1. Introduction
The Harvard Art Museums Bell Krater: Torch Race (1960.344, dated c. 430-420 BCE,
Figure 1a) was one of several Greek terracotta artefacts which received treatment in preparation for display in 2014. After one year of display the cadmium orange restoration paint on the krater had altered in color in some areas from orange to grey (
Figure 1b,c). In comparison, other objects displayed in the same case also in-painted with the same paint showed no sign of alteration. The appearance was deemed unacceptable and re-treatment was required. To appropriately re-treat the krater an understanding of the cause of the alteration was essential and began with understanding the treatment history of the object.
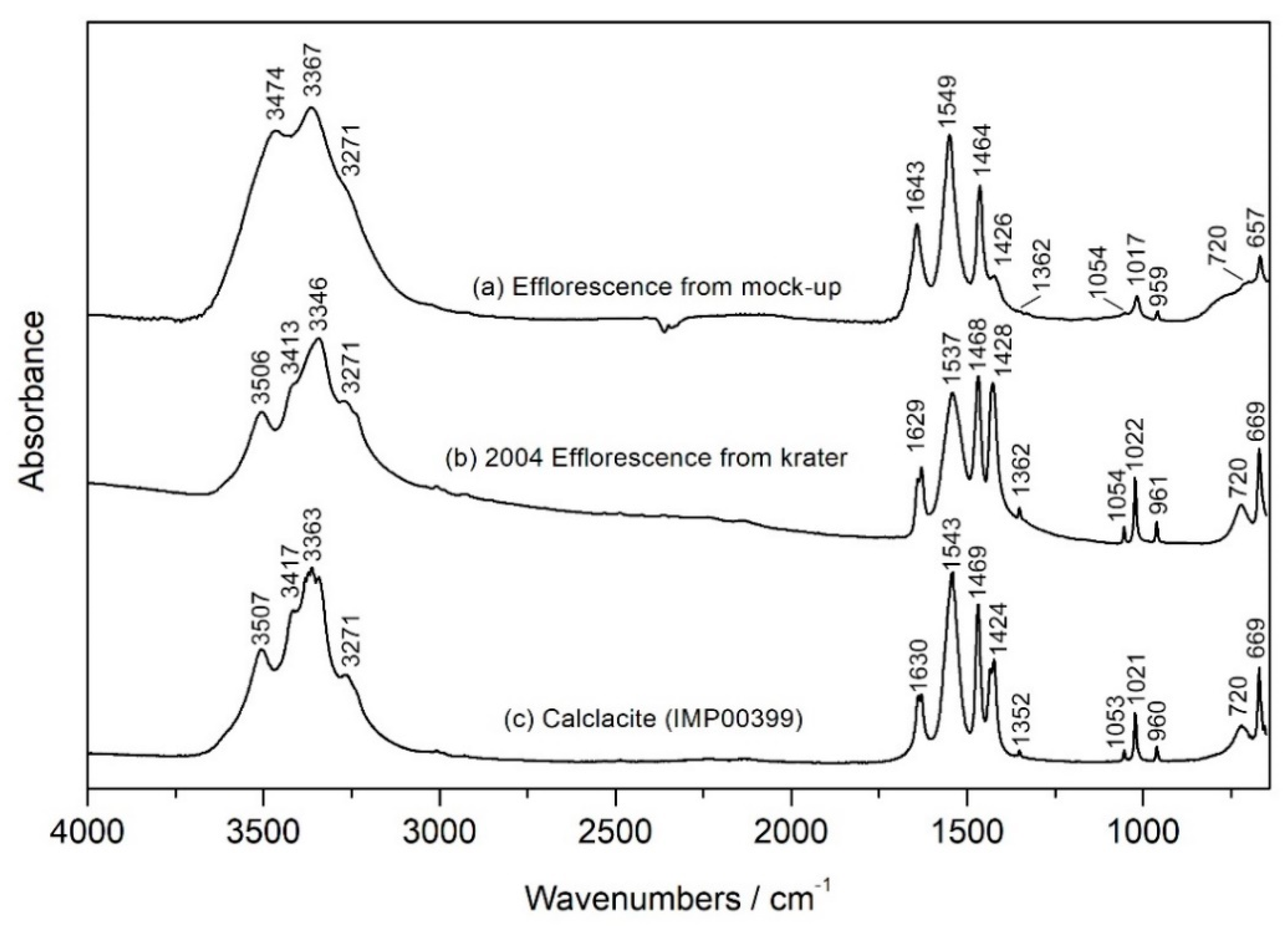
There is no record of any conservation work performed on the krater before its bequest to the museum, however, the object had been re-assembled in the past and this treatment resulted in misplaced joins, abraded surfaces and noticeable over-paint. During a 2004 loan, an efflorescence was observed on the object. The efflorescence was analyzed by SEM-EDS and found to contain calcium and chlorine. FTIR analysis was also performed but the mineral could not be identified with the available spectral databases at that time.
When the spectrum was revisited in the early stages of this investigation, the expansion of the Infrared and Raman Users Group (IRUG) spectral database [
1] led to the identification of calclacite (Ca(CH
3COO)Cl.5H
2O), a calcium chlorine acetate salt (see Figure 5 later). The formation of calclacite has previously been attributed to archaeological ceramics that have residual chlorides from burial or treatment with hydrochloric acid and have been stored in wooden cases [
2]. Hydrochloric acid, along with other acids such as acetic or nitric, were used in the past to remove burial accretions [
3]. For hydrochloric acid, if not completely removed from the object by rinsing, residual chlorine salts can remain and can react with the acid vapors from the wood resulting in the efflorescence. Based on the identification of the efflorescence, the assumption was made that the object underwent incomplete treatment with hydrochloric acid to remove burial accretions at some point in the past.
Desalination was considered during the 2014 treatment of the object but was eventually deemed an unnecessarily risky treatment as restoration joins were considered stable and storage in a controlled environment had prevented the formation of any further efflorescence [
4]. Instead, treatment of the object focused on aesthetic work. Disfiguring paint was removed, restoration fills levelled, and break lines were filled and sealed with acrylic materials, followed by in-painting. Cadmium orange acrylic paint (Golden Heavy Body Artist Acrylics in C.P. Cadmium Orange), containing CdSSe, was chosen for in-painting due to its ability to lighten the darkened, abraded areas of the terracotta. Titan Buff acrylic paint (Golden Heavy Body Artist Acrylics in Titan Buff), containing titanium white (TiO
2), was added as required to adjust the tone. Full details of the materials used for the treatment may be found in a separate publication [
4].
Cadmium pigments offer a broad range of colors from light yellow to deep red. Cadmium yellow is composed of cadmium sulfide (CdS) and the incorporation of Se in increasing amounts changes the color from yellow through orange to red. The alteration of cadmium yellow has been reported in paintings by Henri Matisse [
5,
6,
7,
8,
9], Edvard Munch [
9,
10,
11,
12], Vincent Van Gogh [
13,
14], Pablo Picasso [
13,
15] and others [
13,
16,
17,
18], all dating from the late 19th-early 20th century, before the stability of pigment was improved [
16]. Alteration in areas identified as cadmium sulfide presents itself as either lightening or darkening of the once vibrant pigment.
Researchers have utilized a multitude of analytical tools, and most recently, synchrotron-based radiation techniques, to probe the degradation of the pigment. The observed alteration has been determined to be the result of degradation of the cadmium sulfide pigment through a photo-oxidation process. In the initial stages of the process cadmium sulfate is formed (CdSO
4·xH
2O) which then converts to cadmium carbonate (CdCO
3) and cadmium oxalate (formed from the breakdown of organic components such as binding media [
14], CdC
2O
4). The formation of these white compounds contributes to the observed lightening of the pigment. The formation of cadmium oxide (CdO), a brown compound, has been associated with darkening [
13].
The alteration of cadmium orange (CdSSe) in an artist’s work has not previously been identified, however, in the field of semiconductor chemistry, the photo-oxidation of cadmium selenide (CdSe), has been observed. Studies determined that during irradiation of CdSe in the presence of oxygen, photo-oxidation occurs producing cadmium oxide (CdO) and selenium dioxide (SeO
2) [
19,
20].
Whilst the degradation of artist materials is not unexpected, in any museum, restoration work performed by conservators is expected to last many decades. Increasing access to technical analysis within museum communities has made it easier for appropriate materials to be chosen and those found not to be suitable, such as fugitive colors, can be avoided. In the unlikely event that restoration materials fail quickly, such as in the case presented here, unusual circumstances are likely involved. The theory developed that the krater had received incomplete treatment with hydrochloric acid in the past warranted further investigation in order to determine if this was the cause of the paint alteration and to inform how to proceed with re-treatment.
3. Results and Discussion
3.1. Analysis of Cross-Sections from the Krater
The discoloration of the paint was exclusively observed to occur in areas where the restoration paint was in contact with abraded areas around break lines in the ceramic. No alteration was observed when the paint was applied over the acrylic fills from the 2014 treatment. In
Figure 2a, the terracotta of the krater is present in a cross-section prepared from an area where the paint altered, observable as a very uniform substrate layer. In
Figure 2b, a cross-section prepared from an area where the paint did not alter, the paint is applied to a mixture of restoration materials instead of terracotta. Clearly different from the original terracotta (
Figure 2a), this substrate is consistent with the presence of fill materials applied during the 2014 treatment and from the earlier, undated treatment.
The paint layer, measuring between 3–5 µm, appears almost completely white in the altered cross-section with only a few remaining colored particles. The paint layer is difficult to see in the unaltered cross-section, however with ultraviolet (UV) illumination a distinct green fluorescence from the paint layer is observable.
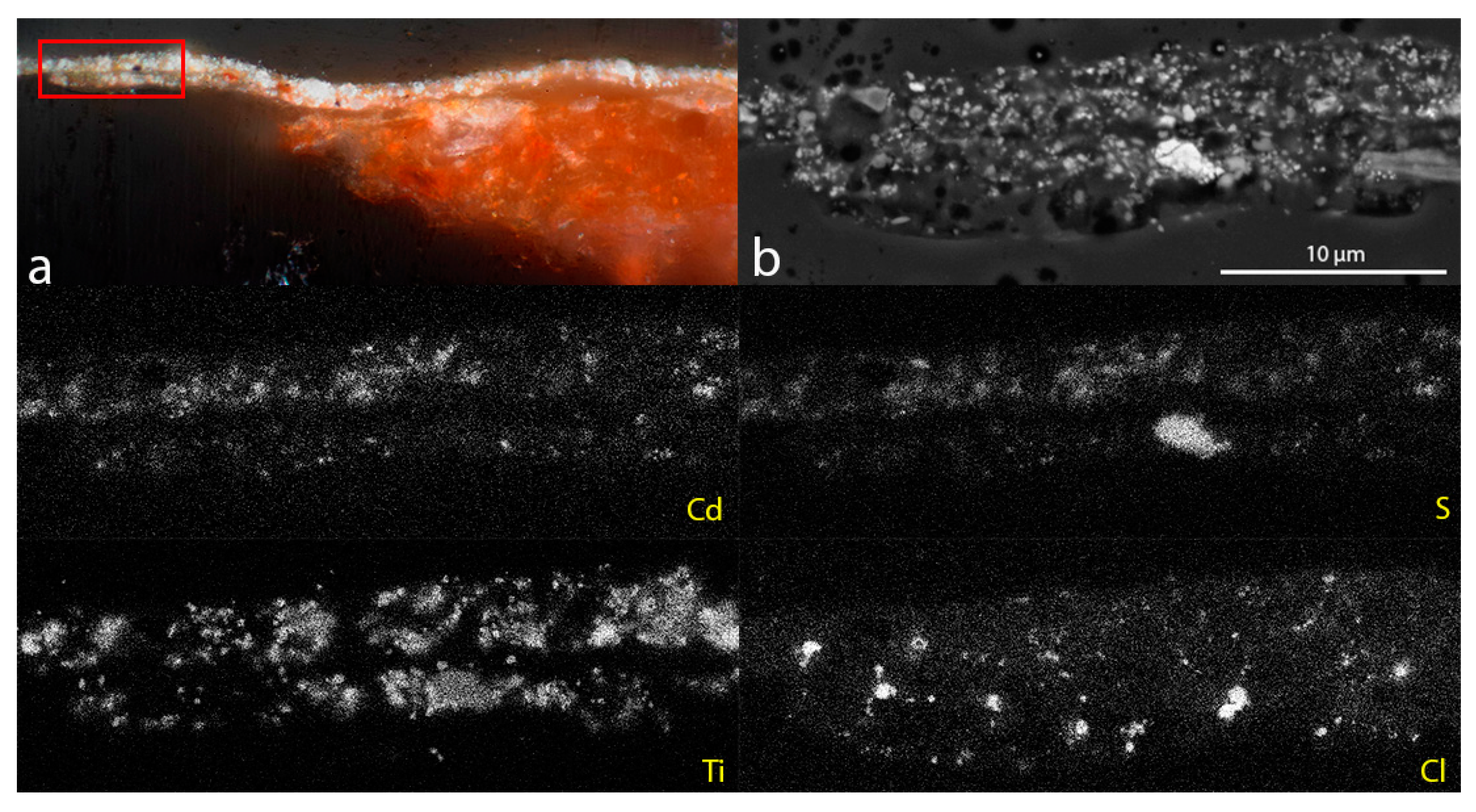
Analysis using SEM-EDS (
Figure 3) confirmed the presence of chlorine (Cl) in the altered paint layer and in the terracotta (not shown in
Figure 3). No Cl was observed in any of the layers in cross-sections prepared from the unaltered paint. This observation, combined with the identification of calclacite from the 2004 efflorescence, suggested that the alteration may be caused by an interaction between the cadmium orange restoration paint and residual chloride ions in the terracotta.
3.2. Characterization of the Fresh Paint
The paint film prepared from Golden Heavy Body Artist Acrylics in C.P. Cadmium Orange is uniformly orange in color with no obvious inclusions. Elemental analysis by XRF of the cadmium orange paint confirmed the presence of cadmium (Cd), sulfur (S) and selenium (Se), consistent with the cadmium orange pigment. Barium (Ba) and zinc (Zn) were additionally identified in the paint.
The Raman spectrum of the cadmium orange paint (
Figure 4a) contains bands assigned to the longitudinal optical (LO) phonon and overtone (2LO) of CdS at 296 and 595 cm
−1, respectively. The LO phonon for CdSe is typically observed at around 200 cm
−1 but may shift in wavenumbers based on the pigment composition, and as such may be accounted for by either or both of the peaks at 192 and 214 cm
−1 [
21]. The presence of barium sulfate, identifiable in
Figure 4a by the peak at 988 cm
−1 representing the symmetric stretching of the SO
4 group, indicates that the paint contains the lithopone version of cadmium orange [
16]. The mineral form of zinc could not be identified from analysis, however, the identification of lithopone may suggest the presence of zinc sulfide (ZnS) and the UV fluorescence observed in the paint, discussed below, may be attributed to the presence of zinc oxide (ZnO).
The cadmium orange paint has the same distinct green UV fluorescence visible in the cross-section images shown in
Figure 2c,d. The fluorescence occurs from very fine particles distributed evenly throughout the paint matrix. Multiple cadmium orange reference samples and pure barium sulfate displayed no fluorescence under the same UV conditions. A reference sample for lithopone contained a scattering of particles which fluoresced pale yellow, typical of zinc oxide (ZnO). Zinc oxide may be present in lithopone in trace amounts, and whilst the fluorescence of zinc oxide is not a match for the green fluorescence observed in the cadmium orange paint film, it is the only pigment identified to have a fluorescence that may be present. It is speculated that the fluorescence color has altered as a result of the paint preparation, however, further study is required.
The paint film prepared from Golden Heavy Body Artist Acrylics in Titan Buff is off-white in color and includes both red and black inclusions. XRF analysis of the paint film showed the presence of titanium (Ti) and Raman analysis identified the presence of rutile (titanium dioxide, TiO
2), based on the presence of B
1g, E
g and A
1g Raman active modes of the of the rutile single crystal at 142, 446 and 610 cm
−1, respectively (
Figure 4b) [
22]. The red and black inclusions were identified as hematite (Fe
2O
3) and rutile, respectively, with Raman. The identification of rutile in the black inclusions suggests the use of the natural form of the mineral in addition to the white synthetic form used for the bulk of the paint [
23].
Importantly, no Cl was identified in either of the paints, supporting the theory that residual chloride ions in the terracotta are involved in the observed alteration of the paint. FTIR analysis confirmed the presence of an acrylic binder in both the cadmium orange and Titan Buff paints, which was identified to contain a combination of methyl methacrylate, n-butyl acrylate and n-butyl methacrylate by pyrolysis-GCMS.
3.3. Mock-Up Study
Mock-ups were created to allow for more extensive study of the paint alteration as the amount of altered material from the krater was limited. The mock-ups were created in pairs with one terracotta piece acidified using 6N hydrochloric acid and the other without. Each mock-up was painted with a swatch of the pure cadmium orange paint and a swatch of the cadmium orange paint mixed with the Titan Buff, mimicking the conservation treatment.
Initial mock-ups were designed to recreate the conditions which are assumed to have contributed to the formation of the efflorescence observed in 2004. One mock-up pair was sealed in a bag with a small volume of acetic acid, approximately 2 mL, to recreate the proposed scenario which caused the formation of the calclacite efflorescence on the krater. White efflorescence formed on the acidified mock-up after three days. The efflorescence was sampled, analyzed by FTIR and identified as calclacite (
Figure 5). No efflorescence was observed on the non-acidified mock-up.
Next, a series of mock-ups were created to study the effects of both chloride ions and light on the alteration of the pigment, aiming to investigate whether display conditions had contributed to the alteration. Mock-up pairs (acidified and non-acidified), were placed in bright natural light (1 and 2), non-direct light (3 and 4) and in the dark (5 and 6). The mock-ups in direct light received direct natural light through a window which filters out the majority of UV radiation. Those placed in indirect light were sheltered from direct natural light, mimicking the closest scenario to the display conditions of the object. The mock-ups which received no light were kept in a closed draw.
During the first three months of exposure, mock-ups in direct light received approximately 9 h of natural daylight per day. The amount of light these mock-ups received during daylight hours varied between 207–2624 footcandles, the equivalent of approximately 2140–28,400 Lux, with the measured UV content of the light varying between 5.7–14.4 mW/Lumen (determined using an ELSEC 765 Environmental Monitor). The lower values are associated with overcast, cloudy days and the higher values with bright, sunny days. The amount of light received by the mock-ups in indirect light during the same time frame remained below 10 footcandles (108 Lux) and UV measurements were consistently zero.
The acidified mock-ups in direct and indirect light (mock-ups 2 and 4, respectively) began to noticeably alter within one month with the cadmium orange darkening to brown and the cadmium orange/Titan Buff mixture changing to grey, matching the observed alteration on the krater. The acidified mock-up placed in the dark (mock-up 6) displayed no visible change after one year and no change was seen on the non-acidified mock-ups at any light level.
Figure 6 shows the mock-ups after one year.
The mock-ups used for this study were treated with 6N hydrochloric acid to produce a quick reaction. A mock-up treated with 1N hydrochloric acid was created for comparison with the more harshly acidified samples. The same alteration was observed on the mock-up acidified with 1N hydrochloric acid, albeit occurring at a reduced rate in comparison to the 6N counterpart taking at least three months for any noticeable alteration to occur.
3.4. Characterization of the Altered Paint from the Mock-Ups and the Krater
A greyed appearance on terracotta ceramics in-painted with acrylic paints has been observed before and was attributed to residual solvent retained in the terracotta substrate migrating to the surface carrying lighter pigments with it [
24]. This was considered as a possibility, however, other terracotta ceramics treated in the same manner as the krater did not show the same alteration and not all of the mock-ups altered as might be expected if this was the case. The migration of pigments could also not be confirmed from SEM-EDS mapping of the paint films in cross-section, with the elements associated with the paint layers remaining relatively evenly distributed across the paint layer (see
Figure 3).
A key observation in the alteration of the paint films on mock-ups 2 and 4 was the formation of dark needle-like structures which are visible only with high magnification in both the cadmium orange paint swatch and the cadmium orange/Titan Buff swatch, the latter shown in
Figure 7a. Whilst these needle-like structures formed on both mock-ups, the formation was more extensive in the mock-up exposed to direct light (mock-up 2), suggesting that high light exposure acts as a catalyst for formation but is not required. The analysis described here is focused on the cadmium orange/Titan Buff altered paint film on mock-up 4 as it most closely resembles the paint mixture and conditions under which alteration occurred on the krater.
A sample of the altered paint from the krater was revisited and the same dark needle-like structures were observed (
Figure 7b). These structures are a little more difficult to see in the visible and UV image (
Figure 7b and d, with some structures highlighted by white arrows), due to their small size which is comparable to the dark inclusions present in the Titan Buff paint.
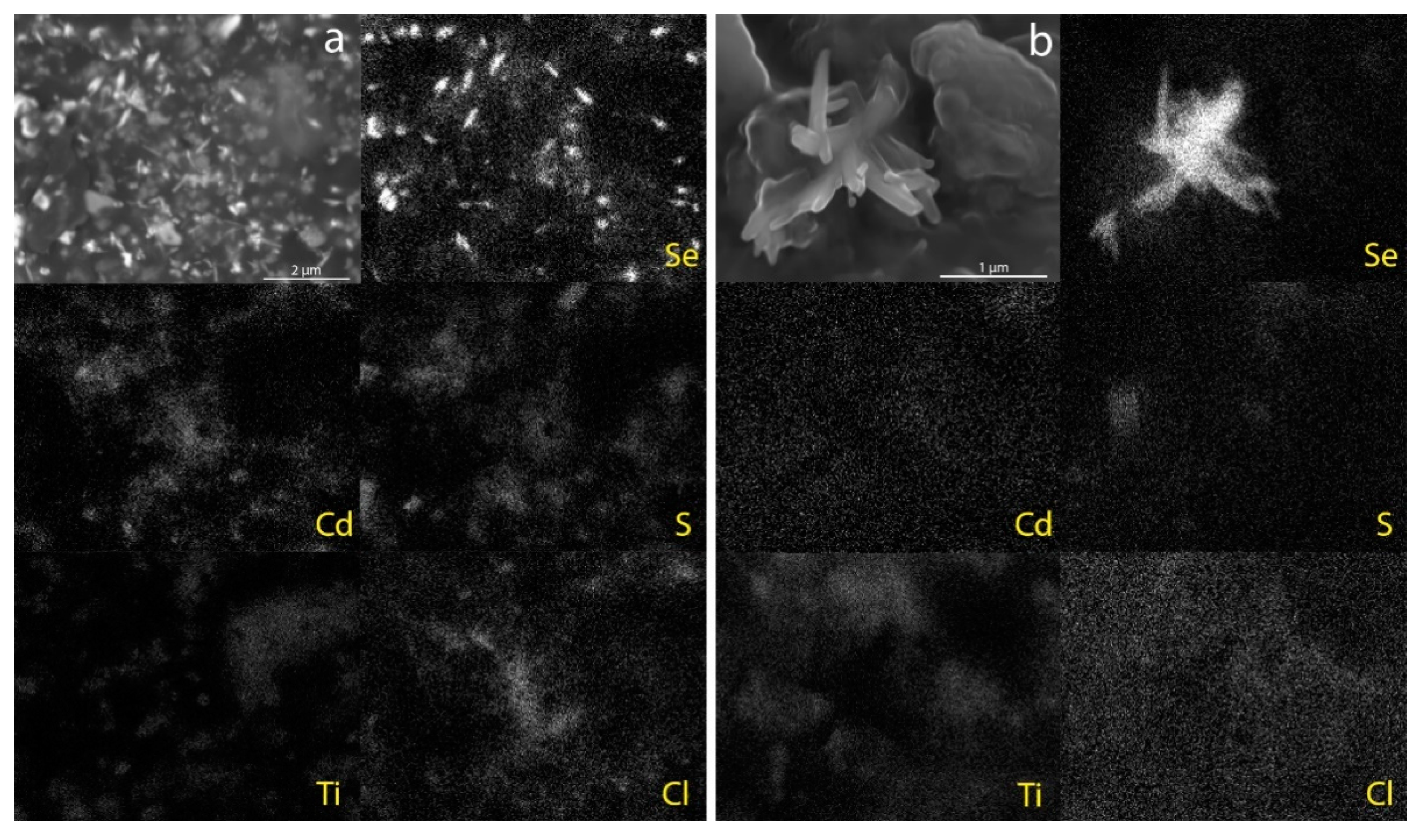
SEM-EDS imaging of the altered paint from mock-up 4 shows that the majority of the dark needle-like structures, which are in the order of 1/2 µm long, can be seen just underneath the surface of the paint film whilst a few protrude slightly from the surface. SEM-EDS mapping revealed that the structures are rich in Se (
Figure 8a). None of the other elements associated with the pigments (Cd, S and Ti) or Cl appear to be associated with the structures. Raman spectra collected from the structures have a unique sharp Raman peak around 234 cm
−1 which is a match to a reference spectrum of Se available through the RRUFF
TM project database (R050656,
Figure 9) [
25]. Comparison with the literature indicates that this Raman band corresponds to the trigonal, polymeric form of Se (
t-Se
n) [
26]. Clusters of dark needle-like structures protruding from the paint surface, similar in size to the Se-rich structures observed in the mock-up paint, were identified in the altered paint taken from the krater with SEM-EDS mapping again revealing that the structures are rich in Se (
Figure 8b) and the Raman spectrum matches the reference for Se already discussed (
Figure 9).
The FTIR spectrum of the altered paint from both the mock-ups and krater remained relatively unchanged in comparison to the spectra of the fresh paint. Whilst the formation of different cadmium compounds (for example CdC
2O
4, CdCO
3) may be identified in the FTIR spectrum [
12,
15], the strong signal from the acrylic binder prevented their identification if present. No apparent degradation of the acrylic binder was observed in the pyrolysis-GCMS chromatogram.
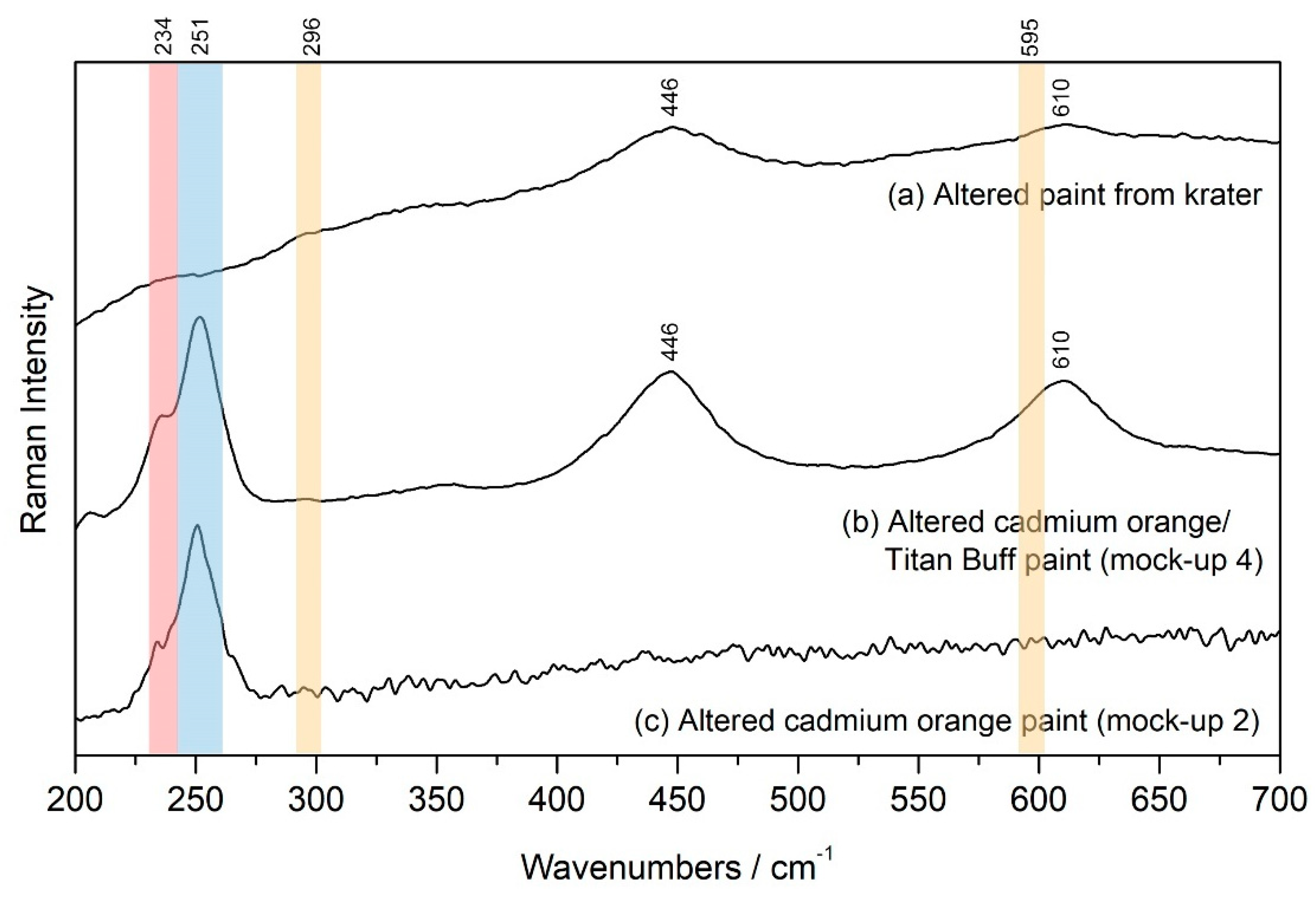
Raman spectra of the paint surrounding the Se-rich structures are shown for the altered paint from the krater, the altered cadmium orange/Titan Buff swatch from mock-up 4 and the altered cadmium orange swatch from mock-up 2 in
Figure 10. The only recognizable feature from the fresh paint is the presence of rutile in the spectra from the krater and mock-up 4 (
Figure 10a,b, respectively). The peaks associated with the cadmium orange pigment (shown in
Figure 4a, with peak locations shaded in orange in
Figure 10) are absent in all three spectra. Instead, the presence of Se remains visible (highlighted by the red shaded area in
Figure 10) and a new peak has formed in the mock-ups at 251 cm
−1, noted in
Figure 10 by the area shaded blue. This peak remains unidentified, seemingly not associated with any of the previously identified degradation products of cadmium pigments (CdSO
4·xH
2O, CdCO
3, CdC
2O
4 or CdO).
4. Conclusions
Using complementary analytical techniques it was determined that the alteration observed for the restoration paint applied to the krater is the result of degradation of the cadmium orange (CdSSe) pigment. This conclusion is supported by the identification of selenium-rich needle-like structures of the trigonal Se
n form within the paint film after alteration occurs, as observed in both the altered paint from the krater and in the mock-ups created to study the cause of the alteration. Based on the behavior of the restoration paint on the ancient Greek krater and on the mock-ups, alteration occurs only in areas of acidified ceramic and in the presence of light. It is hypothesized that the residual chloride ions in the terracotta, resulting from either burial or incomplete treatment with HCl to remove burial accretions, and light exposure act as catalysts for the observed alteration. This is consistent with observations in the literature in which mobile chloride ions have been associated with promoting the oxidation of cadmium sulfide [
12] as well as making the pigment more sensitive to photo-oxidation [
5,
27].
Based on previous research it was anticipated that cadmium would form white (e.g., CdSO4, CdCO3) or brown (CdO) compounds, however, no cadmium-containing compounds were identifiable during this preliminary study. This research will hopefully be extended in the future to fully deduce the cadmium–containing degradation products and further investigate the role of acidity/pH in the alteration. Together, this will allow for a better understanding of the full mechanistic process in which the degradation of cadmium orange occurs.
For the re-treatment of the krater, the altered paint film was removed and non-cadmium containing pigments (Golden Fluid Acrylics cadmium red and yellow) used for in-painting after mock-ups determined them to be stable under the conditions tested during this study [
4]. This same treatment will be used in the future for other ceramics known, or suspected, to have been treated with hydrochloric acid.