Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanolime Synthesis
2.2. Fresco Replicas Preparation
2.3. Consolidants Application
2.4. Instrumentation
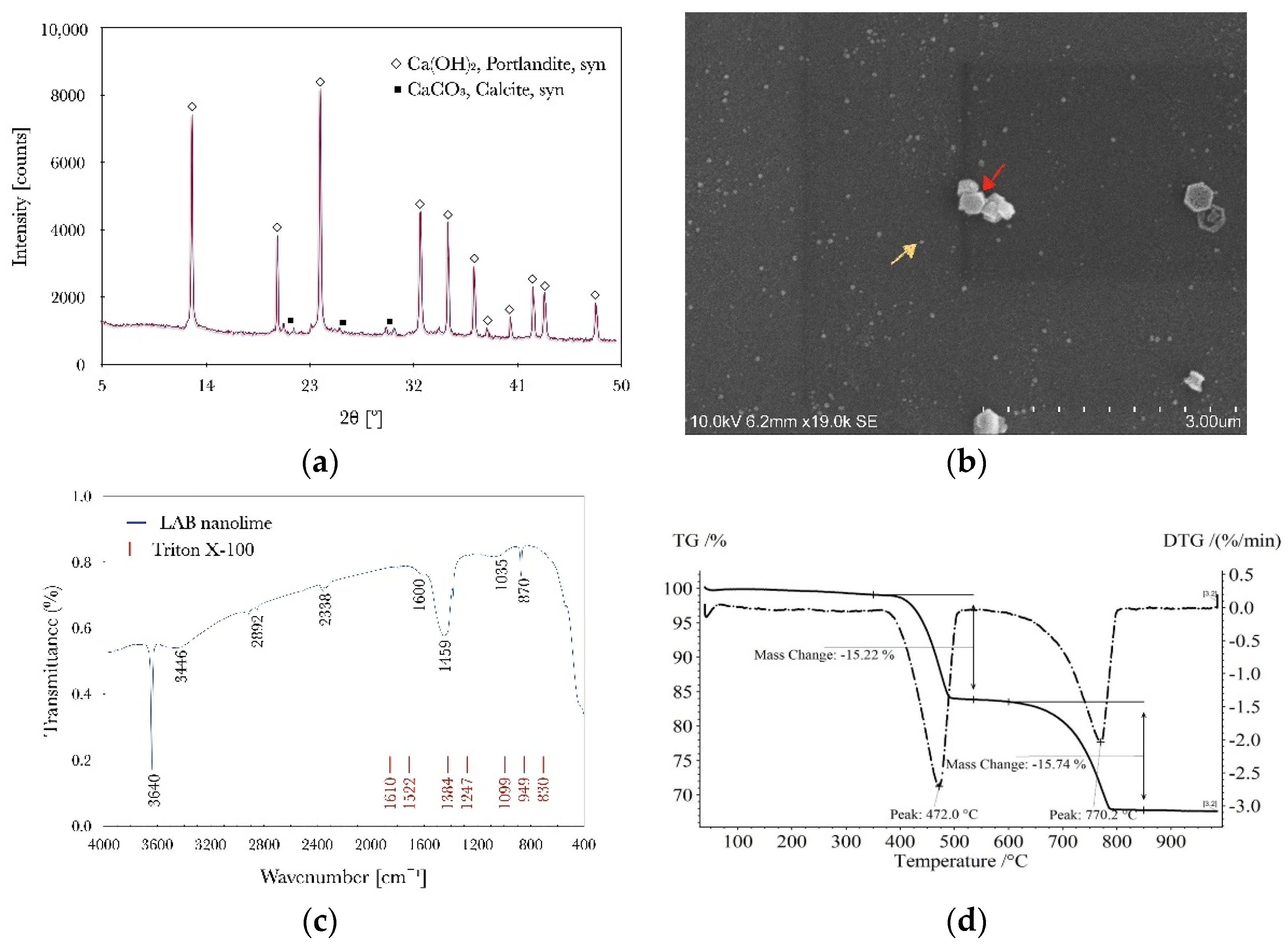
2.4.1. Nanolime Characterization
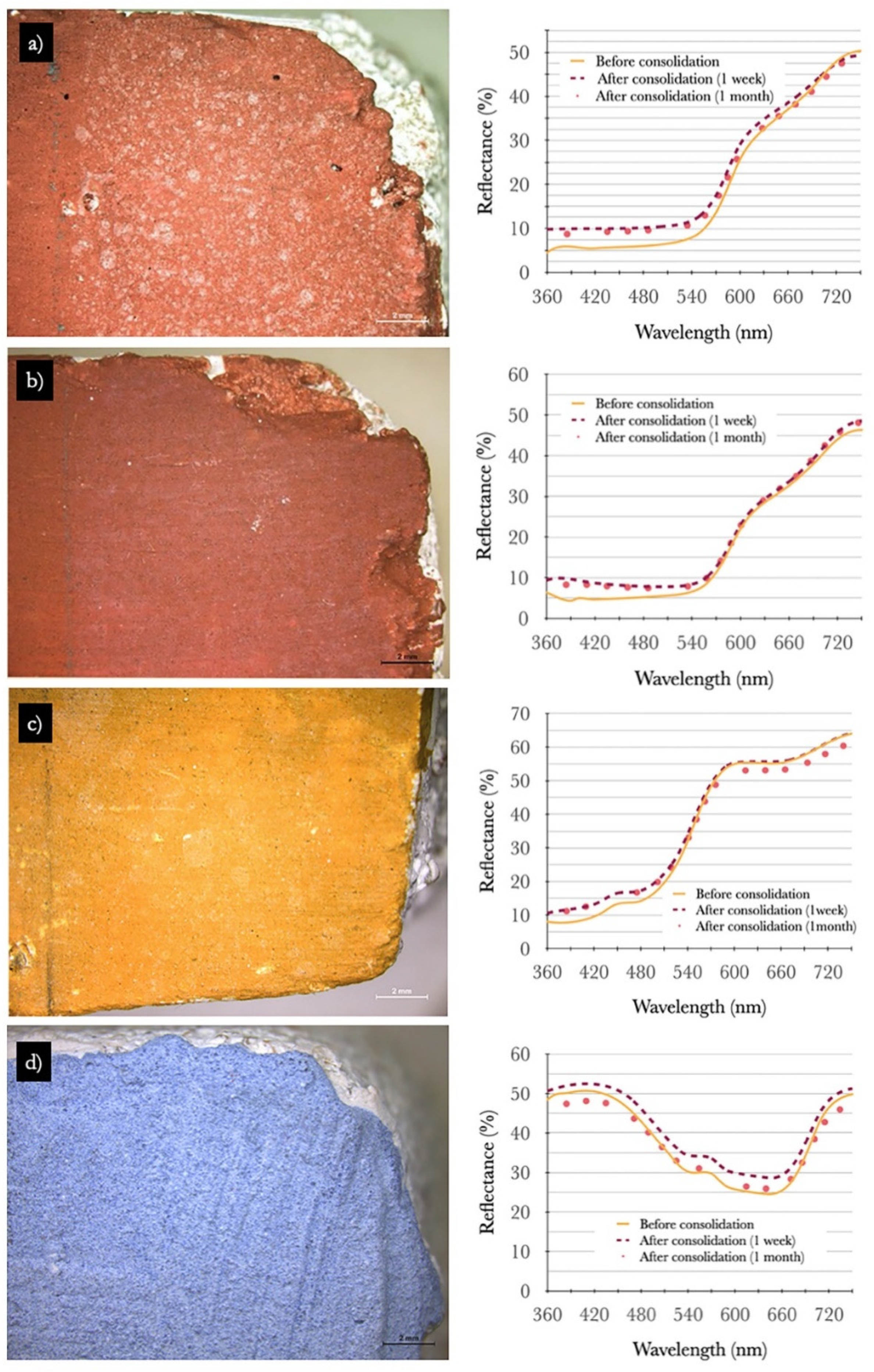
2.4.2. Treatment Evaluation
- (a)
- Technical photography with visible (Vis) and ultraviolet-induced visible fluorescence (UVF) captured with a Canon EOS 800D 24 Mpx. Vis was shot in daylight; for UVF, a XeLED-Ni3UV-R4-365 (120-250VAC) input was used;
- (b)
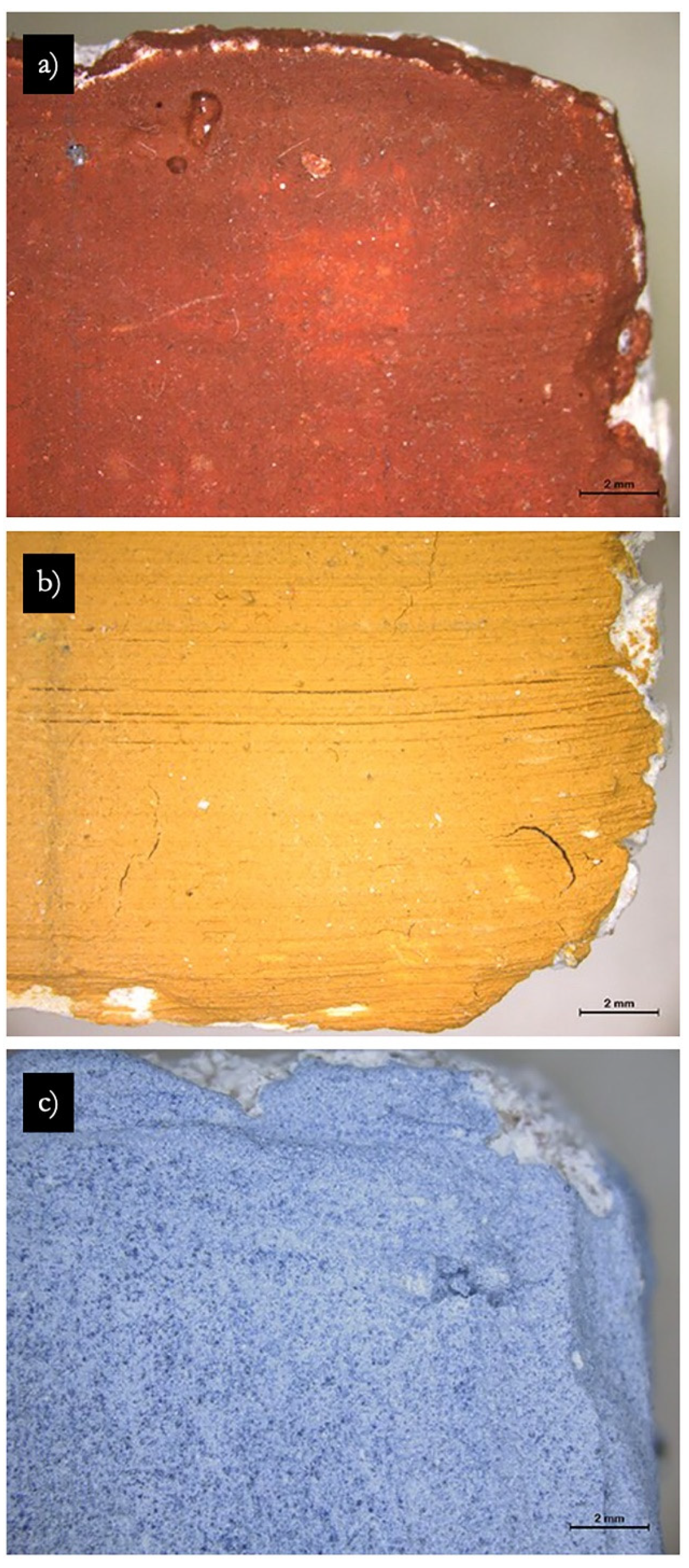
- Optical microscopy (OM-Vis) performed with a stereozoom microscope Leica M205C at 7.8× magnification coupled with a Nikon DS-Fil digital camera;
- (c)
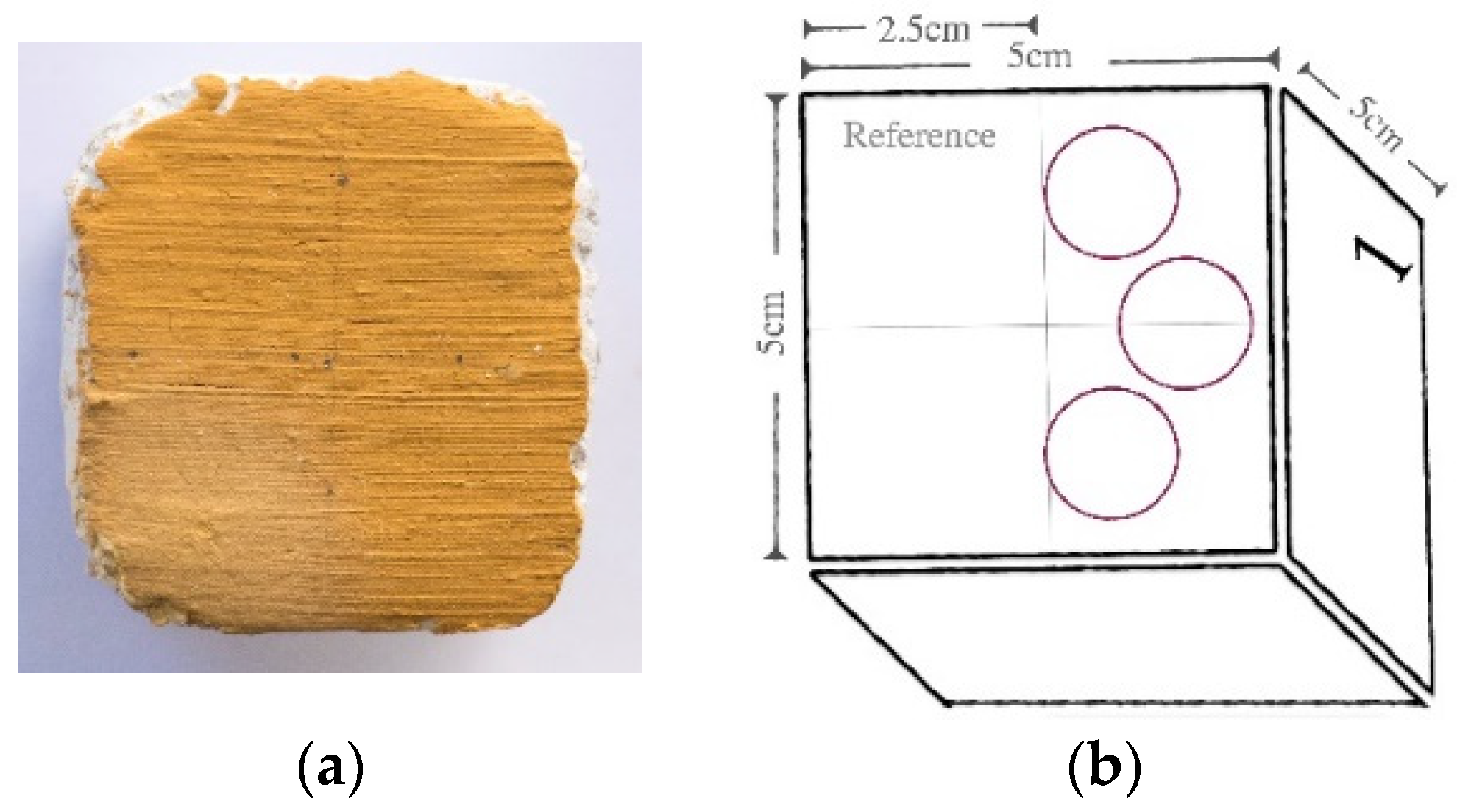
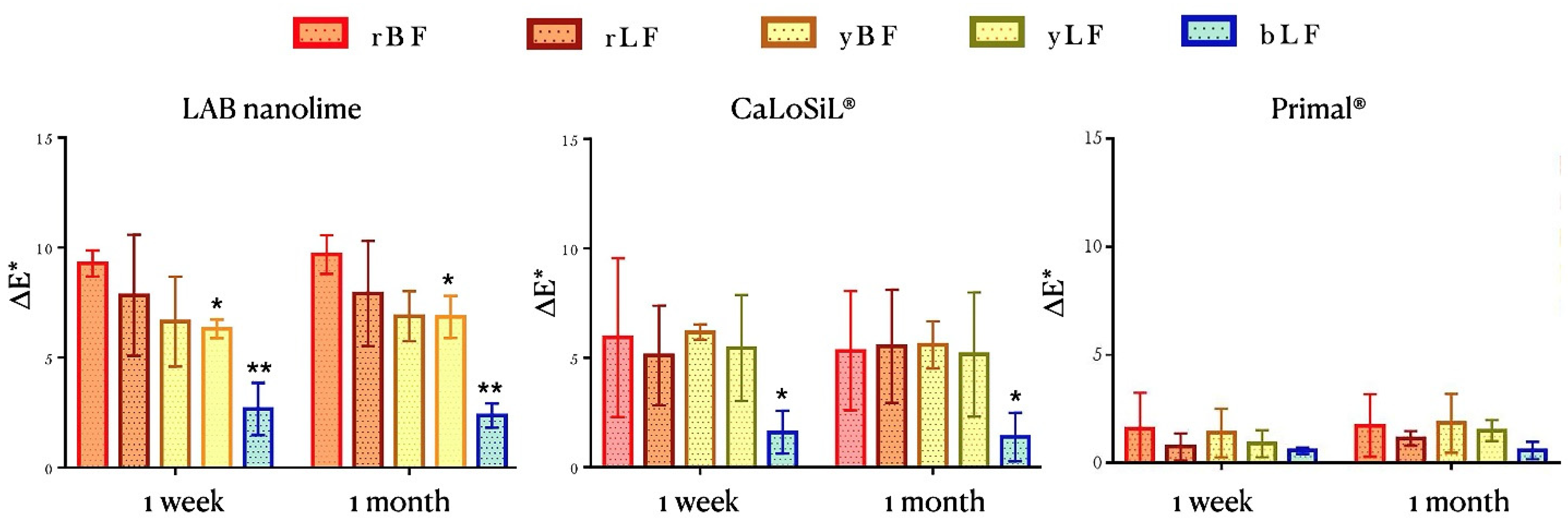
- Colorimetry and spectrophotometry measured with a Data Color Check Plus II, in SCE and Standard Illuminant/Observer D65/10°, and aperture size USAV (⌀ 25 mm). Three replicas of the same pigment/paint technique were measured for each product before, one week and one month after consolidation. The results obtained in CIEL*a*b* chromatic space are the average of three measurements (three light flashes) taken on each of the replicas paint layers in an area of 2.5 cm in diameter (Figure 1). Total color variations (∆E*) and absolute color difference (ΔH) were calculated according to Equations (2) and (3), respectively [36].∆L*, Δa*, and Δb* correspond to variations in the color values in the dark–light, red–green, and yellow–blue axes.where chroma difference ∆C* values were taken by the colorimetry software.
- (d)
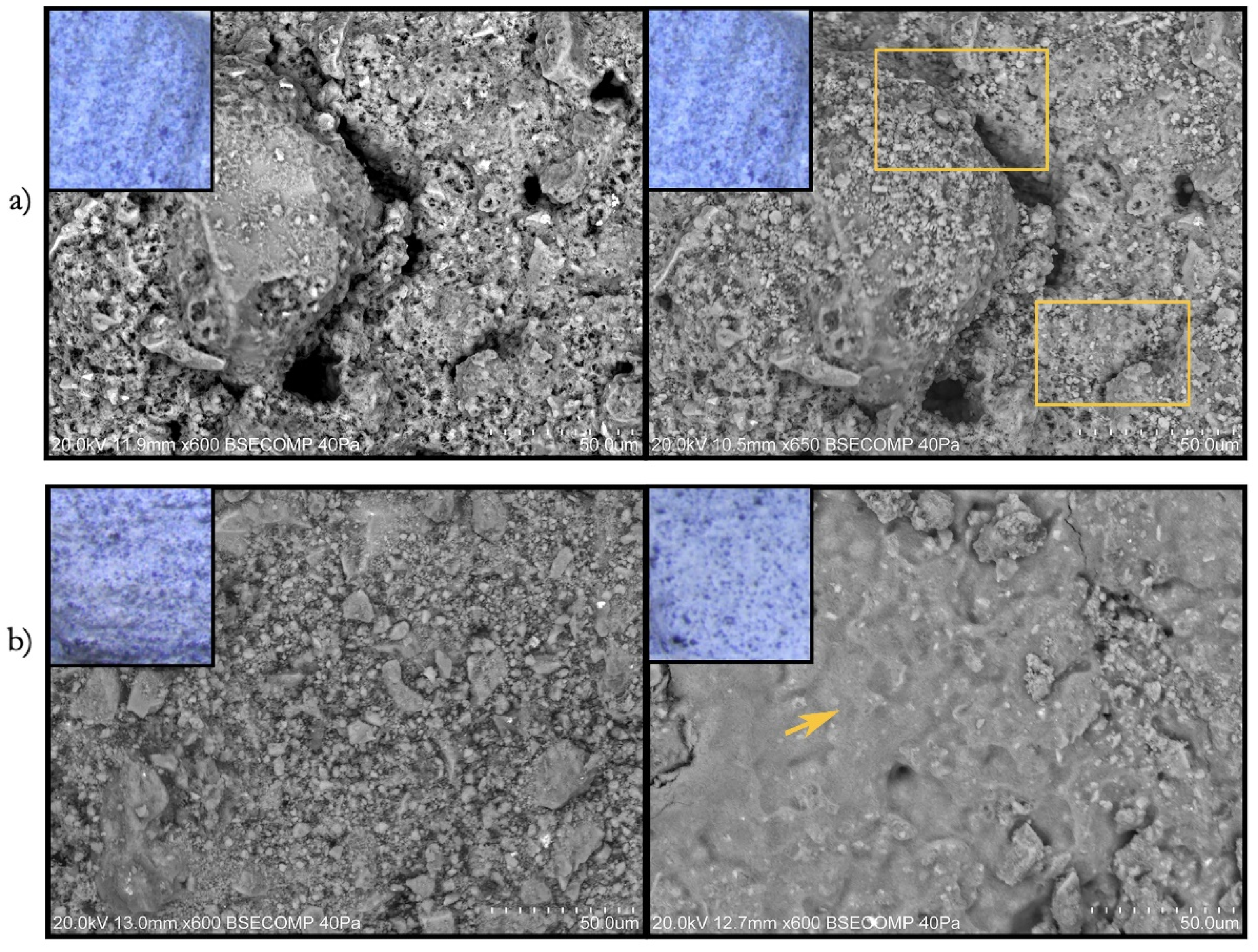
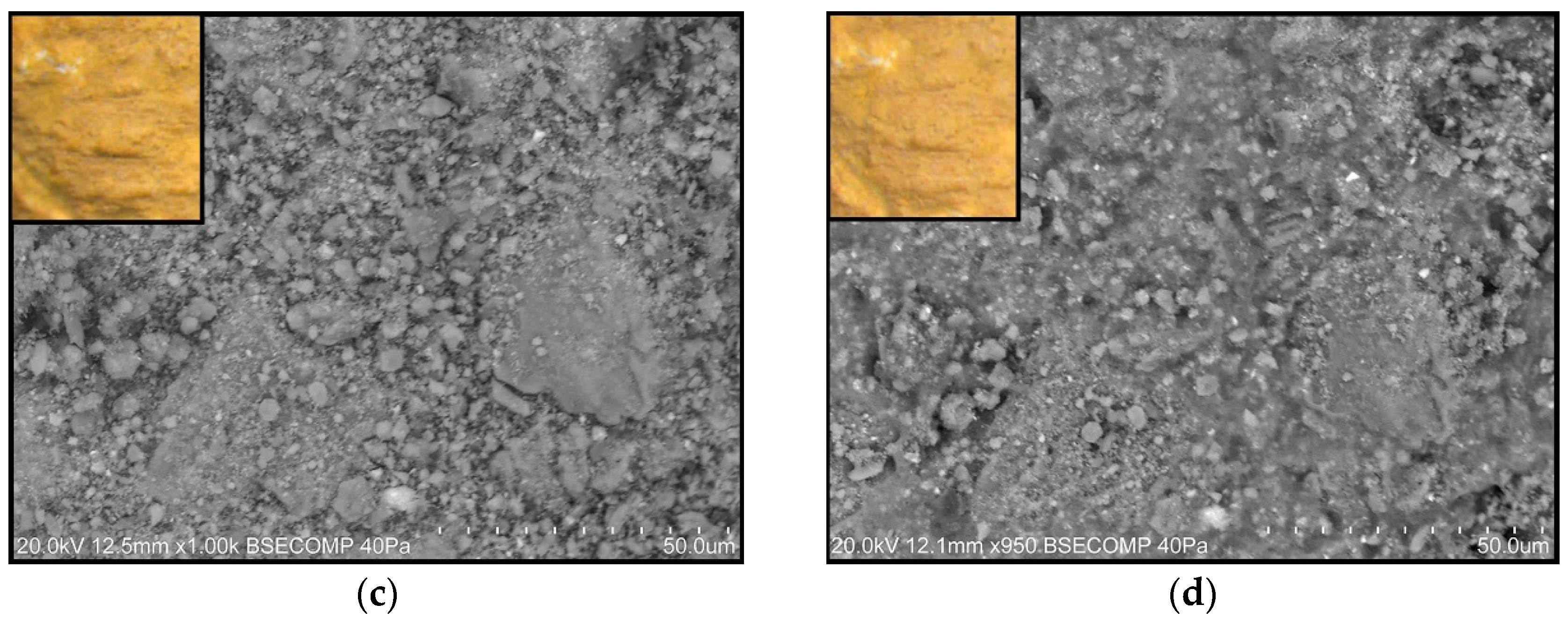
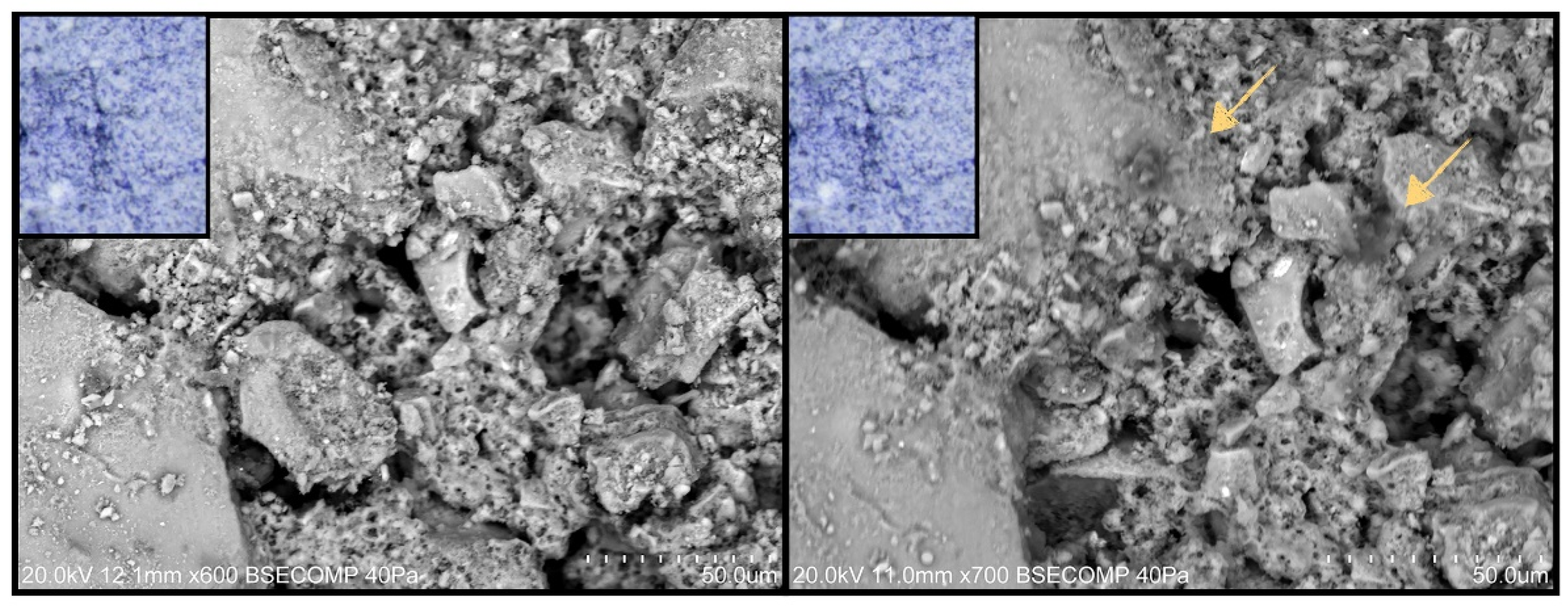
- Painted surfaces were analyzed before and after treatment (one week and one month) by SEM-EDS. SEM imaging was completed in backscattered electron (BSE) mode. EDS analysis and quantification were acquired with Esprit 1.9 software from Brüker corporation.
3. Results and Discussion
3.1. Characterization of the Laboratory-Synthesized Nanolime (LAB Nanolime)
3.2. Evaluation of Treatment
3.2.1. Optical Microscopy and Colorimetry Analysis
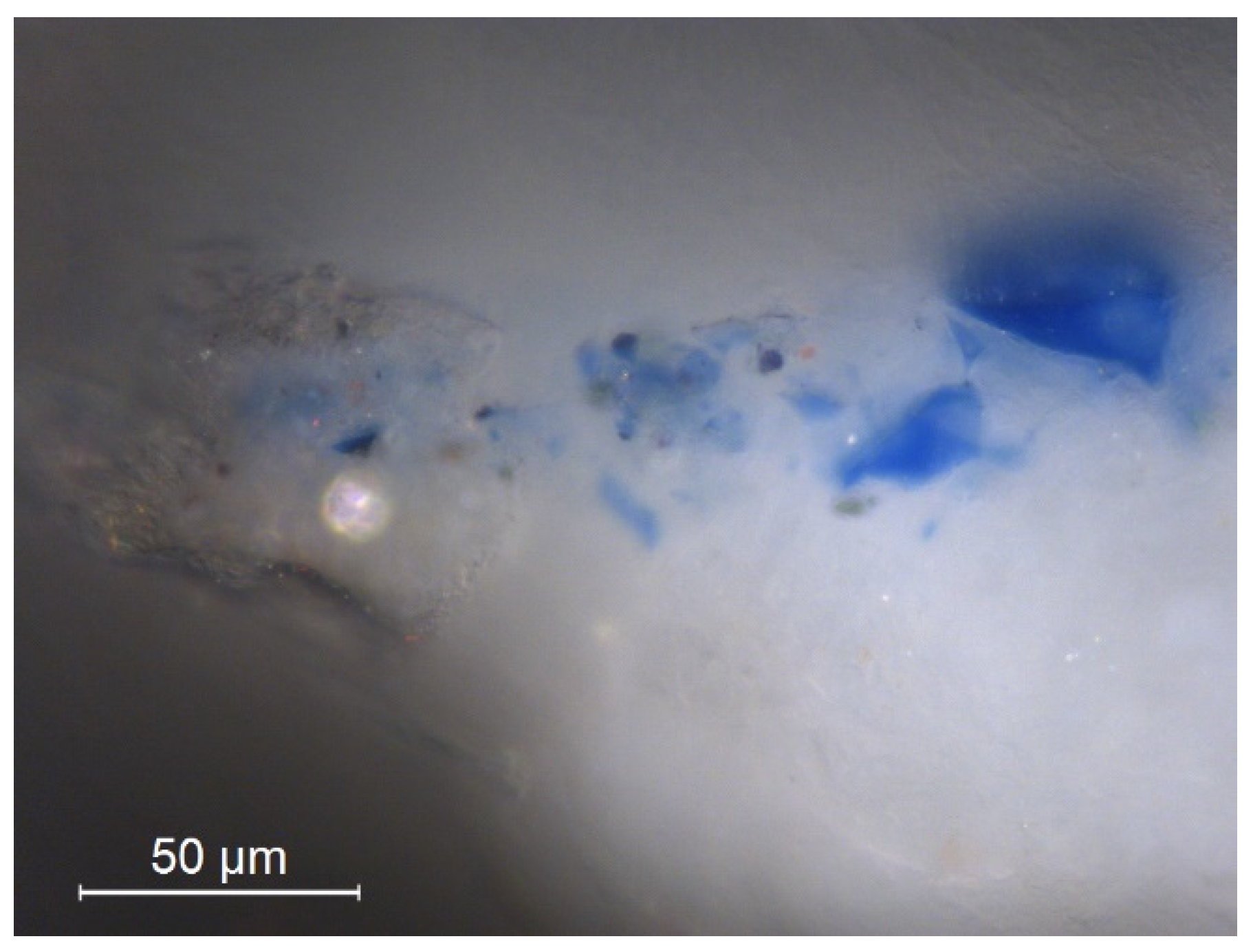
3.2.2. Scanning Electron Microscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Laboratory-Synthesized Nanolime Characterization
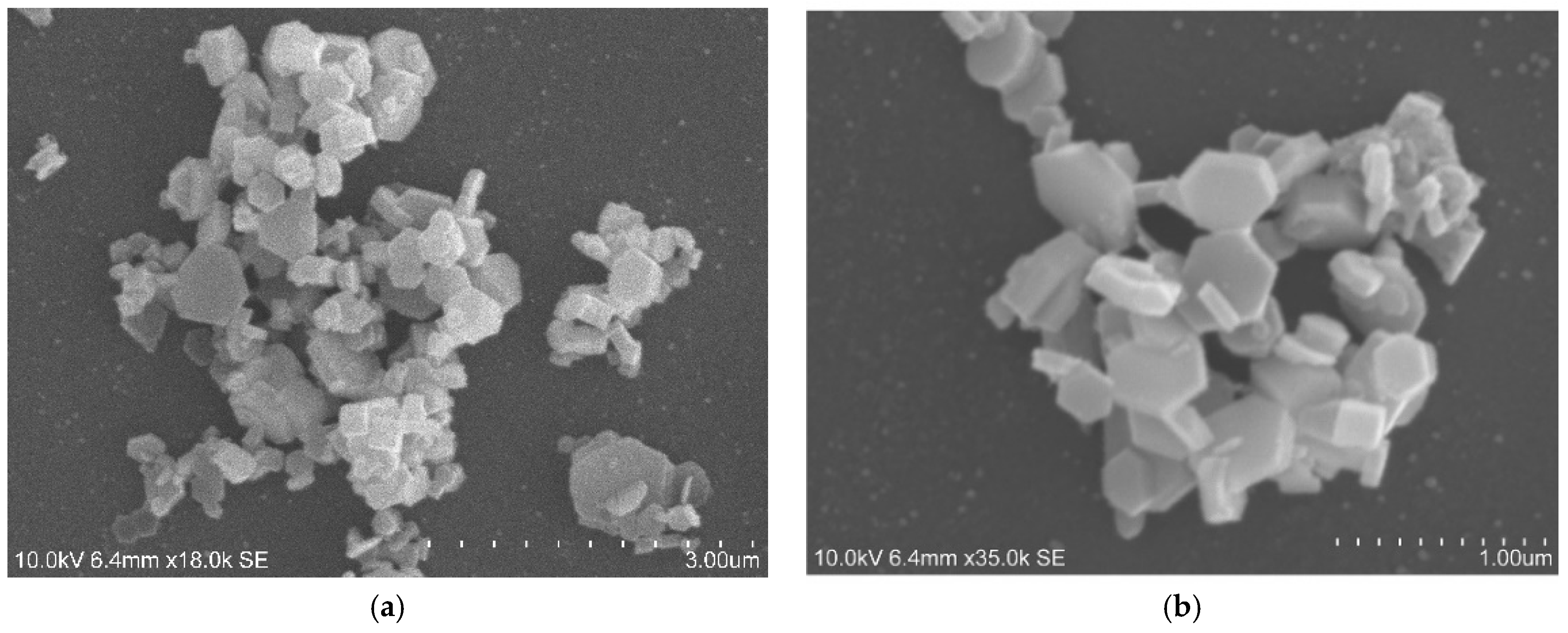
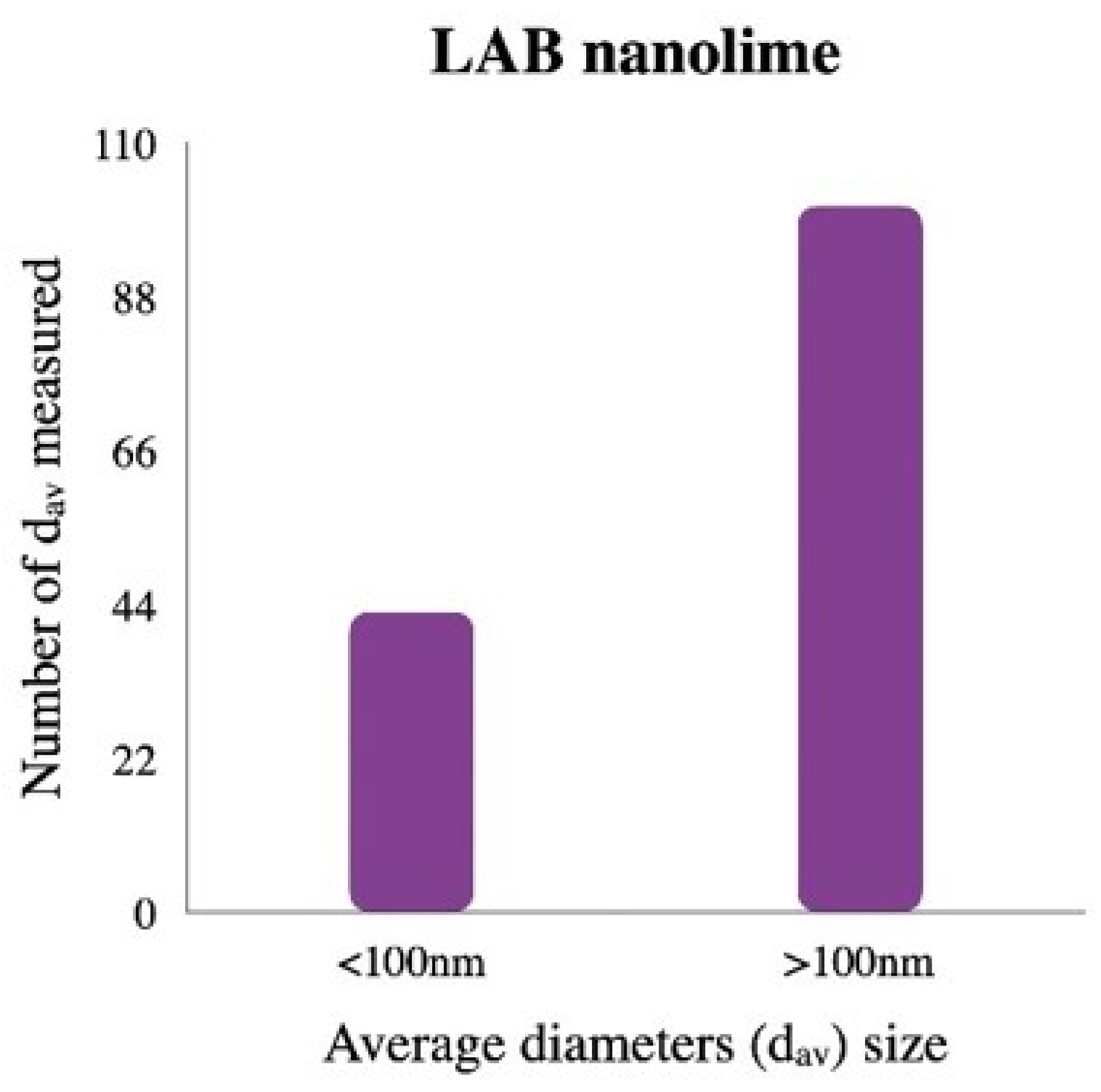
Appendix B. Treatment Evaluation
| Consolidant | Sample Ref. | Color | Painting Technique | ΔL* | ΔE* | ΔH* | ΔL* | ΔE* | ΔH* |
|---|---|---|---|---|---|---|---|---|---|
| One week after | One month after | ||||||||
| LAB nanolime | 3rBF | Red | Buon Fresco | 4.48 | 9.91 | 2.5 | 5.05 | 9.10 | 2.21 |
| 7rBF | Red | Buon Fresco | 3.76 | 8.73 | 1.76 | 5.28 | 10.77 | 2.47 | |
| 11rBF | Red | Buon Fresco | 4.99 | 9.21 | 1.84 | 3.49 | 9.28 | 2.26 | |
| 3rLF | Red | Lime Fresco | 2.49 | 10.48 | 2.8 | 2.17 | 10.17 | 2.89 | |
| 7rLF | Red | Lime Fresco | 3.64 | 8.53 | 2.06 | 3.14 | 8.44 | 2.10 | |
| 11rLF | Red | Lime Fresco | 3.32 | 5.00 | 1.15 | 3.09 | 5.31 | 1.10 | |
| 4yBF | Yellow | Buon Fresco | 0.66 | 8.99 | 1.55 | 1.52 | 8.14 | 2.14 | |
| 7yBF | Yellow | Buon Fresco | 0.51 | 5.36 | 1.92 | 1.20 | 5.91 | 1.87 | |
| 11yBF | Yellow | Buon Fresco | −0.75 | 5.58 | 0.61 | −0.94 | 6.62 | 0.39 | |
| 1yLF | Yellow | Lime Fresco | 0.33 | 5.97 | 0.91 | −0.15 | 5.81 | 0.89 | |
| 7yLF | Yellow | Lime Fresco | 1.11 | 6.20 | 1.15 | −0.06 | 7.69 | 1.19 | |
| 11yLF | Yellow | Lime Fresco | 0.72 | 6.79 | 1.15 | −0.48 | 7.06 | 1.10 | |
| 3bLF | Blue | Lime Fresco | −0.31 | 1.75 | 0.09 | 1.29 | 1.83 | 0.07 | |
| 7bLF | Blue | Lime Fresco | 2.04 | 2.25 | 0.01 | 1.73 | 2.42 | 0.24 | |
| 12bLF | Blue | Lime Fresco | 3.02 | 4.00 | 0.26 | 0.44 | 2.89 | 0.32 | |
| CaLoSiL® IP25 | 12rBF | Red | Buon Fresco | −0.01 | 2.19 | 1.26 | 0.88 | 3.10 | 1.40 |
| 5rBF | Red | Buon Fresco | 2.62 | 9.45 | 5.64 | 2.24 | 8.36 | 4.97 | |
| 9rBF | Red | Buon Fresco | 3.75 | 6.17 | 4.05 | 2.56 | 4.55 | 3.34 | |
| 12rLF | Red | Lime Fresco | 1.8 | 5.31 | 2.98 | 3.83 | 8.03 | 2.42 | |
| 5rLF | Red | Lime Fresco | 1.68 | 7.29 | 4.12 | 1.15 | 5.70 | 3.00 | |
| 9rLF | Red | Lime Fresco | 0.8 | 2.74 | 1.48 | 0.79 | 2.87 | 1.34 | |
| 1yBF | Yellow | Buon Fresco | −1.34 | 6.12 | 1.70 | 0.51 | 6.63 | 1.89 | |
| 5yBF | Yellow | Buon Fresco | −1.65 | 5.85 | 1.86 | 0.35 | 4.47 | 2.01 | |
| 9yBF | Yellow | Buon Fresco | −0.75 | 6.55 | 1.96 | 0.08 | 5.68 | 2.11 | |
| 3yLF | Yellow | Lime Fresco | 0.85 | 3.40 | 1.06 | 0.58 | 2.78 | 0.90 | |
| 5yLF | Yellow | Lime Fresco | 0.32 | 4.85 | 1.15 | 0.05 | 4.39 | 1.38 | |
| 9yLF | Yellow | Lime Fresco | 0.69 | 8.12 | 2.51 | 0.69 | 8.29 | 2.57 | |
| 1bLF | Blue | Lime Fresco | 2.38 | 2.69 | 0.47 | 2.44 | 2.63 | 0.51 | |
| 5bLF | Blue | Lime Fresco | 1.32 | 1.34 | 0.01 | 0.93 | 1.03 | 0.15 | |
| 9bLF | Blue | Lime Fresco | 0.68 | 0.79 | 0.22 | −0.39 | 0.51 | 0.19 | |
| PrimalTM SF-016 ER® | 2rBF | Red | Buon Fresco | −0.41 | 0.44 | 0.16 | −0.35 | 0.99 | 0.18 |
| 6rBF | Red | Buon Fresco | −0.15 | 1.48 | 0.18 | −0.93 | 1.53 | 0.40 | |
| 10rBF | Red | Buon Fresco | 0.14 | 0.34 | 0.02 | 0.15 | 0.93 | 0.43 | |
| 2rLF | Red | Lime Fresco | 0.41 | 1.12 | 0.18 | −0.24 | 2.11 | 0.57 | |
| 6rLF | Red | Lime Fresco | 0.36 | 1.00 | 0.72 | 0.07 | 1.08 | 0.23 | |
| 10rLF | Red | Lime Fresco | 0.18 | 0.36 | 0.05 | −0.03 | 0.76 | 0.21 | |
| 2yBF | Yellow | Buon Fresco | −0.93 | 1.07 | 0.08 | −1.03 | 1.12 | 0.003 | |
| 6yBF | Yellow | Buon Fresco | −0.37 | 2.64 | 0.40 | −0.88 | 3.42 | 0.49 | |
| 10yBF | Yellow | Buon Fresco | 0.45 | 0.46 | 0.10 | −0.14 | 1.01 | 0.90 | |
| 2yLF | Yellow | Lime Fresco | −0.09 | 0.21 | 0.11 | −1.07 | 1.28 | 0.30 | |
| 6yLF | Yellow | Lime Fresco | 0.55 | 1.11 | 0.03 | −0.7 | 1.64 | 0.11 | |
| 10yLF | Yellow | Lime Fresco | −0.71 | 1.40 | 0.41 | −2.63 | 3.44 | 0.43 | |
| 2bLF | Blue | Lime Fresco | 0.45 | 0.47 | 0.14 | −0.21 | 0.22 | 0.06 | |
| 6bLF | Blue | Lime Fresco | 0.52 | 0.52 | 0.64 | 1.01 | 1.01 | 0.03 | |
| 10bLF | Blue | Lime Fresco | −0.68 | 0.75 | 0.14 | −0.51 | 0.53 | 0.09 | |
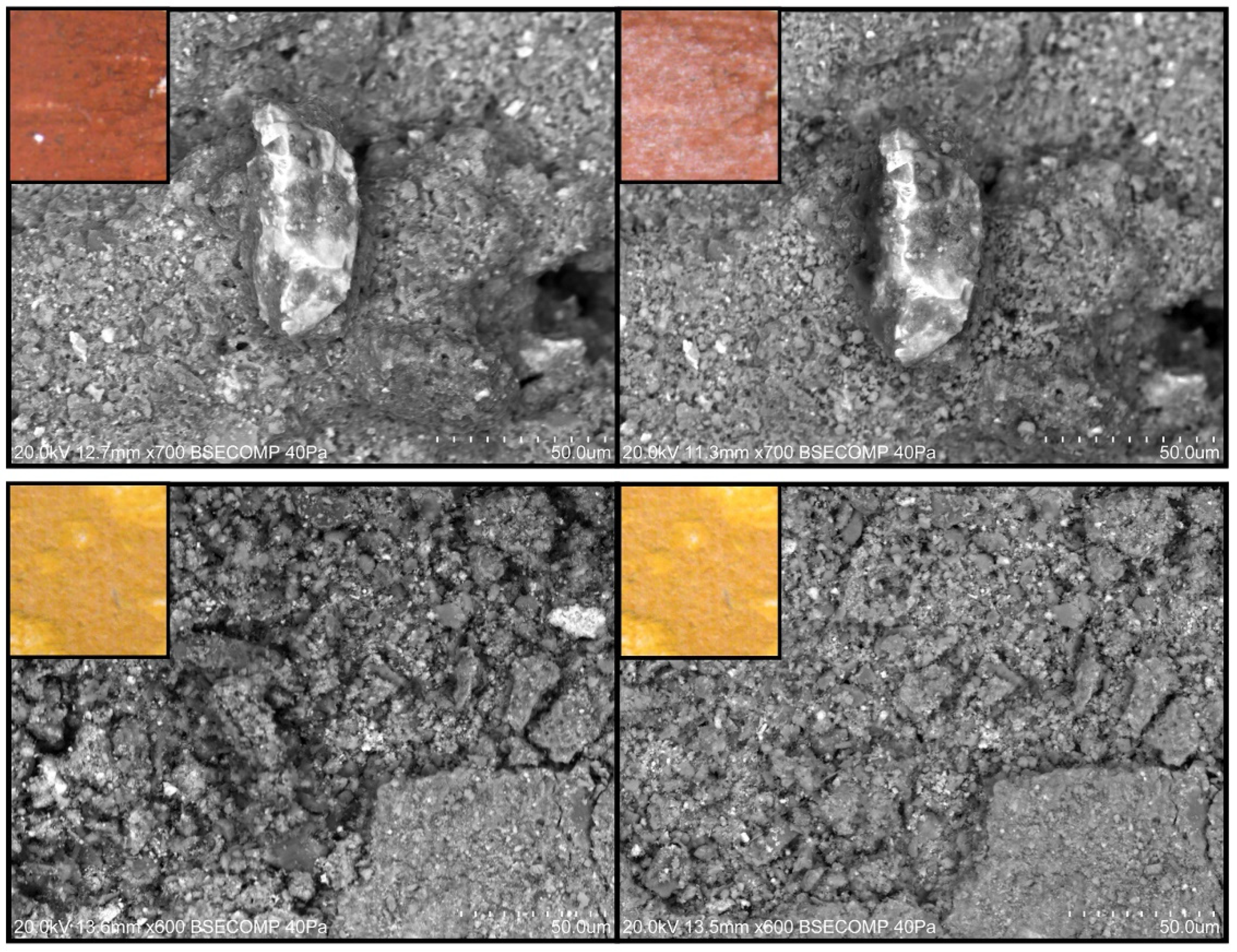
References
- ICOMOS. Principios Para la Preservación, Conservación y Restauración de Pinturas Murales. In Proceedings of the 14ª Asamblea General del ICOMOS, Victoria Falls, Zimbabwe, 27–31 October 2003. [Google Scholar]
- Mora, P.; Mora, L.; Philipott, P. Conservation of Wall Paintings; Butterwoths: Oxford, UK, 1984. [Google Scholar]
- Fichner-Rathus, L. Understanding Art, 10th ed.; Wadsworth: Boston, MA, USA, 2013; ISBN 13-978-1-111-83695-5. [Google Scholar]
- Mora, P. Causes of Deterioration of Mural Paintings; International Centre for the Study of the Preservation and Restoration of Cultural Property (ICCROM): Rome, Italy, 2018. [Google Scholar]
- Spencer, B.N.; Rosenthal, A.; Podany, J.; Larson, J.H.; Zaccari, F. Art Conservation and Restoration, Encyclopaedia Britannica. Available online: https://www.britannica.com/art/art-conservation-and-restoration (accessed on 1 November 2020).
- Gil, M. Conservação de Pintura Mural, Estudo e Consolidação de Argamassas de Cal Aérea e Areia com Falta de Coesão; Laboratório Nacional de Engenharia Civil: Lisboa, Portugal, 2002. [Google Scholar]
- Magaloni, K.D. La pintura mural y su conservación. Arqueol. Mex. 2011, 108, 33–37. [Google Scholar]
- Rodrigues, J.D. Consolidation of decayed stones. A delicate problem with few practical solutions. In Proceedings of the 3rd International Seminar, Guimarães, Portugal, 7–9 November 2001; pp. 3–14. [Google Scholar]
- Zhu, J.; Zhang, P.; Ding, J.; Dong, Y.; Cao, Y.; Dong, W.; Zhao, X.; Li, X.; Camaiti, M. Nano Ca(OH)2: A review on synthesis, properties and applications. J. Cult. Herit. 2021, 50, 25–42. [Google Scholar] [CrossRef]
- Brajer, I.; Kalsbeek, N. Limewater Absorption and Calcite Crystal Formation on a Limewater-Impregnated Secco Wall Painting. Stud. Conserv. 1999, 44, 145–156. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage, a Compendium of Materials and Techniques; Springer: Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Kaszowska, Z.; Kot, M.; Białek-Kostecka, D.; Forczek-Sajdak, A. Application of micro-indentation tests to assess the consolidation procedure of historic wall paintings. J. Cult. Herit. 2018, 36, 286–296. [Google Scholar] [CrossRef]
- Normand, L.; Duchêne, S.; Vergès-Belmin, V.; Dandrel, C.; Giovannacci, D.; Nowik, W. Comparative in Situ Study of Nanolime, Ethyl Silicate and Acrylic Resin for Consolidation of Wall Paintings with High Water and Salt Contents at the Chapter Hall of Chartres Cathedral. Int. J. Arch. Herit. 2020, 14, 1120–1133. [Google Scholar] [CrossRef]
- Baglioni, P.; Carretti, E.; Dei, L.; Giorgi, R. Nanotechnology in wall painting conservation. In Self-Assambly; Robinson, B.H., Ed.; IOS-Press: Amsterdam, The Netherlands, 2013; pp. 32–41. ISBN 1-58603-382-4. [Google Scholar]
- Girginova, P.I.; Galacho, C.; Veiga, R.; Silva, A.S.; Candeias, A. Inorganic Nanomaterials for Restoration of Cultural Heritage: Synthesis Approaches towards Nanoconsolidants for Stone and Wall Paintings. ChemSusChem 2018, 11, 4168–4182. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes filled with MgO for paper reinforcement and deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E. Nanolimes: From synthesis to application. Pure Appl. Chem. 2017, 90, 523–550. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Ortiz, P. Basic Protocol for on-site testing consoli-dant nanoparticles on stone cultural heritage. Heritage 2019, 2, 2712–2724. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Halloysite Nanotubes Loaded with Calcium Hydroxide: Alkaline Fillers for the Deacidification of Waterlogged Archeological Woods. ACS Appl. Mater. Interfaces 2018, 10, 27355–27364. [Google Scholar] [CrossRef]
- Camerini, R.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Hybrid nano-composites for the consolidation of earthen masonry. J. Colloid Interface Sci. 2018, 539, 504–515. [Google Scholar] [CrossRef]
- Gómez-Villalba, L.S.; López-Arce, P.; Fort, R.; Álvarez, M. La aportación de la nanociencia a la conservación de bienes del patrimonio cultural. Patrim. Cult. Esp. 2010, 4, 43–56. [Google Scholar]
- Girginova, P.I.; Galacho, C.; Veiga, R.; Silva, A.S.; Candeias, A. Study of mechanical properties of alkaline earth hydroxide nanoconsolidants for lime mortars. Constr. Build. Mater. 2019, 236, 117520. [Google Scholar] [CrossRef]
- López-Arce, P.; Gómez-Villalba, L.; Martinez-Ramirez, S.; de Buergo, M.; Fort, R. Influence of relative humidity on the carbonation of calcium hydroxide nanoparticles and the formation of calcium carbonate polymorphs. Powder Technol. 2011, 205, 263–269. [Google Scholar] [CrossRef]
- Álvarez, A.E.; Nadal, L.F. Evaluación del proceso de carbonatación de nanocales aplicadas a pinturas murales prehispánicas de origen maya. Interv. Rev. Int. Conserv. Restauración Museol. 2010, 1, 31–41. [Google Scholar] [CrossRef][Green Version]
- Vojtěchovský, J. Surface consolidation of wall paintings using lime nano-suspensions. Acta Polytech. 2017, 57, 139–148. [Google Scholar] [CrossRef]
- Becherini, F.; Durante, C.; Bourguignon, E.; Vigni, M.L.; Detalle, V.; Bernardi, A.; Tomasin, P. Aesthetic compatibility assessment of consolidants for wall paintings by means of multivariate analysis of colorimetric data. Chem. Cent. J. 2018, 12, 98. [Google Scholar] [CrossRef]
- Bourguignon, E.; Tomasin, P.; Detalle, V.; Vallet, J.-M.; Labouré, M.; Olteanu, I.; Favaro, M.; Chiurato, M.A.; Bernardi, A.; Becherini, F. Calcium alkoxides as alternative consolidants for wall paintings: Evaluation of their performance in laboratory and on site, on model and original samples, in comparison to conventional products. J. Cult. Herit. 2018, 29, 54–66. [Google Scholar] [CrossRef]
- Ambrosi, M.; Dei, L.; Giorgi, R.; Neto, C.; Baglioni, P. Colloidal Particles of Ca(OH)2: Properties and Applications to Restoration of Frescoes. Langmuir 2001, 17, 4251–4255. [Google Scholar] [CrossRef]
- Daehne, A.; Herm, C. Calcium hydroxide nanosols for the consolidation of porous building materials-results from EU-STONECORE. Herit. Sci. 2013, 1, 11. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Carretti, E.; Toccafondi, N.; Jaidar, Y. Commercial Ca(OH)2 nanoparticles for the consolidation of immovable works of art. Appl. Phys. A 2013, 114, 723–732. [Google Scholar] [CrossRef]
- Natali, I.; Saladino, M.L.; Andriulo, F.; Martino, D.C.; Caponetti, E.; Carretti, E.; Dei, L. Consolidation and protection by nanolime: Recent advances for the conservation of the graffiti, Carceri dello Steri Palermo and of the 18th century lunettes, SS. Giuda e Simone Cloister, Corniola (Empoli). J. Cult. Herit. 2014, 15, 151–158. [Google Scholar] [CrossRef]
- Giorgi, R.; Bozzi, C.; Dei, L.; Gabbiani, C.; Ninham, A.B.W.; Baglioni, P. Nanoparticles of Mg(OH)2: Synthesis and Application to Paper Conservation. Langmuir 2005, 21, 8495–8501. [Google Scholar] [CrossRef]
- Girginova, P.I.; Galacho, C.; Mirão, J.; Veiga, R.; Silva, A.S.; Candeias, A. Preliminary studies of consolidation of wall paintings: Synthesis and characterisation of nanolime. Conserv. Património 2016, 23, 103–107. [Google Scholar] [CrossRef]
- Gil, M.; Rosado, T.; Ribeiro, I.; Pestana, J.A.; Caldeira, A.T.; Carvalho, M.L.; Dias, L.; Mirão, J.; Candeias, A. Are theyfrescopaintings? Technical and material study ofCasas Pintadasof Vasco da Gama house in Évora (Southern Portugal). X-Ray Spectrom. 2015, 44, 154–162. [Google Scholar] [CrossRef]
- Johnston-Feller, R. Color science in the examination of museum objects: Nondestructive procedures. Color Res. Appl. 2002, 27, 456–457. [Google Scholar] [CrossRef]
- Michalopoulou, A.; Maravelaki, P.-N.; Kilikoglou, V.; Karatasios, I. Morphological characterization of water-based nanolime dispersions. J. Cult. Herit. 2020, 46, 11–20. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Eureste, E. Application of FTIR for Quantitive Lime Analysis. Available online: https://library.ctr.utexas.edu/digitized/texasarchive/phase2/9028-01-1.pdf (accessed on 1 December 2000).
- Kimura, N.; Umemura, J.; Hayashi, S. Polarized FT-IR Spectra of Water in the Middle Phase of Triton X100-Water System. J. Colloid Interface Sci. 1996, 182, 356–364. [Google Scholar] [CrossRef]
- Rajisha, K.R.; Deepa, B.; Pothan, L.A.; Thomas, S. Thermochemical and spectroscopic characterization of natural fibre composites. In Interface Engineering of Natural Fibre Composites for Maximum Performance; Zafeiropoulos, N.E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 241–274. [Google Scholar]
- Rodriguez-Navarro, C.; Suzuki, M.A.; Ruiz-Agudo, E. Alcohol Dispersions of Calcium Hydroxide Nanoparticles for Stone Conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.R.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.; Ortiz, R.; Karapanagiotis, I.; Ortiz, P. Comparison of the performance of a novel nanolime doped with ZnO quantum dots with common consolidants for historical carbonate stone buildings. Appl. Clay Sci. 2020, 195, 105732. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Silva, A.S.; Colla, L.; Fedele, L.; Tomasin, P. Effect of solvent on nanolime transport within limestone: How to improve in-depth deposition. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 171–181. [Google Scholar] [CrossRef]
- Lanzón, M.; De Stefano, V.; Gaitán, J.C.M.; Cardiel, I.B.; Gutiérrez-Carrillo, M.L. Characterisation of earthen walls in the Generalife (Alhambra): Microstructural and physical changes induced by deposition of Ca(OH)2 nanoparticles in original and reconstructed samples. Constr. Build. Mater. 2019, 232, 117202. [Google Scholar] [CrossRef]
- Ševčík, R.; Mácová, P.; Estébanez, M.P.; Viani, A. Influence of additions of synthetic anhydrous calcium carbonate polymorphs on nanolime carbonation. Constr. Build. Mater. 2019, 228, 116802. [Google Scholar] [CrossRef]
- Gherardi, F.; Otero, J.; Blakeley, R.; Colston, B. Application of Nanolimes for the Consolidation of Limestone from the Medieval Bishop’s Palace, Lincoln, UK. Stud. Conserv. 2020, 65, P90–P97. [Google Scholar] [CrossRef]
- Dei, L.; Salvadori, B. Nanotechnology in cultural heritage conservation: Nanometric slaked lime saves architectonic and artistic surfaces from decay. J. Cult. Herit. 2006, 7, 110–115. [Google Scholar] [CrossRef]
- López-Martínez, T.; Otero, J. Preventing the Undesired Surface Veiling after Nanolime Treatments on Wall Paintings: Preliminary Investigations. Coatings 2021, 11, 1083. [Google Scholar] [CrossRef]


| Ariccio | Intonaco |
|---|---|
| 1 part hydrated lime putty; | 1 part hydrated lime putty; |
| 2 parts siliceous sand; | 2 part cream of marble; |
| Buon fresco paint layer | |||
| rBF | yBF | ||
| 4 g red ochre pigment (Kremer French Ochre RTFLES-40020®, Kremer pigmente, Aichstetten, Germany); 10 mL tap water; | 4 g yellow ochre pigment (Kremer French Ochre JCLES-40040®, Kremer pigmente, Aichstetten, Germany); 10 mL tap water; | ||
| Lime fresco paint layer | |||
| rLF | yLF | bLF | |
| 5 g red ochre pigment (Kremer French Ochre RTFLES-40020®) 4 pp of milk lime | 5 g yellow ochre pigment (Kremer French Ochre JCLES -40040®) 4 pp of milk lime | 5 g of smalt pigment (Kremer Smalt-10010®, Kremer pigmente, Aichstetten, Germany) 4 pp of milk lime | |
| Name | Chemical Composition/Manufacturer |
|---|---|
| LAB nanolime | Dispersion of Ca(OH)2 NPs, 25 g/L in Acetone:Ethanol (1:10). Size (SEM): between 18–713 nm |
| CaLoSiL® IP25 | Dispersion of Ca(OH)2 NPs, 25 g/L in iso-propanol (IBZ Salzchemie GmbH & Co. KG, Halsbruecke, Germany) Applied as acquired. Size: between 50–250 nm (Technical Datasheet) |
| PrimalTM SF-016 ER® | Acrylic polymer dispersion (Dow Coating Materials, United States of America): 50–51% solid content. Applied diluted at 2% in tap water, which is a common concentration used in past conservation works [12]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiza, B.; Gil, M.; Galacho, C.; Candeias, A.; Girginova, P.I. Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion. Heritage 2021, 4, 3288-3306. https://doi.org/10.3390/heritage4040183
Baiza B, Gil M, Galacho C, Candeias A, Girginova PI. Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion. Heritage. 2021; 4(4):3288-3306. https://doi.org/10.3390/heritage4040183
Chicago/Turabian StyleBaiza, Berenice, Milene Gil, Cristina Galacho, António Candeias, and Penka I. Girginova. 2021. "Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion" Heritage 4, no. 4: 3288-3306. https://doi.org/10.3390/heritage4040183
APA StyleBaiza, B., Gil, M., Galacho, C., Candeias, A., & Girginova, P. I. (2021). Preliminary Studies of the Effects of Nanoconsolidants on Mural Paint Layers with a Lack of Cohesion. Heritage, 4(4), 3288-3306. https://doi.org/10.3390/heritage4040183









