Investigation towards Laser Cleaning of Corrosion Products from Lead Objects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods and Laser Systems
3. Results
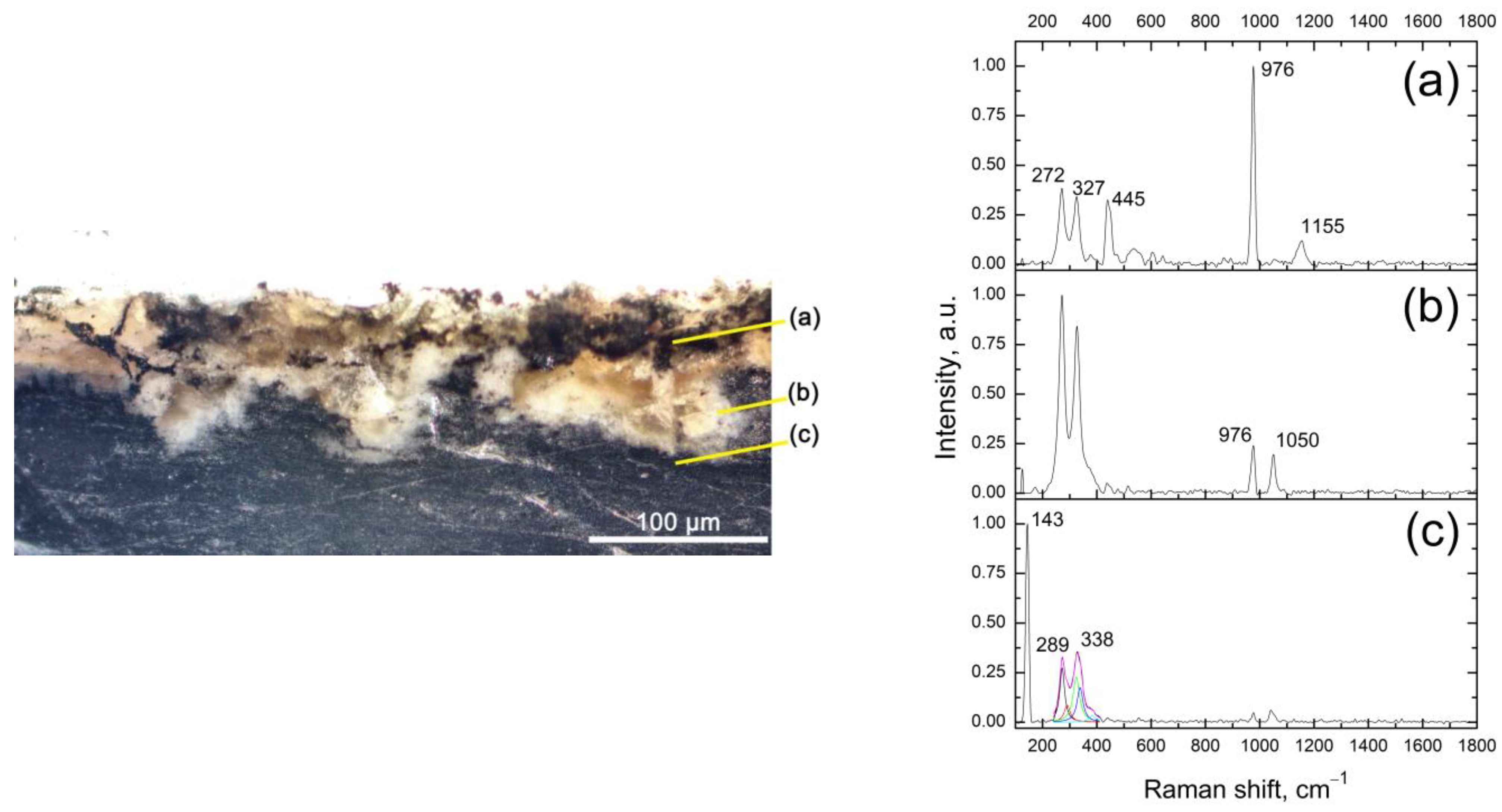
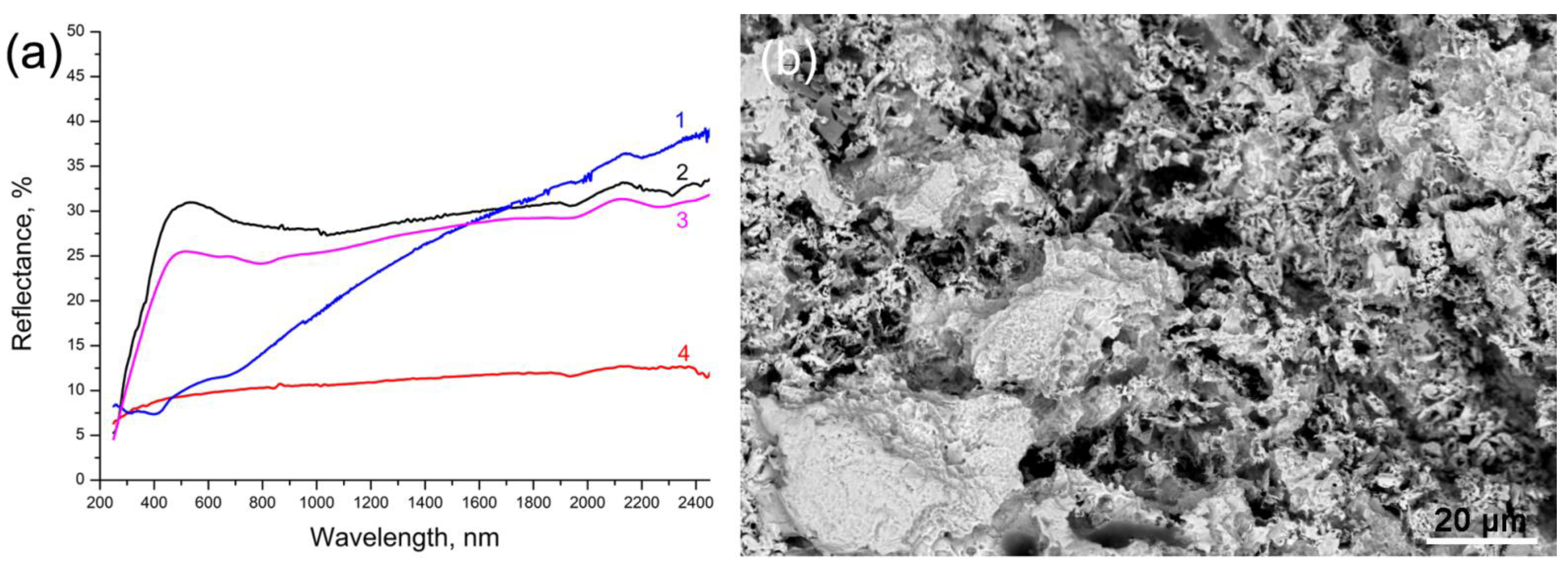
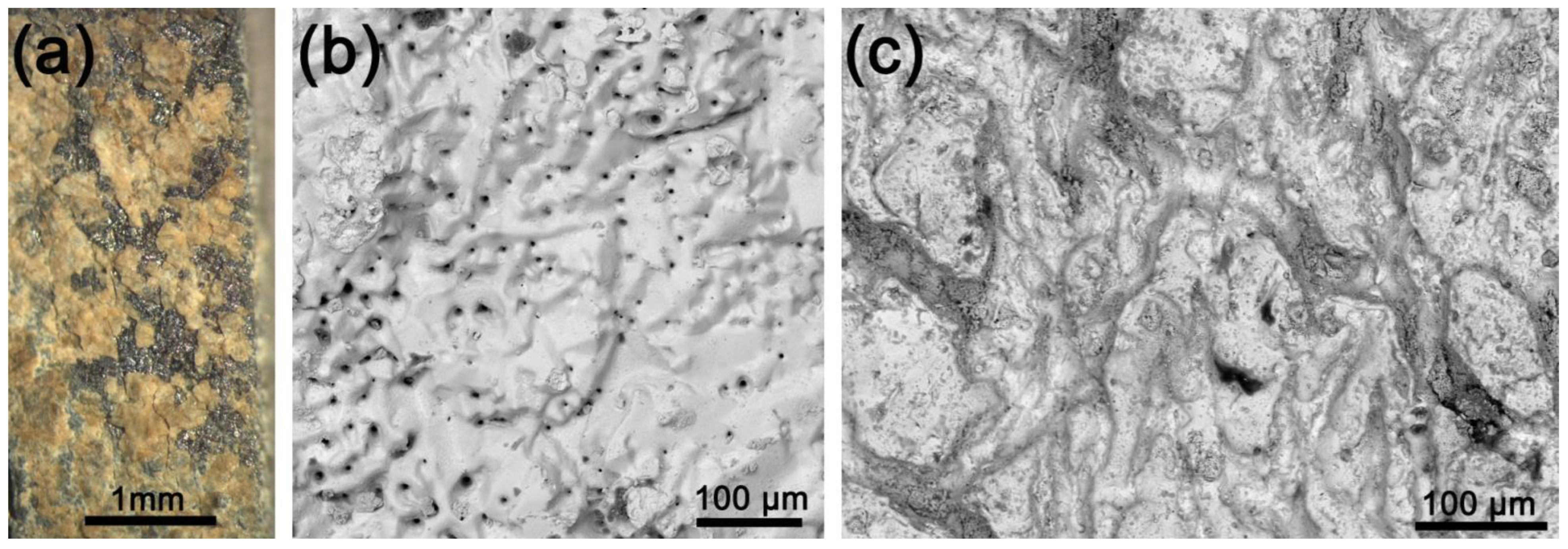
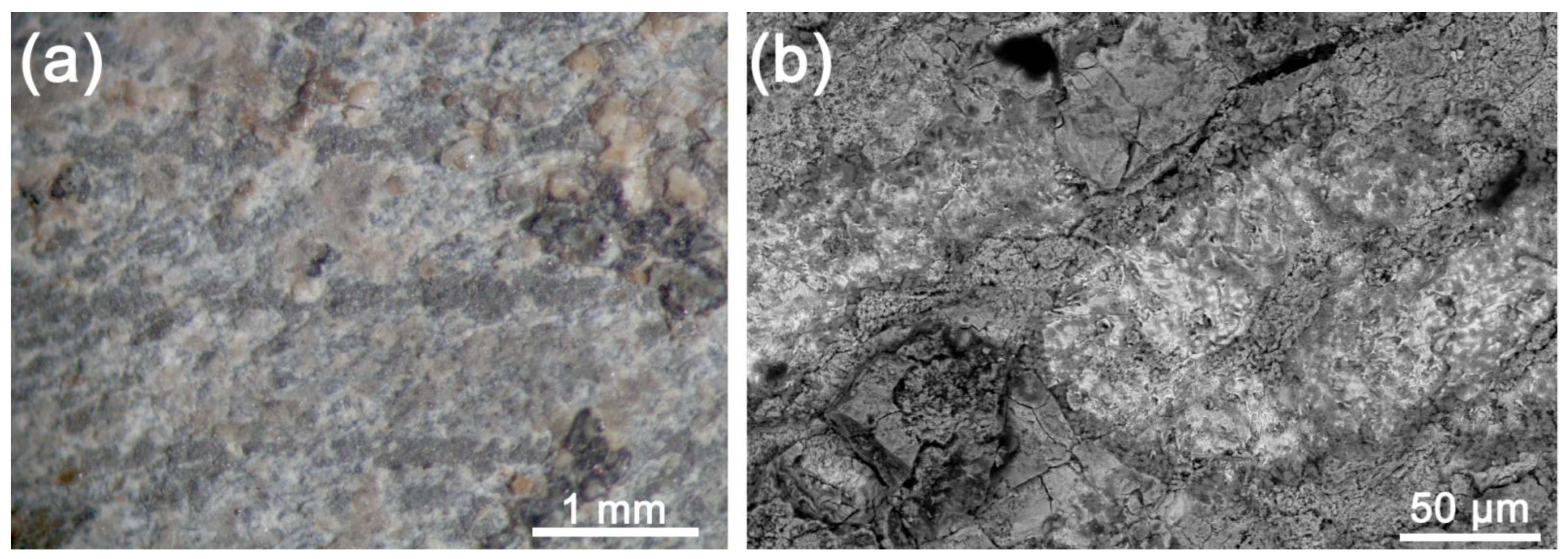
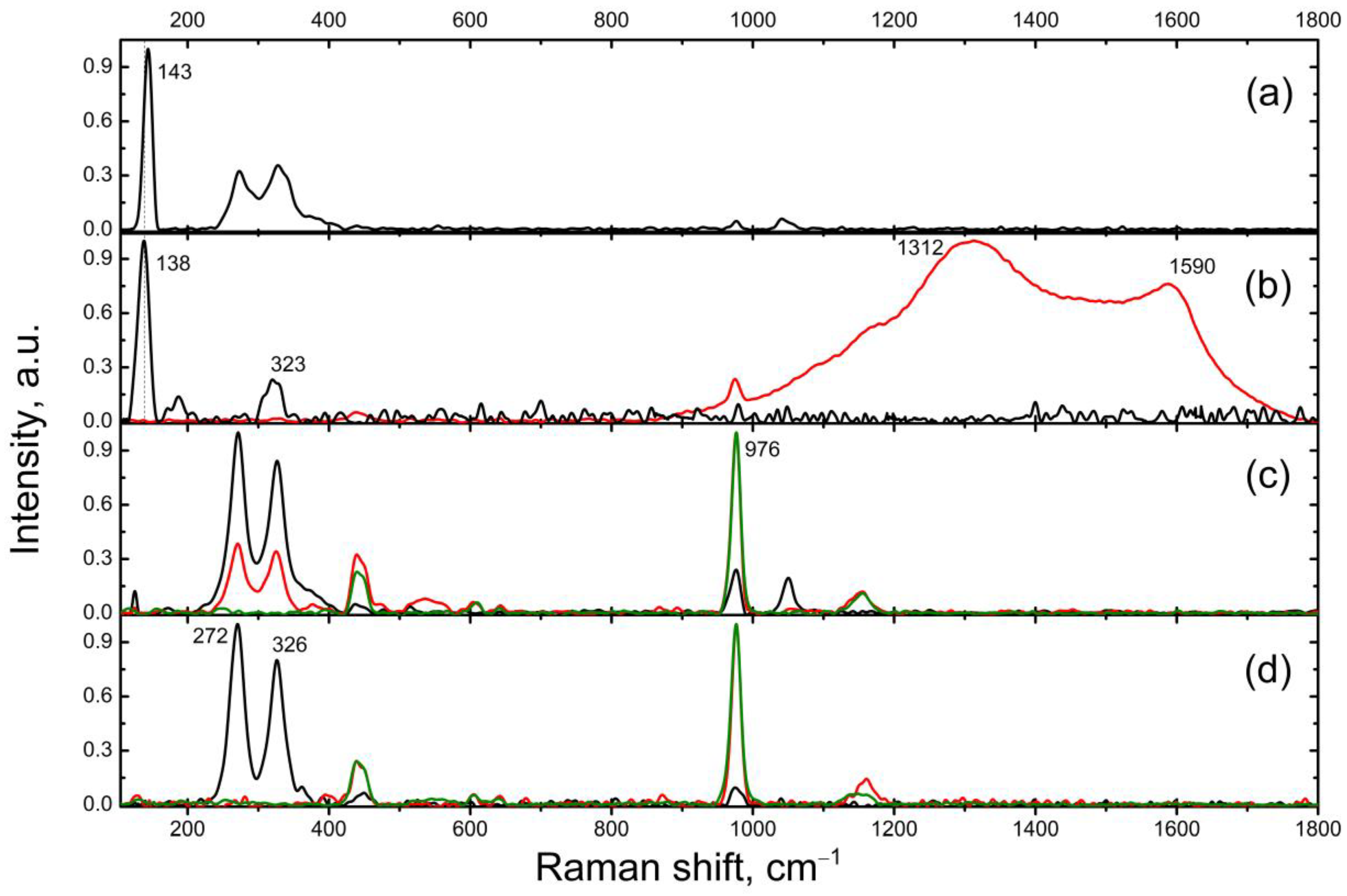
3.1. Samples Characterization
3.2. Laser Cleaning Experiments
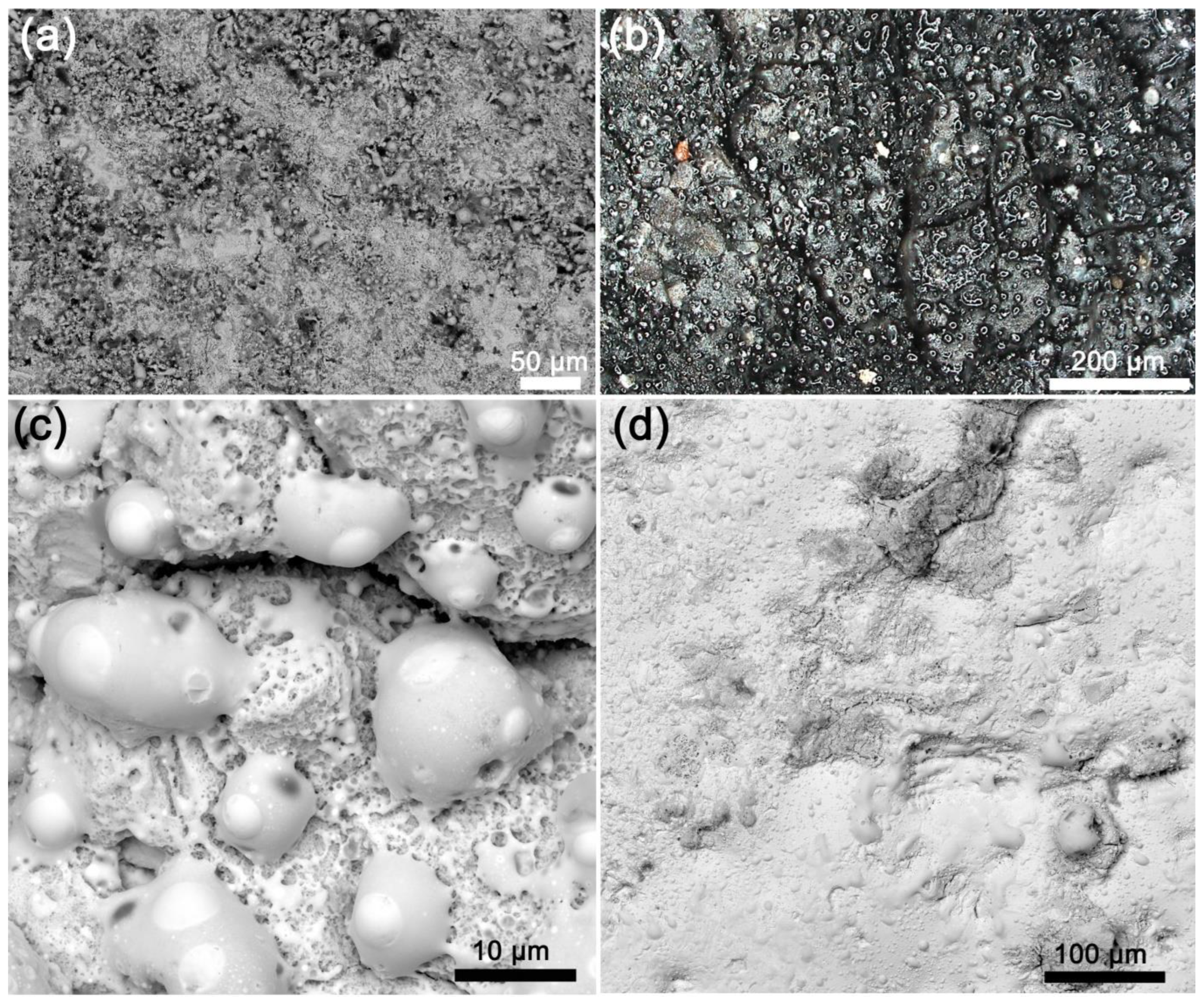
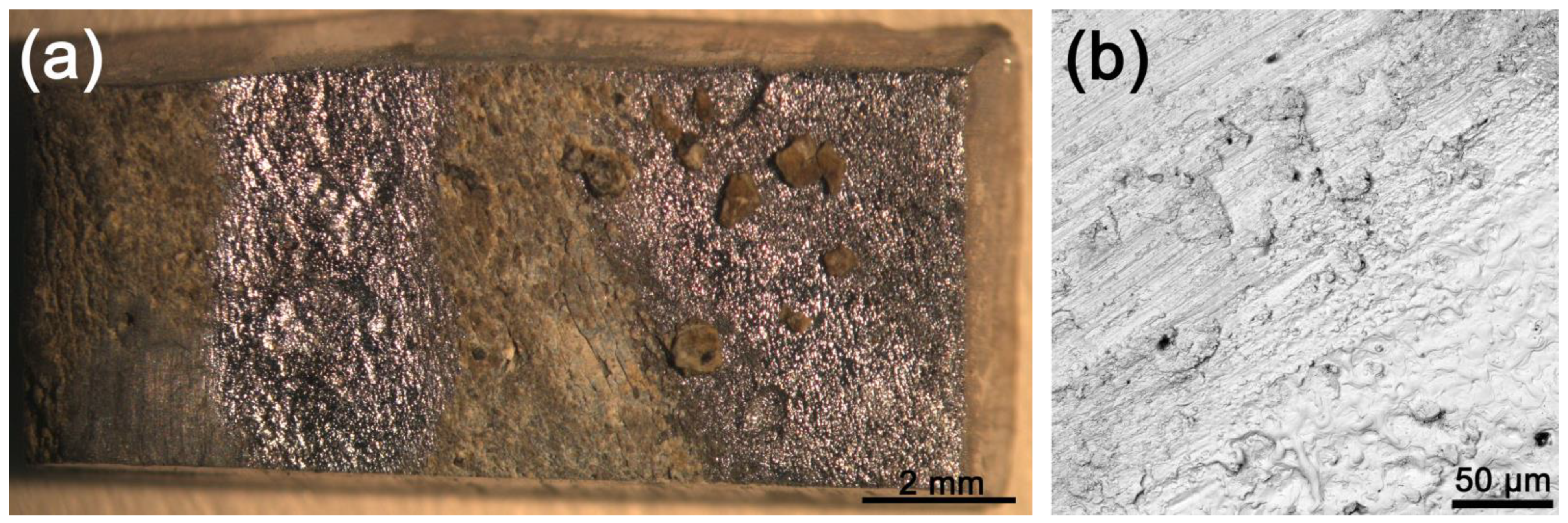
3.2.1. Microsecond Pulses
3.2.2. 100-Nanosecond Pulses
3.2.3. 6–8-Nanosecond Pulses
3.2.4. Picosecond Pulses
532 nm, 63 ps
1064 nm, 76 ps
3.2.5. Femtosecond pulses
4. Discussion
- The spectral reflection coefficients at 1064 nm for metal lead and corrosion layers are almost the same, and approximately 30% (Figure 4). This means that they absorb laser radiation equally well;
- Some part of the incident radiation is effectively scattered and reflected at the surfaces of corrosion layer grains; this leads to a decrease in the absorbed energy;
- Lead has a low melting point (327.5 °C). As the bonding energy of the corrosion layers is quite high, the laser energy used is not sufficient to remove the corrosion layers but generates enough heat to melt the metal;
- The heat capacity of the irradiated materials is quite different. The heat capacity of lead is quite low—0.13 kJ/(kg·K), and a relatively small amount of heat is required to melt it. For comparison, the heat capacities of cerussite and anglesite are (330 J/(kg·K)) and (344 J/(kg·K)), respectively [28];
- The thermal conductivity of lead is relatively low. During the laser heating, the near-surface layer gets overheated and, as the heat does not have time to transfer to the inner layers, this results in metal melting. The thermal conductivity of the corrosion layer is even lower, but due to the optical properties, absorption occurs mainly in the metal.
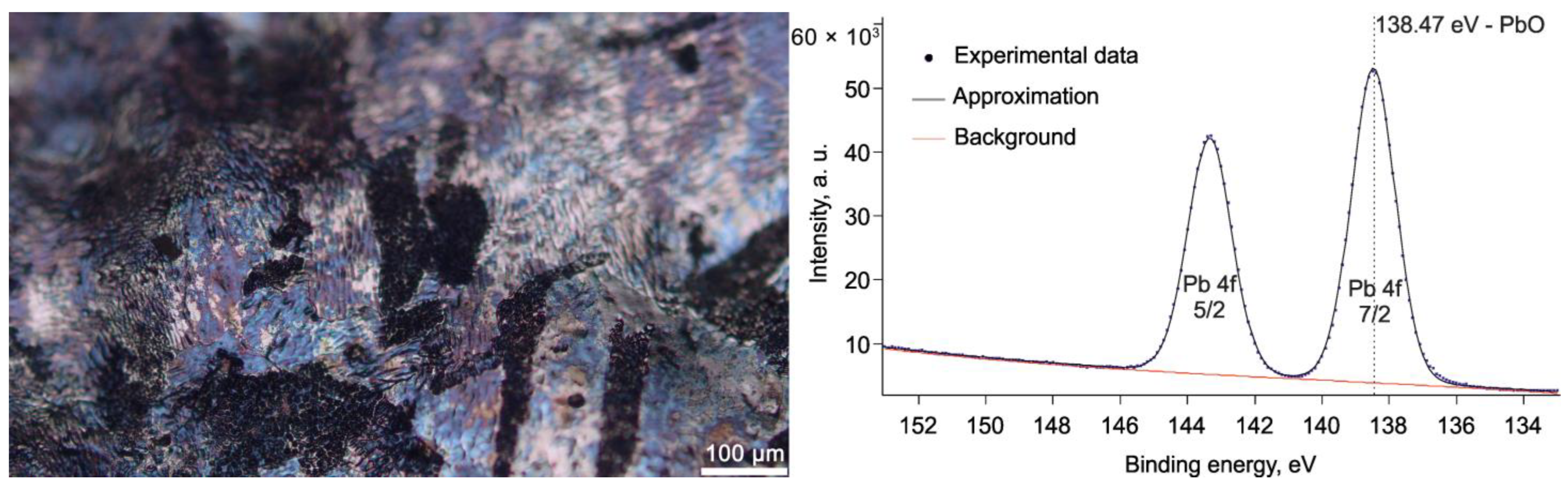
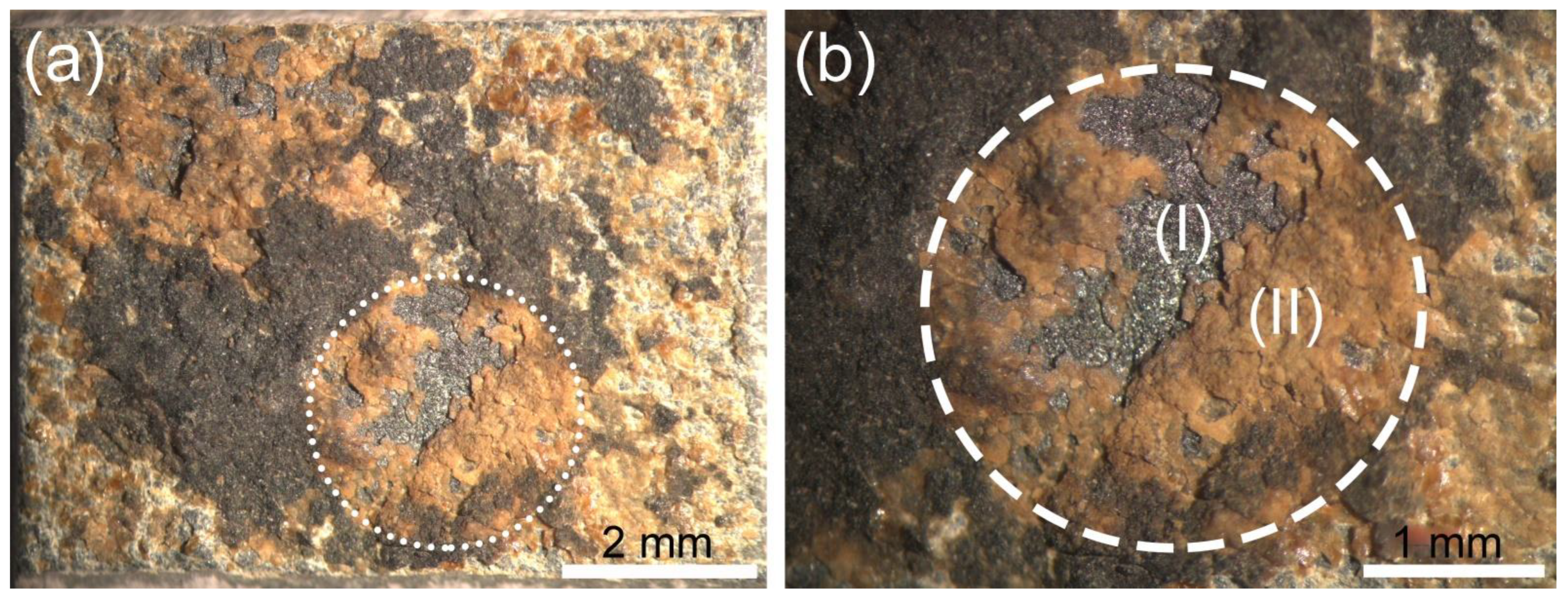
4.1. Surface’s Discoloration
4.1.1. Blue Discoloration
4.1.2. Yellow Discoloration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Keersmaecker, M.; Dowsett, M.; Adriaens, M. How to Preserve Lead Artifacts for Future Generations. In Chemical Interactions between Cultural Artefacts and Indoor Environment; Adriaens, M., Bioletti, S., Rabin, I., Eds.; ACCO: Leuven, Belgium; The Hague, The Netherlands, 2018; pp. 215–244. [Google Scholar]
- Graedel, T.E. Chemical mechanisms for the atmospheric corrosion of lead. J. Electrochem. Soc. 1994, 141, 922–927. [Google Scholar] [CrossRef]
- Costa, V.; Urban, F. Lead and its alloys: Metallurgy, deterioration and conservation. Stud. Conserv. 2005, 50 (Suppl. 1), 48–62. [Google Scholar] [CrossRef]
- Schotte, B.; Adriens, A. The treatment of corroded lead artefacts: An Overview. Stud. Conserv. 2006, 51, 297–304. [Google Scholar] [CrossRef]
- Tranter, G.C. Patination of Lead: An Infra-Red Spectroscopic Study. Br. Corros. J. 1976, 11, 222–224. [Google Scholar] [CrossRef]
- Shemakhanskaya, M.S. Metals and Things; Indrik: Moscow, Russia, 2016; pp. 133–134. (In Russian) [Google Scholar]
- Allen, G.C.; Black, L. Role of organic acids in lead patination. Br. Corros. J. 2000, 35, 39–42. [Google Scholar] [CrossRef]
- Heath, D.; Martin, G. The corrosion of lead and lead/tin alloys occurring on Japanese lacquer objects. Stud. Conserv. 1988, 33 (Suppl. 1), 137–141. [Google Scholar] [CrossRef]
- Niklasson, A.; Johansson, L.-G.; Svensson, J.-E. Atmospheric corrosion of historical organ pipes: Influence of acetic and formic acid vapour and water leaching on lead. In Proceedings of the International Conference on Metals Conservation, Canberra, Australia, 4–8 October 2004; pp. 273–280. [Google Scholar]
- Justo-Estebaranz, A.; Herrera, L.K.; Sigüenza, B.; de Haro, M.C.J.; Justo, A.; Laguna, O. Study of the corrosion products of the lead blocks from the historical organ Jean Pierre Cavaillé of Vinça, France. In Science and Technology for the Conservation of Cultural Heritage, 1st ed.; Rogerio-Candelera, M.A., Lazzari, M., Cano, E., Eds.; CRC Press: London, UK, 2013; pp. 131–134. [Google Scholar]
- Palache, C.; Berman, H.; Frondel, C. The System of Mineralogy of J.D. Dana and E.S. Dana, 7th ed.; Wiley: New York, NY, USA, 1951; p. 322. (In Russian) [Google Scholar]
- Naylor, A. Conservation of the eighteenth century lead statue of George II and the role of laser cleaning. J. Cult. Herit. 2000, 1, 145–149. [Google Scholar] [CrossRef]
- Dickens, J.; Smith, N.; Gerritsen, W. On again, off again: Cathodic protection of a lead and ceramic water closet during desalination. In Proceedings of the International Conference on Metals Conservation, Canberra, Australia, 4–8 October 2004; pp. 465–483. [Google Scholar]
- Asmus, J.F.; Murphy, C.G.; Munk, W.H. Studies on the interaction of laser radiation with art artifacts. In Proceedings of the Developments in Laser Technology II of the Annual Technical Symposium, San Diego, CA, USA, 27–29 August 1974. [Google Scholar] [CrossRef]
- Pini, R.; Siano, S.; Salimbeni, R.; Pasquinucci, M.; Miccio, M. Tests of Laser Cleaning on Archeological Metal Artefacts. J. Cult. Herit. 2000, 1, 129–137. [Google Scholar] [CrossRef]
- Bertholon, R. The location of the original surface, a review of the conservation literature. In Proceedings of the ICOM-CC Metals Working Group, Santiago, Chile, 2–6 April 2001; pp. 167–179. [Google Scholar]
- Korenberg, C.; Baldwin, A. Laser cleaning tests on archaeological copper alloys using an Nd:YAG laser. Laser Chem. 2006, 2006, 075831. [Google Scholar] [CrossRef]
- Buzgar, N.; Buzatu, A.; Sanislav, I.V. The Raman study on certain sulfates. Geology 2009, LV 1, 5–23. [Google Scholar]
- Miyake, M.; Minato, I.; Morikawa, H.; Iwai, S.-I. Crystal structures and sulphate force constants of barite, celestite, and anglesite. Am. Min. 1978, 63, 506–510. [Google Scholar]
- Lee, P.; Huang, E.; Yu, S.; Chen, Y. High-Pressure Raman Study on Anglesite. World J. Condens. Matter Phys. 2013, 3, 28–32. [Google Scholar] [CrossRef]
- Sawchuk, K.; O’Bannon, E.F., III; Vennari, C.; Kavner, A.; Knittle, E.; Williams, Q. An infrared and Raman spectroscopic study of PbSO4-anglesite at high pressures. Phys. Chem. Miner. 2019, 46, 623–637. [Google Scholar] [CrossRef]
- Lutz, H.D.; Beckenkamp, K.; Peter, S. Laurionite-type M(OH)X (M=Ba, Pb; X=Cl, Br, I) and Sr(OH)I. An IR and Raman spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 755–767. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Firtha, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst 2001, 126, 222–227. [Google Scholar] [CrossRef] [PubMed]
- McConachy, T.F.; Yang, K.; Boni, M.; Evans, N.J. Spectral reflectance: Preliminary data on a new technique with potential for non-sulphide base metal exploration. Geochem. Explor. Environ. Anal. 2007, 7, 139–151. [Google Scholar] [CrossRef]
- Black, L.; Allen, G.C. Nature of lead patination. Br. Corros. J. 1999, 34, 192–197. [Google Scholar] [CrossRef]
- Bertasa, M.; Korenberg, C. Successes and challenges in laser cleaning metal artefacts: A review. J. Cult. Herit. 2022, 53, 100–117. [Google Scholar] [CrossRef]
- Rode, A.V.; Baldwin, K.G.H.; Wain, A.; Delaporte, P.H. Ultrafast lasers for conservation of heritage artefacts. AICCM Bull. 2006, 30, 17–26. [Google Scholar] [CrossRef]
- Waples, D.W.; Waples, J.S. A Review and Evaluation of Specific Heat Capacities of Rocks, Minerals, and Subsurface Fluids. Part 1: Minerals and Nonporous Rock. Nat. Resour. Res. 2004, 13, 97–122. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific, Analytical Equipment and Instruments. Available online: https://www.thermofisher.com/ru/ru/home/materials-science/learning-center/periodic-table/other-metal/lead.html (accessed on 30 December 2022).


| Laser System | Wavelength, nm | Pulse Duration |
|---|---|---|
| EOS Combo (El. En., Firenze, Italy) | 1064 | 30–110 μs (FR) |
| 100 ns (LQS) | ||
| SpitLight 2000 (Innolas, Krailling, Germany) | 1064 | 6–8 ns |
| Diode-pumped Nd:YAG laser (Lasers and Optical Systems Co., Ltd., Saint Petersburg, Russia) | 1064 | 76 ps |
| 532 | 63 ps | |
| Ti:Sa laser (Avesta Ltd., Moscow, Russia) | 800 | 100 fs |
| Element/№ | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| O | 7.17 | 7.08 | 7.34 | 17.26 | 17.74 |
| S | n.d.* | n.d. | 0.20 | 7.71 | 8.04 |
| Cl | n.d. | 1.18 | 1.32 | 0.92 | 0.34 |
| Pb | 92.83 | 91.74 | 91.13 | 74.1 | 73.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokuratov, D.; Samokhvalov, A.; Pankin, D.; Vereshchagin, O.; Kurganov, N.; Povolotckaia, A.; Shimko, A.; Mikhailova, A.; Balmashnov, R.; Reveguk, A.; et al. Investigation towards Laser Cleaning of Corrosion Products from Lead Objects. Heritage 2023, 6, 1293-1307. https://doi.org/10.3390/heritage6020071
Prokuratov D, Samokhvalov A, Pankin D, Vereshchagin O, Kurganov N, Povolotckaia A, Shimko A, Mikhailova A, Balmashnov R, Reveguk A, et al. Investigation towards Laser Cleaning of Corrosion Products from Lead Objects. Heritage. 2023; 6(2):1293-1307. https://doi.org/10.3390/heritage6020071
Chicago/Turabian StyleProkuratov, Denis, Andrey Samokhvalov, Dmitry Pankin, Oleg Vereshchagin, Nikolai Kurganov, Anastasia Povolotckaia, Alexander Shimko, Alexandra Mikhailova, Roman Balmashnov, Anastasia Reveguk, and et al. 2023. "Investigation towards Laser Cleaning of Corrosion Products from Lead Objects" Heritage 6, no. 2: 1293-1307. https://doi.org/10.3390/heritage6020071
APA StyleProkuratov, D., Samokhvalov, A., Pankin, D., Vereshchagin, O., Kurganov, N., Povolotckaia, A., Shimko, A., Mikhailova, A., Balmashnov, R., Reveguk, A., Smolyanskaya, O., Redka, D., & Bobrovs, V. (2023). Investigation towards Laser Cleaning of Corrosion Products from Lead Objects. Heritage, 6(2), 1293-1307. https://doi.org/10.3390/heritage6020071






