1. Introduction
The cleaning of artistic surfaces is a tricky question, deeply felt by the operators in the conservation field. In general, cleaning means the removal of deposits of material not belonging to the artwork and accumulated over time, or the removal of degradation products of the original materials. The removal of degraded coatings from the surface of paintings, for example, is a wide field of study that involves many questions.
Free solvents applied with cotton swabs or small brushes are usually employed in the traditional cleaning operations, but these methods are not free of inconvenience, due to the penetration of liquid through the surface that may cause swelling of pictorial layers and leaching of the small molecules produced during aging processes. Leaching causes weakening and thinning of the pictorial layers, with the consequent undesired removal of original materials. Moreover, solvents can be retained in the inner layers and may be toxic for operators [
1,
2].
Since the 1980s, the use of polymer gels as thickeners was introduced with the aim of reducing and preventing solvent drawbacks [
3]. Polymer gels show chemical and physical properties that are attractive for applications such as conservation. In particular, the physical properties of gels are important for confining the cleaning to a specific area of the artwork and to prevent the penetration of the liquid phase into the deep layers. The range of physical properties spans from the rigid (e.g., agar or gellan) to the paste-like (e.g., Carbopol), passing through the viscoelastic systems [
4,
5,
6,
7,
8,
9,
10]. These last systems are conformable to the surface, like the paste-like materials, and show the easy removability and the absence of residues characteristic of rigid gels [
11].
Since the first study on polymer gels, many concerns have been raised about the issue of clearance and neutralization of residues [
12,
13]. The presence and the amount of possible gel residues, how they will react with the materials of the artwork, and how they will themselves change over time are all questions that must be addressed in the research [
14].
It is known that any cleaning method may leave residues after treatments, but their recognition is strongly dependent on the analytical procedure used for detection, as proved by studies where different techniques were used [
15,
16,
17,
18,
19].
Many soft materials have been studied for application in the cleaning of tangible cultural heritage. Poly (vinyl alcohol) (PVA) has received much attention as a gelling agent due to its capability of forming hydrogel systems, its ready availability, and its biodegradability. PVA in aqueous solution reacts with tetraborate ions giving a dynamic crosslinking known as di-diol complexation [
20,
21,
22,
23]. PVA-borate systems have suitable properties to be used for cleaning: they are transparent, stable, and easy to shape and to remove from the surface without the need of strong mechanical or chemical actions. Moreover, they can incorporate organic solvents miscible with water [
24,
25,
26,
27,
28]. In a previous study it was found that the addition of poly (ethylene oxide) (PEO) in proportions of up to 1% can improve mechanical properties modifying the viscoelastic behavior of the gel. Furthermore, the retention of the liquid phase composed by a mixture of water and acetone (70/30) was improved by the presence of PEO, and cleaning tests performed to remove a protective coating of Paraloid B72 from limestone specimens were successful [
29].
Some studies [
30] about the detection of residues after removal of PVA-borax hydrogels were already reported, but in-depth investigations with specific, sensitive, and selective analytical methods have not been performed yet.
In the present study, cleaning tests were carried out on mock-ups treated with PVA-borax gels containing acetone in the liquid phase in order to evaluate both the effectiveness of the removal of an aged varnish and the possible presence of gel residues after cleaning. Different formulations, with and without PEO, were compared. The cleaning efficiency was evaluated visually and by Fourier-transform infrared spectroscopy in the attenuated total reflectance (ATR-FTIR) mode. The analyses of the residues were carried out collecting ATR-FTIR spectra directly on the application area of the gel and by performing pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS) on tiny samples taken from the surfaces after the cleaning test. A Py-GC/MS procedure was specifically developed to detect traces of gel using the acquisition of mass spectra in single-ion-monitoring (SIM) mode. Only the characteristic m/z signals of the gel were registered in order to increase sensitivity and selectivity. Finally, some tests were carried out on real artworks made available by conservators collaborating in the research.
2. Materials and Methods
Poly(vinyl alcohol) (86–89% hydrolyzed, Mw 100,000, Fluka, Milan, Italy), poly(ethylene oxide) (Mw 35,000, Sigma-Aldrich, Milan, Italy), sodium tetraborate decahydrate (Sigma-Aldrich), 2-propanol (Sigma-Aldrich), and acetone (Sigma-Aldrich) were used as received. Gels were prepared with 3 wt.% of PVA and 0.6 wt.% borax according to other literature studies [
29,
30]. One formulation was prepared by adding 1% of PEO, which was found to be effective in improving mechanical properties of the gel and increasing the gel capability to retain the liquid phase [
29]. The formulations of the liquid phase consisted of a 70:30 deionized water/acetone mixture and a 85:15 deionized water/ethanol mixture (only used for tests on real artworks).
The preparation procedure was the same for all formulations: the proper amount of polymers was dissolved in deionized water at 70–80 °C under magnetic stirring until complete dissolution. Sodium tetraborate decahydrate (borax) was solubilized in deionized water at a concentration of 6 wt.%. After polymer dissolution and cooling, the borax solution was added under vigorous stirring until the final salt concentration. The gel forms instantaneously after the addition of borax. When a formulation includes the presence of a cosolvent, the latter is added after cooling of the polymer solution, to prevent evaporation. Formulations were left standing two days before use to allow elimination of any air bubbles.
Mock-ups for tests and evaluation of gel residues were prepared applying a layer of vinyl paint (Maimeri, Italy, with inorganic iron oxide pigment) on a medium-density fiber board (MDF) wood composite support, without priming. The paint layer was then coated with a dammar varnish and exposed to accelerated light aging for 850 h. The vinyl paint was preferred to oil paints because the latter would have required very long cross-linking time. An incomplete cross-linking makes the oil soluble in the liquid phase of the tested gels, introducing unwanted material into the samples to be used for the development of the analytical method. Moreover, it was decided to avoid the presence of polar compounds, such as the fatty acids present in oils, in order to carry out the analyses without derivatizing agents, which is better if the goal is to detect and identify markers of the polymeric gel. Finally, the presence of the vinyl paint, with chemical composition similar to the gel, allowed more severe testing of the analytical method with regard to selectivity, also verifying the possibility of distinguishing two different vinyl polymers.
Light aging was carried out in a solar box (Q-Lab Corporation, Bolton, UK) with a xenon lamp and Window Q filter (120,000 lux), simulating midday, summer sunlight streaming through a window.
Samples for testing the minimum detectable amount of gel in a matrix were prepared by mixing different aliquots of dammar varnish and gel (
Table 1). The samples were prepared by weighing an amount of dried varnish and the necessary quantity of hydrated gel on a suitable support. Dammar varnish was previously aged in a solar box for about 500 h. After completion of the gel drying, the samples consisting of the gel/varnish mixture were introduced into the quartz sample holder for Py-GC/MS analyses.
Dammar varnish was chosen as a matrix rather than the vinyl paint, because dammar is the material with which the gel comes into contact during cleaning. Moreover, a good intervention of varnish removal should not reach the paint layers, therefore during the sampling of the surface, it is more likely to find the varnish rather than the paint layer below.
Cleaning tests were performed by applying on the mock-ups a small amount of gel using a spatula. The gel was then covered with a glass slide to prevent solvent evaporation. After 2 min, the gel was removed with tweezers. All the tests were performed with and without clearing with cotton swabs, after the gel had been mechanically removed. Cotton swabs were soaked with hexane to prevent mechanical damage of the surface, which could occur if dry cotton swabs are used.
After cleaning tests, samples taken from the treated areas were analyzed by Py-GC/MS. Each sample was taken by scratching the surface with a knife on an area of 1 cm2.
For pyrolysis, a Pyroprobe 1500 (CDS Analytical, Oxford, PA, USA), directly connected with the 6890N Network GC System and 5973 Network MASS Selective Detector (Agilent Technologies, Wilmington, DE, USA), was used. The GC was equipped with a methylphenyl–polysiloxane cross-linked, 5% phenyl methyl silicone (30 m, 0.25 mm i.d., 0.25 µm film thickness) capillary column. The oven program was 50 °C (held for 2 min), ramp of 10 °C/min to 300 °C (held for 5 min.) The temperature of the pyrolysis chamber was 600 °C, and the temperature of the interface was 280 °C. The carrier gas was helium (1.0 mL/min), and the split ratio was 1/20 of the total flow. Mass spectra were recorded under electron impact at 70 eV. The interface was kept at 280 °C, ion source at 230 °C, and quadrupole mass analyzer at 150 °C. Mass spectra were collected in total ion counts (TIC) to characterize the materials and in single-ion monitoring (SIM), selecting the characteristic ions of the polymer gel. All instruments were controlled by Enhanced Chem Station (ver. 9.00.00.38) software. The mass spectra assignment was done with the NIST 2008 library and by comparison with literature data [
31].
ATR-FTIR spectra were also collected (FT-IR Nexus Thermo Nicolet, Madison, WI, USA) from the treated area of the mock-ups after cleaning tests. Measurements were performed with 32 scans, resolutions of 4 cm−1, and wavelength range from 500 to 4000 cm−1.
3. Results and Discussion
3.1. Setting up of the Analytical Procedure
In order to develop an effective analytical method to identify the gel residues on the surface of the treated mock-up samples, pyrograms of solid micro-samples taken from the treated surfaces and pyrograms of the gels were collected in TIC mode (
Figure S1, Supplementary Material) and characteristic markers were identified. The aim of this first part of the data analysis was to compare the mass spectra of the compounds characterizing the materials of interest (i.e., PVA gel, dammar varnish, vinyl paint) to decide which selective ions should be chosen for the analyses in SIM mode.
The comparative study of mass spectra allowed the identification of a series of ions (
Table 2) useful for recognizing most of the compounds characterizing the gels. Precisely, fragment ions with
m/z 41, 45, 59, 60, 70, 73, 81, and 106, were chosen as markers of the gel based on two main criteria: good signal abundance and exclusion of ions in common with other reference materials (i.e., dammar varnish and vinyl paint). These ions were then used for the analyses in SIM mode. Comparison between the reference gel pyrogram in TIC and in SIM (
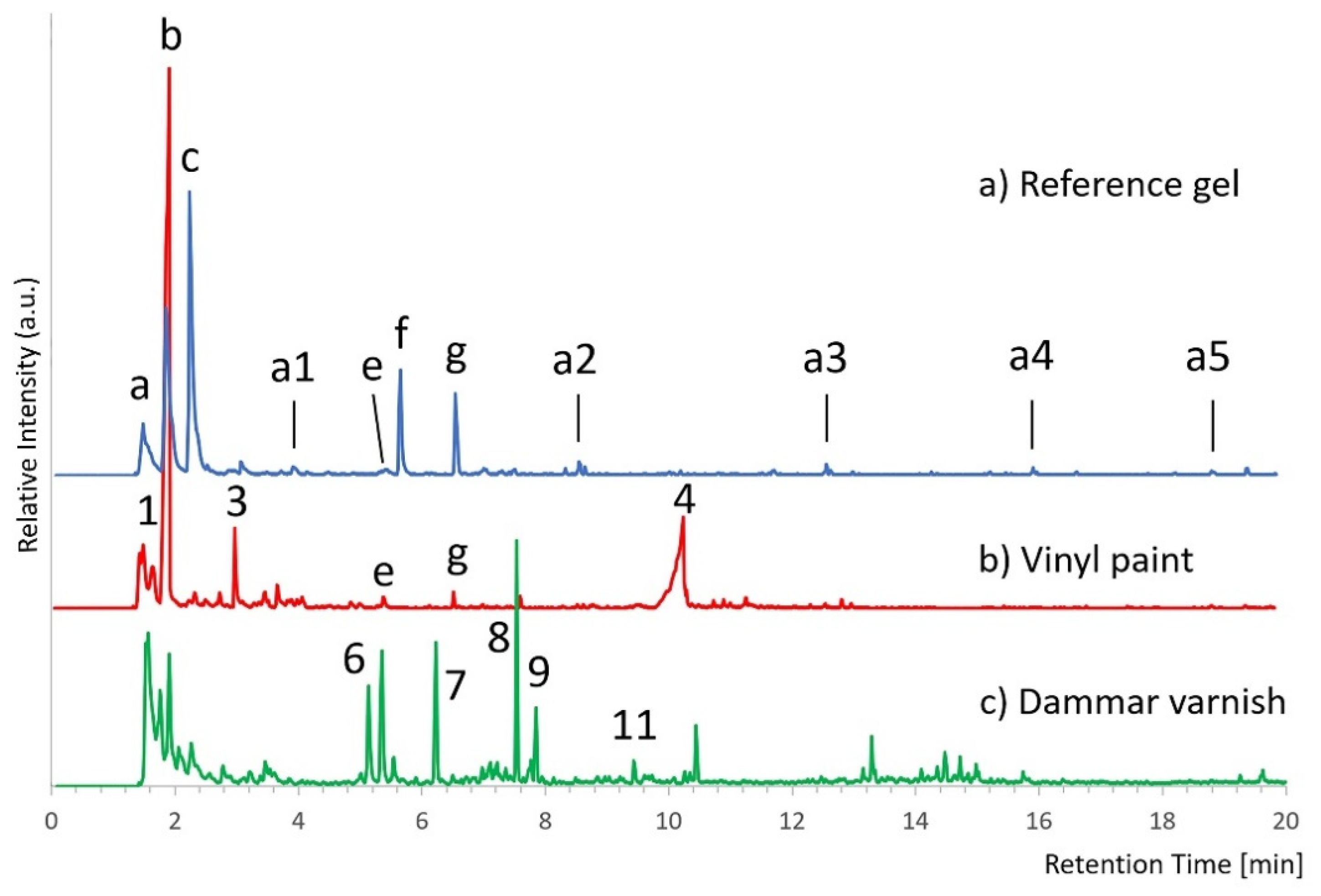
Figure S2, Supplementary Material) shows that the chosen fragment ions are enough to reconstruct the chromatographic profile of the gel. On the other hand, by comparing the SIM curves of gel, dammar varnish, and vinyl paint, it is possible to highlight the presence of some signals, in particular those resulting from the vinyl paint, that could interfere with the recognition of the gel (
Figure 1). Peaks b and g are, respectively, assigned to acetic acid and benzaldehyde and are pyrolysis products common to many vinyl polymers [
21].
In some cases, the analysis of partial mass spectra acquired in SIM allows to distinguish which are the actual signals deriving from the gel. For example, peak c (m/z 41, 70) present in the gel coelutes with a compound present in the dammar varnish, whose partial mass spectrum, however, shows the absence of the m/z 70 ion. Thus, in this case, the absence of the m/z 70 ion allows to exclude the presence of the gel.
3.2. Test of Minimum Detectable Amount
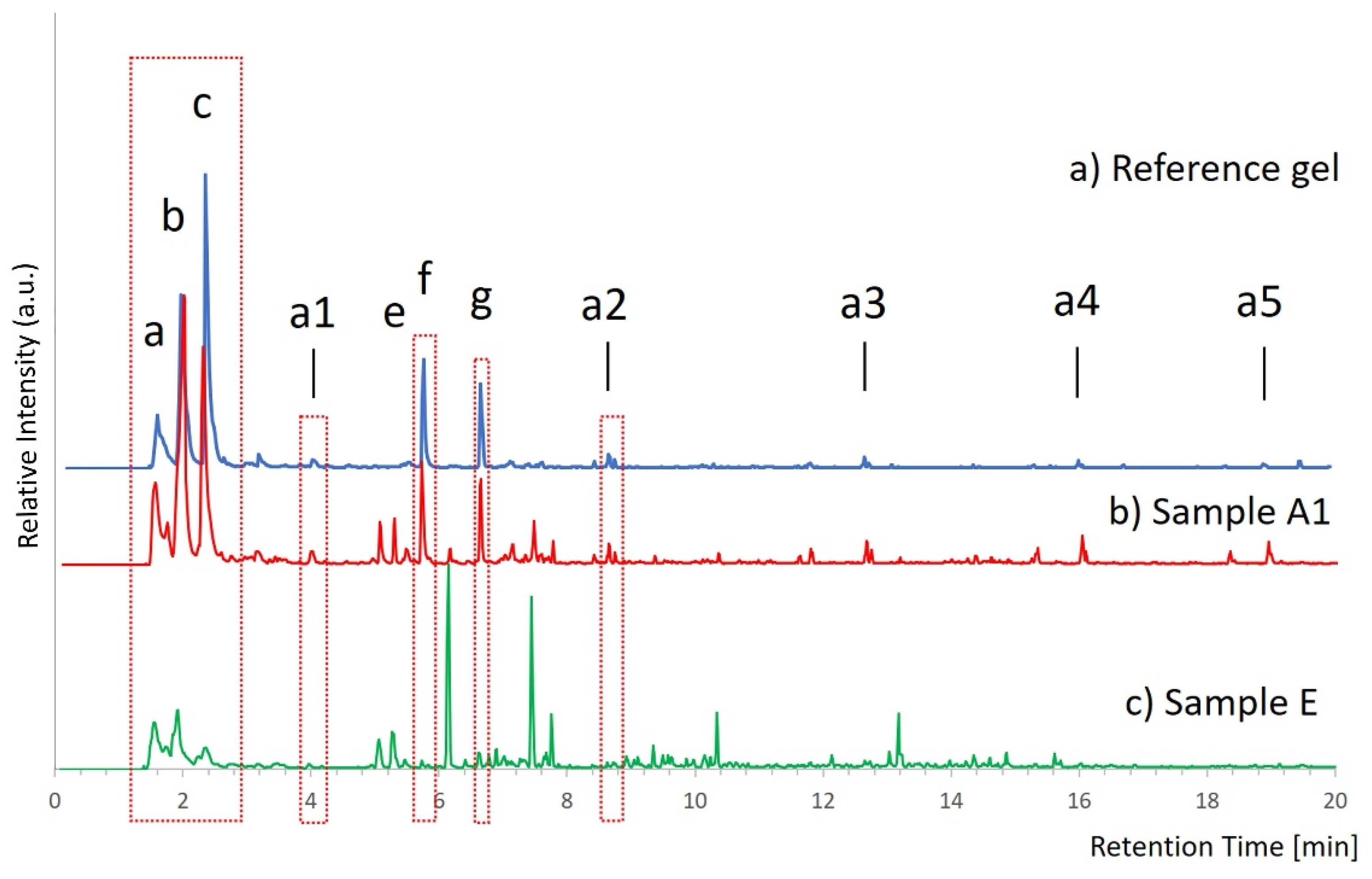
Powder mixtures with a known composition of dried gel and dammar were carefully weighed and analyzed in SIM mode to determine the minimum detectable amount of gel. The pyrograms of sample A (
Table 1), containing about 66% gel, and sample E (
Table 1), which contains about 0.2% gel, corresponding to 2 µg, are reported in
Figure 2. Sample E can be considered representative of any nonvisible residue that may remain on the surface of the artwork after the cleaning.
In sample A (
Figure 2b), the SIM profile of the gel is well recognizable, both with regard to the typical pyrolysis products of PVA, eluted in the first 10 min, and the pyrolysis products of PEO, present at a lower concentration in the gel.
As regards sample E (
Figure 2c), recognition of the characteristic markers of the gel is more difficult, since gel signals have a very low intensity compared to the peaks of the matrix, which constitute the major amount of the sample mass. The main characteristic peaks of the PVA gel are, however, easily identifiable by means of their partial mass spectra and allow detection of the presence of the gel. The signals derived from the PEO additive, eluted after 10 min, are not identifiable and, therefore, cannot be used as diagnostic peaks to detect gel traces. PEO signals are detectable only in the samples containing up to 0.1 mg of gel; therefore, where these signals are absent, it is possible to deduce that the gel amount is below 0.1 mg.
3.3. Cleaning Test and IR and Py-GC/MS Detection of Gel Residues
The gels, both with the addition of PEO and without, have shown good mechanical properties [
29]. They are neither too rigid nor too sticky, are easily spreadable on the surface, and easily removable by peeling. As already observed in previous studies [
25,
26,
27], the gel with PEO is easily moldable, which helps in the application phase, and maintains enough elasticity to ensure its removal. During a series of preliminary tests, it was observed that the gels tend to slide on the surface under slight mechanical stress. In the literature, two possible explanations are proposed for this phenomenon. According to the first theory, the gel solubilizes the surface causing the formation of a liquid layer between the gel and the surface that causes the sliding. The second possible cause of the sliding could be a lowering of the pH due to the acidic nature of the aged paint. The acidic environment determines a shift in the balance of the tetraborate ion responsible for the crosslinking of the gel, so that the gel in contact with the surface becomes liquid [
30].
For cleaning tests, both the gel containing PEO and the gel without PEO were used. The duration of the application, 2 min, was chosen on the basis of preliminary tests and is the best compromise between the need to remove the varnish and to preserve the paint layer underneath. At first, the gel was only mechanically removed after application, without any clearing of the surface. After the gel removal, it was observed that the surface treated with the PEO-added gel dries more quickly and does not leave a whitening halo, instead observed in the gel without PEO. The white halo is probably due to a certain amount of water and/or solvent that remains in the partially solubilized varnish.
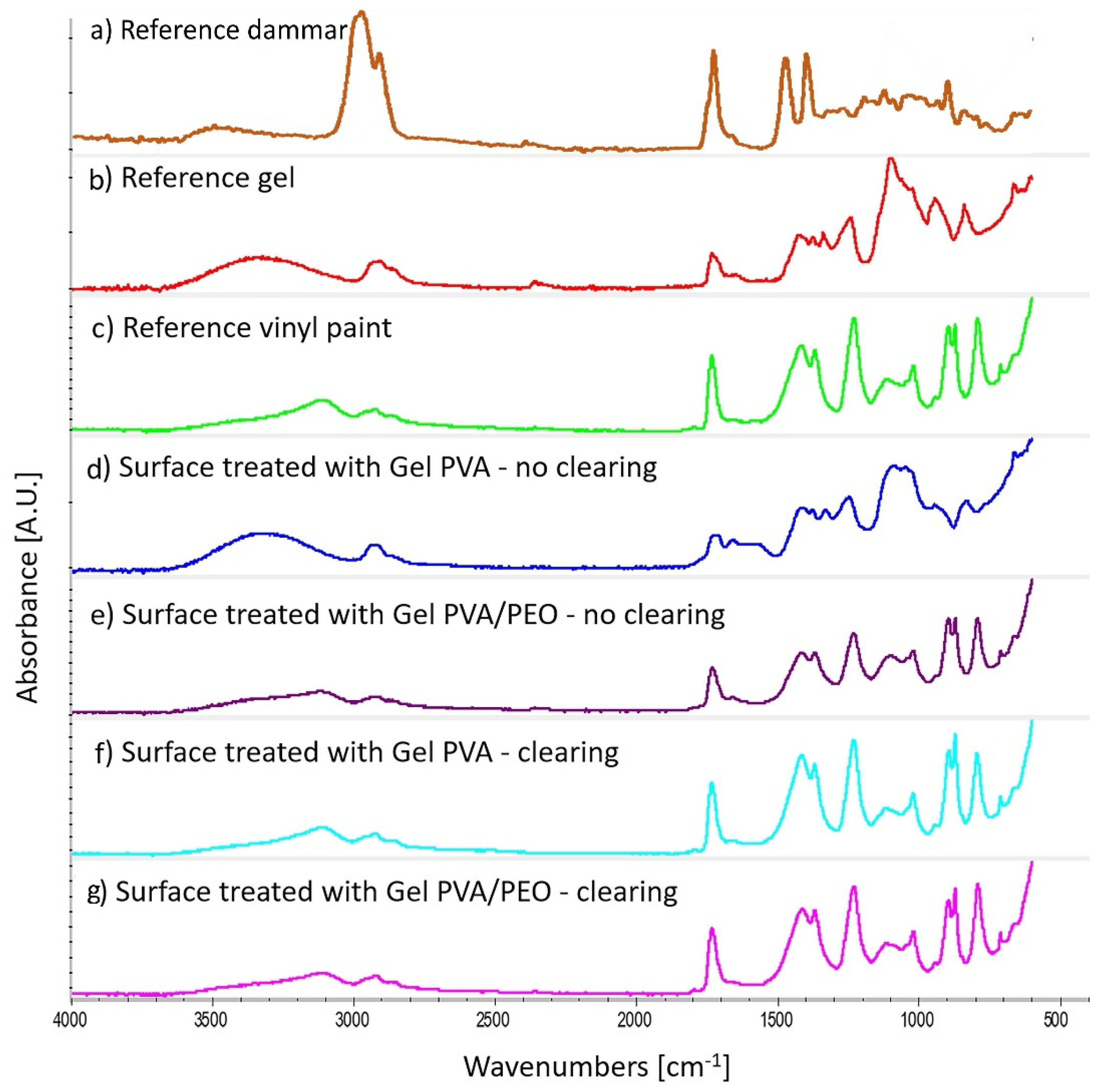
The ATR spectra acquired on the mock-up surfaces (
Figure 3) show that the varnish was completely removed by the gel with PEO. In fact, the IR spectrum of the mock-up sample treated with the gel with PEO (
Figure 3e,g) is the same of the underlying vinyl paint and no signals are observed due to the presence of the gel and of the dammar varnish.
The area treated with the gel without PEO (
Figure 3d) shows an FTIR spectrum nearly superimposable to that of the reference gel, showing how a gel residue is actually present after the removal of the gel and without clearing. Only after clearing, the surface shows the FTIR spectrum of the vinyl paint (
Figure 3f). This confirms that PVA gels containing PEO adhere less to the surface and are easier to remove than gels without PEO.
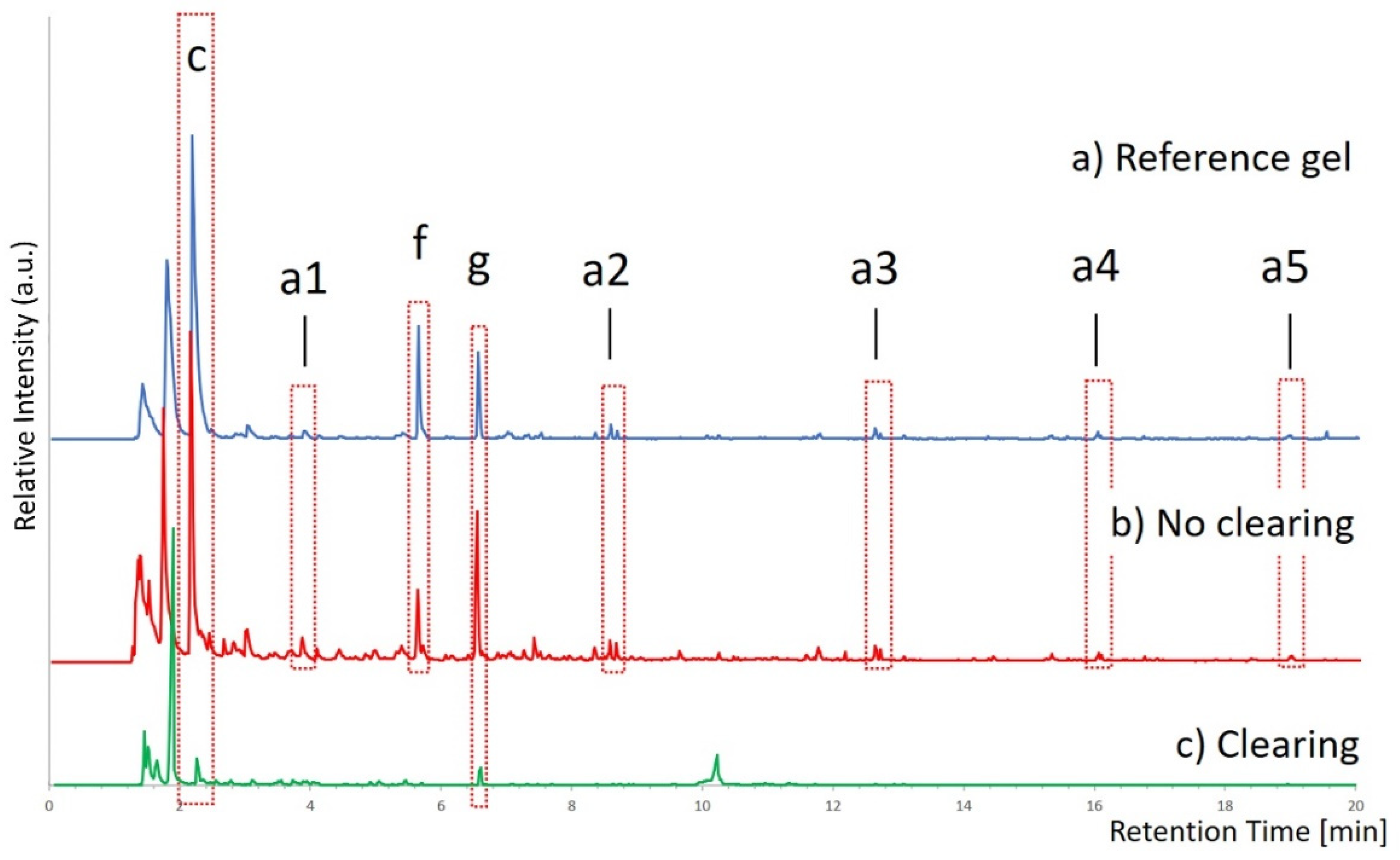
The Py-GC/MS analysis in SIM mode of a sample collected from the mock-up surface treated with the PEO-added gel, apparently free of gel traces based on the results of the FTIR measurement, shows the characteristic signals of the gel (
Figure 4b). PEO signals are also clearly visible (peaks a2–a5) confirming that a significant amount of gel is still present on the mock-up surface, surely higher than the minimum detectable quantity (see the previous paragraph on the detection limit of the Py-GC/MS method).
The same analyses were carried out also after clearing the gel-treated surface, using a cotton swab soaked in hexane to remove any gel residues. Hexane was chosen instead of other apolar solvents more frequently used by restorers, for example white spirit, because hexane is more volatile and, therefore, interferes less with the analyses.
As done previously, also after the hexane clearing, the mock-up surface was preliminarily analyzed by ATR-FTIR. The spectra collected after the hexane clearing (
Figure 3f) are the same as those of the vinyl paint, without traces of gel and dammar varnish.
Subsequently the surfaces were sampled and analyzed by Py-GC/MS in SIM mode.
Figure 4c shows a comparison between the pyrogram of the area treated with the gel containing PEO and cleaned with hexane, and the reference pyrograms of the gel. Unlike the tests performed without clearing, it is much more difficult to recognize a chromatographic pattern comparable to that of the gel. Peaks c and g, present only in the gel and, therefore, indicative of its presence, can be identified only after selective comparison of their partial mass spectra. The detected signals are comparable with those present in sample E (
Table 1), containing about 0.02 mg of gel, and prove the presence of micro-traces of gel residues on the surface of the treated mock-up sample. These residues cannot be seen by the naked eye nor are they detectable by FTIR spectroscopy.
3.4. Case Study on Real Samples
In order to assess the applicability of the proposed procedure, it was applied to two case studies. The first case study is the portrait of a noblewoman, painted by an unknown author in Piedmont (Italy) and belonging to a private collection (
Figure 5). The portrait appeared to be in good condition except for the paint, which appeared to be visibly aged and yellowed. This involved an alteration of the chromatic perception of the entire painting, making it impossible to appreciate the original saturation of the colors. The restoration was aimed at cleaning the painting and replacing the yellowed varnish.
Cleaning tests with a PVA/PEO gel containing 30% of acetone in the liquid phase were performed on the area of the yellow sleeve of the woman’s dress.
The gel was applied using a spatula and was left to work for different times covered by a laboratory slide to minimize the evaporation of the solvent. Once the defined time has elapsed, the gel was removed from the treated surface using tweezers and was cleared with hexane using cotton swabs to remove the solubilized paint and possible gel residues.
In this case, since it was not possible to take a solid sample from the artwork surface, the procedure for the detection of gel residue was applied to the hot water extract of the swabs used for the clearing with hexane. After clearing, the cotton swabs were placed in vials with 3 mL of deionized water and heated to 80 °C for one hour to facilitate the dissolution of the gel possibly collected. After removing the cotton, the vials were left open to evaporate the liquid at room temperature to approximately 0.5 mL of residual solution. Then this solution was placed on a microscopy slide and left at room temperature until completely dry.
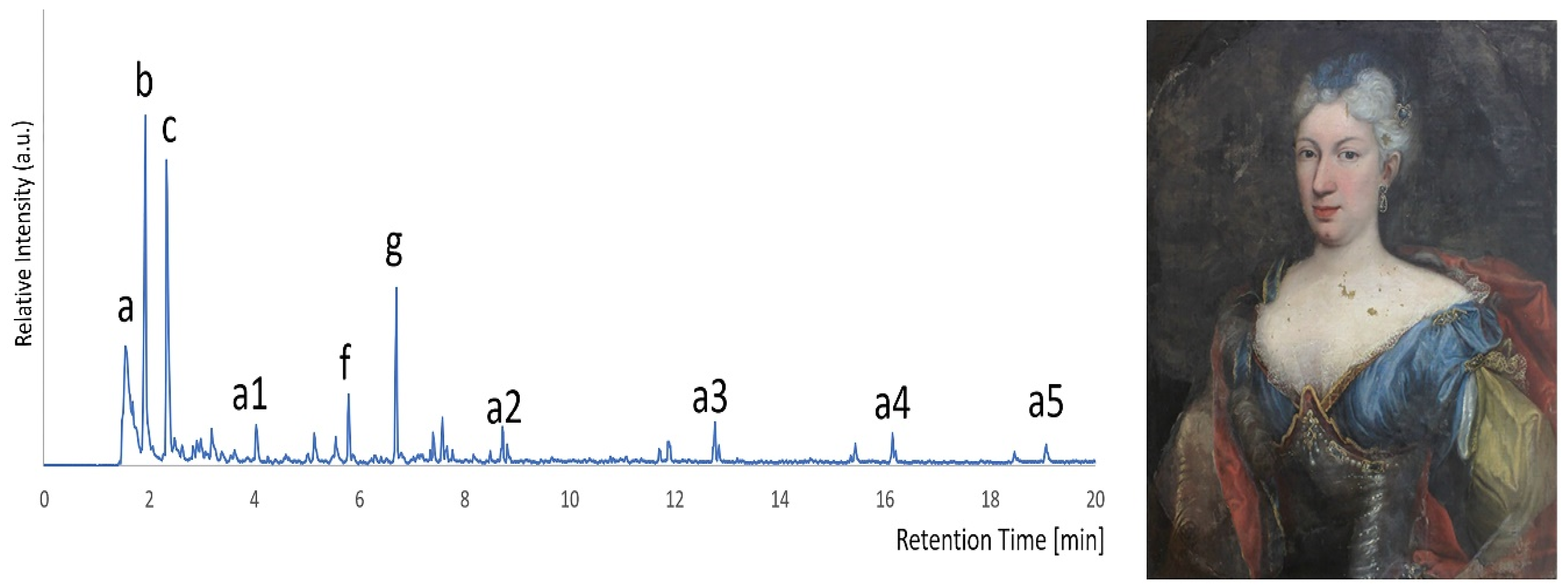
In all the analyzed extracts from cotton swabs that had been used on surfaces left in contact with the gel for longer application time (i.e., 4 min), the signals of the gel were detected. The corresponding pyrogram, shown in
Figure 5, shows many of the peaks present in the reference gel.
At retention time higher than 10 min, the peaks derived from PEO are also evident, indicating that the quantity of gel residues collected by the cotton swab is considerable.
The second case study refers to the restoration of a large painting belonging to an altarpiece of the church of S. Filippo Neri in Biella (Italy), and representing the “Madonna and Child with Angels and the Saints Francesco di Sales and Carlo Borromeo”, painted by Rocco Comaneddi in 1792 (
Figure 6). The work is an oil on canvas of 365 × 200 cm, with a diterpene resin varnish.
During the first phase of the restoration, restorers found that the most suitable solvent for removing the paint was ethanol. The gel used for the cleaning tests was a PVA/PEO gel with 15% ethanol in the liquid phase. Gels were applied for different application times (single application of 2, 3, 4, and 5 min and an application of 2.5 min repeated twice). The tests were carried out both without and with clearing, using swabs soaked in white spirit (
Figure S3).
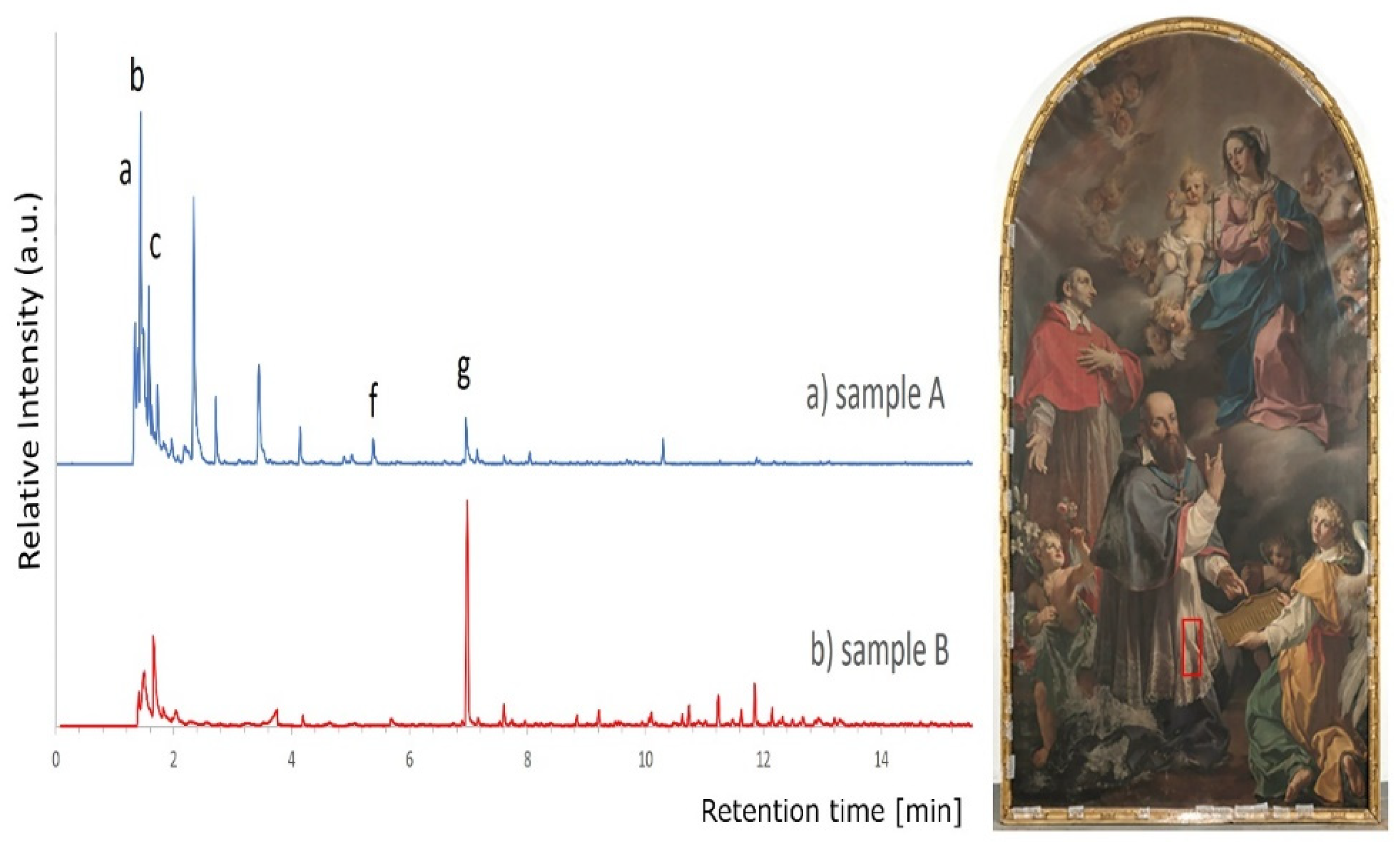
Independently from the application time of the gel, the removal of the varnish was incomplete, proving that the cleaning was rather bland and that it required repeated applications of the gel to completely remove the varnish. This observation, which could be negative from the point of view of the time required for cleaning, especially on a canvas of this size, is instead positive when looking for a cleaning approach that is not too aggressive and does not damage with a single application the surface of the underlying pictorial layers. In addition to cleaning tests, it was also possible to collect micro-samples in the areas treated with gels without and with subsequent clearing. Being very small samples and difficult to handle, they were analyzed directly by Py-GC/MS, to reduce the risk of losing them and having to increase the number of micro-samples to be taken on the painting. The samples were analyzed using the Py-GC/MS method in SIM mode and allowed to detect traces of the gel on the uncleared area (
Figure 6a), while in the area subject to clearing with white spirit no gel signals were detected (
Figure 6b).
4. Conclusions
A Py-GC/MS analytical procedure for the identification of gel residues on paintings after cleaning treatments was developed. The acquisition of mass spectra in SIM mode increases the sensitivity and selectivity of the method, allowing small quantities of material to be identified and the gel signals to be distinguished unequivocally.
The analyses carried out on mixtures of known composition containing different quantities of gel and dammar varnish allowed to establish that the minimum detectable amount of gel is about 2 µg. This quantity is not detectable with the naked eye nor with FTIR spectroscopy.
Analyses performed on gel/varnish mixtures also allowed to determine that when PEO signals are detectable, the amount of gel is at least 0.1 mg. The Py-GC/MS analysis in SIM mode was therefore much more sensitive than FTIR, detecting traces of gels even where IR-related signals did not appear.
The selectivity of the method has proved to be excellent, since it has allowed the detection of the gel in minimal quantities (i.e., 2 µg), even in the presence of a complex matrix, such as the dammar varnish and the vinyl color, which is chemically comparable to the polymeric components of the gel.
The gel containing PEO is effective in removing the aged varnish and leaves less residues than the gel without PEO. However, Py-GC/MS measurements in SIM mode have shown that without a proper clearing operation with apolar solvents, significant quantities of gel remain on the surface. Since the PEO signals have always been identified on the surfaces of the mock-ups treated without clearing, for comparison with the analyses of reference mixtures it is possible to conclude that the amount of gel residue is always higher than 0.1 mg, a quantity about 50 times higher than the minimum detectable amount.
Cleaning tests performed on real artworks showed that, in absence of clearing, it is possible that a certain amount of gel remains on the surface. In the case of the PVA/PEO gel with acetone used on the portrait of a Piedmontese noblewoman, the swabs used for clearing contained clear traces of gel, which if not removed would then remain on the surface. Furthermore, in the case of the altarpiece, in which the PVA/PEO gel contained ethyl alcohol as a co-solvent, the sample collected from the surface treated with the gel but without subsequent clearing showed traces of gel.
Overall, the results obtained show that it is necessary to clear the surfaces with organic solvents to obtain a surface that is as free as possible from gel residues. It is also evident that the possibility of having gel residues depends not only on the type of gel and the solvents it contains but also on the type and state of conservation of the surface on which the gel is applied. Importantly, the strategy proposed for the identification of gel residues can be applied in situations other than that here described (i.e., vinyl paint with dammar paint), taking care to exclude from the list of characteristic fragment ions of the gel, any ions in common with the specific binder or varnish of the painting.