infrared reflectography images that were acquired using an 850 nm filter.
2.2. Documentation and 3D Reconstruction
- Relief 1: a 3D model that was acquired before the safety operation, with the skull in its original position (Figure 1).
- Relief 2: a 3D model that was acquired during the consolidation procedure, after the skull had been overturned and handed.
- Relief 3: a 3D model that was acquired after the consolidation intervention and placement of the skull in the box.
2.3. First Aid Intervention
3. Results
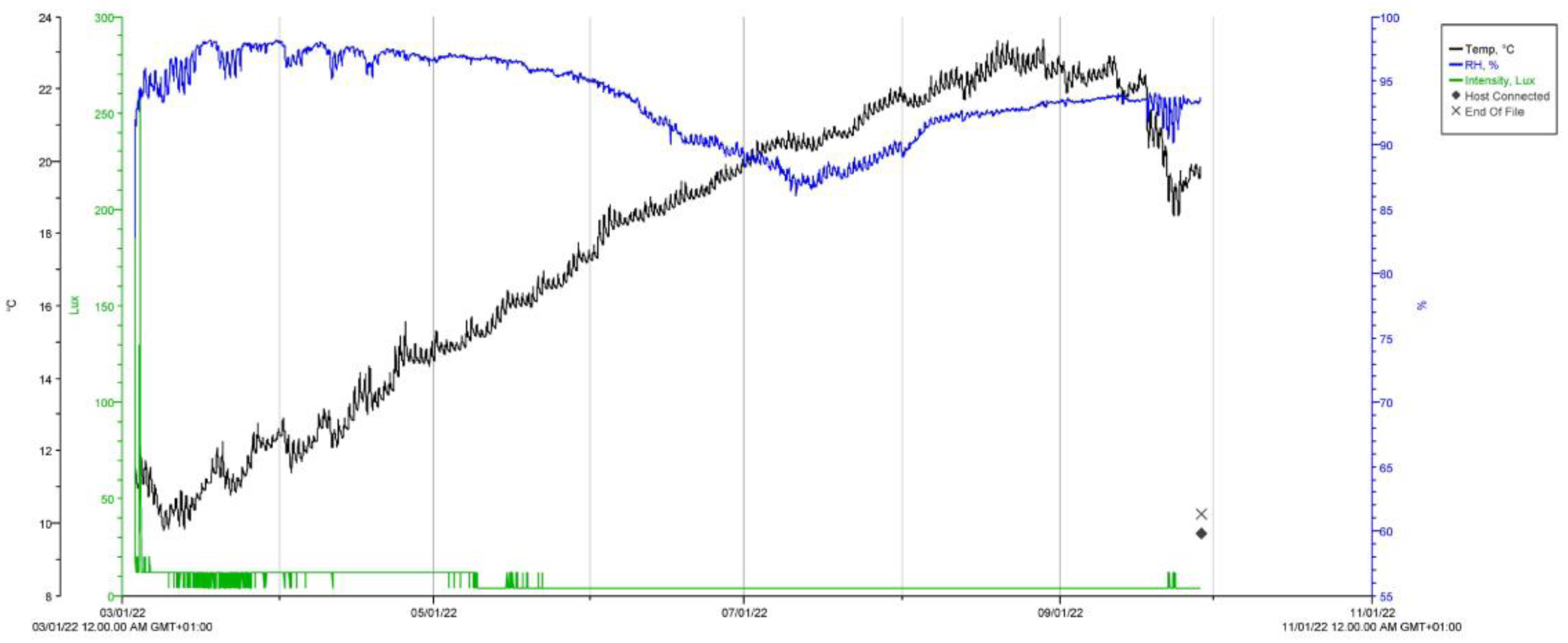
3.1. Crypt Microclimate Monitoring
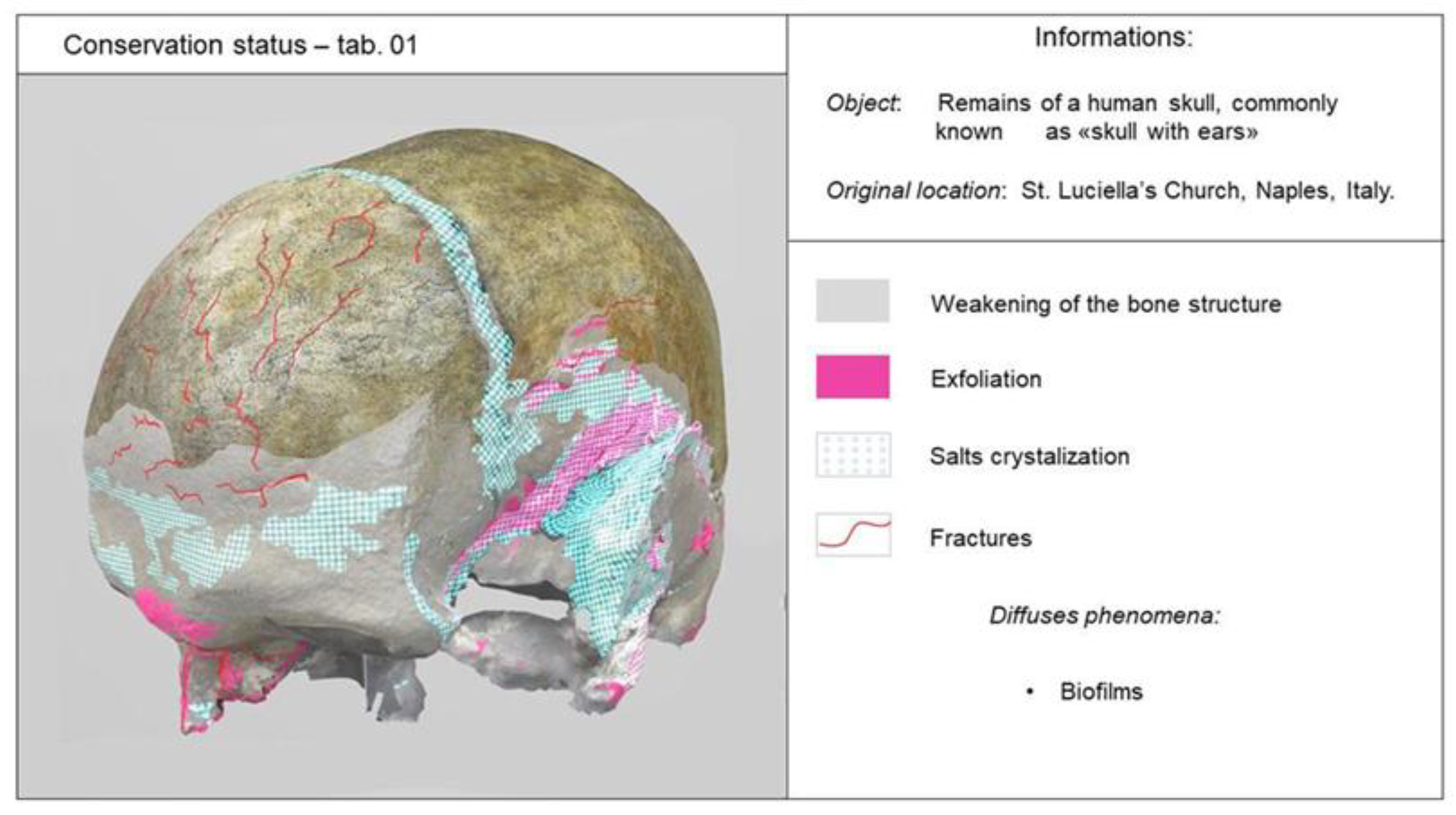
3.2. Definition of Conservation State
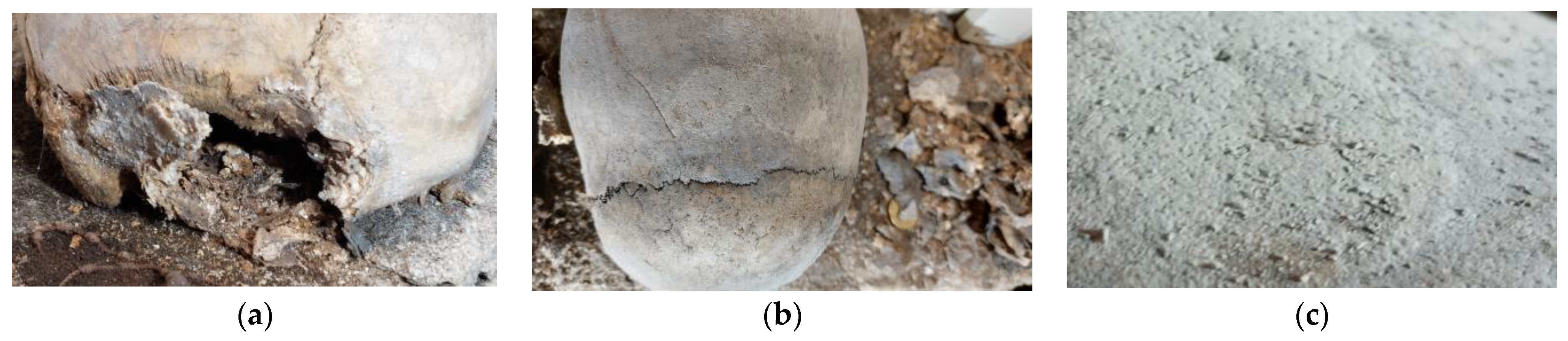
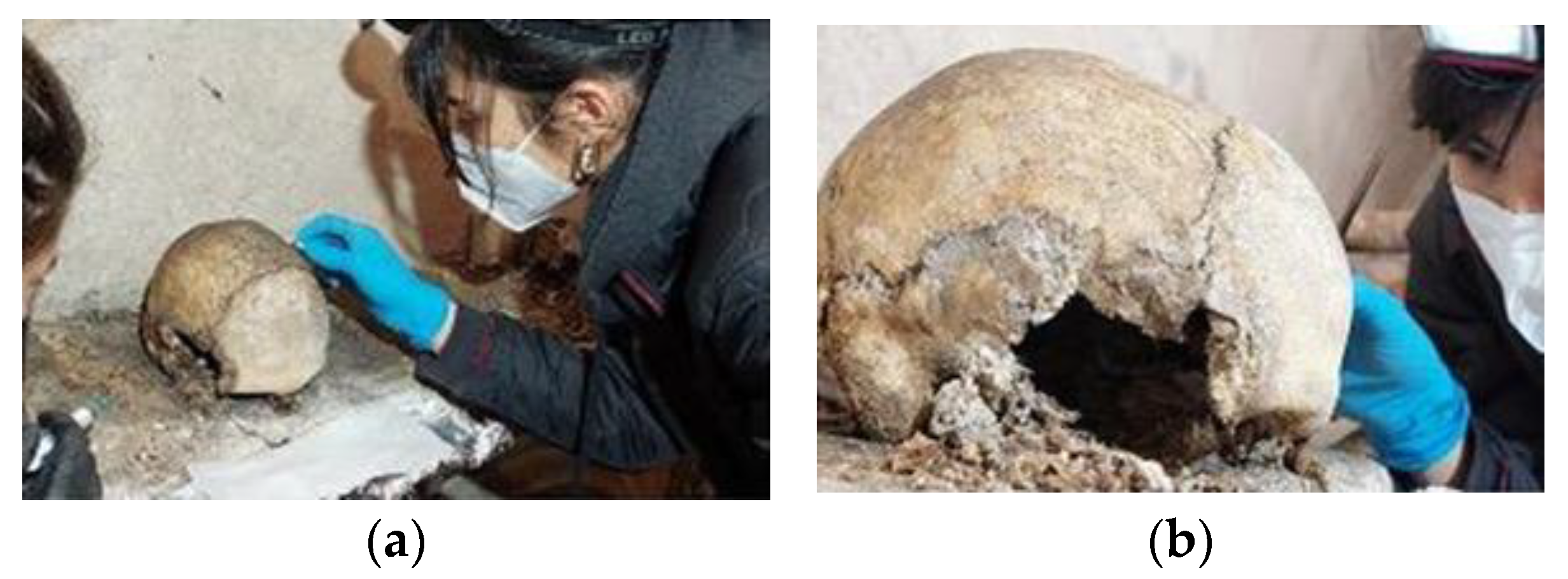
3.2.1. Macroscopic Observations
3.2.2. Anthropological Description
3.2.3. Documentation
3.2.4. Analytical Investigations
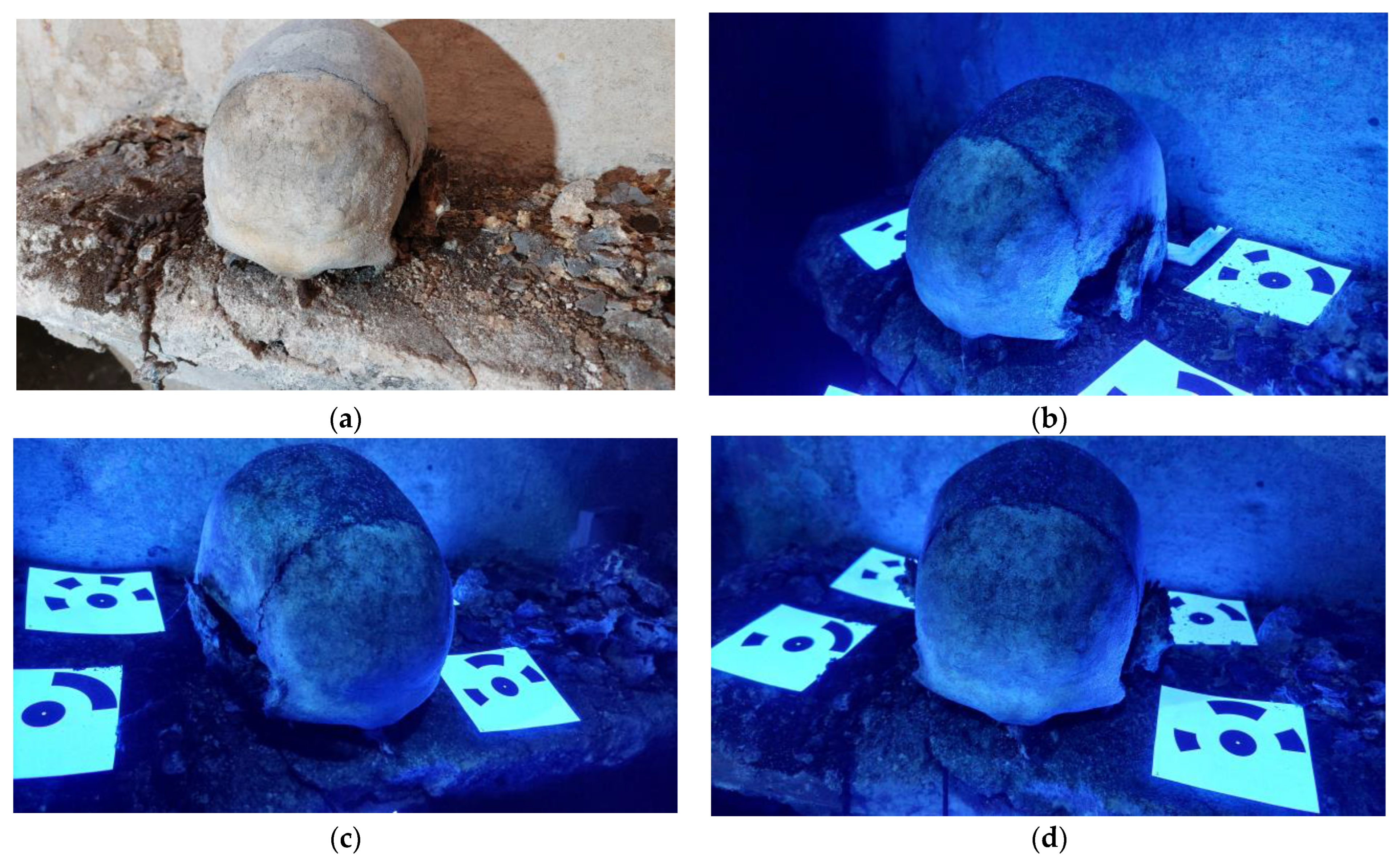
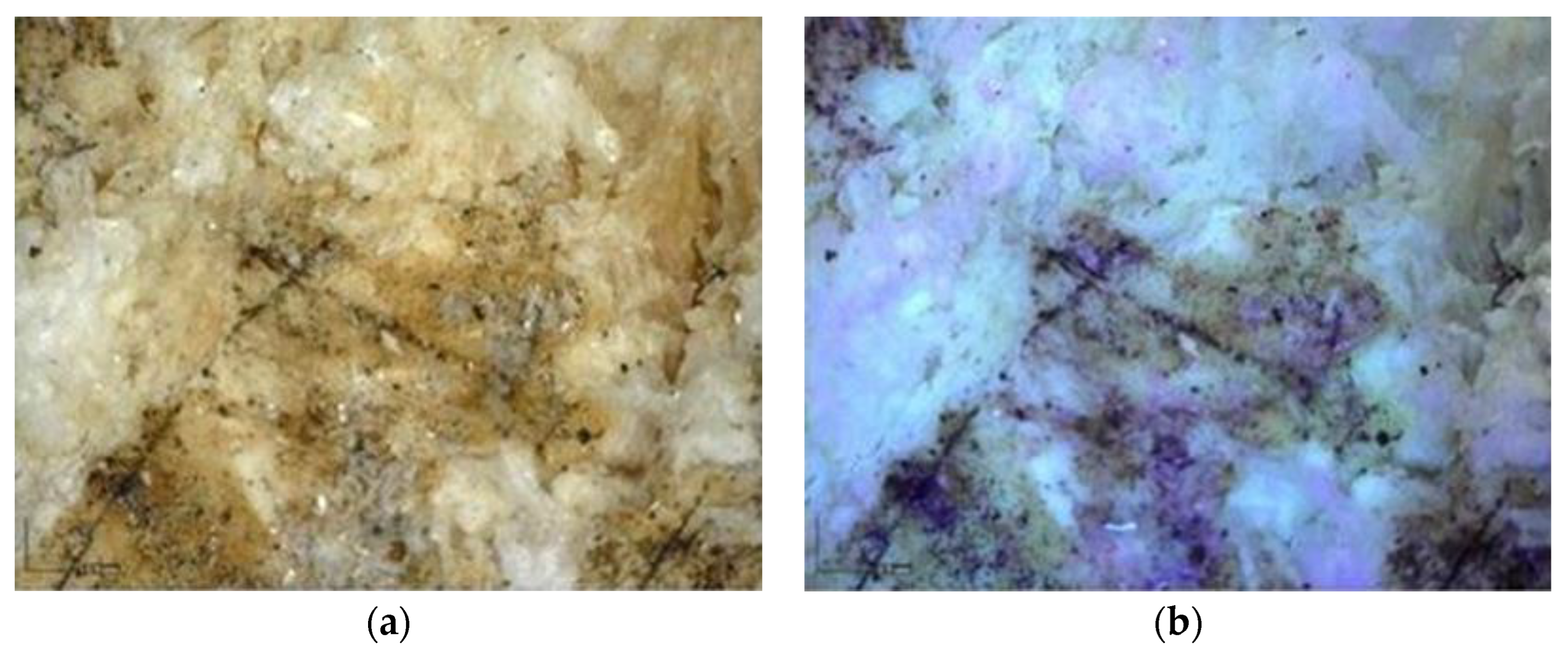
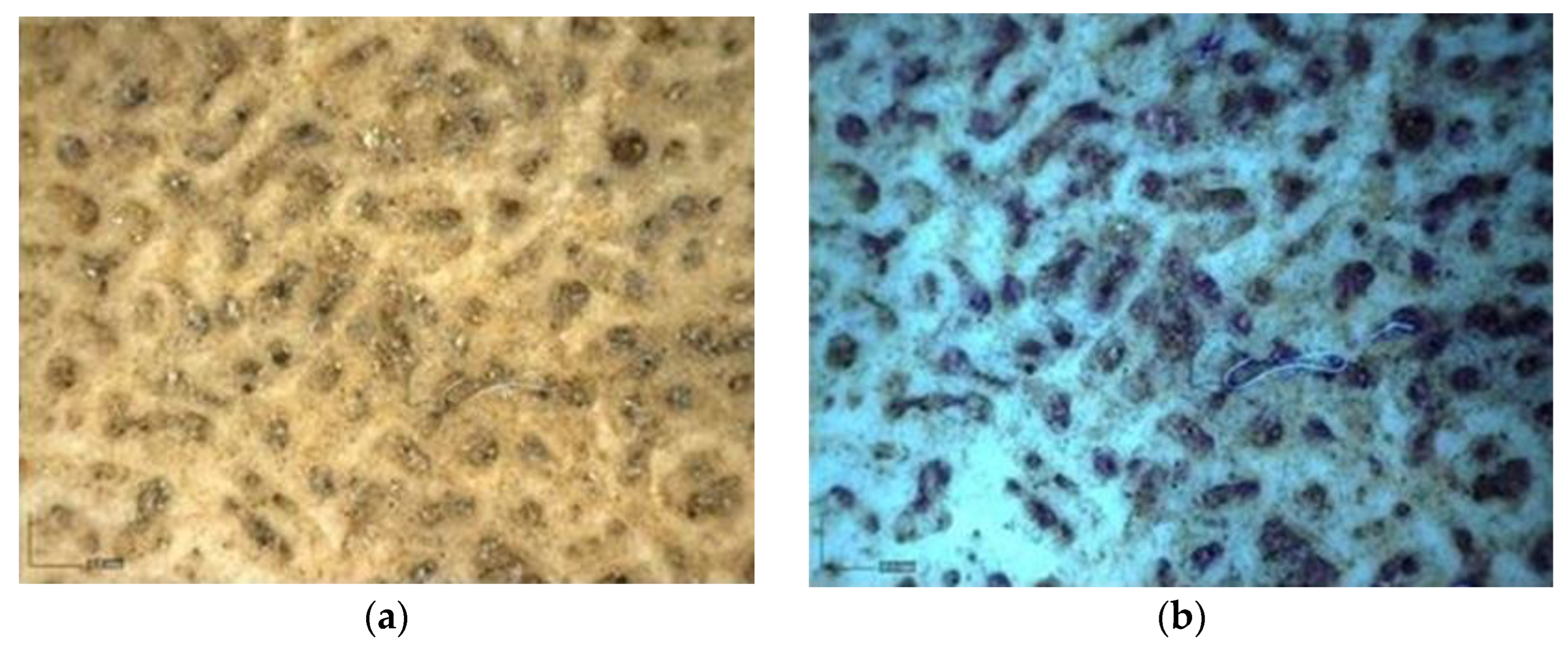
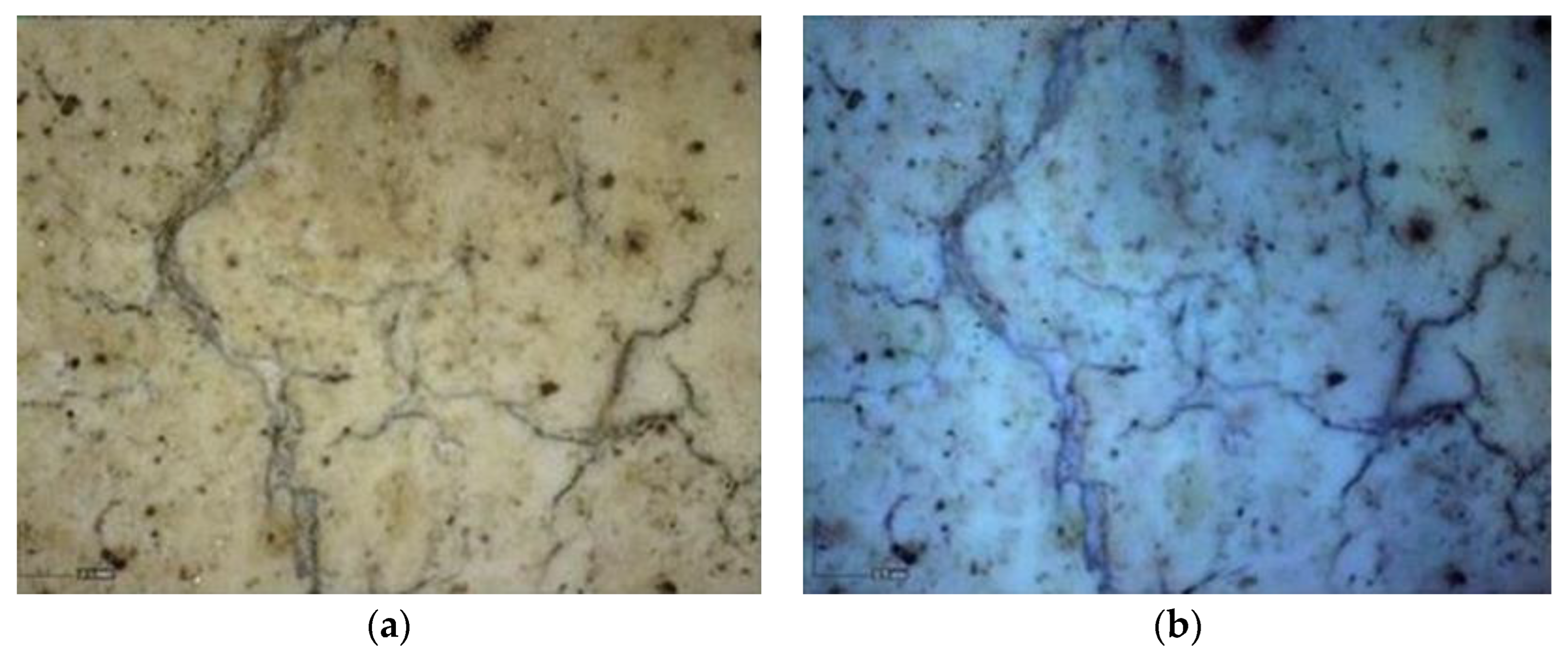
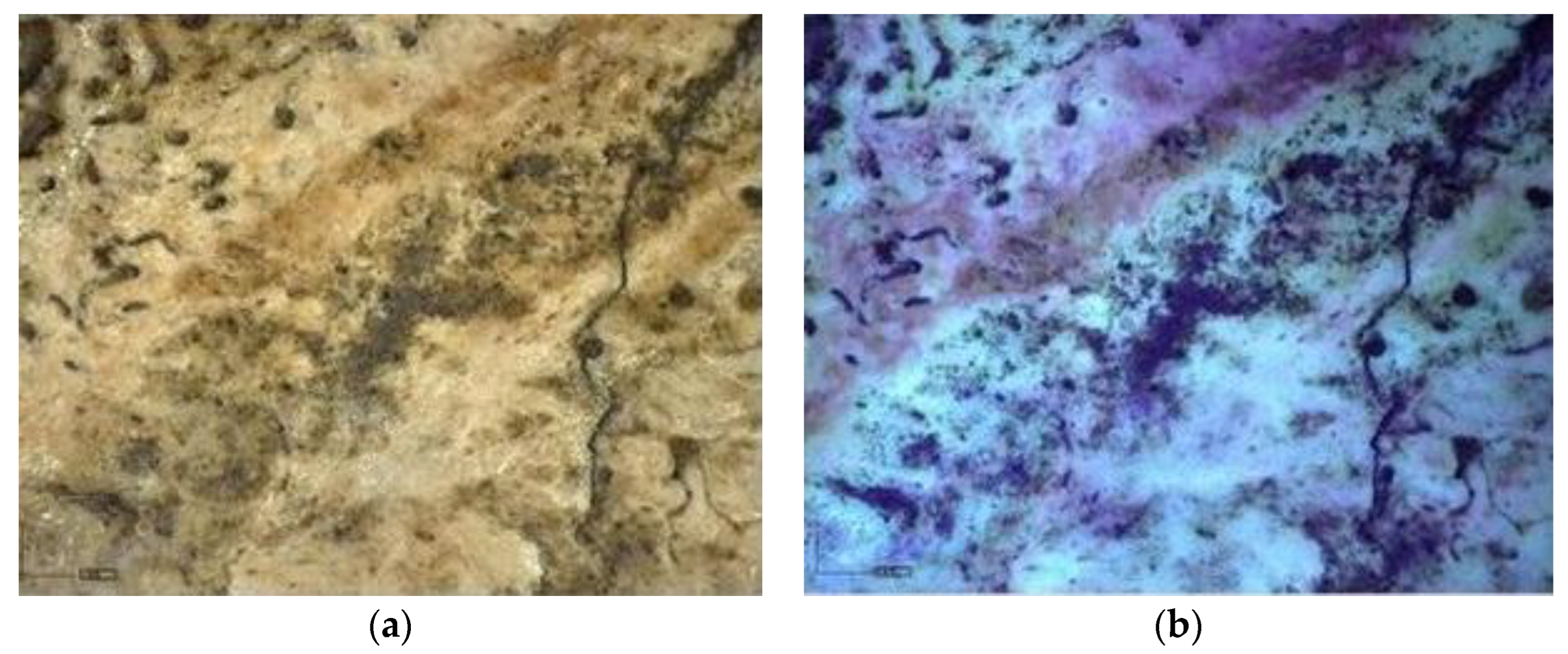
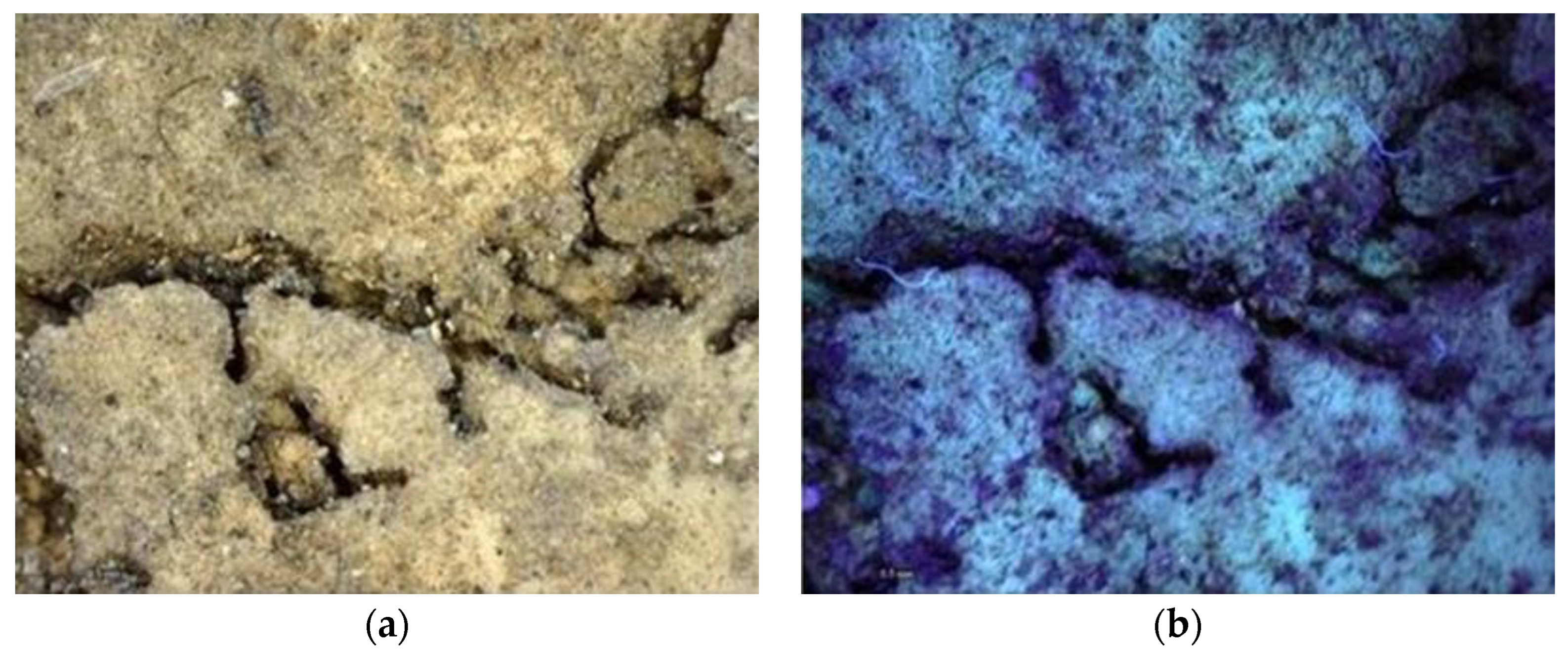
Multispectral Imaging
Digital Microscopy
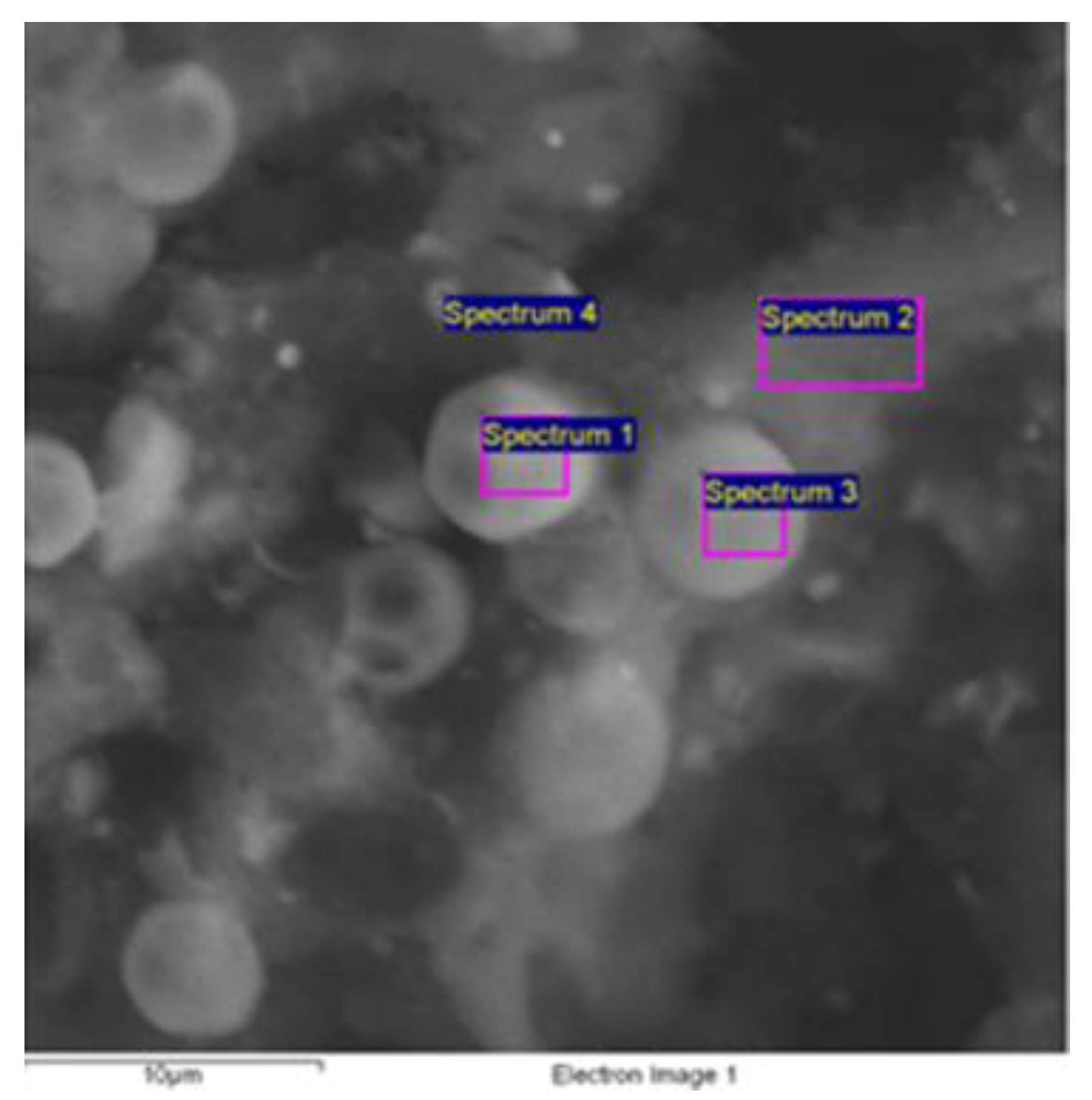
Electron Scanning Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
- (a)
- Spongy bone
- (b)
- Compact bone
- (c)
- Salt efflorescence
- (d)
- Dark patina
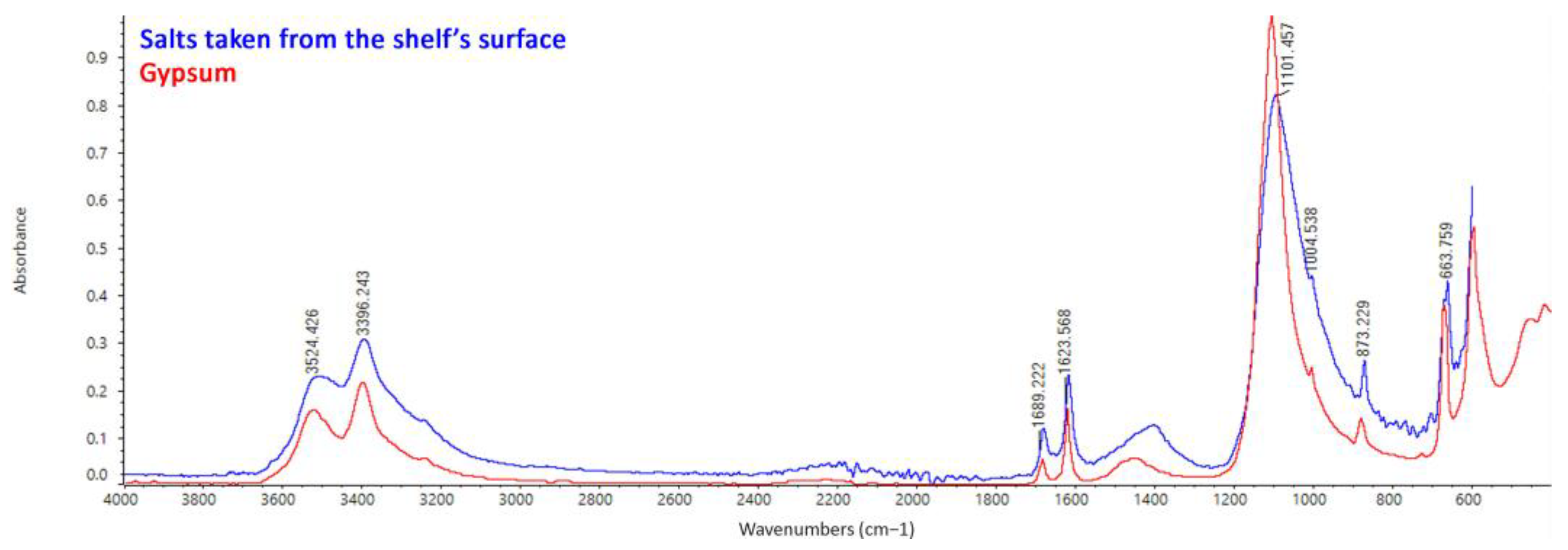
FTIR ATR Spectroscopy
Microbiological Testing
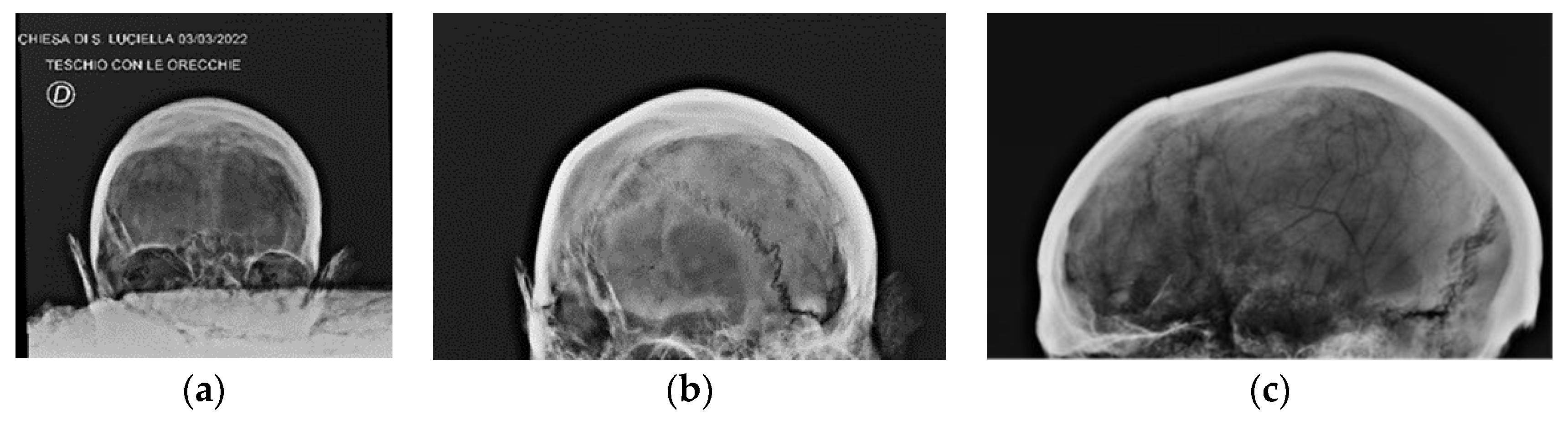
Radiographic Images
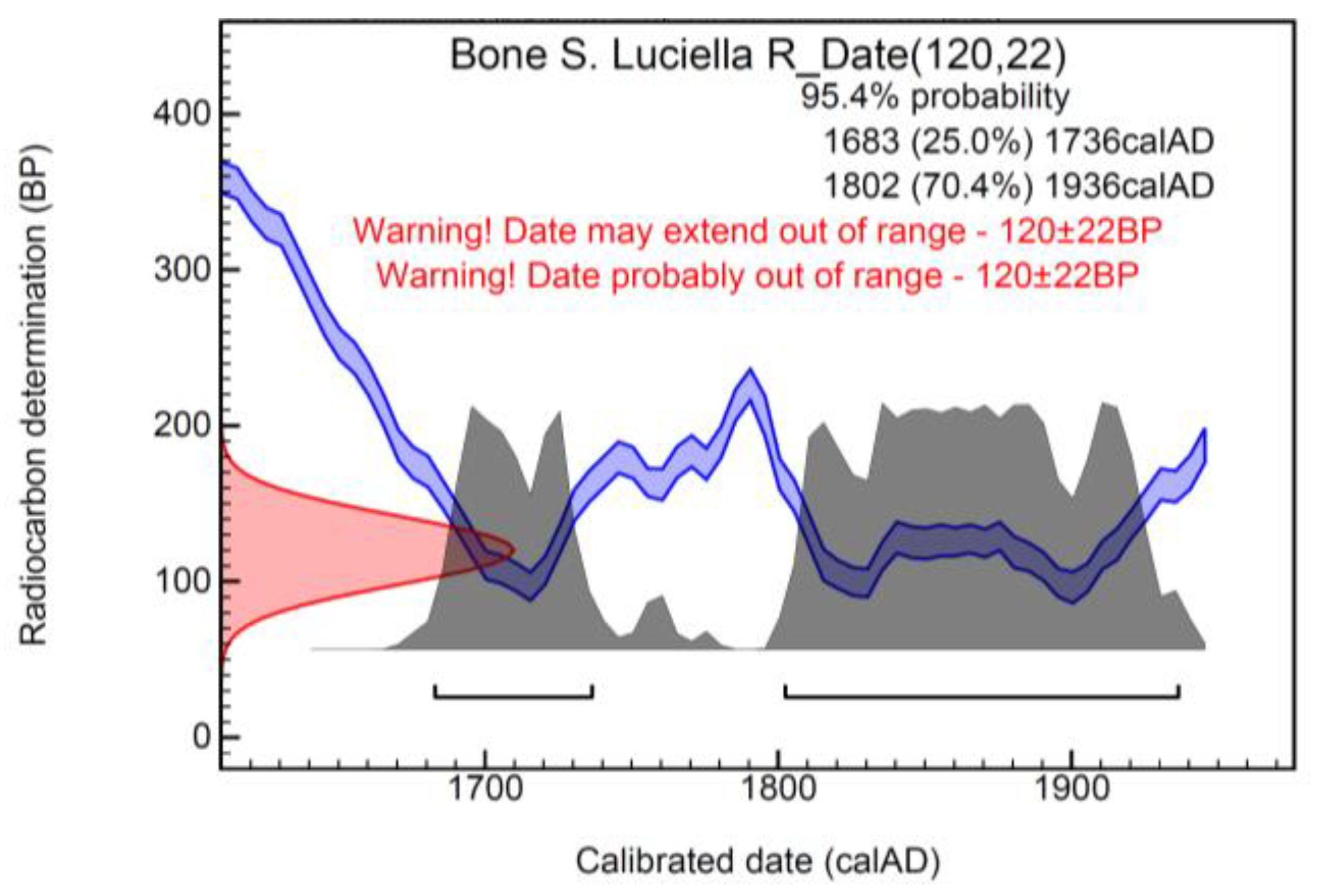
Radiocarbon Dating
3.3. First Aid Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larson, F. Severed: A History of Heads Found; Granta Books: London, UK, 2015. [Google Scholar]
- D’Agostino, E.; Sperduti, A. Skulls. Antenati, Santi e Nemici. In Avo Sapiens. Immagini Dall’oltre Mondo; La Lepre Edizioni: Rome, Italy, 2022; pp. 108–122. [Google Scholar]
- Maiello, G. An Extreme and Massive Expression of the Iconization of Suffering: The Cult of the “Abandoned Souls” in Naples. In The Iconization of Suffering in Literary and Interdisciplinary Perspectives; Constantine the Philosopher University: Nitra, Slovakia, 2019; pp. 145–154. [Google Scholar]
- De Matteis, S. From the anonymous skulls to the collective trance. Ritual representation in the neapolitan underclass. Illuminazioni 2019, 48, 206–277. [Google Scholar]
- Guarino, M. Secondary burials in Naples in the modern and contemporary age: A review. Ethics Med. Public Health 2022, 22, 100793. [Google Scholar] [CrossRef]
- Guarino, M. The hypogea of the churches of Naples: Burials and cult of the dead. Pap. Anthropol. 2022, 31, 7–34. [Google Scholar] [CrossRef]
- Van Gennep, A. The Rites of Passage; The University Press of Chicago: Chicago, IL, USA, 1966. [Google Scholar]
- Vetromile, C.; Spagnuolo, A.; Petraglia, A.; Masiello, A.; di Cicco, M.R.; Lubritto, C. Pre- and post-operam comparison of the energy consumption of a radio base station under energy efficiency actions. Energy Build. 2021, 236, 110772. [Google Scholar] [CrossRef]
- Spagnuolo, A.; Vetromile, C.; Masiello, A.; Alberghina, M.F.; Schiavone, S.; Di Cicco, M.R.; Formato, R.; Lubritto, C. RE-SEMIRTO project: An innovative network of wireless sensors for microclimate monitoring on the Royal Palace of Carditello. J. Phys. Conf. Ser. 2022, 2204, 012076. [Google Scholar] [CrossRef]
- D’Agostino, D.; Macchia, A.; Cataldo, R.; Campanella, L.; Campbell, A. Microclimate and Salt Crystallization in the Crypt of Lecce’s Duomo. Int. J. Archit. Herit. 2015, 9, 290–299. [Google Scholar] [CrossRef]
- Randazzo, L.; Ricca, M.; Pellegrino, D.; La Russa, D.; Macchia, A.; Rivaroli, L.; Marrone, L.; Enei, F.; La Russa, M.F. Anti-fouling additives for the consolidation of archaeological mortars in underwater environment: Efficacy tests performed on the apsidal fishpond of castrumnovum (Rome, Italy). Int. J. Conserv. Sci. 2020, 11, 243–250. [Google Scholar]
- Macchia, A.; Bettucci, O.; Gravagna, E.; Ferro, D.; Albini, R.; Mazzei, B.; Campanella, L. Calcium Hydroxide Nanoparticles and Hypogeum Environment: Test to Understand the Best Way of Application. J. Nanomater. 2014, 2014, 167540. [Google Scholar] [CrossRef]
- Biehler-Gomez, L.; Porta, D.; Mattia, M.; De Angelis, D.; Poppa, P.; Cattaneo, C. ‘Ye must have faith’ how anthropology can contribute to religious heritage: The osteobiography of Italian martyr Saint Nazarius. Int. J. Osteoarchaeol. 2021, 31, 506–512. [Google Scholar] [CrossRef]
- Ministero della Cultura. I Resti Scheletrici Umani: Dallo Scavo, al Laboratorio, al Museo; Istituto Centrale per il Catalogo e la Documentazione/Istituto Centrale per l’Archeologia: Roma, Italy, 2022.
- Swaraldahab, M.A.H.; Christensen, A.M. The Effect of Time on Bone Fluorescence: Implications for Using Alternate Light Sources to Search for Skeletal Remains. J. Forensic Sci. 2016, 61, 442–444. [Google Scholar] [CrossRef]
- Macchia, A.; Biribicchi, C.; Carnazza, P.; Montorsi, S.; Sangiorgi, N.; Demasi, G.; Prestileo, F.; Cerafogli, E.; Colasanti, I.A.; Aureli, H.; et al. Multi-Analytical Investigation of the Oil Painting “Il Venditore di Cerini” by Antonio Mancini and Definition of the Best Green Cleaning Treatment. Sustainability 2022, 14, 3972. [Google Scholar] [CrossRef]
- Macchia, A.; Biribicchi, C.; Rivaroli, L.; Aureli, H.; Cerafogli, E.; Colasanti, I.A.; Carnazza, P.; Demasi, G.; La Russa, M.F. Combined Use of Non-Invasive and Micro-Invasive Analytical Investigations to Understand the State of Conservation and the Causes of Degradation of I Tesori del Mare (1901) by Plinio Nomellini. Methods Protoc. 2022, 5, 52. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Santini, M.; Cristina, M.L. Pseudomonas aeruginosa in the healthcare facility setting. Rev. Med. Microbiol. 2021, 32, 169–175. [Google Scholar] [CrossRef]
- Planet, P.J. Pseudomonas aeruginosa. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2023; pp. 884–889. [Google Scholar]
- Longin, R. New method of collagen extraction for radiocarbon dating. Nature 1971, 230, 241–242. [Google Scholar] [CrossRef] [PubMed]
- DeNiro, M.J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 1985, 317, 806–809. [Google Scholar] [CrossRef]
- Pate, F.D. Bone chemistry and paleodiet. J. Archaeol. Method Theory 1994, 1, 161–209. [Google Scholar] [CrossRef]
- Van Klinken, G.J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 1999, 26, 687–695. [Google Scholar] [CrossRef]
- Ramsey, C.B.; Heaton, T.J.; Schlolaut, G.; Staff, R.A.; Bryant, C.L.; Brauer, A.; Lamb, H.F.; Marshall, M.H.; Nakagawa, T. Reanalysis of the athmospheric radiocarbon calibration record from Lake Suigetsu, Japan. Raiocarbon 2020, 62, 989–999. [Google Scholar] [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.; Ramsey, C.B.; Talamo, S. The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Buikstra, J.E.; Ubelaker, D.H. Standards for data collection from human skeletal remains. Ark. Archaeol. Surv. Res. Ser. 1994, 44. [Google Scholar] [CrossRef]
- Lovejoy, C.O. Dental wear in the Libben population: Its functional pattern and role in the determination of adult skeletal age at death. Am. J. Phys. Anthropol. 1985, 68, 47–56. [Google Scholar] [CrossRef]
- Rivera, F.; Lahr, M.M. New evidence suggesting a dissociated etiology for cribra orbitalia and porotic hyperostosis. Am. J. Phys. Anthropol. 2017, 164, 76–96. [Google Scholar] [CrossRef] [PubMed]
- Bonora, V.; Tucci, G.; Meucci, A.; Pagnini, B. Photogrammetry and 3D Printing for Marble Statues Replicas: Critical Issues and Assessment. Sustainability 2021, 13, 680. [Google Scholar] [CrossRef]
- Alves, E.; Lucas, G.C.; Pozza, E.A.; de Carvalho Alves, M. Scanning Electron Microscopy for Fungal Sample Examination. Lab. Protoc. Fungal Biol. 2013, 133–150. [Google Scholar] [CrossRef]
- Beech, I.; Otlewska, A.; Skòra, J.; Gutarowska, B.; Gaylarde, C. Interactions of fungi with titanium dioxide from paint coating. Indoor Built Environ. 2018, 27, 263–269. [Google Scholar] [CrossRef]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Krivačić, S.; Petrović, Ž.; Mikić, D.; Marciuš, M. Titanium Implant Alloy Modified by Electrochemically Deposited Functional Bioactive Calcium Phosphate Coatings. Coatings 2023, 13, 640. [Google Scholar] [CrossRef]
- Iconaru, S.; Motelica-Heino, M.; Predoi, D. Study on Europium Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Vechietti, F.A.; Marques, D.; Muniz, N.O.; Santos, L.A. Fibers Obtaining and Characterization Using Poly (Lactic-co-Glycolic Acid) and Poly (Isoprene) Containing Hydroxyapatite and α TCP Calcium Phosphate by Electrospinning Method. Key Eng. Mater. 2015, 631, 173–178. [Google Scholar] [CrossRef]
- Dritsa, V.; Pissaridi, K.; Koutoulakis, E.; Mamarelis, I.; Kotoulas, C.; Anastassopoulou, J. An Infrared Spectroscopic Study of Aortic Valve. A Possible Mechanism of Calcification and the Role of Magnesium Salts. In Vivo 2014, 28, 91–98. [Google Scholar]
- Chandrasekaran, A.; Sagadevan, S.; Dakshanamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Kesmez, O. Preparation of Anti-bacterial Biocomposite Nanofibers Fabricated by Electrospinning Method. J. Turk. Chem. Soc. A 2020, 7, 125–142. [Google Scholar] [CrossRef]
- Council of Europe (CoE). Council of Europe Framework Convention on the Value of Cultural Heritage for Society. In Faro Declaration of the Council of Europe’s Strategy for Developing Intercultural Dialogue. 2005. Available online: https://rm.coe.int/1680083746 (accessed on 27 January 2023).














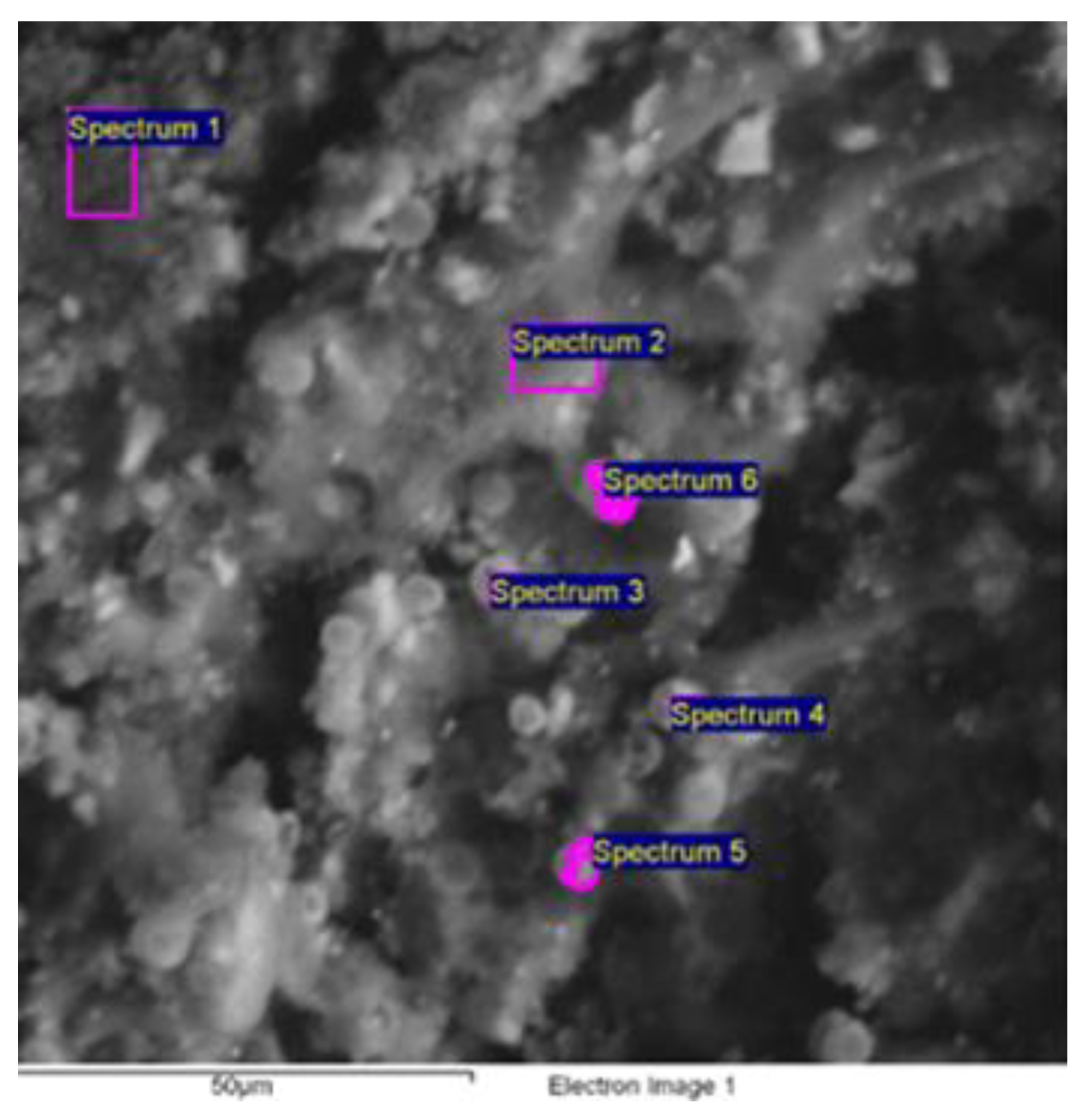











| Spectrum | O * | Na * | Al * | P * | S * | Cl * | Ca * |
|---|---|---|---|---|---|---|---|
| 1 | 53.8 | 1 | --- | 16.7 | 0.4 | 0.3 | 27.9 |
| 2 | 44.5 | --- | 1.6 | 14.3 | 0.4 | 0.5 | 38.7 |
| 3 | 58.7 | --- | --- | 13.7 | --- | --- | 27.6 |
| Spectrum | O * | Na * | Al * | Si * | P * | S * | Cl * | Ca * |
|---|---|---|---|---|---|---|---|---|
| 1 | 46.9 | 0.8 | 0.4 | --- | 17.8 | 0.3 | 0.3 | 33.6 |
| 2 | 40.5 | 0.6 | 0.3 | --- | 17.3 | 0.3 | --- | 39.7 |
| 3 | 40.4 | 0.9 | 0.4 | 0.6 | 18.1 | 1.2 | --- | 38.8 |
| Spectrum | C * | O * | Na * | P * | S * | Cl * | Ca * |
|---|---|---|---|---|---|---|---|
| 1 | --- | 53.4 | 0.8 | 14.5 | 1.3 | 0.3 | 29.7 |
| 2 | --- | 63.7 | --- | 15.8 | 1.4 | --- | 19.1 |
| 3 | 7.5 | 61.1 | --- | 13.7 | 1.4 | --- | 16.4 |
| 4 | 9.1 | 58.2 | --- | 15.1 | 0.3 | --- | 17.4 |
| 5 | 8.7 | 60.9 | --- | 13.4 | 0.4 | --- | 16.1 |
| Spectrum | O * | Mg * | Al * | Si * | P * | S * | Cl * | K * | Ca * | Fe * |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.6 | --- | --- | 0.6 | --- | 0.9 | 0.5 | 82.4 | --- | |
| 2 | 57.3 | --- | 1.3 | 3.4 | 0.7 | 1.2 | 0.6 | 3.8 | 26.5 | 3.2 |
| 3 | 53.6 | --- | 1 | 2.4 | --- | 0.6 | --- | 1.1 | 38.8 | 2.4 |
| 4 | 65.2 | 0.7 | 0.6 | 2.1 | --- | 0.4 | --- | 0.4 | 29.6 | 0.4 |
| 5 | 64.0 | 0.7 | 0.8 | 1.8 | --- | 0.9 | 0.3 | 0.6 | 30.1 | 0.7 |
| 6 | 52.1 | --- | 1 | 2 | 0.4 | 1.7 | --- | 1.1 | 40.4 | 1.3 |
| Spectrum | O * | Mg * | Al * | Si * | S * | Cl * | K * | Ca * | Fe * |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58.3 | 0.5 | 0.6 | 2.1 | 5.5 | 2.0 | 0.6 | 28.8 | 0.4 |
| 2 | 54.3 | 0.9 | 1.0 | 3.4 | 1.3 | 0.7 | 2.8 | 30.7 | 4.7 |
| 3 | 61.6 | 0.7 | 0.5 | 1.6 | 0.4 | --- | 0.5 | 33.8 | 0.8 |
| 4 | 39.8 | --- | 0.8 | 2.3 | --- | --- | 1.8 | 46.6 | 8.4 |
| Sample | Lab. Code | Trc (Years BP) | Calendarial Age AD (2σ) | δ13C vs. PDB (per Mil) | δ15N vs. AIR (per Mil) |
|---|---|---|---|---|---|
| Bone tissue (Skull with ears) | DSH11416-icon25 | 120 ± 22 | Modern | −20.7 | 8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macchia, A.; Montorsi, S.; Salatino, G.; Albini, R.; Cerilli, E.; Biribicchi, C.; Faella, M.; Rogliani, A.; de Caro, T.; Lubritto, C.; et al. Preserving Intangible Heritage through Tangible Finds: The “Skull with Ears”—St. Luciella ai Librai’s Church (Naples, Italy). Heritage 2023, 6, 3541-3566. https://doi.org/10.3390/heritage6040188
Macchia A, Montorsi S, Salatino G, Albini R, Cerilli E, Biribicchi C, Faella M, Rogliani A, de Caro T, Lubritto C, et al. Preserving Intangible Heritage through Tangible Finds: The “Skull with Ears”—St. Luciella ai Librai’s Church (Naples, Italy). Heritage. 2023; 6(4):3541-3566. https://doi.org/10.3390/heritage6040188
Chicago/Turabian StyleMacchia, Andrea, Stefania Montorsi, Giorgia Salatino, Romana Albini, Eugenio Cerilli, Chiara Biribicchi, Massimo Faella, Angela Rogliani, Tilde de Caro, Carmine Lubritto, and et al. 2023. "Preserving Intangible Heritage through Tangible Finds: The “Skull with Ears”—St. Luciella ai Librai’s Church (Naples, Italy)" Heritage 6, no. 4: 3541-3566. https://doi.org/10.3390/heritage6040188
APA StyleMacchia, A., Montorsi, S., Salatino, G., Albini, R., Cerilli, E., Biribicchi, C., Faella, M., Rogliani, A., de Caro, T., Lubritto, C., Vetromile, C., Di Cicco, M. R., Ambrosini, A., & Sperduti, A. (2023). Preserving Intangible Heritage through Tangible Finds: The “Skull with Ears”—St. Luciella ai Librai’s Church (Naples, Italy). Heritage, 6(4), 3541-3566. https://doi.org/10.3390/heritage6040188











